Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus
Abstract
1. Introduction
2. Bioactive Flavonoids from Citrus Fruit Peels
2.1. Flavanones
2.1.1. Didymin
2.1.2. Eriocitrin (Eriomin)
2.1.3. Hesperidin
2.1.4. Naringenin
2.1.5. Naringin
2.1.6. Narirutin
2.1.7. Neohesperidin
2.2. Flavones
2.2.1. Diosmetin
2.2.2. Diosmin
2.2.3. Nobiletin
2.2.4. Sinensetin
2.2.5. Sudachitin
2.2.6. Tangeretin
2.2.7. 3′,4′,3,5,6,7,8Heptamethoxyflavone
2.2.8. 5-Demethylnobiletin
2.2.9. 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone
2.3. Other Flavonoids
2.3.1. Anthocyanins
2.3.2. Cigranoside C, D, E, F
2.3.3. Quercetin
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Singhal, N.; Khurana, L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: Role of dietary fats and oils. J. Am. Coll. Nutr. 2010, 29, 289S–301S. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Xu, J.; Liao, K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J. Biol. Chem. 2004, 279, 35914–35922. [Google Scholar] [CrossRef]
- Giri, S.; Rattan, R.; Haq, E.; Khan, M.; Yasmin, R.; Won, J.S.; Key, L.; Singh, A.K.; Singh, I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr. Metab. 2006, 3, 31. [Google Scholar] [CrossRef]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef]
- Han, H.Y.; Lee, S.K.; Choi, B.K.; Lee, D.R.; Lee, H.J.; Kim, T.W. Preventive Effect of Citrus aurantium Peel Extract on High-Fat Diet-Induced Non-alcoholic Fatty Liver in Mice. Biol. Pharm. Bull. 2019, 42, 255–260. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, H.; Ishida, A.; Watanabe, W.; Narumi, K.; Atsumi, T.; Sugita, C.; Kurokawa, M. Naringenin suppresses macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity. Biochem. Biophys. Res. Commun. 2014, 454, 95–101. [Google Scholar] [CrossRef]
- Asyifah, M.R.; Lu, K.; Ting, H.L.; Zhang, D. Hidden potential of tropical fruit waste components as a useful source of remedy for obesity. J. Agric. Food Chem. 2014, 62, 3505–3516. [Google Scholar] [CrossRef]
- Lu, K.; Han, M.; Ting, H.L.; Liu, Z.; Zhang, D. Scutellarin from Scutellaria baicalensis suppresses adipogenesis by upregulating PPARalpha in 3T3-L1 cells. J. Nat. Prod. 2013, 76, 672–678. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Rashid, A.M.; Lu, K.; Yip, Y.M.; Zhang, D. Averrhoa carambola L. peel extract suppresses adipocyte differentiation in 3T3-L1 cells. Food Funct. 2016, 7, 881–892. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Roowi, S.; Crozier, A. Flavonoids in tropical citrus species. J. Agric. Food Chem. 2011, 59, 12217–12225. [Google Scholar] [CrossRef]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Prevention of Obesity and Type 2 Diabetes with Aged Citrus Peel (Chenpi) Extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Kundusen, S.; Haldar, P.K.; Gupta, M.; Mazumder, U.K.; Saha, P.; Bala, A.; Bhattacharya, S.; Kar, B. Evaluation of Antihyperglycemic Activity of Citrus limetta Fruit Peel in Streptozotocin-Induced Diabetic Rats. ISRN Endocrinol. 2011, 2011, 869273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, J.; Zhang, X.; Zhao, D.G.; Ma, Y.Y.; Li, D.; Ho, C.T.; Huang, Q. Aged citrus peel (chenpi) extract causes dynamic alteration of colonic microbiota in high-fat diet induced obese mice. Food Funct. 2020, 11, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, B.; Malfa, G.A.; La Mantia, A.; Miceli, N.; Sferrazzo, G.; Taviano, M.F.; Di Giacomo, C.; Renis, M.; Acquaviva, R. Anti-adipogenic and anti-oxidant effects of a standardised extract of Moro blood oranges (Citrus sinensis (L.) Osbeck) during adipocyte differentiation of 3T3-L1 preadipocytes. Nat. Prod. Res. 2021, 35, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxid. Med. Cell Longev. 2020, 2020, 1730492. [Google Scholar] [CrossRef]
- Shen, C.Y.; Wan, L.; Wang, T.X.; Jiang, J.G. Citrus aurantium L. var. amara Engl. inhibited lipid accumulation in 3T3-L1 cells and Caenorhabditis elegans and prevented obesity in high-fat diet-fed mice. Pharmacol. Res. 2019, 147, 104347. [Google Scholar] [CrossRef]
- Sathiyabama, R.G.; Rajiv Gandhi, G.; Denadai, M.; Sridharan, G.; Jothi, G.; Sasikumar, P.; Siqueira Quintans, J.S.; Narain, N.; Cuevas, L.E.; Coutinho, H.D.M.; et al. Evidence of insulin-dependent signalling mechanisms produced by Citrus sinensis (L.) Osbeck fruit peel in an insulin resistant diabetic animal model. Food Chem. Toxicol. 2018, 116, 86–99. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chung, Y.M.; Yang, H.T.; Lin, Y.H.; Lin, Y.H.; Hu, W.C.; Chiang, C.F. The potential of immature poken (Citrus reticulata) extract in the weight management, lipid and glucose metabolism. J. Complement. Integr. Med. 2022, 19, 279–285. [Google Scholar] [CrossRef]
- Lu, J.F.; Zhu, M.Q.; Zhang, H.; Liu, H.; Xia, B.; Wang, Y.L.; Shi, X.; Peng, L.; Wu, J.W. Neohesperidin attenuates obesity by altering the composition of the gut microbiota in high-fat diet-fed mice. FASEB J. 2020, 34, 12053–12071. [Google Scholar] [CrossRef]
- Xie, B.; Pan, D.; Liu, H.; Liu, M.; Shi, X.; Chu, X.; Lu, J.; Zhu, M.; Xia, B.; Wu, J. Diosmetin Protects Against Obesity and Metabolic Dysfunctions Through Activation of Adipose Estrogen Receptors in Mice. Mol. Nutr. Food Res. 2021, 65, e2100070. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Salden, B.N.; Troost, F.J.; de Groot, E.; Stevens, Y.R.; Garcés-Rimón, M.; Possemiers, S.; Winkens, B.; Masclee, A.A. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am. J. Clin. Nutr. 2016, 104, 1523–1533. [Google Scholar] [CrossRef]
- Shikishima, Y.; Tsutsumi, R.; Kawakami, A.; Miura, H.; Nii, Y.; Sakaue, H. Sudachi peel extract powder including the polymethoxylated flavone sudachitin improves visceral fat content in individuals at risk for developing diabetes. Food Sci. Nutr. 2021, 9, 4076–4084. [Google Scholar] [CrossRef]
- Saldivar-Gonzalez, F.I.; Aldas-Bulos, V.D.; Medina-Franco, J.L.; Plisson, F. Natural product drug discovery in the artificial intelligence era. Chem. Sci. 2022, 13, 1526–1546. [Google Scholar] [CrossRef]
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar] [CrossRef]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit Juice Industry Wastes as a Source of Bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Ali, M.Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Didymin, a dietary citrus flavonoid exhibits anti-diabetic complications and promotes glucose uptake through the activation of PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Chem. Biol. Interact. 2019, 305, 180–194. [Google Scholar] [CrossRef]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci. Rep. 2014, 4, 3708. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Choi, M.S. Eriocitrin Improves Adiposity and Related Metabolic Disorders in High-Fat Diet-Induced Obese Mice. J. Med. Food 2020, 23, 233–241. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Manthey, J.A.; Nery, M.S.; Spolidorio, L.C.; Cesar, T.B. Low doses of eriocitrin attenuate metabolic impairment of glucose and lipids in ongoing obesogenic diet in mice. J. Nutr. Sci. 2020, 9, e59. [Google Scholar] [CrossRef] [PubMed]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Takamura, N.; Shuto, T.; Ogata, K.; Tokunaga, J.; Kawai, K.; Kai, H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-alpha in mouse adipocytes. Biochem. Biophys. Res. Commun. 2010, 394, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, Y.; Shen, S.; Zhi, Z.; Cheng, H.; Chen, S.; Ye, X. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chem. 2020, 326, 126785. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P.; Natraj, P.; Ranaweera, S.S.; Dayarathne, L.A.; Lee, Y.J.; Han, C.H. Anti-adipogenic effect of the flavonoids through the activation of AMPK in palmitate (PA)-treated HepG2 cells. J. Vet. Sci. 2022, 23, e4. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Chen, J.F.; Shen, L.M.; Zhou, J.; Wang, C.F. Anti-atherosclerotic effect of hesperidin in LDLr(-/-) mice and its possible mechanism. Eur. J. Pharmacol. 2017, 815, 109–117. [Google Scholar] [CrossRef]
- Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Alcaide-Hidalgo, J.M.; Canela, N.; Arola, L.; Mayneris-Perxachs, J. Metabolomics Elucidates Dose-Dependent Molecular Beneficial Effects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet. Antioxidants 2020, 9, 79. [Google Scholar] [CrossRef]
- Nishikawa, S.; Hyodo, T.; Nagao, T.; Nakanishi, A.; Tandia, M.; Tsuda, T. α-Monoglucosyl Hesperidin but Not Hesperidin Induces Brown-Like Adipocyte Formation and Suppresses White Adipose Tissue Accumulation in Mice. J. Agric. Food Chem. 2019, 67, 1948–1954. [Google Scholar] [CrossRef]
- Yoshida, H.; Tsuhako, R.; Sugita, C.; Kurokawa, M. Glucosyl Hesperidin Has an Anti-diabetic Effect in High-Fat Diet-Induced Obese Mice. Biol. Pharm. Bull. 2021, 44, 422–430. [Google Scholar] [CrossRef]
- Tsuhako, R.; Yoshida, H.; Sugita, C.; Kurokawa, M. Naringenin suppresses neutrophil infiltration into adipose tissue in high-fat diet-induced obese mice. J. Nat. Med. 2020, 74, 229–237. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, W.; Oomagari, H.; Tsuruta, E.; Shida, M.; Kurokawa, M. Citrus flavonoid naringenin inhibits TLR2 expression in adipocytes. J. Nutr. Biochem. 2013, 24, 1276–1284. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARalpha, PPARgamma and LXRalpha. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef]
- Richard, A.J.; Amini-Vaughan, Z.; Ribnicky, D.M.; Stephens, J.M. Naringenin inhibits adipogenesis and reduces insulin sensitivity and adiponectin expression in adipocytes. Evid. Based Complement. Alternat. Med. 2013, 2013, 549750. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Lau, F.H.; Lin, Y.; Stephens, J.M.; Johnson, W.D.; Coulter, A.A. Naringenin Promotes Thermogenic Gene Expression in Human White Adipose Tissue. Obesity 2019, 27, 103–111. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Shi, X.; Tan, X.; Si, Q. Naringenin activates beige adipocyte browning in high fat diet-fed C57BL/6 mice by shaping the gut microbiota. Food Funct. 2022, 13, 9918–9930. [Google Scholar] [CrossRef]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Drangova, M.; Huff, M.W. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr(-/-) mice. J. Lipid. Res. 2018, 59, 1714–1728. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Sutherland, B.G.; DiMattia, A.S.; Khami, M.; Koppes, J.B.; Sawyez, C.G.; Whitman, S.C.; Huff, M.W. Naringenin decreases progression of atherosclerosis by improving dyslipidemia in high-fat-fed low-density lipoprotein receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 742–748. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Felder, S.L.; Chhoker, S.; Edwards, J.Y.; Gros, R.; Huff, M.W. Naringenin prevents cholesterol-induced systemic inflammation, metabolic dysregulation, and atherosclerosis in Ldlr-/-; mice. J. Lipid. Res. 2013, 54, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.C.; Telford, D.E.; Edwards, J.Y.; Sutherland, B.G.; Sawyez, C.G.; Huff, M.W. Naringenin Supplementation to a Chow Diet Enhances Energy Expenditure and Fatty Acid Oxidation, and Reduces Adiposity in Lean, Pair-Fed Ldlr(-/-) Mice. Mol. Nutr. Food Res. 2019, 63, e1800833. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Markle, J.M.; Hegele, R.A.; Huff, M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Huff, M.W. Naringenin enhances the regression of atherosclerosis induced by a chow diet in Ldlr(-/-) mice. Atherosclerosis 2019, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Abdel Azeem, M.N. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.Y.; Banh, T.; Hsiao, Y.H.; Cole, R.M.; Straka, S.R.; Yee, L.D.; Belury, M.A. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Snoke, D.B.; Nishikawa, Y.; Cole, R.M.; Ni, A.; Angelotti, A.; Vodovotz, Y.; Belury, M.A. Dietary Naringenin Preserves Insulin Sensitivity and Grip Strength and Attenuates Inflammation but Accelerates Weight Loss in a Mouse Model of Cancer Cachexia. Mol. Nutr. Food Res. 2021, 65, e2100268. [Google Scholar] [CrossRef]
- Chen, J.; Mo, H.; Guo, R.; You, Q.; Huang, R.; Wu, K. Inhibition of the leptin-induced activation of the p38 MAPK pathway contributes to the protective effects of naringin against high glucose-induced injury in H9c2 cardiac cells. Int. J. Mol. Med. 2014, 33, 605–612. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Yan, Y.; Chen, Q.; Luo, F.; Zhu, X.; Li, X.; Chen, K. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012, 135, 1471–1478. [Google Scholar] [CrossRef]
- Jia, S.; Hu, Y.; Zhang, W.; Zhao, X.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-A(y) mice. Food Funct. 2015, 6, 878–886. [Google Scholar] [CrossRef]
- Meephat, S.; Prasatthong, P.; Rattanakanokchai, S.; Bunbupha, S.; Maneesai, P.; Pakdeechote, P. Diosmetin attenuates metabolic syndrome and left ventricular alterations via the suppression of angiotensin II/AT1 receptor/gp(91phox)/p-NF-κB protein expression in high-fat diet fed rats. Food Funct. 2021, 12, 1469–1481. [Google Scholar] [CrossRef]
- Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory effect of diosmetin on inflammation and lipolysis in coculture of adipocytes and macrophages. J. Food Biochem. 2020, 44, e13261. [Google Scholar] [CrossRef]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef]
- Tung, Y.C.; Li, S.; Huang, Q.; Hung, W.L.; Ho, C.T.; Wei, G.J.; Pan, M.H. 5-Demethylnobiletin and 5-Acetoxy-6,7,8,3′,4′-pentamethoxyflavone Suppress Lipid Accumulation by Activating the LKB1-AMPK Pathway in 3T3-L1 Preadipocytes and High Fat Diet-Fed C57BL/6 Mice. J. Agric. Food Chem. 2016, 64, 3196–3205. [Google Scholar] [CrossRef]
- Miyata, Y.; Tanaka, H.; Shimada, A.; Sato, T.; Ito, A.; Yamanouchi, T.; Kosano, H. Regulation of adipocytokine secretion and adipocyte hypertrophy by polymethoxyflavonoids, nobiletin and tangeretin. Life Sci. 2011, 88, 613–618. [Google Scholar] [CrossRef]
- Kanda, K.; Nishi, K.; Kadota, A.; Nishimoto, S.; Liu, M.C.; Sugahara, T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim. Biophys. Acta 2012, 1820, 461–468. [Google Scholar] [CrossRef]
- Lone, J.; Parray, H.A.; Yun, J.W. Nobiletin induces brown adipocyte-like phenotype and ameliorates stress in 3T3-L1 adipocytes. Biochimie 2018, 146, 97–104. [Google Scholar] [CrossRef]
- Morrow, N.M.; Burke, A.C.; Samsoondar, J.P.; Seigel, K.E.; Wang, A.; Telford, D.E.; Sutherland, B.G.; O’Dwyer, C.; Steinberg, G.R.; Fullerton, M.D.; et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J. Lipid. Res. 2020, 61, 387–402. [Google Scholar] [CrossRef]
- Tsuboi, T.; Lu, R.; Yonezawa, T.; Watanabe, A.; Woo, J.T.; Abe-Dohmae, S.; Yokoyama, S. Molecular mechanism for nobiletin to enhance ABCA1/G1 expression in mouse macrophages. Atherosclerosis 2020, 297, 32–39. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Choi, S.S.; Choi, B.K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 2013, 24, 156–162. [Google Scholar] [CrossRef]
- Bunbupha, S.; Pakdeechote, P.; Maneesai, P.; Prasarttong, P. Nobiletin alleviates high-fat diet-induced nonalcoholic fatty liver disease by modulating AdipoR1 and gp91(phox) expression in rats. J. Nutr. Biochem. 2021, 87, 108526. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Saito, K.; Yamakawa, H.; Choi, S.S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar] [CrossRef]
- Zhang, M.; Xin, Y.; Feng, K.; Yin, B.; Kan, Q.; Xiao, J.; Cao, Y.; Ho, C.T.; Huang, Q. Comparative Analyses of Bioavailability, Biotransformation, and Excretion of Nobiletin in Lean and Obese Rats. J. Agric. Food Chem. 2020, 68, 10709–10718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X.; Zhu, J.; Zhao, D.G.; Ma, Y.Y.; Li, D.; Ho, C.T.; Huang, Q. Bidirectional interaction of nobiletin and gut microbiota in mice fed with a high-fat diet. Food Funct. 2021, 12, 3516–3526. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yoon, D.S.; Jung, U.J. Efficacy of nobiletin in improving hypercholesterolemia and nonalcoholic fatty liver disease in high-cholesterol diet-fed mice. Nutr. Res. Pract. 2021, 15, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Shin, H.S.; Ko, H.C.; Kim, S.J. Effects of sinensetin on lipid metabolism in mature 3T3-L1 adipocytes. Phytother. Res. 2013, 27, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Yu, Y.; Lee, J.; Jeong, W.S.; Ho, C.T.; Jun, M. Polymethoxyflavones: Novel beta-Secretase (BACE1) Inhibitors from Citrus Peels. Nutrients 2017, 9, 973. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef]
- Xu, W.; Miyamoto, L.; Aihara, H.; Yamaoka, T.; Tanaka, N.; Tsuchihashi, Y.; Ikeda, Y.; Tamaki, T.; Kashiwada, Y.; Tsuchiya, K. Methanol extraction fraction from Citrus Sudachi peel exerts lipid reducing effects in cultured cells. J. Med. Investig. 2018, 65, 225–230. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Yoshida, T.; Nii, Y.; Okahisa, N.; Iwata, S.; Tsukayama, M.; Hashimoto, R.; Taniguchi, Y.; Sakaue, H.; Hosaka, T.; et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014, 11, 32. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, L.; Qiu, C. Tangeretin protects mice from diet-induced metabolic inflammation via activating adipose lactate accumulation and macrophage M2 polarization. Biochem. Biophys. Res. Commun. 2022, 630, 16–23. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chao, T.Y.; Hsu, H.J.; Wang, C.Y.; Lin, C.Y.; Gao, W.Y.; Wu, M.J.; Yen, J.H. The Lipid-Modulating Effect of Tangeretin on the Inhibition of Angiopoietin-like 3 (ANGPTL3) Gene Expression through Regulation of LXRα Activation in Hepatic Cells. Int. J. Mol. Sci. 2021, 22, 9853. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.; Ren, W.; Zhu, Y.; Zhao, Q.; Zhang, K.; Su, D.; Qiu, C.; Zhang, W.; Li, K. Citrus flavone tangeretin is a potential insulin sensitizer targeting hepatocytes through suppressing MEK-ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2020, 529, 277–282. [Google Scholar] [CrossRef]
- Nery, M.; Ferreira, P.S.; Gonçalves, D.R.; Spolidorio, L.C.; Manthey, J.A.; Cesar, T.B. Physiological effects of tangeretin and heptamethoxyflavone on obese C57BL/6J mice fed a high-fat diet and analyses of the metabolites originating from these two polymethoxylated flavones. Food Sci. Nutr. 2021, 9, 1997–2009. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Yin, L.; Zhu, M.; Wang, F.; Zhang, L.; Wang, H.; Zhou, Z.; Zhu, H.; Huang, C.; et al. Tangeretin promotes lifespan associated with insulin/insulin-like growth factor-1 signaling pathway and heat resistance in Caenorhabditis elegans. Biofactors 2022, 48, 442–453. [Google Scholar] [CrossRef]
- Kim, H.I.; Jeong, Y.U.; Kim, J.H.; Park, Y.J. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Flavonoid, Inhibits Collagenase Activity and Induces Type I Procollagen Synthesis in HDFn Cells. Int. J. Mol. Sci. 2018, 19, 620. [Google Scholar] [CrossRef]
- Sawamoto, A.; Nakanishi, M.; Okuyama, S.; Furukawa, Y.; Nakajima, M. Heptamethoxyflavone inhibits adipogenesis via enhancing PKA signaling. Eur. J. Pharmacol. 2019, 865, 172758. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, P.S.; Chen, Y.F.; Ho, C.T.; Pan, M.H. Suppression of Adipogenesis by 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone from Orange Peel in 3T3-L1 Cells. J. Med. Food 2016, 19, 830–835. [Google Scholar] [CrossRef]
- Lai, C.S.; Ho, M.H.; Tsai, M.L.; Li, S.; Badmaev, V.; Ho, C.T.; Pan, M.H. Suppression of adipogenesis and obesity in high-fat induced mouse model by hydroxylated polymethoxyflavones. J. Agric. Food Chem. 2013, 61, 10320–10328. [Google Scholar] [CrossRef]
- Deng, M.; Dong, L.; Jia, X.; Huang, F.; Chi, J.; Muhammad, Z.; Ma, Q.; Zhao, D.; Zhang, M.; Zhang, R. The flavonoid profiles in the pulp of different pomelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars and their in vitro bioactivity. Food Chem. X 2022, 15, 100368. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Gu, Y.; Jiang, Z.; Zhou, Z. Tangeretin prevents obesity by modulating systemic inflammation, fat browning, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. J. Nutr. Biochem. 2022, 101, 108943. [Google Scholar] [CrossRef]
- Sung, J.; Suh, J.H.; Wang, Y. Effects of heat treatment of mandarin peel on flavonoid profiles and lipid accumulation in 3T3-L1 adipocytes. J. Food Drug Anal. 2019, 27, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.L.; Li, S.Z.; Lai, C.J.; Wei, M.Y.; Chen, B.Z.; Li, P.; Zheng, G.D.; Liu, E.H. Evaluation of anti-lipase activity and bioactive flavonoids in the Citri Reticulatae Pericarpium from different harvest time. Phytomedicine 2018, 43, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Song, S.; Lee, J.; Chang, H.; Lee, S. Clinical Investigations of the Effect of Citrus unshiu Peel Pellet on Obesity and Lipid Profile. Evid. Based Complement. Alternat. Med. 2018, 2018, 4341961. [Google Scholar] [CrossRef] [PubMed]
- Pla-Pagà, L.; Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Foguet-Romero, E.; Catalán, Ú.; Mayneris-Perxachs, J.; Canela, N.; Valls, R.M.; Arola, L.; et al. Proteomic Analysis of Heart and Kidney Tissues in Healthy and Metabolic Syndrome Rats after Hesperidin Supplementation. Mol. Nutr. Food Res. 2020, 64, e1901063. [Google Scholar] [CrossRef]
- Schneider, P.; Walters, W.P.; Plowright, A.T.; Sieroka, N.; Listgarten, J.; Goodnow, R.A., Jr.; Fisher, J.; Jansen, J.M.; Duca, J.S.; Rush, T.S.; et al. Rethinking drug design in the artificial intelligence era. Nat. Rev. Drug Discov. 2020, 19, 353–364. [Google Scholar] [CrossRef]
- King, R.D.; Rowland, J.; Oliver, S.G.; Young, M.; Aubrey, W.; Byrne, E.; Liakata, M.; Markham, M.; Pir, P.; Soldatova, L.N.; et al. The automation of science. Science 2009, 324, 85–89. [Google Scholar] [CrossRef]

| Flavonoids | Constituent | Fruit Source | Chemical Structure | Mechanism | Molecular Pathways (Ref.) | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
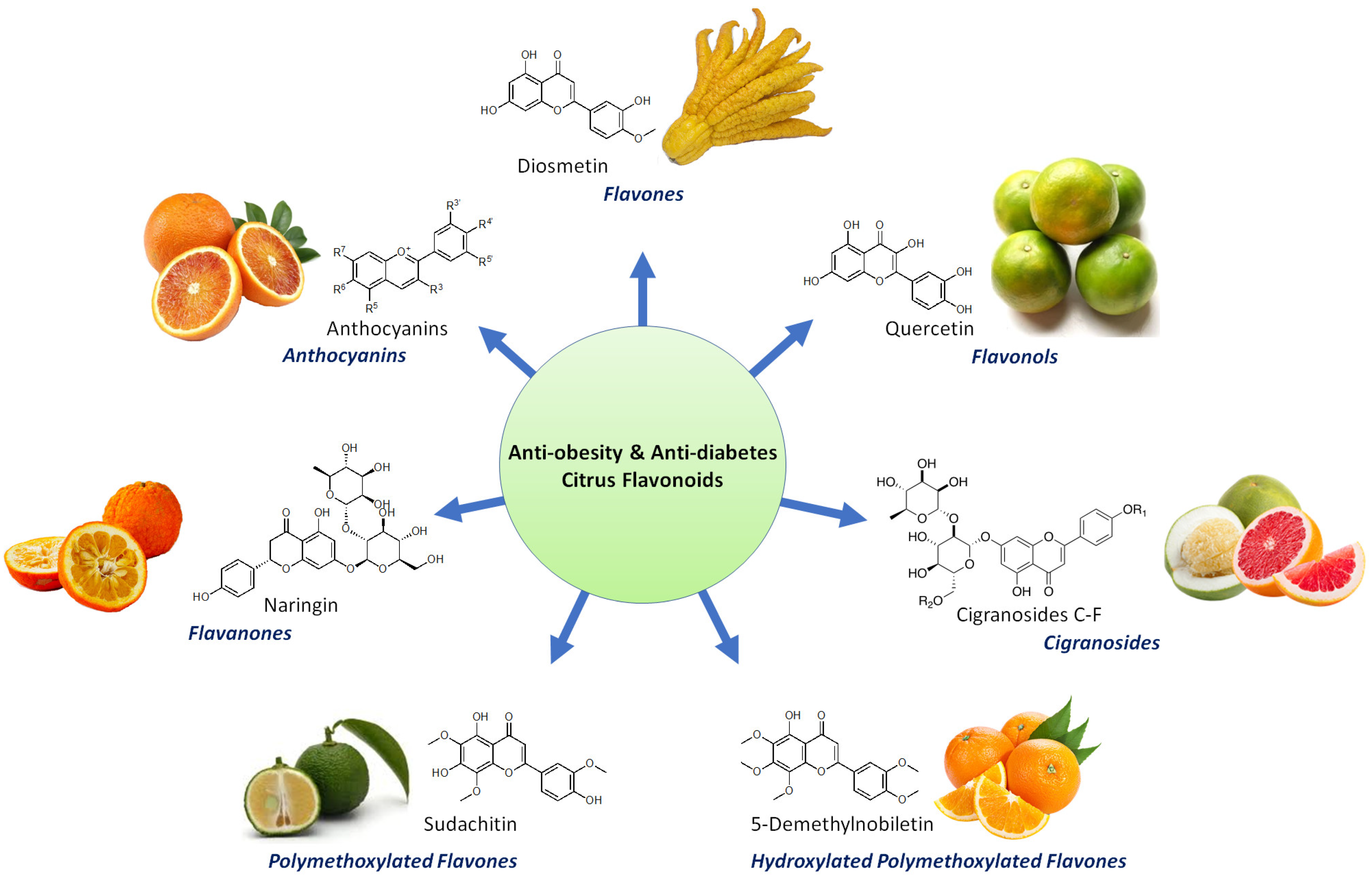
| Didymin | Flavanone | C. reticulata, etc. | 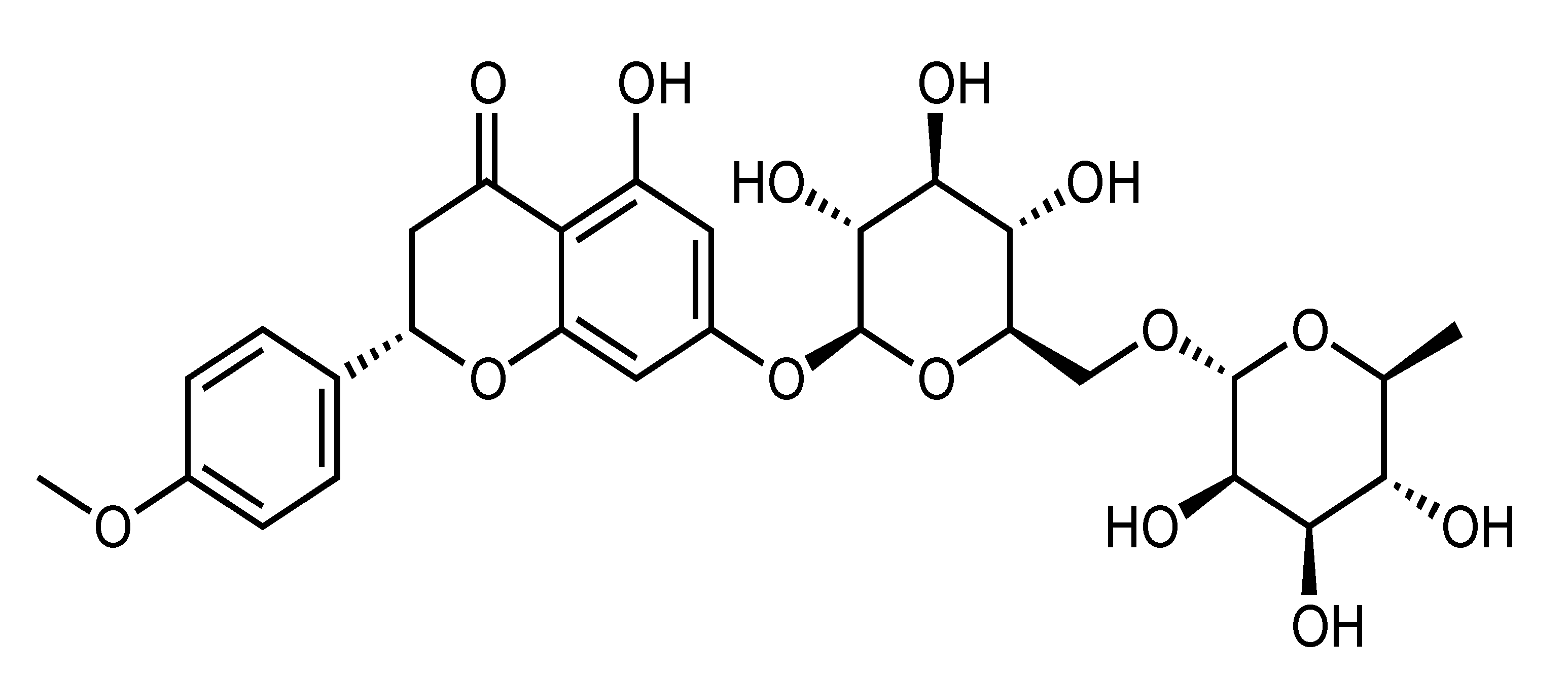 | Improvement of insulin sensitivity, Amelioration of hyperglycemia | α-glucosidase, PTP1B, RLAR, HRAR, AGE, ONOO–, ROS, IRS-1, PI3K, Akt, GSK-3, PEPCK and G6Pase [38] | Insulin-resistant HepG2 [38] | |
| Eriocitrin (Eriomin) | Flavanone | C. latifolia, C. limon, C. leiocarpa, C. grandis cv Hirado, etc. | 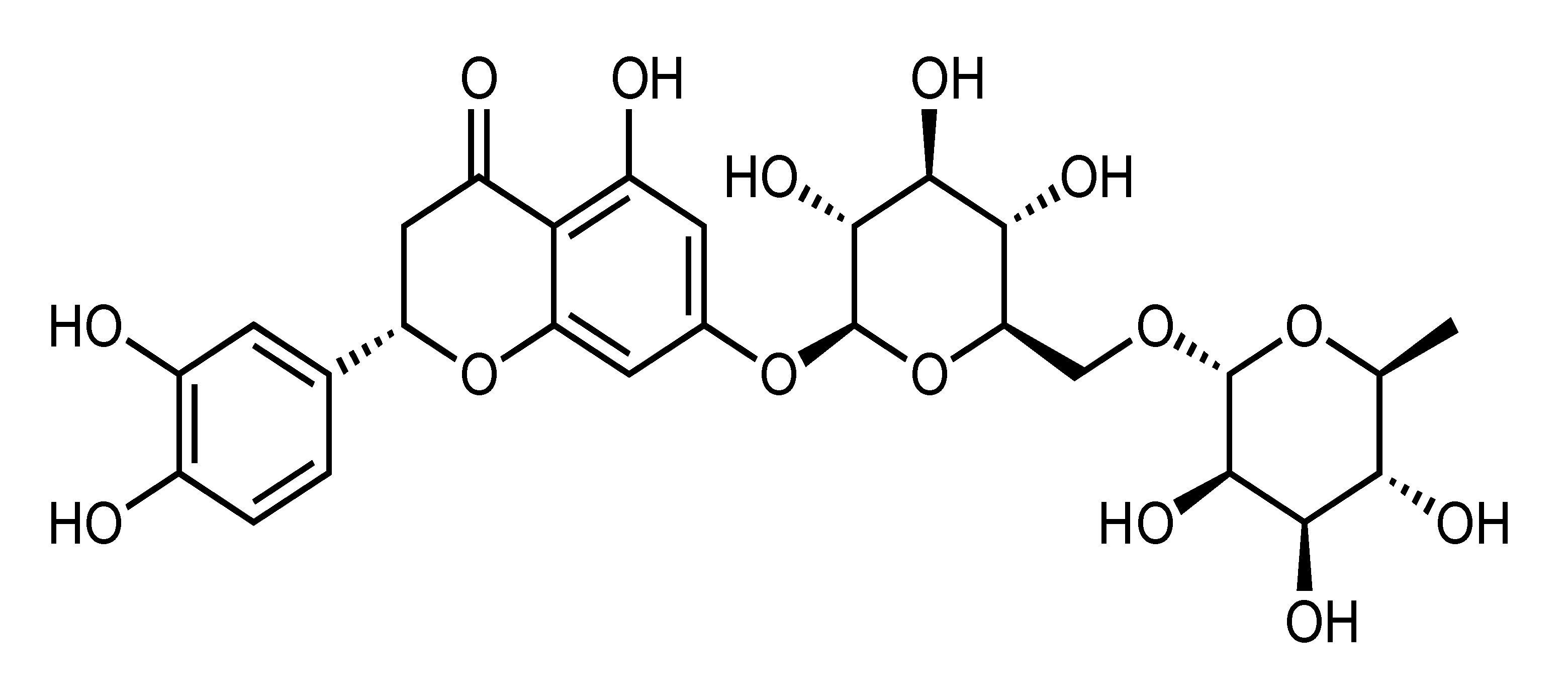 | Suppression of adipogenesis, oxidative stress and inflammation, Amelioration of dyslipidemia and hepatic steatosis, Reduction of liver lipid accumulation, Activation of mitochondrial biogenesis, Enhancement of FA oxidation, Improvement of insulin sensitivity, Reduction of body weight | PPARα, NRF1, ATP5J, COX4l1 [39], ACC, PPARα, PPARγ, UCP1, PGC1α, SREBP1, SREBP2 [40], Resistin, Leptin [41], GLP-1, TNF-α, IL-6 [42] | HepG2 [39] | DIO-zebrafish [39], HFD-fed and HCD-fed obese rats [39], HFD-fed obese mice [40,41] |
| Hesperidin | Flavanone | C. tangerina, C. suhuiensis, C. kinokuni, C. succosa, etc. | 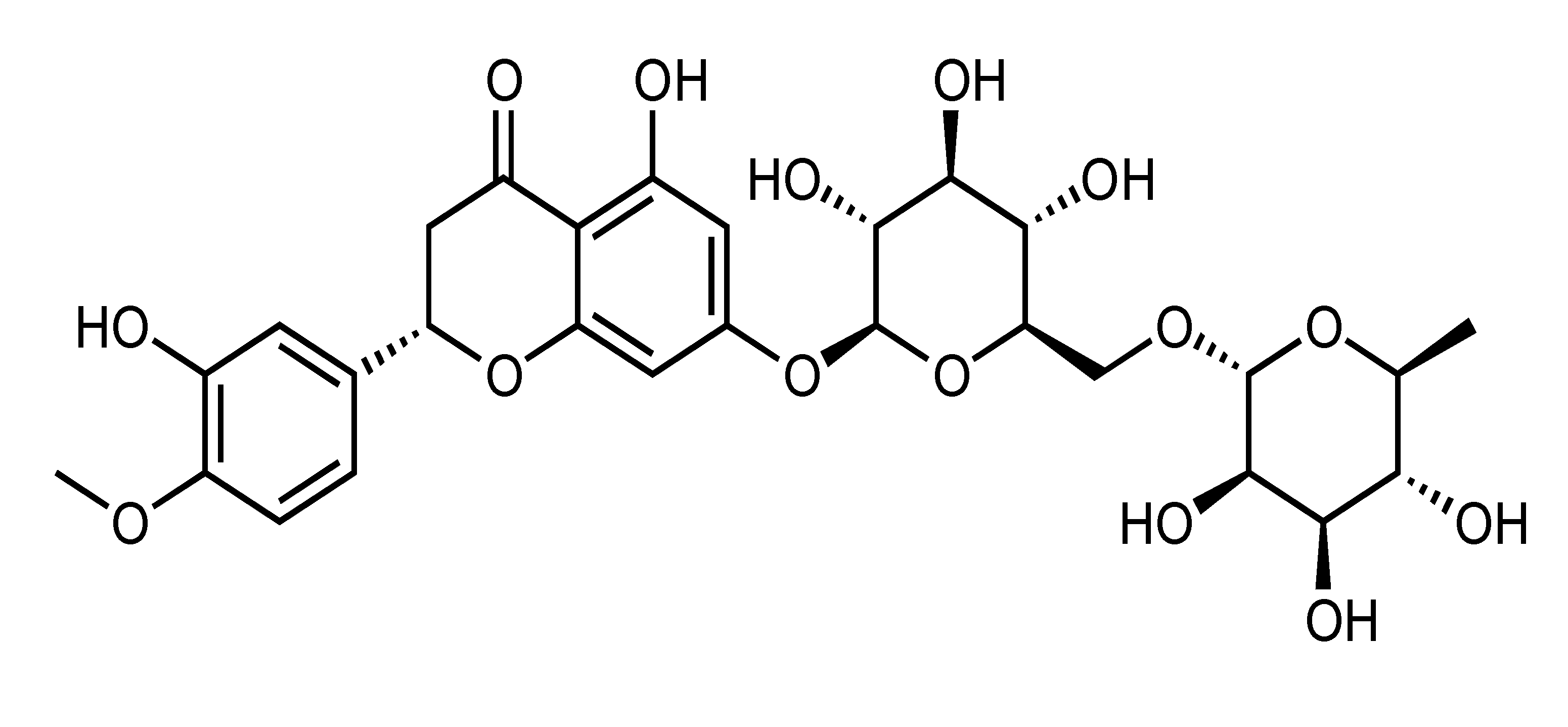 | Suppression of adipogenesis, TG accumulation, oxidative stress and inflammation, Reduction of body weight and liver glycogen content, Improvement of insulin sensitivity and endothelial function, Amelioration of hyperglycemia and hyperlipidemia, Suppression of serum insulin and C-peptide, Inhibition of macrophage foam cell formation and TNF-α-stimulated FFA secretion | AMPK, ACC, GSK3β, SREBP-2, HMGCR [45], ACCα, FAS, ABCG8, ABCA1, ABCG1, ROS [46], GPx, GST, SOD, GLUT-4, insulin receptor β-subunit [25], PL [44], NF-κB, TNF-α, IL-6, ERK, perilipin and PDE3B [43], Akt, AMPK, eNOS, NO, VCAM-1 [31], VCAM-1, ICAM-1, selectin [32] | PA-treated HepG2 [45], HepG2 [44], 3T3-L1 [43], Primary bovine aortic endothelial cells [31] | HFD-fed LDLr(-/-) mice [46], NA/STZ-induced diabetic rats [25], CAF-fed obese rat [47] |
| Flavonoids | Constituent | Fruit Source | Chemical Structure | Mechanism | Molecular Pathways (Ref.) | In Vitro | In Vivo |
| Naringenin | Flavanone | C. aurantium, etc. |  | Suppression of adipogenesis, Amelioration of hyperglycemia, hyperinsulinemia and dyslipidemia, Reduction of adipose tissue inflammation, Reduction of body weight, Inhibition of TNF-α-stimulated FFA secretion, Inhibition of muscle strength loss, Improvement of endothelial function, Amelioration of metabolic syndrome and atherosclerosis, Enhancement of atherosclerosis regression, Induction of insulin resistance, Increase of energy expenditure | PPARγ, TLR2, NF-κB, JNK, TNF-α and MCP-1 [51], AMPKα, Cyclin D1, MCP-1, IL-6, leptin [64], NF-κB, TNF-α, IL-6, ERK, perilipin and PDE3B [43], adiponectin, PGC1α, IL-6 [65], iHSP70, HSF1, Akt, AS160, NF-κB, IL-6, VCAM-1, ICAM-1 [55], Pnpla2, PGC1α, CPT1A [61], adiponectin, IRS-1 [53], TNF-α, IL1b, CCL2, CCL3 [58], PPARα, PGC1α, SREBP-1 [60], UCP1, PGC1α, PGC1β, adiponectin, ChREBP, GLUT4 [54], MCP-1, JNK [12], MCP-1, MCP-3 [50], IR, GLUT4, adiponectin [63] | 3T3-L1, 3T3-L1 and RAW264 macrophages coculture [12,50,51], HUVEC [55], hADSC [54] | HFD-fed obese mice [50,51], HFD-fed obese ovariectomized mice [64], Colon-26 cancer cachexia mice [65], HFD-fed LDLr(-/-) mice [56,57,58,60,61], HFD-fed Fgf21(-/-) mice [62], NA/STZ-induced diabetic rats [63] |
| Flavonoids | Constituent | Fruit Source | Chemical Structure | Mechanism | Molecular Pathways (Ref.) | In Vitro | In Vivo |
| Naringin | Flavanone | C. aurantium, C. natsudaidai, C. paradisi, C. grandis cv. Hirado, etc. | 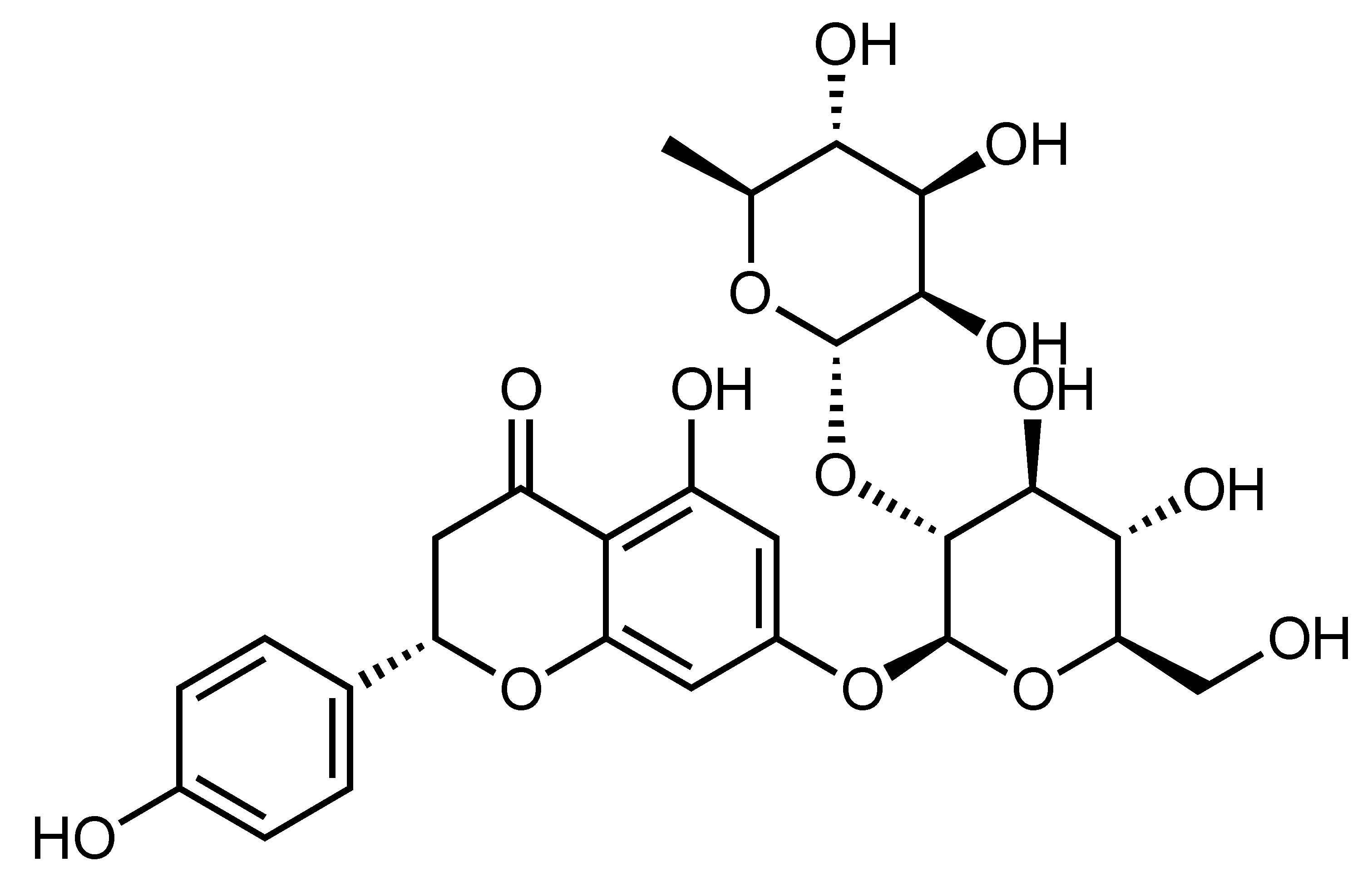 | Inhibition of high glucose-induced cardiomyocyte injury, Suppression of apoptosis, oxidative stress and mitochondrial damage, Improvement of insulin sensitivity | p38, ROS [66], IR, GLUT4, adiponectin [63] | H9c2 [66] | NA/STZ-induced diabetic rats [63] |
| Narirutin | Flavanone | C. shunkokan, C. sulcata, C. leiocarpa, C. nobilis var Knep, etc. | 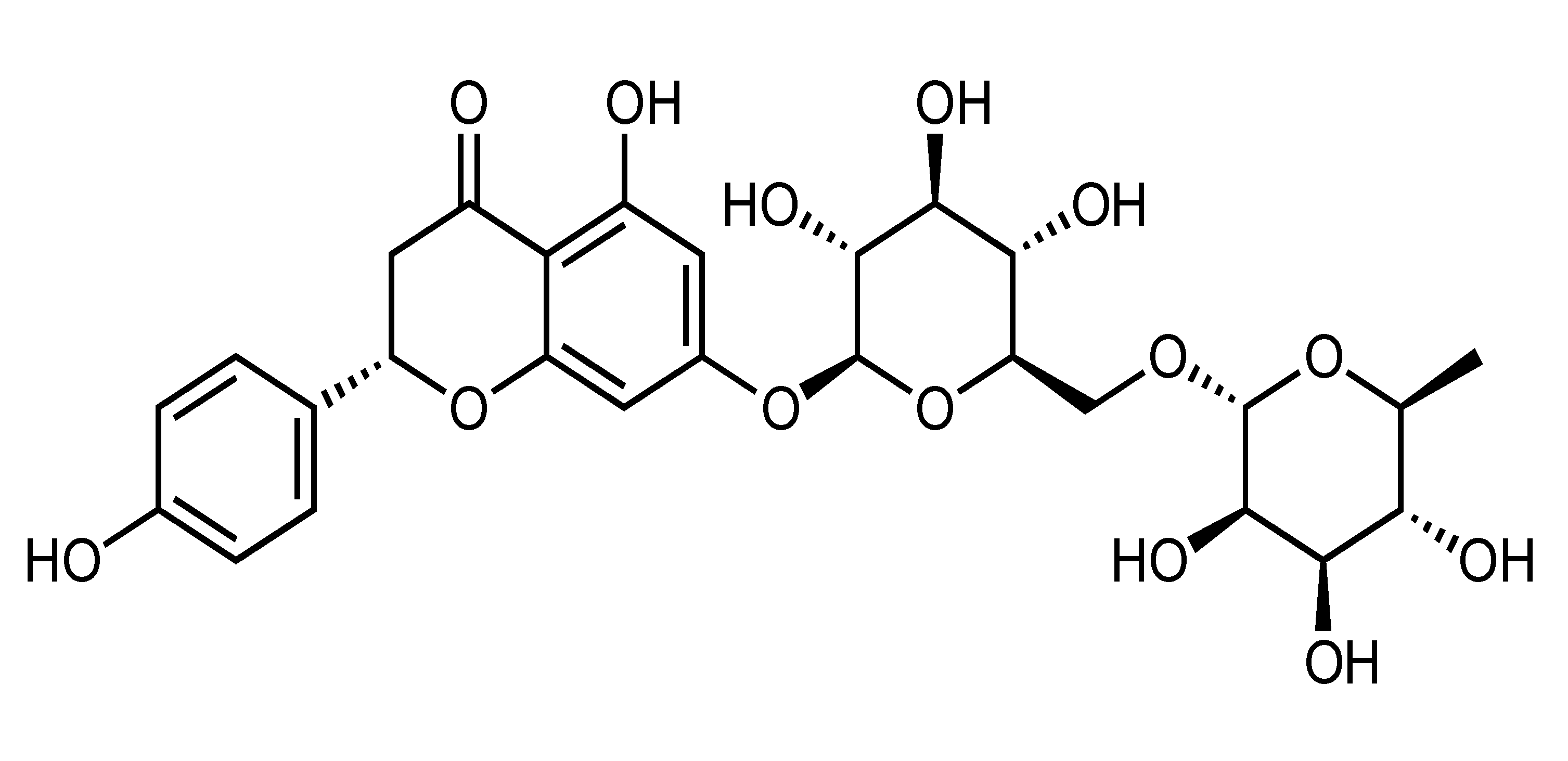 | Suppression of adipogenesis, Suppression of TG accumulation | AMPK, ACC, GSK3β, SREBP-2, HMGCR [45] | PA-treated HepG2 [45] | |
| Neohesperidin | Flavanone | C. aurantium, C. bergamia, C. glaberrima, C. hassaku, C. changshanensis, etc. | 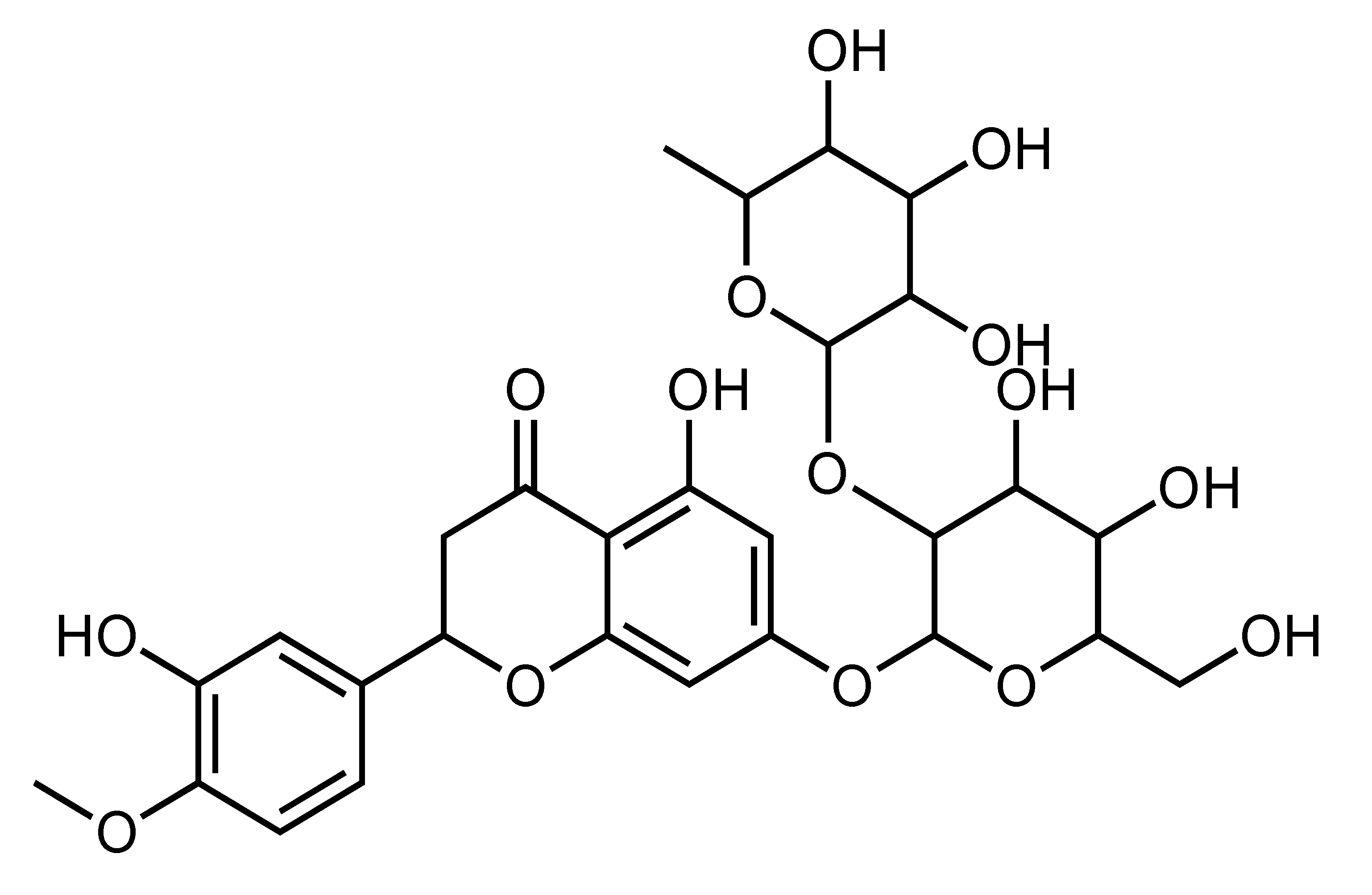 | Suppression of TG accumulation, Improvement of insulin sensitivity, Amelioration of hyperglycemia and hyperlipidemia, Reduction of body weight and inflammation | AMPK [67], SCD-1, FAS, ACOX [68] | HepG2 [67] | Diabetic KK-A(y) mice [68], HFD-fed obese mice [29] |
| Diosmetin | Flavone | C. suhuiensis, C. medica var. 2, etc. | 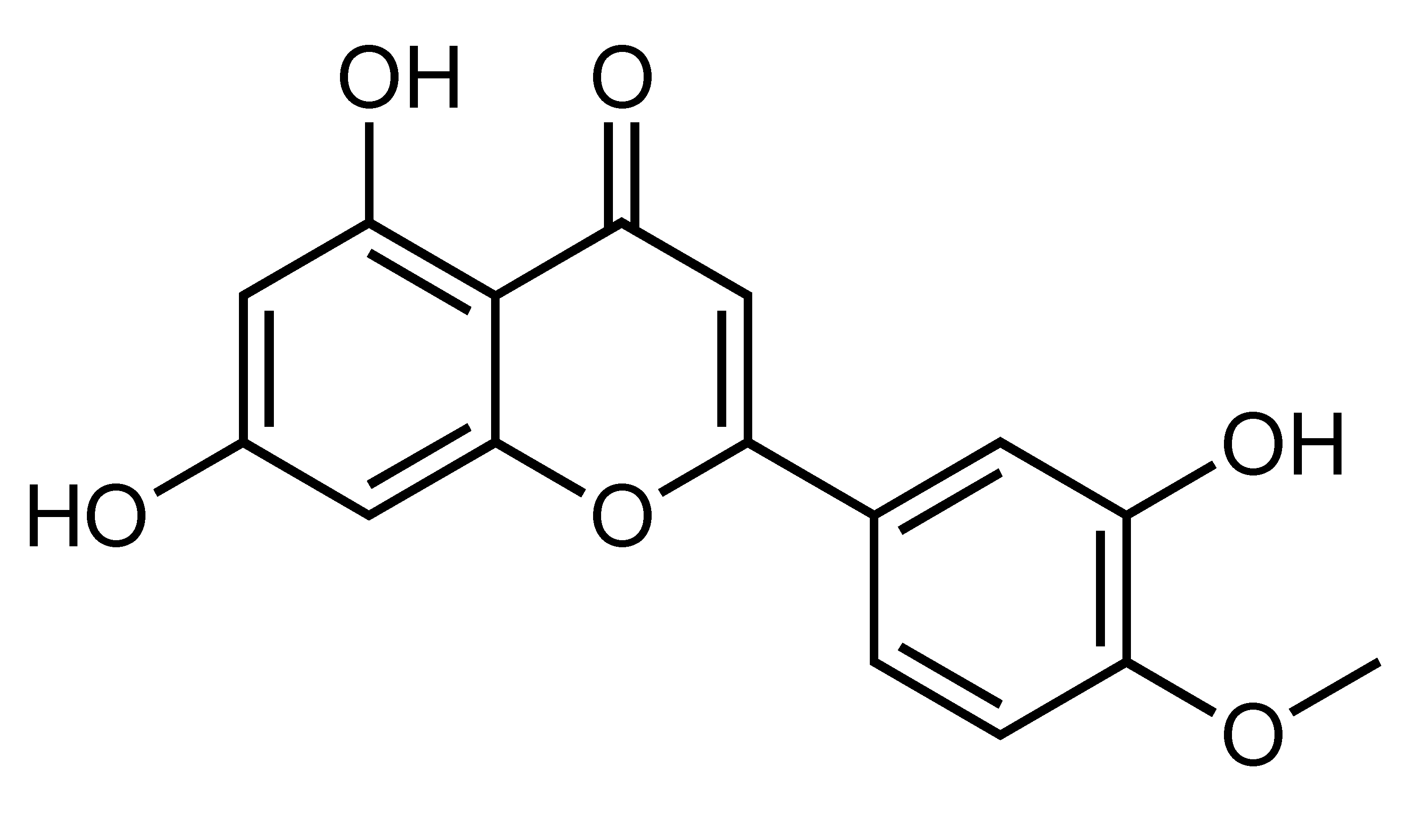 | Amelioration of hypertension, hyperglycemia, insulin resistance, dyslipidemia, LV hypertrophy and fibrosis, Reduction of body weight and fat mass, Improvements of insulin sensitivity, Inhibition of inflammation, Inhibition of lipolysis | Ang II, AT1 receptor, gp91phox, p-NF-κB [69], ERs [30], iNOS, MAPKs, NF-kB, TNF-α, p50, MCP-1 [70] | 3T3-L1 [30], 3T3-L1 and RAW264 macrophages coculture [70] | HFD-fed SD rats [69], HFD-fed obese mice [30] |
| Flavonoids | Constituent | Fruit Source | Chemical Structure | Mechanism | Molecular Pathways (Ref.) | In Vitro | In Vivo |
| Diosmin | Flavone | C. montana, C. latifolia, C. lumia, C. kinokuni, etc. |  | Prevention of diabetic neuropathy progression | NO, SOD [71] | HFD/STZ-induced diabetic rats [71] | |
| Nobiletin | Polymethoxylated flavone | C. reticulata, C. tangerina, C. suhuiensis, C. tachibana, etc. |  | Suppression of adipogenesis, Suppression of TG accumulation, Induction of apoptosis, Modulation of gut microbiota, Suppression of FA synthesis and enhancement of FA oxidation, Reduction of obesity, hepatic steatosis, and dyslipidemia, Improvement of insulin sensitivity, Amelioration of hyperglycemia, hypercholesterolemia and NAFLD, Amelioration of metabolic syndrome and atherosclerosis, Increase of cellular cholesterol release | adiponectin, MCP-1, resistin, and caspase 3 [73], AMPK, ACC, GSK3β, SREBP-2, HMGCR [45], AMPK, ACC [76], LDL, IL-1β, IL-6 [83], ABCA1, ABCG1, PPARγ, LXRα [77], AdipoR1, gp91 [79], adiponectin, MCP-1, IL-6, PPARγ, GLUT4, GLUT1, Akt [78,80], C/EBPβ, PPARδ, PPARα, PGC-1α, UCP1, AMPK, JNK [75], PPARγ, CREB, STAT5 [74] | 3T3-L1 [72,73,74,75], PA-treated HepG2 [45], HepG2, Ampkβ1-/- primary mouse hepatocyte [76], J774.1 mouse macrophages [77] | HFD-fed obese mice [72,82], Ampkβ1-/-, AccDKI, and iβ1β2AKO mice [76], HFD-fed obese rats [79,81], HCD-fed nonobese mice [83], HFD-fed LDLr(-/-) mice [56], diabetic ob/ob mice [80] |
| Flavonoids | Constituent | Fruit Source | Chemical Structure | Mechanism | Molecular Pathways (Ref.) | In Vitro | In Vivo |
| Sinensetin | Polymethoxylated flavone | C. sunki, C. sinensis cv Valencia, C. tachibana, C. tangerina, etc. | 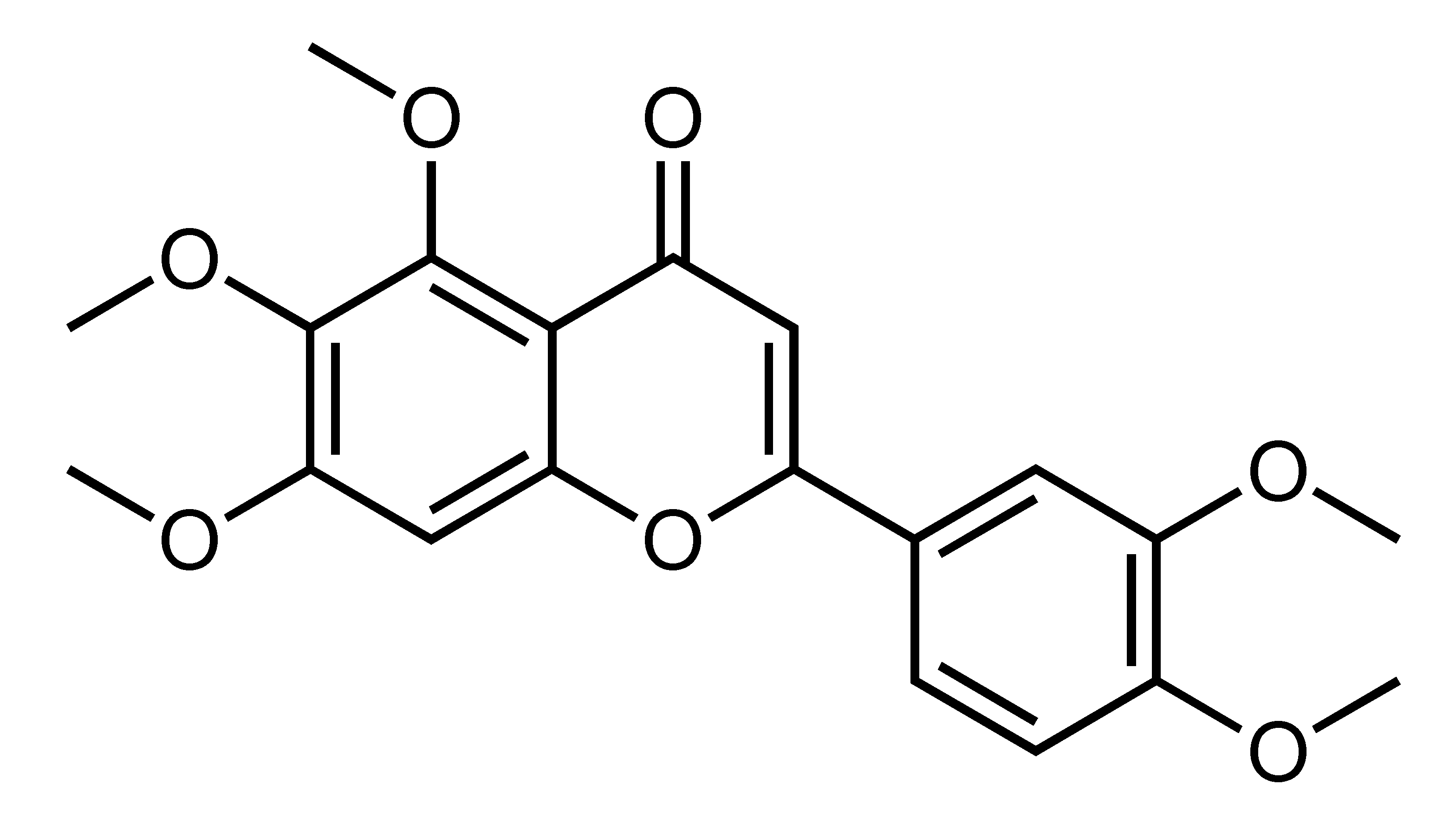 | Suppression of adipogenesis, Suppression of TG accumulation | AMPK, ACC, GSK3β, SREBP-2, HMGCR [45], SREBP1c, PKA, IRS, Akt, AMPK, ACC [84] | PA-treated HepG2 [45], 3T3-L1 [84] | |
| Sudachitin | Polymethoxylated flavone | C. sudachi, etc. |  | Suppression of adipogenesis, Improvement of insulin sensitivity, Amelioration of hyperglycemia and dyslipidemia, Reduction of body weight, Increase of energy expenditure | Sirt1, PGC1α [88] | db/db mice [88], HFD-fed obese mice [88] | |
| Tangeretin | Polymethoxylated flavone | C. reticulata, C. tangerina, C. succosa, C. suhuiensis, etc. | 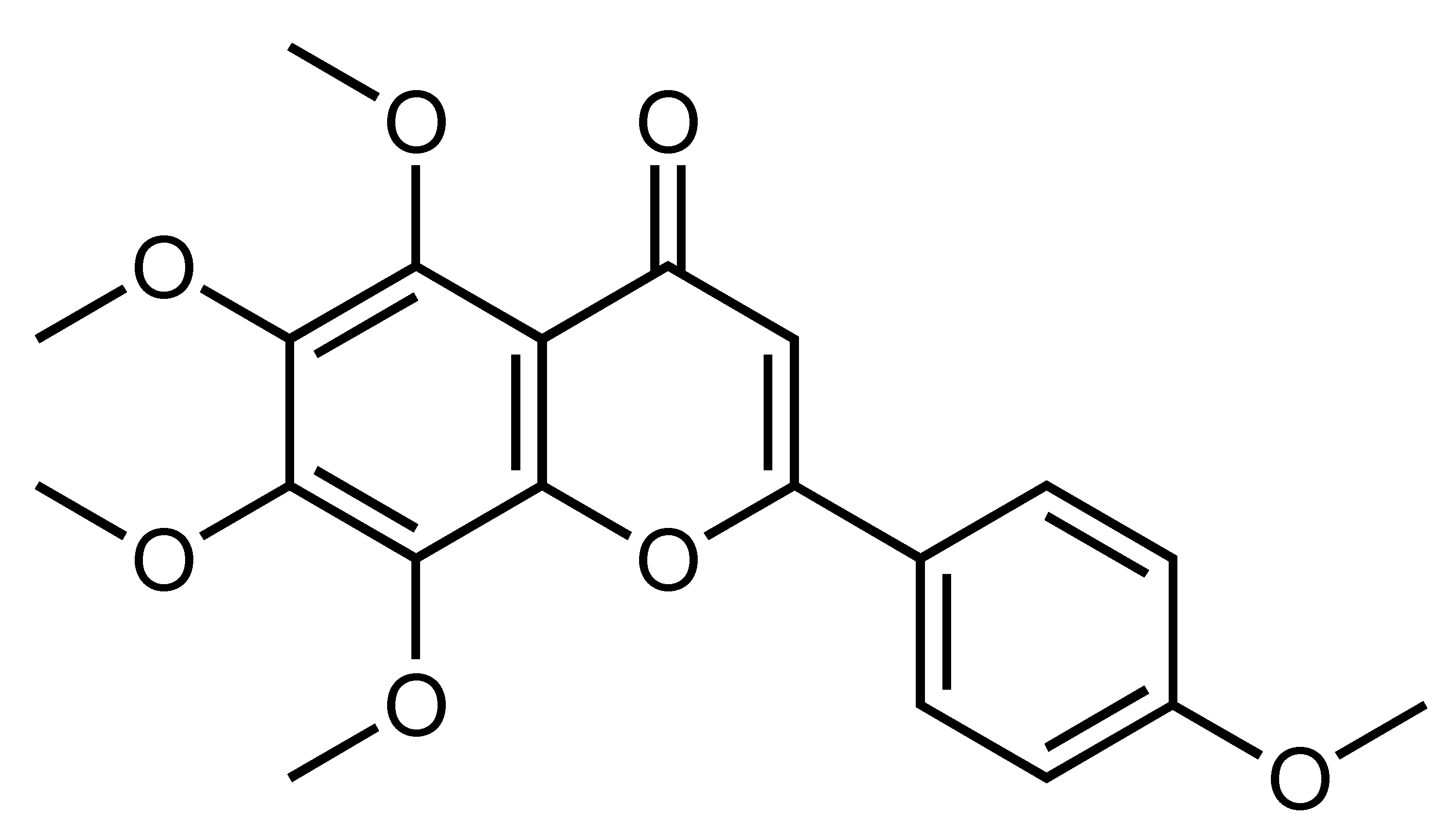 | Suppression of adipogenesis, Suppression of TG accumulation, Improvement of liver insulin sensitivity and glucose homeostasis, Reduction of body weight and inflammation, Suppression of gut microbiota dysbiosis, Prolongation of life span | adiponectin and MCP-1 [73], AMPK, ACC, GSK3β, SREBP-2, HMGCR [45], MEK, ERK1/2 [91], ANGPTL3, LPL, LXRα [90], IL-10 [92], DAF-16, HSP-16.2, HSP-16.49 [93], LXRα, PPARγ, PPARα, PGC1α [52] | 3T3-L1 [73], PA-treated HepG2 [45], Primary mouse hepatocytes [91], HepG2, primary rat hepatocytes and Huh-7 [52,90], BMDM, BMDM and adipocyte coculture [89] | db/db mice [91], HFD-fed obese mice [92,100], Caenorhabditis elegans [93] |
| Flavonoids | Constituent | Fruit Source | Chemical Structure | Mechanism | Molecular Pathways (Ref.) | In Vitro | In Vivo |
| 3′,4′,3,5,6,7,8-Heptamethoxyflavone | Polymethoxylated flavone | C. yatsusiro, C. oto, C. clementina, C. nobilis var Knep, etc. | 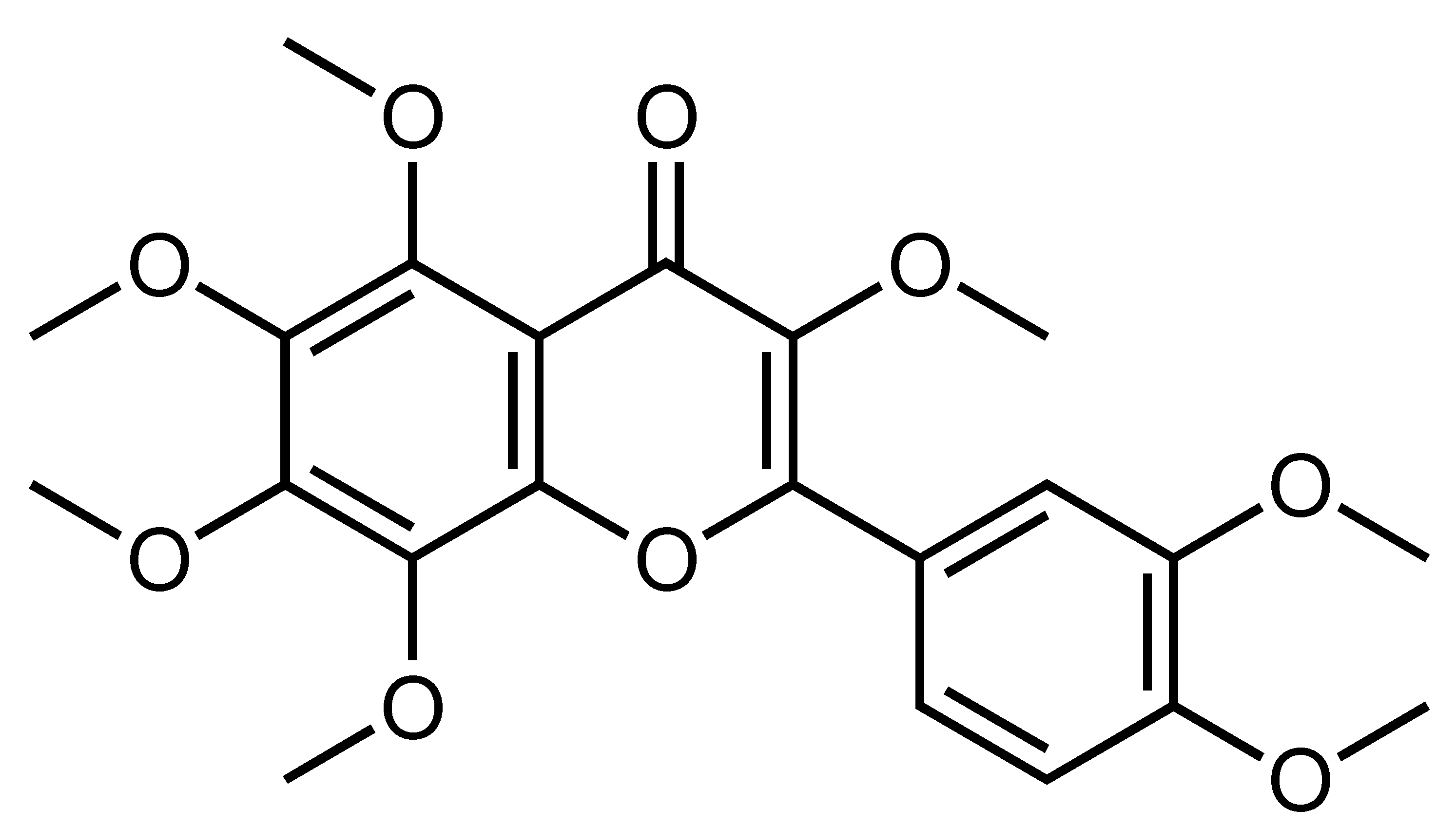 | Suppression of adipogenesis, Amelioration of hyperglycemia and hyperlipidemia | PPARγ, C/EBPα, SREBP1, PKACα, AMPK, ACC [95], IL-10 [92] | 3T3-L1 [95] | HFD-fed obese mice [92] |
| 5-Demethylnobiletin | Hydroxylated polymethoxylated flavone | C. reticulata, etc. | 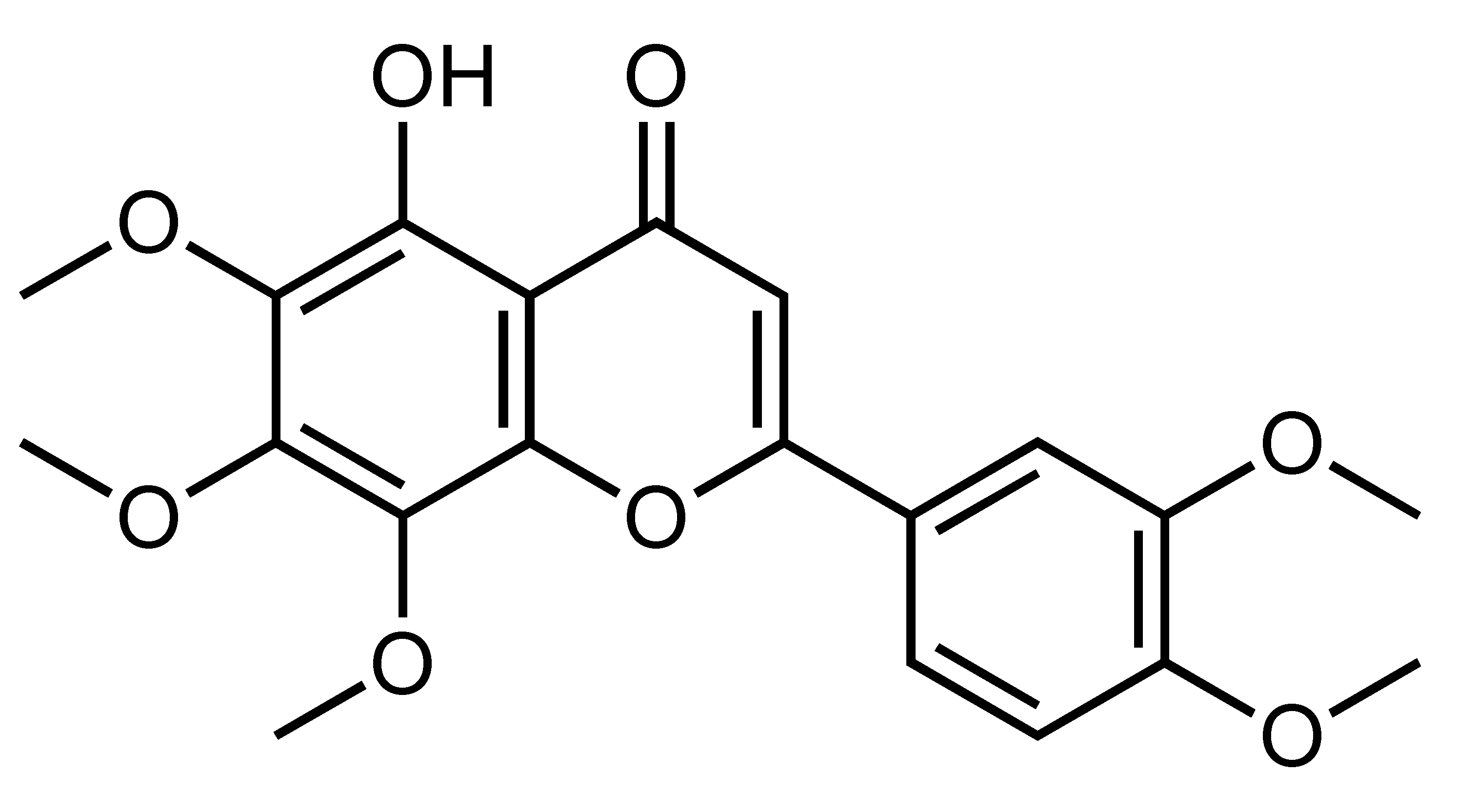 | Suppression of adipogenesis, Suppression of TG accumulation | 3T3-L1 [72] | ||
| 5-Hydroxy-3,6,7,8,3′,4′-Hexamethoxyflavone | Hydroxylated polymethoxylated flavone | C. sinensis, C. reticulate, etc. | 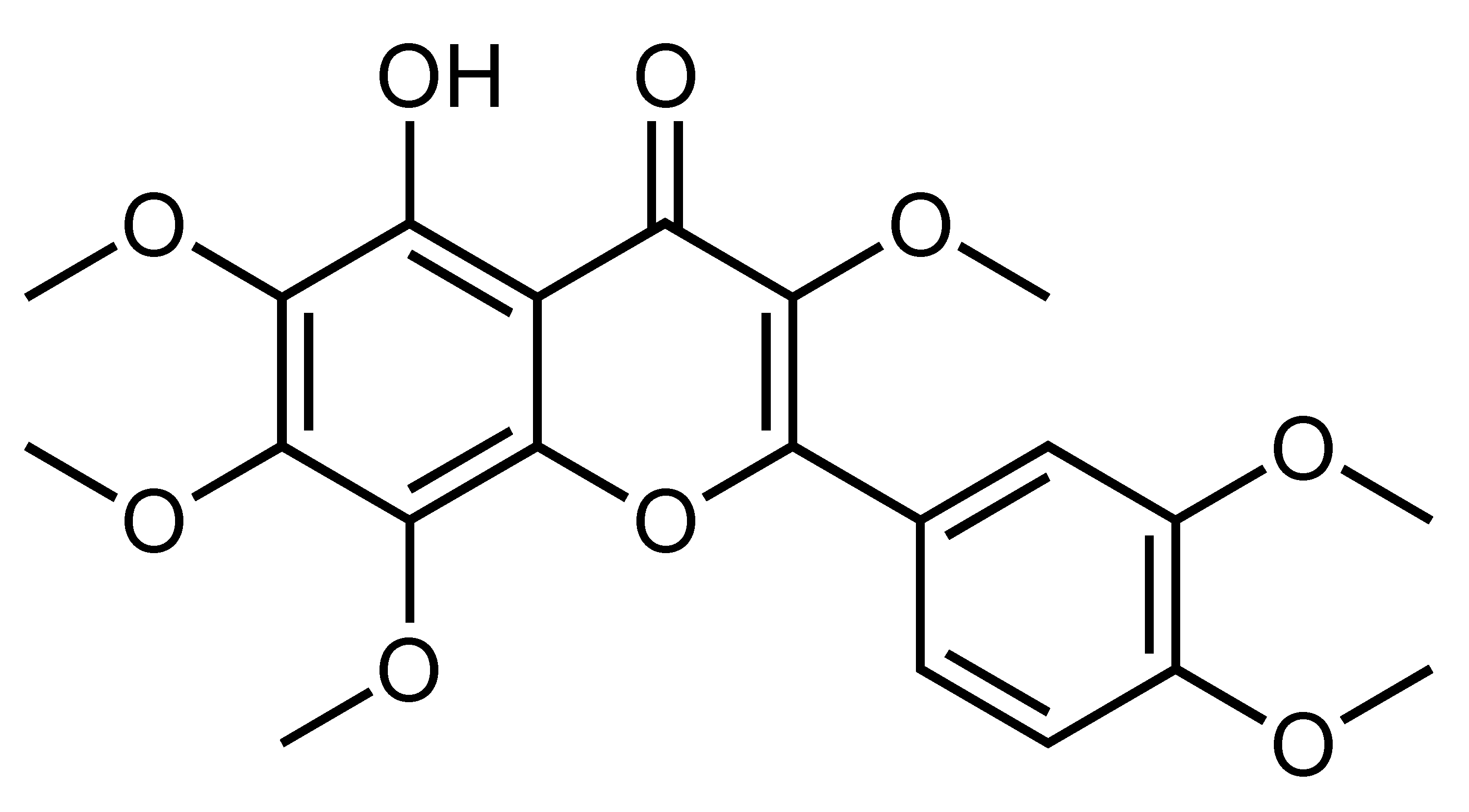 | Suppression of adipogenesis, Delay of cell cycle progression, Suppression of hepatic lipid accumulation | PPARγ, SREBP1, aP2, FAS, ACC, AMPK [97], C/EBPs, SIRT1 [96] | 3T3-L1 [96,97] | HFD-fed obese mice [97] |
| Anthocyanins | Flavonoids | C. sinensis L. Osbeck, etc. | 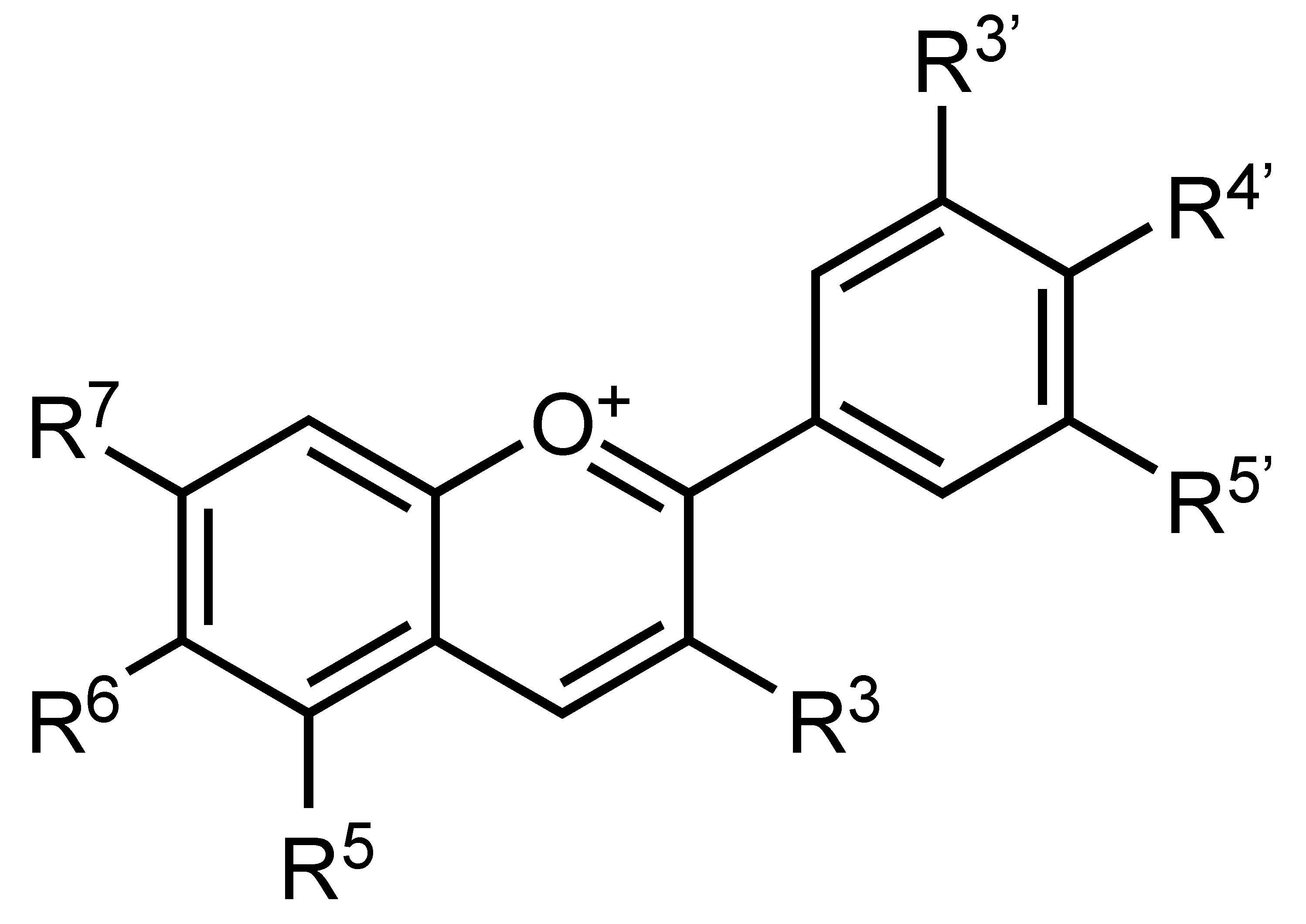 | Suppression of adipogenesis, Suppression of TG accumulation | ROS, adipokine, PPARγ, C/EBPα, SREBP-1c, ACCα, FAS and CSA [24] | 3T3-L1 [24] | |
| Cigranoside C, D, E, F | Flavonoids | C. grandis L. Osbeck, C. grandis Shatianyu, C. paradisi Mcfad, etc. | 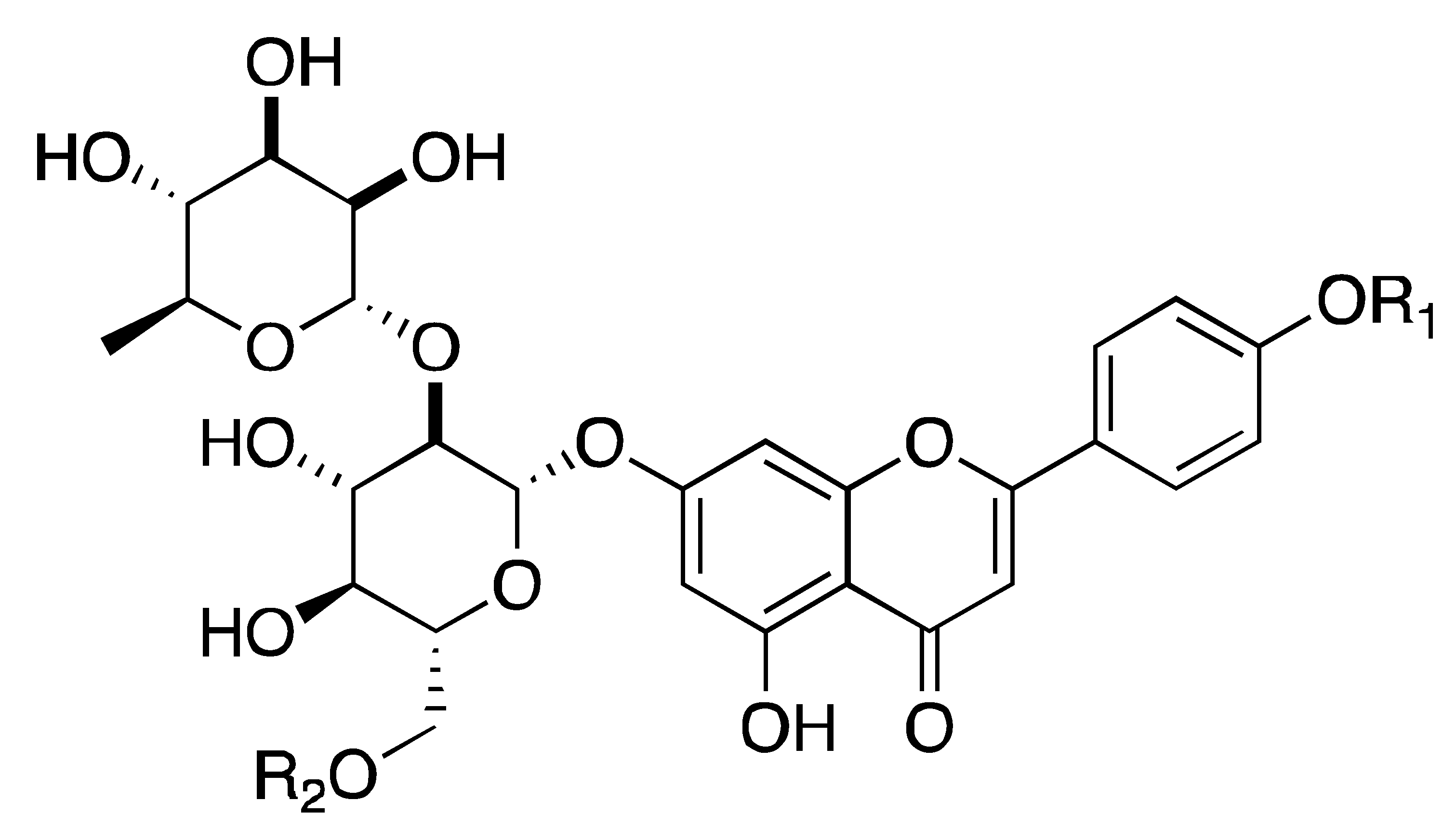 | Suppression of α-amylase, α-glucosidase, and PL | α-amylase, α-glucosidase, PL [98] | cell-free assay [98] | |
| Flavonoids | Constituent | Fruit Source | Chemical Structure | Mechanism | Molecular Pathways (Ref.) | In Vitro | In Vivo |
| Quercetin | Flavonol | C. reticulata, etc. | 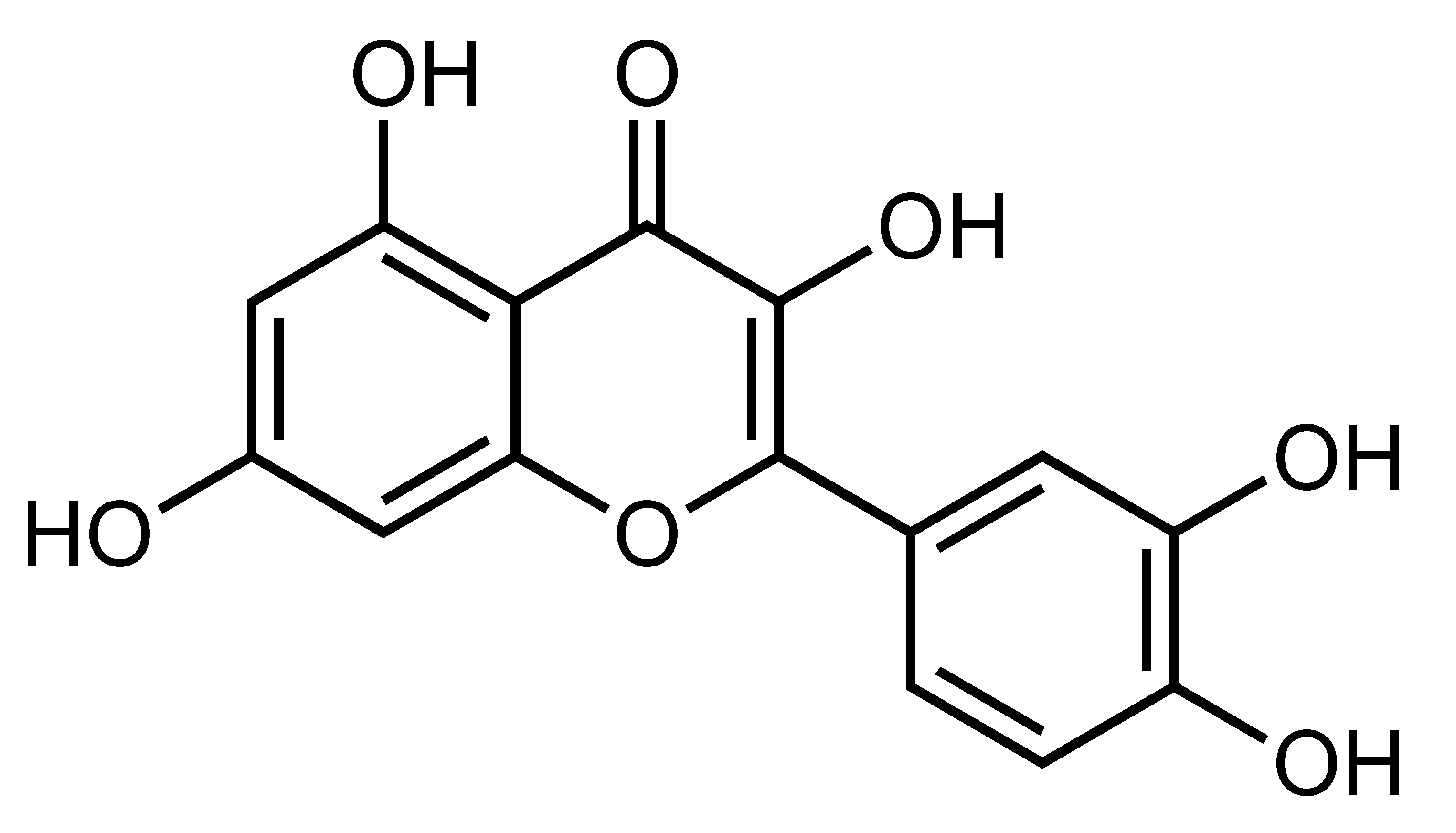 | Improvement of insulin sensitivity, Amelioration of hyperglycemia and hyperlipidemia, Suppression of serum insulin and C-peptide, Reduction of oxidative stress, Reduction of liver glycogen content | adiponectin, GPx, GST, SOD, GLUT-4, insulin receptor β-subunit [25], AMPK, p38, CaMKK, GLUT4 [99] | L6 myotubes [99] | NA/STZ-induced diabetic rats [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; Yip, Y.M. Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus. Future Pharmacol. 2023, 3, 14-37. https://doi.org/10.3390/futurepharmacol3010002
Lu K, Yip YM. Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus. Future Pharmacology. 2023; 3(1):14-37. https://doi.org/10.3390/futurepharmacol3010002
Chicago/Turabian StyleLu, Kaihui, and Yew Mun Yip. 2023. "Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus" Future Pharmacology 3, no. 1: 14-37. https://doi.org/10.3390/futurepharmacol3010002
APA StyleLu, K., & Yip, Y. M. (2023). Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus. Future Pharmacology, 3(1), 14-37. https://doi.org/10.3390/futurepharmacol3010002






