Anxiolytic/Sedative Effect of Monoterpene (–)-Borneol in Mice and In Silico Molecular Interaction with GABAA Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Elevated Plus Maze Test
2.4. Open Field Test
2.5. Light-Dark Box Test
2.6. Thiopental Sodium Induced Sleeping Time Test
2.7. Statistical Analysis
2.8. Molecular Docking
3. Results
3.1. Elevated Plus Maze Test
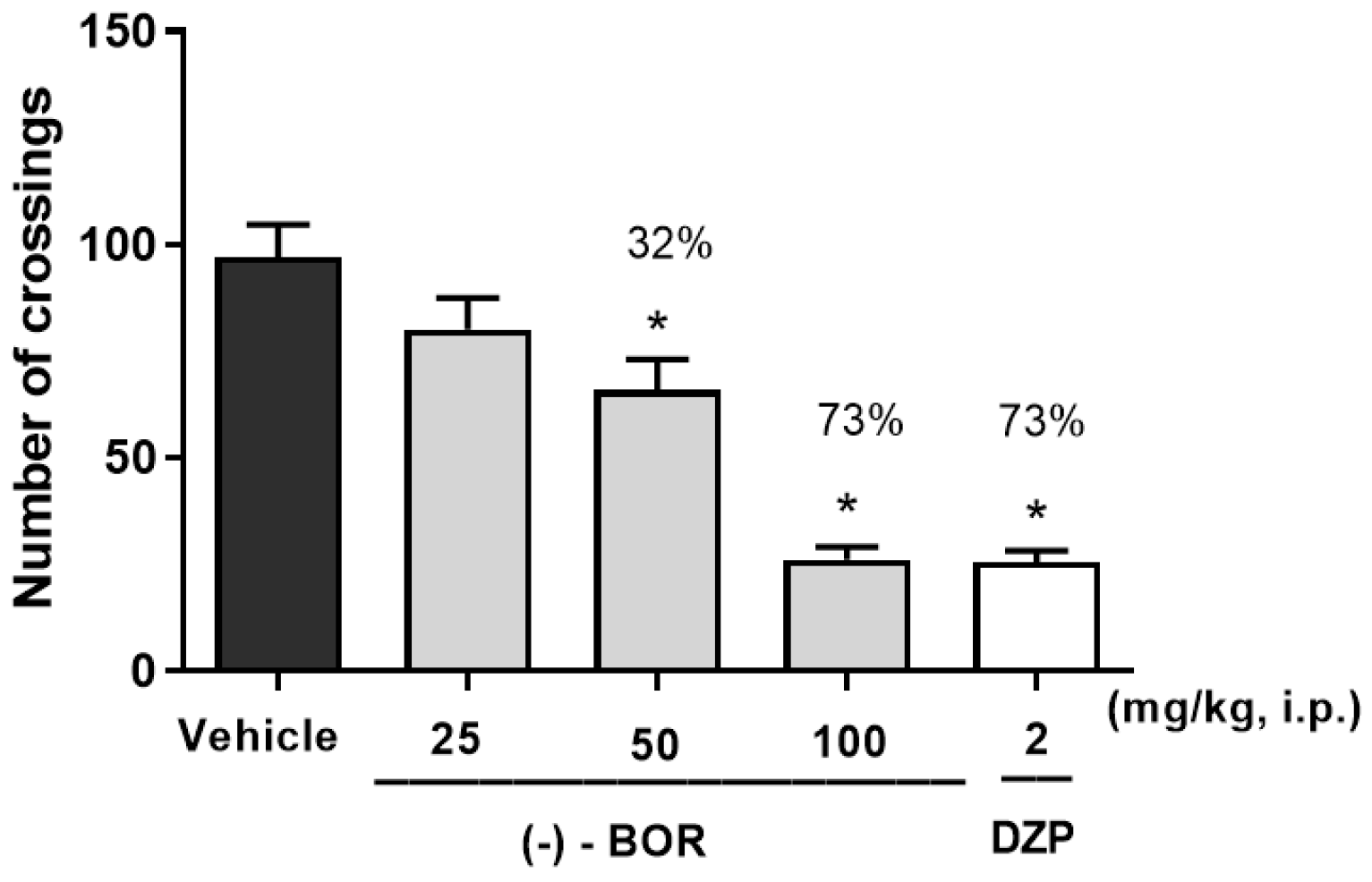
3.2. Open Field Testing
3.3. Light-Dark Box Test
3.4. Thiopental Sodium Induced Sleeping Time Test
3.5. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagatin, M.C.; Tozatti, C.S.S.; Abiko, L.A.; Yamazaki, D.A.S.; Silva, P.R.A.; Perego, L.M.; Audi, E.A.; Seixas, F.A.V.; Basso, E.A.; De Freitas Gauze, G. Molecular Docking and Panicolytic Effect of 8-Prenylnaringenin in the Elevated T-Maze. Pharm. Soc. Japan. 2014, 62, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Doukkali, Z.; Taghzouti, K.; Bouidida, E.L.H.; Nadjmouddine, M.; Cherrah, Y.; Alaoui, K. Evaluation of anxiolytic activity of methanolic extract of Urtica urens in a mice model. Behav. Brain Funct. 2015, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, N.E.; Barrow, J.C. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem. Neurosci. 2014, 5, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Liebrenz, M.; Gehring, M.-T.; Buadze, A.; Caflisch, C. High-dose benzodiazepine dependence: A qualitative study of patients’ perception on cessation and withdrawal. BMC Psychiatry 2015, 15, 116. [Google Scholar] [CrossRef]
- Zwenger, S.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol Rev. 2008, 3, 1–7. [Google Scholar]
- Cheng, A.-X.; Lou, Y.-G.; Mao, Y.-B.; Lu, S.; Wang, L.-J.; Chen, X.-Y. Plant Terpenoids: Biosynthesis and Ecological Functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Daglia, M.; Jafari, S. Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol. Rep. 2016, 68, 671–679. [Google Scholar] [CrossRef]
- Chen, B.T.; Hopf, F.W.; Bonci, A. Synaptic plasticity in the mesolimbic system. Ann. N. Y. Acad. Sci. 2010, 1187, 129–139. [Google Scholar] [CrossRef]
- Granger, R.E.; Campbell, E.L.; Johnston, G.A.R. (+)- And (–)-borneol: Efficacious positive modulators of GABA action at human recombinant GABAA receptors. Biochem. Pharmacol. 2005, 69, 1101–1111. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.; Guimarães, A.; Araújo, B.; Oliveira, G.; Santana, M.; Moreira, F.; Santos, M.; Cavalcanti, S.; Júnior, W.L.; Botelho, M.; et al. Carvacrol, borneol and citral reduce convulsant activity in rodents. Afr. J. Biotechnol. 2010, 9, 6566–6572. [Google Scholar]
- Silva-Filho, J.C.; Oliveira, N.N.P.M.; Arcanjo, D.D.R.; Quintans, L.; Cavalcanti, S.C.H.; Santos, M.R.V.; Oliveira, R.D.C.M.; Oliveira, A. Investigation of Mechanisms Involved in (−)-Borneol-Induced Vasorelaxant Response on Rat Thoracic Aorta. Basic Clin. Pharmacol. Toxicol. 2011, 110, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Sherkheli, M.A.; Schreiner, B.; Haq, R.U.; Werner, M.; Hatt, H. Borneol inhibits TRPA1, a proinflammatory and noxious pain-sensing cation channel. Pak. J. Pharm. Sci. 2015, 28, 1357–1363. [Google Scholar] [PubMed]
- El Ganouni, S.; Tazi, A.; Hakkou, F. Potential serotonergic interactions with the anxiolytic-like effects of calcium channel antagonists. Pharmacol. Biochem. Behav. 1998, 60, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Rev. Drug Discov. 2016, 15, 19–34. [Google Scholar] [CrossRef]
- Bergmann, R.; Kongsbak, K.; Sørensen, P.L.; Sander, T.; Balle, T. A Unified Model of the GABAA Receptor Comprising Agonist and Benzodiazepine Binding Sites. PLoS ONE 2013, 8, e52323. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus maze to measure anxiey in the mouse. Psychopharmacology 1987, 25, 180–185. [Google Scholar]
- Archer, J. Tests for emotionality in rats and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Crawley, J.N. Neuropharmacologic specificity of a simple model for the behavioural actions of benzodiazepines. Pharmacol. Biochem. Behav. 1981, 15, 695–699. [Google Scholar] [CrossRef]
- Carlini, E.; Contar, J.D.D.; Silva-Filho, A.R.; Da Silveira-Filho, N.G.; Frochtengarten, M.L.; Bueno, O.F. Pharmacology of lemongrass (Cymbopogon citratus Stapf). I. Effects of teas prepared from the leaves on laboratory animals. J. Ethnopharmacol. 1986, 17, 37–64. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Capelle, K. A bird’s-eye view of density-functional theory. Braz. J. Phys. 2006, 36, 1318–1343. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Combs, H.; Markman, J. Anxiety Disorders in Primary Care. Med. Clin. N. Am. 2014, 98, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; Silva, O.A.; De Almeida, A.A.C.; Cerqueira, G.S.; De Sousa, D.P.; De Freitas, R.M. Anxiolytic-like effects of carvacryl acetate, a derivative of carvacrol, in mice. Pharmacol. Biochem. Behav. 2013, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Souto-Maior, F.N.; De Carvalho, F.L.; De Morais, L.C.S.L.; Netto, S.M.; De Sousa, D.P.; De Almeida, R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.; Hanrahan, J. Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus Officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef]
- Moniruzzaman; Bhattacharjee, P.S.; Pretty, M.R.; Hossain, S. Sedative and Anxiolytic-Like Actions of Ethanol Extract of Leaves ofGlinus oppositifolius (Linn.) Aug. DC. Evid.-Based Complement. Altern. Med. 2016, 2016, 2565320. [Google Scholar] [CrossRef]
- Rodgers, R.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef]
- Colla, A.R.; Rosa, J.M.; Cunha, M.P.; Rodrigues, A.L.S. Anxiolytic-like effects of ursolic acid in mice. Eur. J. Pharmacol. 2015, 758, 171–176. [Google Scholar] [CrossRef]
- Bourin, M. Animal models for screening anxiolytic-like drugs: A perspective. Dialogues Clin. Neurosci. 2015, 17, 295–303. [Google Scholar] [CrossRef]
- Silva, M.I.G.; Neto, M.R.D.A.; Neto, P.F.T.; Moura, B.A.; Amaral, J.F.D.; De Sousa, D.P.; Vasconcelos, S.M.M.; De Sousa, F.C.F. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol. Biochem. Behav. 2007, 88, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaca, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal models of anxiety disorders and stress. Rev. Bras. Psiquiatr. 2013, 35, S101–S111. [Google Scholar] [CrossRef] [PubMed]
- Aman, U.; Subhan, F.; Shahid, M.; Akbar, S.; Ahmad, N.; Ali, G.; Fawad, K.; Sewell, R.D.E. Passiflora incarnata attenuation of neuropathic allodynia and vulvodynia apropos GABA-ergic and opioidergic antinociceptive and behavioural mechanisms. BMC Complement. Altern. Med. 2016, 16, 77. [Google Scholar] [CrossRef]
- Hussin, A.T.; Fraser, L.M.; Ramos, A.; Brown, R.E. The effect of chlordiazepoxide on measures of activity and anxiety in Swiss-Webster mice in the triple test. Neuropharmacology 2012, 63, 883–889. [Google Scholar] [CrossRef]
- Islam, N.U.; Khan, I.; Rauf, A.; Muhammad, N.; Shahid, M.; Shah, M.R. Antinociceptive, muscle relaxant and sedative activities of gold nanoparticles generated by methanolic extract of Euphorbia milii. BMC Complement. Altern. Med. 2015, 15, 6–11. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, J.B.I.; Lee, H.L.; Yoon, S.Y.; Kim, G.H.; Lee, Y.S.; Cheong, J.H. The involvement of magnoflorine in the sedative and anxiolytic effects of Sinomeni Caulis et Rhizoma in mice. J. Nat. Med. 2013, 67, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Ci, S.; Ren, T.; Su, Z. Investigating the Putative Binding-mode of GABA and Diazepam within GABAA Receptor Using Molecular Modeling. Protein J. 2008, 27, 71–78. [Google Scholar] [CrossRef]
- Raihan, O.; Habib, R.; Brishti, A.; Rahman, M.; Saleheen, M.; Manna, M. Sedative and anxiolytic effects of the methanolic extract of Leea indica (Burm. f.) Merr. leaf. Drug Discov. Ther. 2011, 5, 185–189. [Google Scholar] [CrossRef]
- Mula, M. Using anxiolytics in epilepsy: Neurobiological, neuropharmacological and clinical aspects. Epileptic Disord. 2016, 18, 217–227. [Google Scholar] [CrossRef]
- Richter, L.; De Graaf, C.; Sieghart, W.; Varagic, Z.; Mörzinger, M.; De Esch, I.; Ecker, G. t UKPMC Funders Group benzodiazepine binding-site ligands. Nat. Chem Biol. 2012, 8, 455–464. [Google Scholar] [CrossRef]
- Benke, D.; Barberis, A.; Kopp, S.; Altmann, K.-H.; Schubiger, M.; Vogt, K.E.; Rudolph, U.; Möhler, H. GABAA receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology 2009, 56, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.C.; Venâncio, E.T.; De Sousa, D.P.; De Franca Fonteles, M.M.; De Vasconcelos, S.M.M.; Viana, G.S.B.; De Sousa, F.C.F. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: Involvement with GABAergic transmission. Fundam Clin. Pharmacol. 2010, 24, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Chebib, M.; Johnston, G.A.R. GABA-activated ligand gated ion channels: Medicinal chemistry and molecular biology. J. Med. Chem. 2000, 20, 1427–1447. [Google Scholar] [CrossRef] [PubMed]






| Conformation | Binding Free Energy (Kcal/mol) | Inhibitory Constant Ki (μM) | GABAAR Amino Acid Residues | Binding between (–)-BOR and Val50 | Energy (Kcal/mol) | Length (Å) |
|---|---|---|---|---|---|---|
| 1 | −4.96 | 231.31 | Lys274, Val50, Gln185, Phe186, Met49 | Hydrogen bridge | 1.41 | 2.045 |
| 2 | −4.96 | 230.41 | Lys274, Val50, Gln185, Phe186, Met49 | Hydrogen bridge | −1.43 | 2.056 |
| 3 | −4.89 | 259.46 | Val50, Met 49, Pro184, Gln185, Lys274 | Hydrogen bridge | −1.72 | 1.915 |
| Molecule | Linking Subunit | Amino Acid Residue | References |
|---|---|---|---|
| (-)-BOR | ___-__ | Lys274, Val50, Gln185, Phe186, Met49 | This work |
| DZP | α1/γ2 | Lys105, Tyr160, Tyr210, Val212/ Phe77 | (Ci et al., 2008; Bergmann et al., 2013) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, M.P.d.M.d.; Silva Junior, M.P.d.; Lima, F.d.C.A.; Gutierrez, S.J.C.; Arcanjo, D.D.R.; Oliveira, R.d.C.M. Anxiolytic/Sedative Effect of Monoterpene (–)-Borneol in Mice and In Silico Molecular Interaction with GABAA Receptor. Future Pharmacol. 2023, 3, 132-141. https://doi.org/10.3390/futurepharmacol3010009
Amaral MPdMd, Silva Junior MPd, Lima FdCA, Gutierrez SJC, Arcanjo DDR, Oliveira RdCM. Anxiolytic/Sedative Effect of Monoterpene (–)-Borneol in Mice and In Silico Molecular Interaction with GABAA Receptor. Future Pharmacology. 2023; 3(1):132-141. https://doi.org/10.3390/futurepharmacol3010009
Chicago/Turabian StyleAmaral, Maurício Pires de Moura do, Marcelo Pereira da Silva Junior, Francisco das Chagas Alves Lima, Stanley Juan Chavez Gutierrez, Daniel Dias Rufino Arcanjo, and Rita de Cássia Meneses Oliveira. 2023. "Anxiolytic/Sedative Effect of Monoterpene (–)-Borneol in Mice and In Silico Molecular Interaction with GABAA Receptor" Future Pharmacology 3, no. 1: 132-141. https://doi.org/10.3390/futurepharmacol3010009
APA StyleAmaral, M. P. d. M. d., Silva Junior, M. P. d., Lima, F. d. C. A., Gutierrez, S. J. C., Arcanjo, D. D. R., & Oliveira, R. d. C. M. (2023). Anxiolytic/Sedative Effect of Monoterpene (–)-Borneol in Mice and In Silico Molecular Interaction with GABAA Receptor. Future Pharmacology, 3(1), 132-141. https://doi.org/10.3390/futurepharmacol3010009







