Unraveling the Impact of Salbutamol Polytherapy: Clinically Relevant Drug Interactions
Abstract
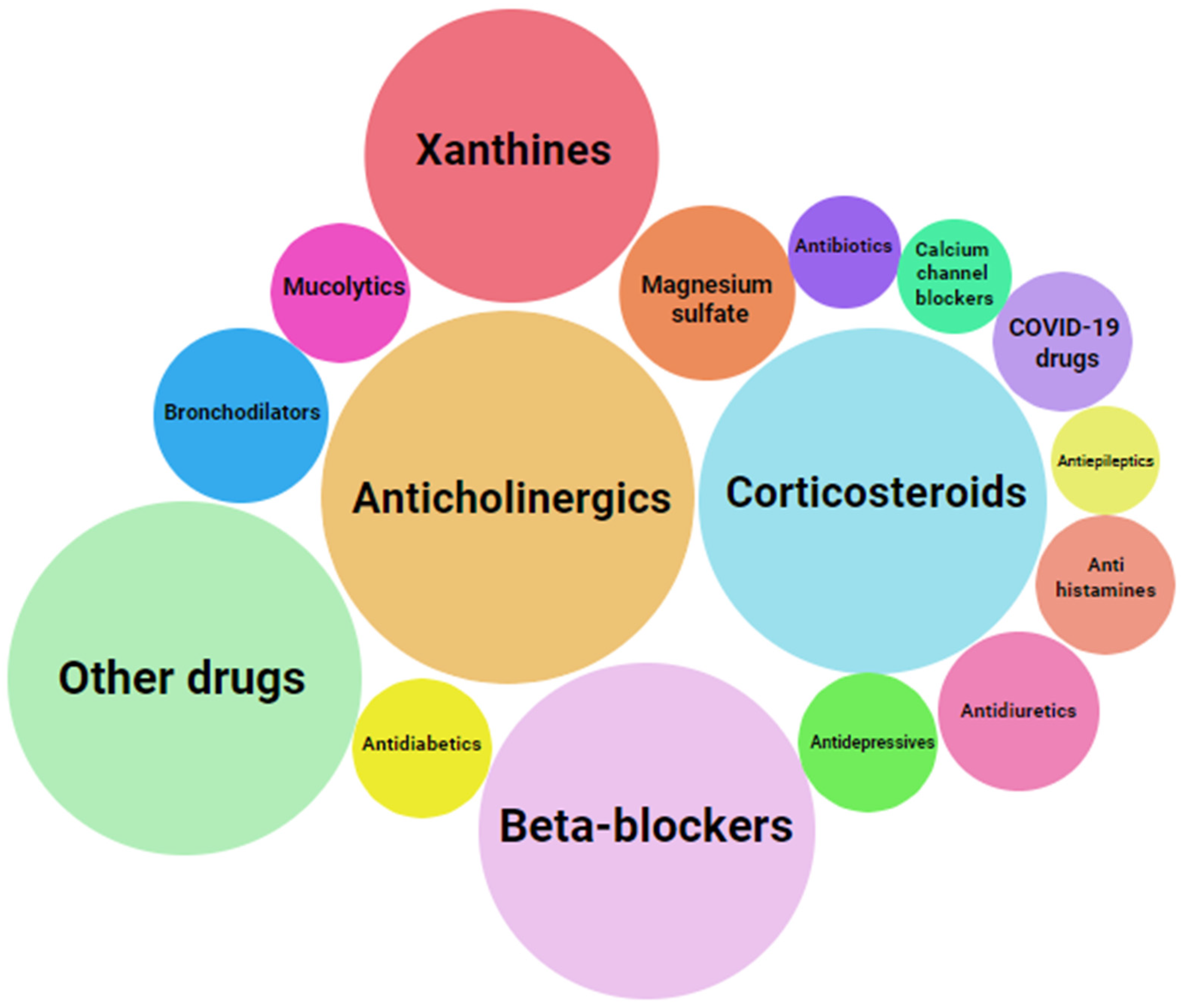
1. Introduction
2. DDIs of Salbutamol
2.1. Interaction with Other SABAs/LABAs
2.2. Interaction with Anticholinergics
2.3. Interaction with Corticosteroids
2.4. Interaction with Magnesium Sulfate
2.5. Interaction with Xanthines
2.6. Interaction with Mucolytics
2.7. Interaction with β-Blockers
2.8. Interaction with Antiepileptic Drugs
2.9. Interaction with Antidepressant Drugs
2.10. Interaction with Antihistamines
2.11. Interaction with Antidiabetic Drugs
2.12. Interaction with COVID-19 Drugs
2.13. Interaction with Diuretics
2.14. Other Interactions
3. Pharmacogenetics Considerations in DDI of Salbutamol
4. Future Perspectives: The Need of PK DDI Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.; Cheng, Z.; Xie, F. Evaluation of Pharmacokinetic Drug-Drug Interactions: A Review of the Mechanisms, In Vitro and In Silico Approaches. Metabolites 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Straubinger, R.M.; Mager, D.E. Pharmacodynamic Drug–Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1395–1406. [Google Scholar] [CrossRef]
- Haschke, M. Functional Interactions between P-Glycoprotein and CYP3A in Drug Metabolism. Expert Opin. Drug Metab. Toxicol. 2005, 1, 641–654. [Google Scholar] [CrossRef]
- Sikka, R.; Magauran, B.; Ulrich, A.; Shannon, M. Bench to Bedside: Pharmacogenomics, Adverse Drug Interactions, and the Cytochrome P450 System. Acad. Emerg. Med. 2005, 12, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Russell, D.W. Clinical Importance of the Cytochromes P450. Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Thiers, B.H. The Challenge of Managing Drug Interactions in Elderly People. Yearb. Dermatol. Dermatol. Surg. 2008, 2008, 250–251. [Google Scholar] [CrossRef]
- Calzetta, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. Dual LABA/LAMA Bronchodilators in Chronic Obstructive Pulmonary Disease: Why, When, and How. Expert Rev. Respir. Med. 2018, 12, 261–264. [Google Scholar] [CrossRef]
- Rogliani, P.; Ritondo, B.L.; Zerillo, B.; Matera, M.G.; Calzetta, L. Drug Interaction and Chronic Obstructive Respiratory Disorders. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100009. [Google Scholar] [CrossRef]
- Calzetta, L.; Matera, M.G.; Cazzola, M. Pharmacological Interaction between LABAs and LAMAs in the Airways: Optimizing Synergy. Eur. J. Pharmacol. 2015, 761, 168–173. [Google Scholar] [CrossRef]
- DrugBank Online. Salbutamol: Uses, Interactions, Mechanism of Action—DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01001 (accessed on 7 October 2022).
- Ahrens, R.C.; Smith, G.D.; Pharm, D. Albuterol: An Adrenergic Agent for Use in the Treatment of Asthma Pharmacology, Pharmacokinetics and Clinical Use. Pharmacotherapy 1984, 4, 105–120. [Google Scholar] [CrossRef]
- Morgan, D.; Paull, J.; Richmond, B.; Wilson-Evered, E.; Ziccone, S. Pharmacokinetics of Intravenous and Oral Salbutamol and Its Sulphate Conjugate. Br. J. Clin. Pharmacol. 1986, 22, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Schmekel, B.; Rydberg, I.; Norlander, B.; Sjöswärd, K.N.; Ahlner, J.; Andersson, R.G.G. Stereoselective Pharmacokinetics of S-Salbutamol after Administration of the Racemate in Healthy Volunteers. Eur. Respir. J. 1999, 13, 1230–1235. [Google Scholar] [CrossRef]
- Werner, H.A. Status Asthmaticus in Children: A Review. Chest 2001, 119, 1913–1929. [Google Scholar] [CrossRef]
- Gallelli, L.; Colosimo, M.; Pirritano, D.; Ferraro, M.; De Fazio, S.; Marigliano, N. Retrospective Evaluation of Adverse Drug Reactions Induced by Nonsteroidal Anti-inflammatory Drugs. Clin. Drug Investig. 2007, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Ferreri, G.; Colosimo, M.; Pirritano, D.; Flocco, M.; Pelaia, G. Retrospective Analysis of Adverse Drug Reactions to Bronchodilators Observed in Two Pulmonary Divisions of Catanzaro, Italy. Pharm. Res. 2003, 48, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Ferreri, G.; Colosimo, M.; Pirritano, D.; Guadagnino, L.; Pelaia, G. Adverse Drug Reactions to Antibiotics Observed in Two Pulmonology Divisions of Catanzaro, Italy: A Six-year Retrospective Study. Pharm. Res. 2002, 46, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic Drug-Drug Interaction and Their Implication in Clinical Management. J. Res. Med. Sci. 2013, 18, 600–609. [Google Scholar]
- Ajimura, C.M.; Jagan, N.; Morrow, L.E.; Malesker, M.A. Drug Interactions With Oral Inhaled Medications. J. Pharm. Technol. 2018, 34, 273–280. [Google Scholar] [CrossRef]
- 2022 GINA Main Report – Global Initiative for Asthma Guidelines – GINA. Available online: https://ginasthma.org/gina-reports/ (accessed on 15 October 2022).
- Looijmans-van den Akker, I.; Werkhoven, A.; Verheij, T. Over-Prescription of Short-Acting Beta Agonists in the Treatment of Asthma. Fam. Pract. 2021, 38, 612–616. [Google Scholar] [CrossRef]
- Smyth, E.T.; Pavord, I.D.; Wong, C.S.; Wisniewski, A.F.Z.; Williams, J.; Tattersfield, A.E. Interaction and Dose Equivalence of Salbutamol and Salmeterol in Patients with Asthma. Br. Med. J. 1993, 306, 543–545. [Google Scholar] [CrossRef]
- Blom, M.W.; Sommers, D.K. Comparative Pharmacology of Salmeterol and Formoterol and Their Interaction with Salbutamol in Healthy Volunteers. Clin. Drug Investig. 1997, 14, 400–404. [Google Scholar] [CrossRef]
- Adler, A.; Uziel, Y.; Mei-Zahav, M.; Horowitz, I. Formoterol Induces Tolerance to the Bronchodilating Effect of Salbutamol Following Methacholine-Provocation Test in Asthmatic Children. Pulm. Pharmacol. Ther. 2006, 19, 281–285. [Google Scholar] [CrossRef]
- DrugBank Online. Indacaterol: Uses, Interactions, Mechanism of Action—DrugBank Online. Available online: https://go.drugbank.com/drugs/DB05039 (accessed on 7 March 2023).
- Cazzola, M.; Rogliani, P.; Ruggeri, P.; Segreti, A.; Proietto, A.; Picciolo, S.; Matera, M.G. Chronic Treatment with Indacaterol and Airway Response to Salbutamol in Stable COPD. Respir. Med. 2013, 107, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Huang, Y.; Jiang, H.; Wang, P.; Zhang, L.; Ren, L.; Han, P. Albuterol combined with tiotropium bromide to improve the conditions and pulmonary functions of patients with acute exacerbation of chronic obstructive pulmonary disease. All Life 2020, 13, 668–674. [Google Scholar] [CrossRef]
- Ducharme, F.M.; Davis, G.M. Randomized Controlled Trial of Ipratropium Bromide and Frequent Low Doses of Salbutamol in the Management of Mild and Moderate Acute Pediatric Asthma. J. Pediatr. 1998, 133, 479–485. [Google Scholar] [CrossRef]
- Horner, D. Towards Evidence Based Emergency Medicine: Best BETs from the Manchester Royal Infirmary. Emerg. Med. J. 2018, 35, 270. [Google Scholar] [CrossRef]
- Reisman, J.; Galdes-Sebalt, M.; Kazim, F.; Canny, G.; Levison, H. Frequent Administration by Inhalation of Salbutamol and Ipratropium Bromide in the Initial Management of Severe Acute Asthma in Children. J. Allergy Clin. Immunol. 1988, 81, 16–20. [Google Scholar] [CrossRef]
- Chakraborti, A.; Lodha, R.; Pandey, R.M.; Kabra, S.K. Randomized Controlled Trial of Ipratropium Bromide and Salbutamol versus Salbutamol Alone in Children with Acute Exacerbation of Asthma. Indian J. Pediatr. 2006, 73, 979–983. [Google Scholar] [CrossRef]
- Harumdini, M.; Supriyatno, B.; Sekartini, R. Efficacy of Salbutamol—Ipratropium Bromide Nebulization Compared to Salbutamol Alone in Children with Mild to Moderate Asthma Attacks. Paediatr. Indones. 2012, 52, 200. [Google Scholar] [CrossRef]
- O’Driscoll, B.R.; Horsley, M.G.; Taylor, R.J.; Chambers, D.K.; Bernstein, A. Nebulised Salbutamol With and Without Ipratropium Bromide in Acute Airflow Obstruction. Lancet 1989, 333, 1418–1420. [Google Scholar] [CrossRef]
- Iramain, R.; López-Herce, J.; Coronel, J.; Spitters, C.; Guggiari, J.; Bogado, N. Inhaled Salbutamol plus Ipratropium in Moderate and Severe Asthma Crises in Children. J. Asthma 2011, 48, 298–303. [Google Scholar] [CrossRef]
- Hong, J.; Bao, Y.; Chen, A.; Li, C.; Xiang, L.; Liu, C.; Chen, Z.; Zhao, D.; Fu, Z.; Shang, Y. Chinese Guidelines for Childhood Asthma 2016: Major Updates, Recommendations and Key Regional Data. J. Asthma 2018, 55, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Karadag, B.; Ceran, O.; Guven, G.; Dursun, E.; Ipek, I.O.; Karakoc, F.; Ersu, R.H.; Bozaykut, A.; Inan, S.; Dagli, E. Efficacy of salbutamol and ipratropium bromide in the management of acute bronchiolitis--a clinical trial. Respiration 2008, 76, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the Diagnosis and Management of Asthma 2007 (EPR-3)—NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma (accessed on 7 October 2022).
- Griffiths, B.; Ducharme, F.M. Combined Inhaled Anticholinergics and Short-Acting Beta2-Agonists for Initial Treatment of Acute Asthma in Children. Paediatr. Respir. Rev. 2013, 14, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tong, L.; Gao, P.; Hu, Y.; Wang, H.; Chen, Z.; Fang, L. Combination of Ipratropium Bromide and Salbutamol in Children and Adolescents with Asthma: A Meta-Analysis. PLoS ONE 2021, 16, e0237620. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, S.W.; Vandenberghe, C.; Voaklander, B.; Nikel, T.; Campbell, S.; Rowe, B.H. Combined Inhaled Beta-Agonist and Anticholinergic Agents for Emergency Management in Adults with Asthma. Cochrane Database Syst. Rev. 2017, 2017, CD001284. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Congleton, J.; Page, R.L.; Pearson, S.B.; Muers, M.F. Comparison of Nebulised Salbutamol and Ipratropium Bromide with Salbutamol Alone in the Treatment of Chronic Obstructive Pulmonary Disease. Thorax 1995, 50, 834–837. [Google Scholar] [CrossRef]
- Shrestha, M.; O’Brien, T.; Haddox, R.; Scott Gourlay, H.; Reed, G. Decreased Duration of Emergency Department Treatment of Chronic Obstructive Pulmonary Disease Exacerbations with the Addition of Ipratropium Bromide to β-Agonist Therapy. Ann. Emerg. Med. 1991, 20, 1206–1209. [Google Scholar] [CrossRef]
- Taitinen, L.A.; Poppius, H. Combination of Oxitropium Bromide and Salbutamol in the Treatment of Asthma with Pressurized Aerosols. Br. J. Dis. Chest 1986, 80, 179–186. [Google Scholar] [CrossRef]
- Wempe, J.; Postma, D.S.; Breederveld, N.; Alting-Hebing, D.; Van Der Mark, T.W.; Koëter, G.H. Separate and Combined Effects of Corticosteroids and Bronchodilators on Airflow Obstruction and Airway Hyperresponsiveness in Asthma. J. Allergy Clin. Immunol. 1992, 89, 679–687. [Google Scholar] [CrossRef]
- Methacholine Challenge Test. American Lung Association. Available online: https://www.lung.org/lung-health-diseases/lung-procedures-and-tests/methacholine-challenge-test (accessed on 14 January 2023).
- Chipps, B.E.; Albers, F.C.; Reilly, L.; Johnsson, E.; Cappelletti, C.; Papi, A. Efficacy and Safety of As-Needed Albuterol/Budesonide versus Albuterol in Adults and Children Aged ≥4 Years with Moderate-to-Severe Asthma: Rationale and Design of the Randomised, Double-Blind, Active-Controlled MANDALA Study. BMJ Open Respir. Res. 2021, 8, e001077. [Google Scholar] [CrossRef]
- Papi, A.; Chipps, B.E.; Beasley, R.; Panettieri, R.A.; Israel, E.; Cooper, M.; Dunsire, L.; Jeynes-Ellis, A.; Johnsson, E.; Rees, R.; et al. Albuterol–Budesonide Fixed-Dose Combination Rescue Inhaler for Asthma. New Engl. J. Med. 2022, 386, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Tang, Y.; Liu, G.; Chen, Q. Effects of Budesonide Combined with Salbutamol on Pulmonary Function and Peripheral Blood Eosinophiles and IgE in Patients with Acute Attack of Bronchial Asthma. Pak. J. Med. Sci. 2022, 38, 1495. [Google Scholar] [CrossRef] [PubMed]
- Quan-Jun, Y.; Jian-Ping, Z.; Jian-Hua, Z.; Yong-Long, H.; Bo, X.; Jing-Xian, Z.; Bona, D.; Yuan, Z.; Cheng, G. Distinct Metabolic Profile of Inhaled Budesonide and Salbutamol in Asthmatic Children during Acute Exacerbation. Basic Clin. Pharmacol. Toxicol. 2017, 120, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Aldrey, O.E.; Añez, H.; Deibis, L.; Tassinari, P.; Isturiz, G.; Bianco, N.E. A Double-Blind, Cross-over Study Using Salbutamol, Beclomethasone, and a Combination of Both in Bronchial Asthma. J. Asthma 1995, 32, 21–28. [Google Scholar] [CrossRef]
- Chhabra, S. A Comparison of Inhaled Salbutamol with a Combination of Salbutamol and Beclomethasone Dipropionate in Moderately Severe Asthma. Indian J. Chest Dis. Allied Sci. 1994, 36, 119–124. [Google Scholar]
- Descalzi, D.; Folli, C.; Nicolini, G.; Riccio, A.M.; Gamalero, C.; Scordamaglia, F.; Canonica, G.W. Anti-Proliferative and Anti-Remodelling Effect of Beclomethasone Dipropionate, Formoterol and Salbutamol Alone or in Combination in Primary Human Bronchial Fibroblasts. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 432–437. [Google Scholar] [CrossRef]
- Estrada-Reyes, E.; Del Río-Navarro, B.E.; Rosas-Vargas, M.A.; Nava-Ocampo, A.A. Co-Administration of Salbutamol and Fluticasone for Emergency Treatment of Children with Moderate Acute Asthma. Pediatr. Allergy Immunol. 2005, 16, 609–614. [Google Scholar] [CrossRef]
- Silvanus, M.T.; Groeben, H.; Peters, J. Corticosteroids and Inhaled Salbutamol in Patients with Reversible Airway Obstruction Markedly Decrease the Incidence of Bronchospasm after Tracheal Intubation. Anesthesiology 2004, 100, 1052–1057. [Google Scholar] [CrossRef]
- Hurme, P.; Homil, K.; Lehtinen, P.; Turunen, R.; Vahlberg, T.; Vuorinen, T.; Camargo, C.A.; Gern, J.E.; Jartti, T. Efficacy of Inhaled Salbutamol with and without Prednisolone for First Acute Rhinovirus-Induced Wheezing Episode. Clin. Exp. Allergy 2021, 51, 1121–1132. [Google Scholar] [CrossRef]
- Taylor, D.R.; Wilkins, G.T.; Herbison, G.P.; Flannery, E.M. Interaction between Corticosteroid and β-Agonist Drugs; Biochemical and Cardiovascular Effects in Normal Subjects. Chest 1992, 102, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Kose, M.; Ozturk, M.A.; Poyrazoğlu, H.; Elmas, T.; Ekinci, D.; Tubas, F.; Kurt, T.; Goktas, M.A. The Efficacy of Nebulized Salbutamol, Magnesium Sulfate, and Salbutamol/Magnesium Sulfate Combination in Moderate Bronchiolitis. Eur. J. Pediatr. 2014, 173, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, H.A.; El-Garhy, O.H.; Ali, M.A.; Youssef, N.A. The Efficacy of Nebulized Magnesium Sulfate Alone and in Combination with Salbutamol in Acute Asthma. Drug Des. Devel. Ther. 2016, 10, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Sweeney, J.; Rumantir, M.; Coates, A.L.; Willan, A.R.; Stephens, D.; Atenafu, E.G.; Finkelstein, Y.; Thompson, G.; Zemek, R.; et al. Effect of Nebulized Magnesium vs Placebo Added to Albuterol on Hospitalization among Children with Refractory Acute Asthma Treated in the Emergency Department: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2020, 324, 2038–2047. [Google Scholar] [CrossRef]
- Diao, M.; Min, J.; Guo, F.; Zhang, C.L. Effects of Salbutamol Aerosol Combined with Magnesium Sulfate on T-Lymphocyte Subgroup and Th1/Th2 Cytokines of Pediatric Asthma. Exp. Ther. Med. 2017, 13, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Rogala, B.; Bozek, A.; Gluck, J.; Jarzab, J. Prevalence of IgE-Mediated Allergy and Evaluation of Th1/Th2 Cytokine Profiles in Patients with Severe Bronchial Asthma. Adv. Dermatol. Allergol. 2015, 4, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alokail, M.S.; Draz, H.M.; Abd-Alrahman, S.H.; Yakout, S.M.; Clerici, M. Th1/Th2 Cytokine Pattern in Arab Children with Severe Asthma. Int. J. Clin. Exp. Med. 2014, 7, 2286–2291. [Google Scholar]
- Yamada, Y.; Matsumoto, K.; Hashimoto, N.; Saikusa, M.; Homma, T.; Yoshihara, S.; Saito, H. Effect of Th1/Th2 Cytokine Pretreatment on RSV-Induced Gene Expression in Airway Epithelial Cells. Int. Arch. Allergy Immunol. 2011, 154, 185–194. [Google Scholar] [CrossRef]
- Taylor, D.R.; Buick, B.; Kinney, C.; Lowry, R.C.; McDevitt, D.G. The Efficacy of Orally Administered Theophylline, Inhaled Salbutamol, and a Combination of the Two as Chronic Therapy in the Management of Chronic Bronchitis with Reversible Air-Flow Obstruction. Am. Rev. Respir. Dis. 1985, 131, 747–751. [Google Scholar] [CrossRef]
- Nishimura, K.; Koyama, H.; Ikeda, A.; Sugiura, N.; Kawakatsu, K.; Izumi, T. The Additive Effect of Theophylline on a High-Dose Combination of Inhaled Salbutamol and Ipratropium Bromide in Stable COPD. Chest 1995, 107, 718–723. [Google Scholar] [CrossRef]
- Thomas, P.; Pugsley, J.A.; Stewart, J.H. Theophylline and Salbutamol Improve Pulmonary Function in Patients with Irreversible Chronic Obstructive Pulmonary Disease. Chest 1992, 101, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.; Whiting, B.; Meredith, P.; Addis, G. Theophylline-salbutamol Interaction: Bronchodilator Response to Salbutamol at Maximally Effective Plasma Theophylline Concentrations. Br. J. Clin. Pharmacol. 1981, 11, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.P.; Fergusson, D.M. Effects of Oral Theophylline and Oral Salbutamol. Arch. Dis. Child. 1982, 57, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Whyte, K.; Reid, C.; Addis, G.; Whitesmith, R.; Reid, J. Salbutamol Induced Hypokalaemia: The Effect of Theophylline Alone and in Combination with Adrenaline. Br. J. Clin. Pharmacol. 1988, 25, 571–578. [Google Scholar] [CrossRef]
- Eidelman, D.H.; Sami, M.H.; McGregor, M.; Cosio, M.G. Combination of Theophylline and Salbutamol for Arrhythmias in Severe COPD. Chest 1987, 91, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Ezeamuzie, C.I.; Shihab, P.K. Interactions between Theophylline and Salbutamol on Cytokine Release in Human Monocytes. J. Pharmacol. Exp. Ther. 2010, 334, 302–309. [Google Scholar] [CrossRef]
- Amirav, I.; Amitai, Y.; Avital, A.; Godfrey, S. Enhancement of Theophylline Clearance by Intravenous Albuterol. Chest 1988, 94, 444–445. [Google Scholar] [CrossRef]
- Rivington, R.N.; Boulet, L.P.; Cote, J.; Kreisman, H.; Small, D.I.; Alexander, M.; Day, A.; Harsanyi, Z.; Darke, A.C. Efficacy of Uniphyl®, Salbutamol, and Their Combination in Asthmatic Patients on High-Dose Inhaled Steroids. Am. J. Respir. Crit. Care Med. 1995, 151, 325–332. [Google Scholar] [CrossRef]
- Gupta, R.; Wadhwa, R. Mucolytic Medications; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Wang, Y.; Lu, J.; Li, T.; Zhao, S.; Yang, W.; Liu, L.; Shi, X.; Ding, L. Investigation of a Potential Drug-Drug Interaction between Salbutamol and Ambroxol and Bioequivalence of a New Fixed-Dose Combination Containing These Two Drugs in Healthy Chinese Subjects. Int. J. Pharmacol. Ther. 2018, 56, 247. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Li, T.; Zhao, S.; Yang, W.; Liu, L.; Shi, X.; Michael, M.; Ding, L. Pharmacokinetics and Safety of Salbutamol/Ambroxol Fixed-Dose Combination Granules in Healthy Chinese Subjects. Int. J. Pharmacol. Ther. 2018, 56, 597. [Google Scholar] [CrossRef]
- Bennett, M.; Chang, C.L.; Tatley, M.; Savage, R.; Hancox, R.J. The Safety of Cardioselective Β1-Blockers in Asthma: Literature Review and Search of Global Pharmacovigilance Safety Reports. ERJ Open Res. 2021, 7, 00801. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, M.J.; Weinacker, A.B. β-Adrenergic-Blocking Agents in Bronchospastic Diseases: A Therapeutic Dilemma. Pharmacotherapy 1999, 19, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Chang, C.L.; Tuffery, C.; Hopping, S.; Hancox, R.J. The Impact of Regular Bisoprolol on the Response to Salbutamol in Asthma: A Double-Blind Randomized Placebo-Controlled Crossover Trial. Respirology 2021, 26, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Minton, N.A.; Baird, A.R.; Henry, J.A. Modulation of the Effects of Salbutamol by Propranolol and Atenolol. Eur. J. Clin. Pharmacol. 1989, 36, 449–453. [Google Scholar] [CrossRef]
- Anavekar, S.; Barter, C.; Adam, W.; Doyle, A. A Double-Blind Comparison of Verapamil and Labetalol in Hypertensive Patients with Coexisting Chronic Obstructive Airways Disease. J. Cardiovasc. Pharmacol. 1982, 4, S374–S377. [Google Scholar] [CrossRef]
- Tucker, W.; Sankar, P.; Theetha Kariyanna, P. Selective Beta-1-Blockers; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Salpeter, S.R.; Ormiston, T.M.; Salpeter, E.E. Cardioselective β-Blockers in Patients with Reactive Airway Disease: A Meta-Analysis. Ann. Intern. Med. 2002, 137, 715–725. [Google Scholar] [CrossRef]
- Sheppard, D.; DiStefano, S.; Byrd, R.C.; Eschenbacher, W.L.; Bell, V.; Steck, J.; Laddu, A. Effects of Esmolol on Airway Function in Patients with Asthma. J. Clin. Pharmacol. 1986, 26, 169–174. [Google Scholar] [CrossRef]
- Horn, J.R.; Hansten, P.D. Beta-Blockers and Beta-Agonists: What Is the Risk? Pharm. Times 2013, 79, 412–418. [Google Scholar]
- Adam, W.; Meagher, E.; Barter, C. Labetalol, Beta Blockers, and Acute Deterioration of Chronic Airway Obstruction. Clin. Exp. Hypertens. A 1982, 4, 1419–1428. [Google Scholar] [CrossRef]
- Dunn, T.L.; Gerber, M.J.; Shen, A.S.; Fernandez, E.; Iseman, M.D.; Cherniack, R.M. The Effect of Topical Ophthalmic Instillation of Timolol and Betaxolol on Lung Function in Asthmatic Subjects. Am. Rev. Respir. Dis. 1986, 133, 264–268. [Google Scholar] [CrossRef]
- Buckley, M.M.T.; Goa, K.L.; Clissold, S.P. Ocular Betaxolol: A Review of Its Pharmacological Properties, and Therapeutic Efficacy in Glaucoma and Ocular Hypertension. Drugs 1990, 40, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E. Addressing the Burden of Epilepsy: Many Unmet Needs. Pharmacol. Res. 2016, 107, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Świąder, M.; Zakrocka, I.; Świąder, K.; Zawadzki, A.; Łuszczki, J.J.; Czuczwar, S.J.; Munir, D. Influence of Salbutamol on the Anticonvulsant Potency of the Antiepileptic Drugs in the Maximal Electroshock-Induced Seizures in Mice. Pharmacol. Rep. 2019, 71, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.; Vale, N. Salbutamol in the Management of Asthma: A Review. Int. J. Mol. Sci. 2022, 23, 14207. [Google Scholar] [CrossRef]
- Uysalol, M.; Yildiz, R.; Ozunal, Z.G. Is Seizure an Adverse Effect of Salbutamol in the Pediatric Population? Balk. Med. J. 2022, 39, 340. [Google Scholar] [CrossRef] [PubMed]
- Drug Interaction Report: Endep, Ventolin. Available online: https://www.drugs.com/interactions-check.php?drug_list=168-3168,109-2463 (accessed on 7 March 2023).
- RxList.com. Ventolin HFA Side Effects Center. Available online: https://www.rxlist.com/ventolin-hfa-side-effects-drug-center.htm#overview (accessed on 14 January 2023).
- Camargos, P.A.M.; Rodrigues, M.E.S.M.; Solé, D.; Scheinmann, P. Asma e Rinite Alérgica Como Expressão de Uma Única Doença: Um Paradigma Em Construção. J. Pediatr. 2002, 78, 123–128. [Google Scholar] [CrossRef]
- Carpentiere, G.; Marino, S.; Castello, F.; Bonanno, C.T.; Baldanza, C. Effect of Ketotifen on the Bronchodilatation Induced by Salbutamol. Respiration 1988, 54, 190–192. [Google Scholar] [CrossRef]
- Advenier, C.; Candenas, M.L.; Naline, E.; De Vos, C. The Effect of Cetirizine on the Human Isolated Bronchus: Interaction with Salbutamol. J. Allergy Clin. Immunol. 1991, 88, 104–113. [Google Scholar] [CrossRef]
- Torres, R.M.; Souza, M.D.S.; Coelho, A.C.C.; De Mello, L.M.; Souza-Machado, C. Association between Asthma and Type 2 Diabetes Mellitus: Mechanisms and Impact on Asthma Control—A Literature Review. Can. Respir. J. 2021, 2021, 8830439. [Google Scholar] [CrossRef]
- Fogli, S.; Stefanelli, F.; Picchianti, L.; Del Re, M.; Mey, V.; Bardelli, C.; Danesi, R.; Breschi, M.C. Synergistic Interaction between PPAR Ligands and Salbutamol on Human Bronchial Smooth Muscle Cell Proliferation. Br. J. Pharmacol. 2013, 168, 266–275. [Google Scholar] [CrossRef]
- Fogli, S.; Pellegrini, S.; Adinolfi, B.; Mariotti, V.; Melissari, E.; Betti, L.; Fabbrini, L.; Giannaccini, G.; Lucacchini, A.; Bardelli, C.; et al. Rosiglitazone Reverses Salbutamol-Induced β 2-Adrenoceptor Tolerance in Airway Smooth Muscle. Br. J. Pharmacol. 2011, 162, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.A.; Tangiisuran, B.; Kharshid, A.M.; Abdul-Aziz, N.; Hassan, Y.; Aziz, N.A.; Elsayed, T.M. Drug-Drug Interaction-Related Uncontrolled Glycemia. J. Pharm. Bioallied Sci. 2017, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 and Asthma: What Patients Need to Know. Available online: https://www.aaaai.org/Tools-for-the-Public/Conditions-Library/Asthma/covid-prevent (accessed on 14 January 2023).
- If You Get COVID 19 and Have Asthma. Available online: https://www.asthma.org.uk/advice/triggers/coronavirus-covid-19/covid-19-and-asthma/ (accessed on 14 January 2023).
- Azanza, J.R.; Mensa, J.; Castillo, J.G.; Del Rufo, M.L.; Molero, J.M.; Valle, N.M.; Barberán, J. Interactions Listed in the Paxlovid Fact Sheet, Classified According to Risks, Pharmacological Groups, and Consequences. Rev. Esp. Quimioter. 2022, 35, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Anrys, P.; Petit, A.E.; Thevelin, S.; Sallevelt, B.; Drenth, C.; Soiza, R.L.; Correa-Pérez, A.; Dalleur, O.; De Brauwer, I.; Petrovic, M.; et al. An International Consensus List of Potentially Clinically Significant Drug-Drug Interactions in Older People. J. Am. Med. Dir. Assoc. 2021, 22, 2121–2133.e24. [Google Scholar] [CrossRef]
- Saba, M.; Davoodabadi, A.; Ghaffari, A.; Gilasi, H.; Haghpanah, B. Combination Adjunctive Nebulized Furosemide and Salbutamol versus Single Agent Therapy in COPD Patients: A Randomized Controlled Trial. Ann. Med. Surg. 2020, 57, 85–90. [Google Scholar] [CrossRef]
- Bebitoğlu, B.T.; Oğuz, E.; Nuhoğlu, Ç.; Dalkılıç, A.E.K.; Çirtlik, P.; Temel, F.; Hodzic, A. Evaluation of Potential Drug-Drug Interactions in a Pediatric Population. Turk. Arch. Pediatr. 2020, 55, 30–38. [Google Scholar] [CrossRef]
- GlaxoSmithKline. Ventolin ® HFA. Cps 2014, 1–24. [Google Scholar]
- Karttunen, P.; Tukiainen, H.; Silvasti, M.; Kolonen, S. Antitussive Effect of Dextromethorphan and Dextromethorphan-Salbutamol Combination in Healthy Volunteers with Artificially Induced Cough. Respiration 1987, 4, 88–100. [Google Scholar] [CrossRef]
- Silvasti, M.; Karttunen, P.; Happonen, P.; Mykkanen, M.; Romppanen, T.; Tukiainen, H. Pharmacokinetic Comparison of a Dextromethorphan-Salbutamol Combination Tablet and a Plain Dextromethorphan Tablet. Int. J. Clin. Pharm. Ther. Toxicol. 1990, 28, 268–272. [Google Scholar]
- Albuterol Drug Interactions—Drugs.Com. Available online: https://www.drugs.com/drug-interactions/albuterol-index.html (accessed on 16 January 2023).
- Furusho, K.; Nishikawa, K.; Sasaki, S.; Akasaka, T.; Arita, M.; Edwards, A. The Combination of Nebulized Sodium Cromoglycate and Salbutamol in the Treatment of Moderate-to-Severe Asthma in Children. Pediatr. Allergy Immunol. 2002, 13, 209–216. [Google Scholar] [CrossRef]
- Pfleger, A.; Eber, E.; Weinhandl, E.; Zach, M.S. Effects of Nedocromil and Salbutamol on Airway Reactivity in Children with Asthma. Eur. Respir. J. 2002, 20, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, G.L.; Trinquet, G.; Harper, A.E. Compliance with Nedocromil Sodium and a Nedocromil Sodium/Salbutamol Combination. Eur. Respir. J. 1996, 9, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.G.; Brunel, P.; Nell, G.; Quinn, G.; Kaik, G.A. Benazepril, an Angiotensin Converting Enzyme Inhibitor: Drug Interaction with Salbutamol and Bronchial Response to Histamine in Normal Subjects. Br. J. Clin. Pharmacol. 1997, 44, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Q.; Liu, Z.; Li, R.J.; Pan, P.W.; Hou, Y.Y.; Lu, W.G.; Bai, G. The Synergistic Anti-Asthmatic Effects of Glycyrrhizin and Salbutamol. Acta Pharmacol. Sin. 2010, 31, 443–449. [Google Scholar] [CrossRef]
- Bethke, T.; Giessmann, T.; Westphal, K.; Weinbrenner, A.; Hauns, B.; Hauschke, D.; David, M.; Lahu, G.; Zech, K.; Hermann, R.; et al. Roflumilast, a Once-Daily Oral Phosphodiesterase 4 Inhibitor, Lacks Relevant Pharmacokinetic Interactions with Inhaled Salbutamol When Co-Administered in Healthy Subjects. Int. J. Clin. Pharm. Ther. 2006, 44, 572–579. [Google Scholar] [CrossRef]
- Murdoch, R.D.; Cowley, H.; Kelly, J.; Higgins, R.; Webber, D. Cilomilast (Ariflo®) Does Not Potentiate the Cardiovascular Effects of Inhaled Salbutamol. Pulm. Pharmacol. Ther. 2002, 15, 521–527. [Google Scholar] [CrossRef]
- HAUNS, B. Pharmacokinetics of the Selective PDE4 Inhibitor Roflumilast and Its Active Metabolite Roflumilast N-Oxide Are Not Affected by Concomitant Budesonide, Salbutamol, or Erythromycin*1. J. Allergy Clin. Immunol. 2004, 113, S222. [Google Scholar] [CrossRef]
- Calzetta, L.; Page, C.P.; Spina, D.; Cazzola, M.; Rogliani, P.; Facciolo, F.; Matera, M.G. Effect of the Mixed Phosphodiesterase 3/4 Inhibitor RPL554 on Human Isolated Bronchial Smooth Muscle Tone. J. Pharmacol. Exp. Ther. 2013, 346, 414–423. [Google Scholar] [CrossRef]
- Singh, D.; Abbott-Banner, K.; Bengtsson, T.; Newman, K. The Short-Term Bronchodilator Effects of the Dual Phosphodiesterase 3 and 4 Inhibitor RPL554 in COPD. Eur. Respir. J. 2018, 52, 1801074. [Google Scholar] [CrossRef]
- Gugger, M.; Marco Crivelli, A.; Galeazzi, R.L.; Bachofen, H. Effects of the Calcium Antagonist Tiapamil on Pulmonary Gas Exchange in Patients with Chronic Obstructive Pulmonary Disease Who Receive Salbutamol. Clin. Pharmacol. Ther. 1985, 38, 96–100. [Google Scholar] [CrossRef]
- Rolla, G.; Arossa, W.; Bucca, C.; Bugiani, M. Effect of Nifedipine on Salbutamol-Induced Bronchodilation in Partially Reversible Airway Obstruction. Int. J. Clin. Pharm. Res. 1986, 6, 409–413. [Google Scholar]
- Rao, C.; Shenoy, V.; Udaykumar, P. Potential Drug-Drug Interactions in the Pediatric Intensive Care Unit of a Tertiary Care Hospital. J. Pharmacol. Pharmacother. 2019, 10, 63–68. [Google Scholar] [CrossRef]
- Spanakis, M.; Patelarou, A.; Patelarou, E.; Tzanakis, N. Drug Interactions for Patients with Respiratory Diseases Receiving Covid-19 Emerged Treatments. Int. J. Environ. Res. Public Health 2021, 18, 11711. [Google Scholar] [CrossRef] [PubMed]
- Mahboobipour, A.A.; Baniasadi, S. Clinically Important Drug–Drug Interactions in Patients Admitted to Hospital with COVID-19: Drug Pairs, Risk Factors, and Management. Drug Metab. Pers. Ther. 2021, 36, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, J.E.; Kelly, R.S.; Lutz, S.M.; Lasky-Su, J.; Wu, A.C. Pharmacogenetics of Bronchodilator Response: Future Directions. Curr. Allergy Asthma Rep. 2021, 21, 47. [Google Scholar] [CrossRef]
- Aneesh, T.P.; Sekhar, S.; Jose, A.; Chandran, L.; Zachariah, S.M. The Right Drug to the Right Person. J. Clin. Med. Res. 2009, 1, 191–194. [Google Scholar] [CrossRef]
- Pharmacogenomics: What Does It Mean for Your Health?—CDC. Available online: https://www.cdc.gov/genomics/disease/pharma.htm (accessed on 20 January 2023).
- Hikino, K.; Kobayashi, S.; Ota, E.; Mushiroda, T.; Urayama, K.Y.; Kobayashi, T. A Meta-Analysis of the Influence of ADRB2 Genetic Polymorphisms on Albuterol (Salbutamol) Therapy in Patients with Asthma. Br. J. Clin. Pharmacol. 2021, 87, 1708–1716. [Google Scholar] [CrossRef]
- Ortega, V.E.; Meyers, D.A.; Bleecker, E.R. Asthma Pharmacogenetics and the Development of Genetic Profiles for Personalized Medicine. Pharmgenom. Pers. Med. 2015, 8, 9–22. [Google Scholar] [CrossRef]
- Broadley, K.J. β-Adrenoceptor Responses of the Airways: For Better or Worse? Eur. J. Pharmacol. 2006, 533, 15–27. [Google Scholar] [CrossRef]
- García-Menaya, J.M.; Cordobés-Durán, C.; García-Martín, E.; Agúndez, J.A.G. Pharmacogenetic Factors Affecting Asthma Treatment Response. Potential Implications for Drug Therapy. Front. Pharmacol. 2019, 10, 520. [Google Scholar] [CrossRef]
- Hall, I.P.; Wheatley, A.; Wilding, P.; Liggett, S.B. Association of Glu 27 Β2-Adrenoceptor Polymorphism with Lower Airway Reactivity in Asthmatic Subjects. Lancet 1995, 345, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R. β-Adrenergic Receptor Polymorphisms: Relationship to the β-Agonist Controversy and Clinical Implications. Expert Opin. Pharmacother. 2007, 8, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Israel, E.; Drazen, J.M.; Liggett, S.B.; Boushey, H.A.; Cherniack, R.M.; Chinchilli, V.M.; Cooper, D.M.; Fahy, J.V.; Fish, J.E.; Ford, J.G.; et al. The Effect of Polymorphisms of the Beta(2)-Adrenergic Receptor on the Response to Regular Use of Albuterol in Asthma (International Archives of Allergy and Immunology (2000) 162 (75–80)). Int. Arch. Allergy Immunol. 2012, 157, 212. [Google Scholar] [CrossRef]
- Taylor, D.R.; Drazen, J.M.; Herbison, G.P.; Yandava, C.N.; Hancox, R.J.; Town, G.I. Asthma Exacerbations during Long Term β Agonist Use: Influence of Β2 Adrenoceptor Polymorphism. Thorax 2000, 55, 762–767. [Google Scholar] [CrossRef]
- Corvol, H.; Burchard, E.G. Pharmacogenetic Response to Albuterol among Asthmatics. Pharmacogenomics 2008, 9, 505–510. [Google Scholar] [CrossRef]
- Sears, M.R.; Taylor, D.R.; Print, C.G.; Lake, D.C.; Li, Q.; Flannery, E.M.; Yates, D.M.; Lucas, M.K.; Herbison, G.P. Regular Inhaled Beta-Agonist Treatment in Bronchial Asthma. Lancet 1990, 336, 1391–1396. [Google Scholar] [CrossRef]
- Whalen, E.J.; Foster, M.W.; Matsumoto, A.; Ozawa, K.; Violin, J.D.; Que, L.G.; Nelson, C.D.; Benhar, M.; Keys, J.R.; Rockman, H.A.; et al. Regulation of β-Adrenergic Receptor Signaling by S-Nitrosylation of G-Protein-Coupled Receptor Kinase 2. Cell 2007, 129, 511–522. [Google Scholar] [CrossRef]
- Ozawa, K.; Whalen, E.J.; Nelson, C.D.; Mu, Y.; Hess, D.T.; Lefkowitz, R.J.; Stamler, J.S. S-Nitrosylation of β-Arrestin Regulates β-Adrenergic Receptor Trafficking. Mol. Cell 2008, 31, 395–405. [Google Scholar] [CrossRef]
- Moore, P.E. Influence of Gene-Gene Interactions on Response to Albuterol Therapy. Pharmacogenomics 2011, 12, 1–3. [Google Scholar] [CrossRef]
- Choudhry, S.; Que, L.G.; Yang, Z.; Liu, L.; Eng, C.; Kim, S.O.; Kumar, G.; Thyne, S.; Chapela, R.; Rodriguez-Santana, J.R.; et al. GSNO Reductase and Β2-Adrenergic Receptor Gene-Gene Interaction: Bronchodilator Responsiveness to Albuterol. Pharmacogenet. Genom. 2010, 20, 351–358. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Matera, M.G. Pharmacogenomic Response of Inhaled Corticosteroids for the Treatment of Asthma: Considerations for Therapy. Pharmgenom. Pers. Med. 2020, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Tang, Y.; Liu, J. Pharmacogenomics Genetic Variation in the Glucocorticoid. Pharmacogenomics 2017, 18, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, G.A.; Lazarus, R.; Smith, R.S.; Tantisira, K.G.; Meyers, D.A.; Peters, S.P.; Weiss, S.T.; Bleecker, E.R. The Glucocorticoid Receptor Heterocomplex Gene STIP1 Is Associated with Improved Lung Function in Asthmatic Subjects Treated with Inhaled Corticosteroids. J. Allergy Clin. Immunol. 2009, 123, 1376–1383.e7. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Yamada-Yamasawa, W.; Kudoh, H.; Matsuzaki, T.; Nakajima, T.; Hakuno, H.; Hiraoka, R.; Fukunaga, K.; Oguma, T.; Sayama, K.; et al. Association between β-Adrenoceptor Gene Polymorphisms and Relative Response to β 2-Agonists and Anticholinergic Drugs in Japanese Asthmatic Patients. Respirology 2010, 15, 849–854. [Google Scholar] [CrossRef] [PubMed]

 | Absorption | Systemic levels are initially undetectable; after 2–3 h, a low plasma concentration is observed (Cmax: 3 ng/mL and Tmax: 0.42 h). |
| Distribution | The volume of distribution is 156 ± 38 L. | |
| Metabolism | Mainly metabolized by sulfate conjugation (by sulfotransferase enzymes), but cytochrome P450 (CYP) enzymes are also involved in metabolism. | |
| Excretion | Salbutamol is excreted in the urine within 24 h (t1/2: 2.7—5 h and CL: 272 ± 38 mL/min). |
| Drug | Drug Class | Effect | Interaction Level | Reference |
|---|---|---|---|---|
| Dextromethorphan | Antitussive | More effective antitussive action | L | [109,110,111] |
| Sodium cromoglycate | Mast cell stabilizer | Better control of asthma symptoms | M | [111,112] |
| Nedocromil sodium | Benzopyrone | Decreased excretion rate of salbutamol | M | [111,113,114] |
| Benazepril | Antiseptic | No interaction | L | [111,115] |
| Glycyrrhizin | Saponin | Enhancement of anti-inflammatory effects | L | [111,116] |
| Cilomilast | Phosphodiesterase inhibitors | No relevant PK interactions | L | |
| Roflumilast | [111,117,118,119,120,121] | |||
| Ensifentrine | ||||
| Nifedipine | Calcium channel blockers | Increased heart rate; normal lung function | L | [111,122,123] |
| Tiapamil | ||||
| Phenylephrine | Nasal decongestant | Hypokalemia | M | [111,124] |
| Digoxin | Digitalis glycosides | Decreased digoxin serum levels | M | [111,113] |
| Gentamicin | Aminoglycoside Antibiotic | QT prolongation | M | [111,125] |
| Azithromycin | Macrolide antibiotic | QT prolongation | M | [111,125] |
| Chloroquine | Antimalarial | QT prolongation | M | [111,125] |
| Promethazine | Phenothiazine | M | [111,126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, L.; Vale, N. Unraveling the Impact of Salbutamol Polytherapy: Clinically Relevant Drug Interactions. Future Pharmacol. 2023, 3, 296-316. https://doi.org/10.3390/futurepharmacol3010019
Marques L, Vale N. Unraveling the Impact of Salbutamol Polytherapy: Clinically Relevant Drug Interactions. Future Pharmacology. 2023; 3(1):296-316. https://doi.org/10.3390/futurepharmacol3010019
Chicago/Turabian StyleMarques, Lara, and Nuno Vale. 2023. "Unraveling the Impact of Salbutamol Polytherapy: Clinically Relevant Drug Interactions" Future Pharmacology 3, no. 1: 296-316. https://doi.org/10.3390/futurepharmacol3010019
APA StyleMarques, L., & Vale, N. (2023). Unraveling the Impact of Salbutamol Polytherapy: Clinically Relevant Drug Interactions. Future Pharmacology, 3(1), 296-316. https://doi.org/10.3390/futurepharmacol3010019







