Aspirin Therapy, Cognitive Impairment, and Dementia—A Review
Abstract
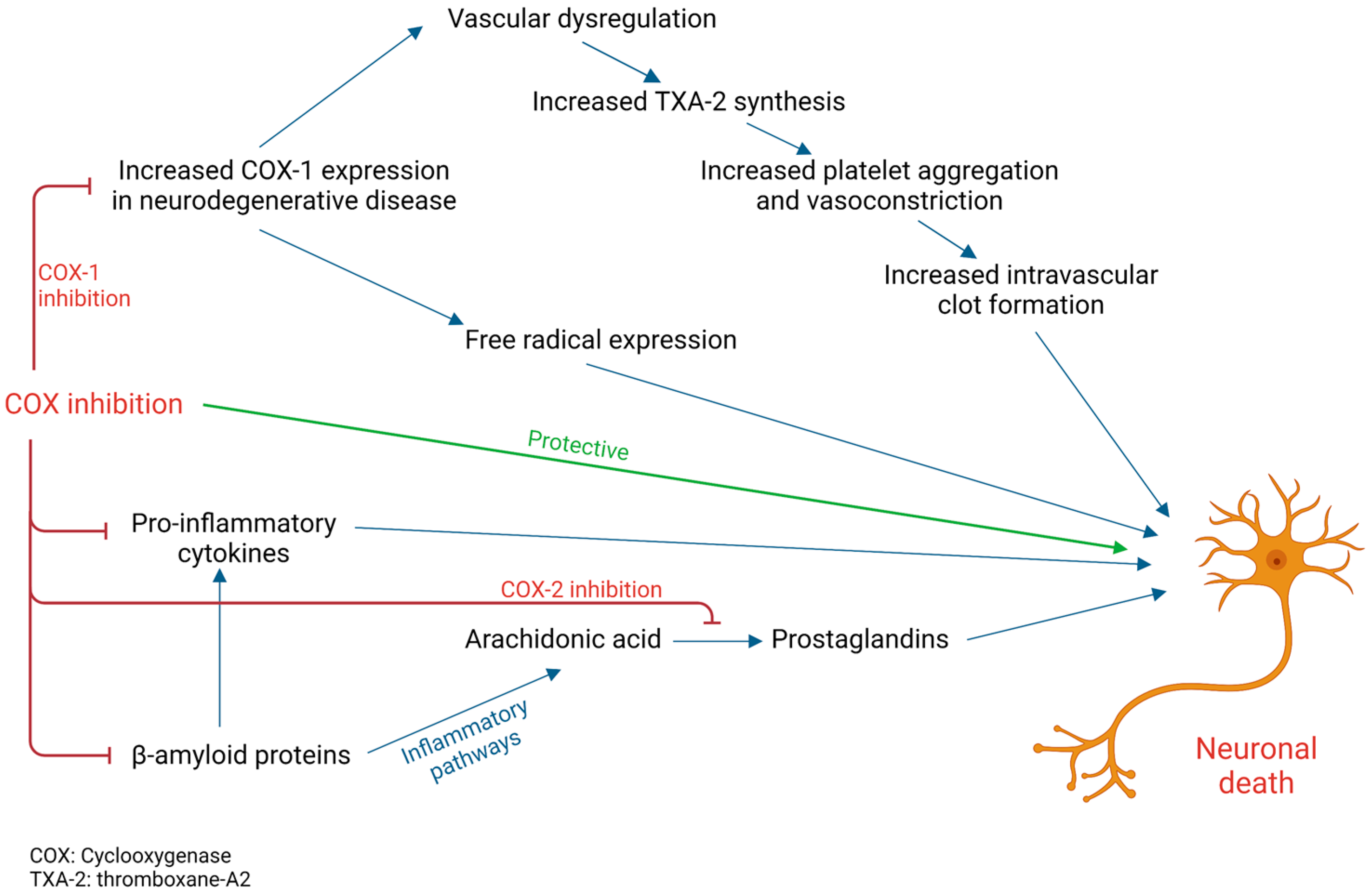
1. Introduction
2. Materials and Methods
3. Results
3.1. Primary Prevention of Cognitive Impairment and Dementia
3.2. Aspirin and Cognitive Decline in Alzheimer’s Disease (AD)
3.3. Aspirin and Cognitive Decline in Vascular Dementia (VD)
3.4. Aspirin and Cognitive Decline in Cerebral Small Vessel Disease (CSVD)
3.5. Aspirin and Dementia in Patients with Coronary Heart Disease (CHD)
3.6. Aspirin and Dementia in Patients with Late-Onset Depression (LOD)
3.7. Aspirin in Women
3.8. Impact of Age
3.9. The Role of High-Dose Aspirin
3.10. Aspirin and Cerebral Haemorrhage
3.11. Dual- or Triple-Antiplatelet Therapy: Aspirin in Combination with Dipyridamole or Clopidogrel
3.12. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geldmacher, D.S.; Whitehouse, P.J. Evaluation of Dementia. N. Engl. J. Med. 1996, 335, 330–336. [Google Scholar] [CrossRef]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Love, S. Neuropathological investigation of dementia: A guide for neurologists. J. Neurol. Neurosurg. Psychiatry 2005, 76, v8–v14. Available online: http://jnnp.bmj.com/content/76/suppl_5/v8.abstract (accessed on 5 November 2022). [CrossRef] [PubMed]
- Mufson, E.J.; Binder, L.; Counts, S.E.; DeKosky, S.T.; Detoledo-Morrell, L.; Ginsberg, S.D.; Ikonomovic, M.D.; Perez, S.E.; Scheff, S.W. Mild cognitive impairment: Pathology and mechanisms. Acta Neuropathol. 2011, 123, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V. Stroke and potentially preventable dementias proclamation: Updated world stroke day proclamation. Stroke 2015, 46, 3039–3040. [Google Scholar] [CrossRef]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013, 136, 2697–2706. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Grosser, T.; Fries, S.; Fitzgerald, G.A. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J. Clin. Investig. 2005, 116, 4–15. [Google Scholar] [CrossRef]
- Hoozemans, J.J.M.; Rozemuller, A.J.M.; Janssen, I.; De Groot, C.J.A.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol. 2001, 101, 2–8. [Google Scholar] [CrossRef]
- Yermakova, A.V.; Rollins, J.; Callahan, L.M.; Rogers, J.; O’Banion, M.K. Cyclooxygenase-1 in Human Alzheimer and Control Brain: Quantitative Analysis of Expression by Microglia and CA3 Hippocampal Neurons. J. Neuropathol. Exp. Neurol. 1999, 58, 1135–1146. [Google Scholar] [CrossRef]
- Choi, S.-H.; Aid, S.; Bosetti, F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications for translational research. Trends Pharmacol. Sci. 2009, 30, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Bosetti, F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to β-amyloid. Aging 2009, 1, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Langenbach, R.; Bosetti, F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide- induced inflammatory response and brain injury. FASEB J. 2008, 22, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Teeling, J.; Cunningham, C.; Newman, T.; Perry, V. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1. Brain Behav. Immun. 2010, 24, 409–419. Available online: https://www.sciencedirect.com/science/article/pii/S0889159109005170 (accessed on 8 November 2022). [CrossRef] [PubMed]
- Gómez-Isla, T.; Blesa, R.; Boada, M.; Clarimón, J.; Del Ser, T.; Domenech, G.; Ferro, J.M.; Gómez-Ansón, B.; Manubens, J.M.; Martínez-Lage, J.M.; et al. A randomized, double-blind, placebo controlled-trial of triflusal in mild cognitive impairment: The TRIMCI study. Alzheimer Dis. Assoc. Disord. 2008, 22, 21–29. [Google Scholar] [CrossRef]
- Clarke, R.; Harrison, G.; Richards, S. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. J. Intern. Med. 2003, 254, 67–75. [Google Scholar]
- Martinez-Ramirez, S.; Greenberg, S.M.; Viswanathan, A. Cerebral microbleeds: Overview and implications in cognitive impairment. Alzheimer’s Res. Ther. 2014, 6, 33. [Google Scholar] [CrossRef]
- Naka, H.; Nomura, E.; Kitamura, J.; Imamura, E.; Wakabayashi, S.; Matsumoto, M. Antiplatelet Therapy as a Risk Factor for Microbleeds in Intracerebral Hemorrhage Patients: Analysis Using Specific Antiplatelet Agents. J. Stroke Cerebrovasc. Dis. 2013, 22, 834–840. [Google Scholar] [CrossRef]
- Parish, S.; Mafham, M.; Offer, A.; Barton, J.; Wallendszus, K.; Stevens, W.; Buck, G.; Haynes, R.; Collins, R.; Bowman, L.; et al. Effects of aspirin on dementia and cognitive function in diabetic patients: The ASCEND trial. Eur. Heart J. 2022, 43, 2010–2019. [Google Scholar] [CrossRef]
- Ogawa, H.; Nakayama, M.; Morimoto, T.; Uemura, S.; Kanauchi, M.; Doi, N.; Jinnouchi, H.; Sugiyama, S.; Saito, Y.; for the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) Trial. Investigators Low-Dose Aspirin for Primary Prevention of Atherosclerotic Events in Patients with Type 2 Diabetes: A Randomized Controlled Trial. JAMA 2008, 300, 2134–2141. [Google Scholar] [CrossRef]
- Price, J.F.; Stewart, M.C.; Deary, I.J.; Murray, G.D.; Sandercock, P.; Butcher, I.; Fowkes, F.G.R.; Trialists, O.B.O.T.A. Low dose aspirin and cognitive function in middle aged to elderly adults: Randomised controlled trial. BMJ 2008, 337, a1198. Available online: https://www.bmj.com/content/337/bmj.a1198 (accessed on 10 November 2022). [CrossRef] [PubMed]
- Ryan, J.; Storey, E.; Murray, A.M.; Woods, R.L.; Wolfe, R.; Reid, C.M.; Nelson, M.R.; Chong, T.T.; Williamson, J.D.; Ward, S.A.; et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology 2020, 95, e320–e331. [Google Scholar] [CrossRef] [PubMed]
- Bentham, P.; Gray, R.; Sellwood, E.; Hills, R.; Crome, P.; Raftery, J. Aspirin in Alzheimer’s disease (AD2000): A randomised open-label trial. Lancet Neurol. 2008, 7, 41–49. [Google Scholar] [PubMed]
- Almeida, O.P.; Alfonso, H.; Jamrozik, K.; Hankey, G.J.; Flicker, L. Aspirin use, depression, and cognitive impairment in later life: The health in men study. J. Am. Geriatr. Soc. 2010, 58, 990–992. [Google Scholar] [CrossRef]
- Chang, C.-W.; Horng, J.-T.; Hsu, C.-C.; Chen, J.-M. Mean Daily Dosage of Aspirin and the Risk of Incident Alzheimer’s Dementia in Patients with Type 2 Diabetes Mellitus: A Nationwide Retrospective Cohort Study in Taiwan. J. Diabetes Res. 2016, 2016, 9027484. [Google Scholar] [CrossRef]
- Ferrari, C.; Lombardi, G.; Polito, C.; Lucidi, G.; Bagnoli, S.; Piaceri, I.; Nacmias, B.; Berti, V.; Rizzuto, D.; Fratiglioni, L.; et al. Alzheimer’s Disease Progression: Factors Influencing Cognitive Decline. J. Alzheimer’s Dis. 2017, 61, 785–791. [Google Scholar] [CrossRef]
- Broe, G.A.; Grayson, D.A.; Creasey, H.M.; Waite, L.M.; Casey, B.J.; Bennett, H.P.; Brooks, W.S.; Halliday, G.M. Anti-inflammatory Drugs Protect Against Alzheimer Disease at Low Doses. Arch. Neurol. 2000, 57, 1586–1591. [Google Scholar] [CrossRef]
- Weng, J.; Zhao, G.; Weng, L.; Guan, J.; Initiative, F.T.A.D.N. Aspirin using was associated with slower cognitive decline in patients with Alzheimer’s disease. PLoS ONE 2021, 16, e0252969. [Google Scholar] [CrossRef]
- Meyer, J.S.; Rogers, R.L.; McClintic, K.; Mortel, K.F.; Lotfi, J. Randomized clinical trial of daily aspirin therapy in multi-infarct dementia: A pilot study. J. Am. Geriatr. Soc. 1989, 37, 549–555. [Google Scholar] [CrossRef]
- Raum, E.; Rothenbacher, D.; Löw, M.; Stegmaier, C.; Ziegler, H.; Brenner, H. Changes of cardiovascular risk factors and their implications in subsequent birth cohorts of older adults in Germany: A life course approach. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 809–814. [Google Scholar] [CrossRef]
- Allen, N.; Sudlow, C.; Downey, P.; Peakman, T.; Danesh, J.; Elliott, P.; Gallacher, J.; Green, J.; Matthews, P.; Pell, J.; et al. UK Biobank: Current status and what it means for epidemiology. Health Policy Technol. 2012, 1, 123–126. Available online: https://www.sciencedirect.com/science/article/pii/S2211883712000597 (accessed on 13 November 2022). [CrossRef]
- Szekely, C.A.; Breitner, J.; Fitzpatrick, A.L.; Rea, T.D.; Psaty, B.M.; Kuller, L.H.; Zandi, P.P. NSAID use and dementia risk in the Cardiovascular Health Study*: Role of APOE and NSAID type. Neurology 2007, 70, 17–24. [Google Scholar] [CrossRef]
- Hébert, R.; Lindsay, J.; Verreault, R.; Rockwood, K.; Hill, G.; Dubois, M.-F. Vascular Dementia. Stroke 2000, 31, 1487–1493. [Google Scholar] [CrossRef]
- Akoudad, S.; Wolters, F.J.; Viswanathan, A.; De Bruijn, R.F.; Van Der Lugt, A.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A.; Vernooij, M.W. Association of Cerebral Microbleeds With Cognitive Decline and Dementia. JAMA Neurol. 2016, 73, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Youn, Y.C.; Jeong, J.H.; Han, H.J.; Kim, J.H.; Lee, J.-H.; Park, K.H.; Park, K.W.; Kim, E.-J.; Oh, M.S.; et al. Cilostazol Versus Aspirin on White Matter Changes in Cerebral Small Vessel Disease: A Randomized Controlled Trial. Stroke 2022, 53, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, I.; Altieri, M.; Di Clemente, L.; Vicenzini, E.; Pantano, P.; Raz, E.; Silvestrini, M.; Provinciali, L.; Paolino, I.; Marini, C.; et al. Longitudinal Study on Low-Dose Aspirin versus Placebo Administration in Silent Brain Infarcts: The Silence Study. Stroke Res. Treat. 2018, 2018, 7532403. [Google Scholar] [CrossRef]
- Nguyen, T.N.M.; Chen, L.-J.; Trares, K.; Stocker, H.; Holleczek, B.; Beyreuther, K.; Brenner, H.; Ben Schöttker, B. Long-term low-dose acetylsalicylic use shows protective potential for the development of both vascular dementia and Alzheimer’s disease in patients with coronary heart disease but not in other individuals from the general population: Results from two large cohort studies. Alzheimer’s Res. Ther. 2022, 14, 75. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Chiu, C.-C.; Teng, H.-W.; Huang, C.-T.; Liu, C.-Y.; Huang, L.-J. Aspirin and Risk of Dementia in Patients with Late-Onset Depression: A Population-Based Cohort Study. BioMed Res. Int. 2020, 2020, 1704879. [Google Scholar] [CrossRef]
- Matsumoto, C.; Ogawa, H.; Saito, Y.; Okada, S.; Soejima, H.; Sakuma, M.; Masuda, I.; Nakayama, M.; Doi, N.; Jinnouchi, H.; et al. Sex Difference in Effects of Low-Dose Aspirin on Prevention of Dementia in Patients With Type 2 Diabetes: A Long-term Follow-up Study of a Randomized Clinical Trial. Diabetes Care 2020, 43, 314–320. [Google Scholar] [CrossRef]
- Kern, S.; Skoog, I.; Östling, S.; Kern, J.; Börjesson-Hanson, A. Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk? A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women. BMJ Open 2012, 2, e001288. [Google Scholar] [CrossRef]
- Kang, J.H.; Cook, N.; Manson, J.; Buring, J.E.; Grodstein, F. Low dose aspirin and cognitive function in the women’s health study cognitive cohort. BMJ 2007, 334, 987. Available online: https://www.bmj.com/content/334/7601/987 (accessed on 16 November 2022). [CrossRef]
- Kang, J.H.; Grodstein, F. Regular use of nonsteroidal anti-inflammatory drugs and cognitive function in aging women. Neurology 2003, 60, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.E.; Johansson, B.; Takkinen, S.; Zarit, S.; McClearn, G.; Melander, A.; Berg, S. Does aspirin protect against Alzheimer’s dementia? A study in a Swedish population-based sample aged ≥ 80 years. Eur. J. Clin. Pharmacol. 2003, 59, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Jonker, C.; Comijs, H.; Smit, J. Does aspirin or other NSAIDs reduce the risk of cognitive decline in elderly persons? Results from a population-based study. Neurobiol. Aging 2003, 24, 583–588. [Google Scholar] [CrossRef]
- Stewart, W.F.; Kawas, C.; Corrada, M.; Metter, E.J. Risk of Alzheimer’s disease and duration of NSAID use. Neurology 1997, 48, 626–632. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Woods, R.L.; Nelson, M.R.; Reid, C.M.; Kirpach, B.; Wolfe, R.; Storey, E.; Shah, R.C.; Lockery, J.E.; Tonkin, A.M.; et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1499–1508. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Maggi, S.; Thompson, T.; Schofield, P.; Muller, C.; Tseng, P.; Lin, P.; Carvalho, A.F.; Solmi, M. Low-Dose Aspirin Use and Cognitive Function in Older Age: A Systematic Review and Meta-analysis. J. Am. Geriatr. Soc. 2017, 65, 1763–1768. [Google Scholar] [CrossRef]
- Hachinski, V.; Einhäupl, K.; Ganten, D.; Alladi, S.; Brayne, C.; Stephan, B.C.M.; Sweeney, M.D.; Zlokovic. B.; Iturria-Medina, Y.; Iadecola. C.; et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimers Dement. 2019, 15, 961–984. [Google Scholar] [CrossRef]
- Kinney, J.W.; BeMiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Cribbs, D.H.; Berchtold, N.C.; Perreau, V.; Coleman, P.D.; Rogers, J.; Tenner, A.J.; Cotman, C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J. Neuroinflamm. 2012, 9, 179. [Google Scholar] [CrossRef]
- Casolini, P.; Catalani, A.; Zuena, A.R.; Angelucci, L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J. Neurosci. Res. 2002, 68, 337–343. [Google Scholar] [CrossRef]
- Zotova, E.; Nicoll, J.A.; Kalaria, R.; Holmes, C.; Boche, D. Inflammation in Alzheimer’s disease: Relevance to pathogenesis and therapy. Alzheimer’s Res. Ther. 2010, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Manly, J.J.; Schupf, N.; Tang, M.X.; Mayeux, R.; Luchsinger, J. Association of C-Reactive Protein with Cognitive Impairment. Arch. Neurol. 2010, 67, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Simen, A.A.; Bordner, K.A.; Martin, M.P.; Moy, L.A.; Barry, L. Cognitive dysfunction with aging and the role of inflammation. Ther. Adv. Chronic Dis. 2011, 2, 175–195. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Iturria-Medina, Y.I.; Sotero, R.C.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef]
- Dregan, A.; Chowienczyk, P.; Armstrong, D. Patterns of anti-inflammatory drug use and risk of dementia: A matched case-control study. Eur. J. Neurol. 2015, 22, 1421–1428. [Google Scholar] [CrossRef]
- Zandi, P.P.; Anthony, J.C.; Hayden, K.; Mehta, K.; Mayer, L.; Breitner, J.C. Reduced incidence of AD with NSAID but not H2 receptor antagonists: The Cache County Study. Neurology 2002, 59, 880–886. [Google Scholar] [CrossRef]
- Breitner, J.; Gau, B.A.; Welsh, K.A.; Plassman, B.L.; McDonald, W.M.; Helms, M.J.; Anthony, J.C. Inverse association of anti-inflammatory treatments and Alzheimer’s disease: Initial results of a co-twin control study. Neurology 1994, 44, 227. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Grodstein, F.; Bienias, J.L.; Schneider, J.A.; Wilson, R.S.; Kelly, J.F.; Evans, D.A.; Bennett, D.A. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology 2008, 70, 2219–2225. [Google Scholar] [CrossRef]
- Cornelius, C.; Fastbom, J.; Winblad, B.; Viitanen, M. Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology 2004, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kaida-Yip, F.; Zabel, M. NSAID Use and the Prevention of Alzheimer’s Disease: A Meta-Analysis (P6.184). Neurology 2018, 90, P6.184. Available online: http://n.neurology.org/content/90/15_Supplement/P6.184.abstract (accessed on 10 November 2022).
- Etminan, M.; Gill, S.; Samii, A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: Systematic review and meta-analysis of observational studies. BMJ 2003, 327, 128. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, L.; Wang, H.-F.; Tan, C.-C.; Meng, X.-F.; Wang, C.; Tang, S.-W.; Yu, J.-T. Anti-Inflammatory Drugs and Risk of Alzheimer’s Disease: An Updated Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2015, 44, 385–396. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. Available online: https://www.sciencedirect.com/science/article/pii/S0140673615604615 (accessed on 13 November 2022). [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems: Alphabetical Index; World Health Organization: Geneva, Switzerland, 2004; Volume 3. [Google Scholar]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef]
- Veld, B.A.I.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; van Duijn, C.M.; Stijnen, T.; Breteler, M.M.; Stricker, B.H. Nonsteroidal Antiinflammatory Drugs and the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2001, 345, 1515–1521. [Google Scholar] [CrossRef]
- Raju, N.; Sobieraj-Teague, M.; Hirsh, J.; O’Donnell, M.; Eikelboom, J. Effect of Aspirin on Mortality in the Primary Prevention of Cardiovascular Disease. Am. J. Med. 2011, 124, 621–629. Available online: https://www.sciencedirect.com/science/article/pii/S0002934311002634 (accessed on 13 November 2022). [CrossRef]
- Emdin, C.A.; Rothwell, P.M.; Salimi-Khorshidi, G.; Kiran, A.; Conrad, N.; Callender, T.; Mehta, Z.; Pendlebury, S.T.; Anderson, S.G.; Mohseni, H.; et al. Blood Pressure and Risk of Vascular Dementia: Evidence From a Primary Care Registry and a Cohort Study of Transient Ischemic Attack and Stroke. Stroke 2016, 47, 1429–1435. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Bordet, R.; Ihl, R.; Korczyn, A.; Lanza, G.; Jansa, J.; Hoerr, R.; Guekht, A. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: A consensus report. BMC Med. 2017, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [PubMed]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Jacova, C.; Pearce, L.; Costello, R.; McClure, L.A.; Holliday, S.L.; Hart, R.G.; Benavente, O.R.; Ms, L.A.P. Cognitive impairment in lacunar strokes: The SPS3 trial. Ann. Neurol. 2012, 72, 351–362. [Google Scholar] [CrossRef]
- Van Leijsen, E.M.; De Leeuw, F.-E.; Tuladhar, A.M. Disease progression and regression in sporadic small vessel disease–insights from neuroimaging. Clin. Sci. 2017, 131, 1191–1206. [Google Scholar] [CrossRef]
- Smith, E.E.; Saposnik, G.; Biessels, G.J.; Doubal, F.N.; Fornage, M.; Gorelick, P.B.; Greenberg, S.M.; Higashida, R.T.; Kasner, S.E.; Seshadri, S. Prevention of Stroke in Patients with Silent Cerebrovascular Disease: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2017, 48, e44–e71. [Google Scholar] [CrossRef]
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement Diagn. Assess Dis Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Tahsili-Fahadan, P.; Geocadin, R.G. Heart–Brain Axis. Circ. Res. 2017, 120, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Leto, L.; Feola, M. Cognitive impairment in heart failure patients. J. Geriatr. Cardiol. 2014, 11, 316–328. [Google Scholar]
- Davis, K.K.; Mintzer, M.; Himmelfarb, C.D.; Hayat, M.J.; Rotman, S.; Allen, J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur. J. Heart Fail. 2012, 14, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Vogels, R.L.C.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Heart Fail. 2007, 9, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zheng, F.; Yan, L.; Zhong, B. Cognitive Decline before and after Incident Coronary Events. J. Am. Coll. Cardiol. 2019, 73, 3041–3050. Available online: https://www.sciencedirect.com/science/article/pii/S0735109719349289 (accessed on 14 November 2022). [CrossRef] [PubMed]
- Stefanidis, K.B.; Askew, C.; Greaves, K.; Summers, M.J. The Effect of Non-Stroke Cardiovascular Disease States on Risk for Cognitive Decline and Dementia: A Systematic and Meta-Analytic Review. Neuropsychol. Rev. 2017, 28, 1–15. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Sabia, S.; Lajnef, M.; Ferrie, J.E.; Nabi, H.; Britton, A.R.; Marmot, M.G.; Shipley, M.J. History of coronary heart disease and cognitive performance in midlife: The Whitehall II study. Eur. Heart J. 2008, 29, 2100–2107. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Britton, A.R.; Marmot, M. Vascular Disease and Cognitive Function: Evidence from the Whitehall II Study. J. Am. Geriatr. Soc. 2003, 51, 1445–1450. [Google Scholar] [CrossRef]
- Schievink, S.H.; van Boxtel, M.P.; Deckers, K.; van Oostenbrugge, R.J.; Verhey, F.R.; Köhler, S. Cognitive changes in prevalent and incident cardiovascular disease: A 12-year follow-up in the Maastricht Aging Study (MAAS). Eur. Heart J. 2022, 43, e2–e9. [Google Scholar] [CrossRef]
- Zhu, L.; Viitanen, M.; Guo, Z.; Winblad, B.; Fratiglioni, L. Blood Pressure Reduction, Cardiovascular Diseases, and Cognitive Decline in the Mini-Mental State Examination in a Community Population of Normal Very Old People: A Three-Year Follow-up. J. Clin. Epidemiol. 1998, 51, 385–391. [Google Scholar] [CrossRef]
- Petrovitch, H.; White, L.; Masaki, K.H.; Ross, G.; Abbott, R.D.; Rodriguez, B.L.; Lu, G.; Burchfiel, C.M.; Blanchette, P.L.; Curb, J. Influence of Myocardial Infarction, Coronary Artery Bypass Surgery, and Stroke on Cognitive Impairment in Late Life. Am. J. Cardiol. 1998, 81, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.B. Illnesses Causing Dementia in the Very Elderly. N. Engl. J. Med. 1993, 328, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Eller, T.; Vasar, V.; Shlik, J.; Maron, E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Jung, H.-G.; Myint, A.-M.; Kim, H.; Park, S.-H. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007, 104, 91–95. [Google Scholar] [CrossRef]
- Berk, M.; Agustini, B.; Woods, R.L.; Nelson, M.R.; Shah, R.C.; Reid, C.M.; Storey, E.; Fitzgerald, S.M.; Lockery, J.E.; Wolfe, R.; et al. Effects of aspirin on the long-term management of depression in older people: A double-blind randomised placebo-controlled trial. Mol. Psychiatry 2021, 26, 5161–5170. [Google Scholar] [CrossRef]
- Kjeldsen, S.E.; Kolloch, R.E.; Leonetti, G.; Malliond, J.-M.; Zanchetti, A.; Elmfeldt, D.; Warnold, I.; Hansson, L. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. J. Hypertens. 2000, 18, 629–642. [Google Scholar] [CrossRef]
- Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N. Engl. J. Med. 1989, 321, 129–135. [Google Scholar] [CrossRef]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cook, N.R.; Lee, I.-M.; Gordon, D.; Gaziano, J.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N. Engl. J. Med. 2005, 352, 1293–1304. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Yamaguchi, T.; Watanabe, S.; Yamamoto, T. Involvement of arachidonic acid cascade in working memory impairment induced by interleukin-1 beta. Neuropharmacology 2004, 46, 1195–1200. [Google Scholar] [CrossRef]
- Andreasson, K.I.; Savonenko, A.; Vidensky, S.; Goellner, J.J.; Zhang, Y.; Shaffer, A.; Kaufmann, W.E.; Worley, P.F.; Isakson, P.; Markowska, A.L. Age-Dependent Cognitive Deficits and Neuronal Apoptosis in Cyclooxygenase-2 Transgenic Mice. J. Neurosci. 2001, 21, 8198–8209. [Google Scholar] [CrossRef] [PubMed]
- Uchikado, H.; Akiyama, H.; Kondo, H.; Ikeda, K.; Tsuchiya, K.; Kato, M.; Oda, T.; Togo, T.; Iseki, E.; Kosaka, K. Activation of vascular endothelial cells and perivascular cells by systemic inflammation—An immunohistochemical study of postmortem human brain tissues. Acta Neuropathol. 2004, 107, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Ek, M.; Engblom, D.; Saha, S.; Blomqvist, A.; Jakobsson, P.J.; Ericsson-Dahlstrand, A. Inflammatory response: Pathway across the blood-brain barrier. Nature 2001, 410, 430–431. [Google Scholar] [CrossRef]
- Montine, T.J.; Sidell, K.R.; Crews, B.C.; Markesbery, W.R.; Marnett, L.J.; Roberts, L.J., 2nd; Morrow, J.D. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology 1999, 53, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Combrinck, M.; Williams, J.; De Berardinis, M.A.; Warden, D.; Puopolo, M.; Smith, A.D.; Minghetti, L. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 85–88. [Google Scholar] [CrossRef]
- Cowley, T.R.; Fahey, B.; O’Mara, S.M. COX-2, but not COX-1, activity is necessary for the induction of perforant path long-term potentiation and spatial learning in vivo. Eur. J. Neurosci. 2008, 27, 2999–3008. [Google Scholar] [CrossRef]
- Teather, L.A.; Packard, M.G.; Bazan, N.G. Post-Training Cyclooxygenase-2 (COX-2) Inhibition Impairs Memory Consolidation. Learn. Mem. 2002, 9, 41–47. [Google Scholar] [CrossRef]
- Sang, N.; Zhang, J.; Marcheselli, V.; Bazan, N.G.; Chen, C. Postsynaptically Synthesized Prostaglandin E2 (PGE2) Modulates Hippocampal Synaptic Transmission via a Presynaptic PGE2 EP2 Receptor. J. Neurosci. 2005, 25, 9858–9870. [Google Scholar] [CrossRef]
- Aisen, P.S.; Thal, L.J.; Ferris, S.H.; Assaid, C.; Nessly, M.L.; Giuliani, M.J.; Lines, C.R.; Norman, B.A.; Potter, W.Z. Rofecoxib in patients with mild cognitive impairment: Further analyses of data from a randomized, double-blind, trial. Curr. Alzheimer Res. 2008, 5, 73–82. [Google Scholar] [CrossRef]
- Reines, S.A.; Block, G.A.; Morris, J.C.; Liu, G.; Nessly, M.L.; Lines, C.R.; Norman, B.A.; Baranak, C.C. Rofecoxib. Neurology 2004, 62, 66–71. Available online: http://n.neurology.org/content/62/1/66.abstract (accessed on 16 November 2022). [CrossRef]
- Group, A.R. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007, 68, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Soininen, H.; West, C.; Robbins, J.; Niculescu, L. Long-Term Efficacy and Safety of Celecoxib in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2006, 23, 8–21. [Google Scholar] [CrossRef]
- Leoutsakos, J.-M.S.; Muthen, B.O.; Breitner, J.C.; Lyketsos, C.G. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: Findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. Int. J. Geriatr. Psychiatry 2012, 27, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schafer, K.A.; Grundman, M.; Pfeiffer, E.; Sano, M.; Davis, K.L.; Farlow, M.R.; Jin, S.; Thomas, R.G.; Thal, L.J.; et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA 2003, 289, 2819–2826. [Google Scholar] [CrossRef]
- Akoudad, S.; Portegies, M.L.; Koudstaal, P.J.; Hofman, A.; Van Der Lugt, A.; Ikram, M.A.; Vernooij, M.W. Cerebral Microbleeds Are Associated With an Increased Risk of Stroke. Circulation 2015, 132, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Halpin, A.; Towfighi, A.; Gilson, A.; Busl, K.; Rost, N.; Smith, E.E.; Greenberg, M.S.; Rosand, J.; Viswanathan, A. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010, 75, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-L.; Liu, C.-H.; Chang, Y.-M.; Lin, T.-Y.; Chien, C.-Y.; Chen, C.-H.; Tsai, K.-J.; Lin, S.-H.; Sung, P.-S. The Impact of Antiplatelet Use on the Risk of Intracerebral Hemorrhage in Patients with Alzheimer’s Disease: A Nationwide Cohort Study1. J. Alzheimer’s Dis. 2020, 73, 297–306. [Google Scholar] [CrossRef]
- Jeong, S.-W.; Jung, K.-H.; Chu, K.; Bae, H.-J.; Lee, S.-H.; Roh, J.-K. Clinical and Radiologic Differences between Primary Intracerebral Hemorrhage with and without Microbleeds on Gradient-Echo Magnetic Resonance Images. Arch. Neurol. 2004, 61, 905–909. [Google Scholar] [CrossRef]
- Wang, Z.; Soo, Y.O.; Mok, V.C. Cerebral Microbleeds. Stroke 2014, 45, 2811–2817. [Google Scholar] [CrossRef]
- Wong, K.; Chan, Y.; Liu, J.; Gao, S.; Lam, W.W. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology 2003, 60, 511–513. [Google Scholar] [CrossRef]
- Ge, L.; Niu, G.; Han, X.; Gao, Y.; Wu, Q.; Wu, H.; Zhang, Y.; Guo, D. Aspirin Treatment Increases the Risk of Cerebral Microbleeds. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 2011, 38, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Ueba, T.; Kajiwara, M.; Miyamatsu, N.; Yamashita, K. Cerebral Microbleeds in Patients With Intracerebral Hemorrhage Are Associated With Previous Cerebrovascular Diseases and White Matter Hyperintensity, but Not With Regular Use of Antiplatelet Agents. Neurol. Med.-Chir. 2009, 49, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.A.; Raniga, P.; Ferris, N.J.; Woods, R.L.; Storey, E.; Bailey, M.J.; Brodtmann, A.; Yates, P.; Donnan, G.; Trevak, R.; et al. ASPREE-NEURO study protocol: A randomized controlled trial to determine the effect of low-dose aspirin on cerebral microbleeds, white matter hyperintensities, cognition, and stroke in the healthy elderly. Int. J. Stroke Off. J. Int. Stroke Soc. 2017, 12, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.D. Dipyridamole is neuroprotective for cultured rat embryonic cortical neurons. Biochem. Biophys. Res. Commun. 2004, 314, 501–504. [Google Scholar] [CrossRef]
- Farinelli, S.E.; Greene, L.A.; Friedman, W.J. Neuroprotective Actions of Dipyridamole on Cultured CNS Neurons. J. Neurosci. 1998, 18, 5112–5123. [Google Scholar] [CrossRef] [PubMed]
- Aldandashi, S.; Noor, R.; Wang, C.X.; Uddin, G.; Shuaib, A. Combination treatment with dipyridamole, aspirin, and tPA in an embolic model of stroke in rats. Exp. Neurol. 2007, 205, 563–568. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.Y.; Lee, W.S.; Hong, K.W. Lack of antiapoptotic effects of antiplatelet drug, aspirin and clopidogrel, and antioxidant, MCI-186, against focal ischemic brain damage in rats. Neurol. Res. 2005, 27, 483–492. [Google Scholar] [CrossRef]
- Diener, H.-C.; Sacco, R.L.; Yusuf, S.; Cotton, D.; Ôunpuu, S.; Lawton, W.A.; Palesch, Y.; Martin, R.H.; Albers, G.W.; Bath, P.; et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: A double-blind, active and placebo-controlled study. Lancet Neurol. 2008, 7, 875–884. [Google Scholar] [CrossRef]
- Hankey, G.J.; Eikelboom, J.W. What do the results of the PRoFESS trial teach us? Lancet Neurol. 2008, 7, 860–862. [Google Scholar] [CrossRef]
- Pearce, L.A.; McClure, L.A.; Anderson, D.C.; Jacova, C.; Sharma, M.; Hart, R.G.; Benavente, O.R. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: A secondary analysis from the SPS3 randomised trial. Lancet Neurol. 2014, 13, 1177–1185. [Google Scholar] [CrossRef]
- Investigators, S.; Benavente, O.R.; Hart, R.G.; McClure, L.A.; Szychowski, J.M.; Coffey, C.S. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N. Engl. J. Med. 2012, 367, 817–825. [Google Scholar] [CrossRef]
- Douiri, A.; McKevitt, C.; Emmett, E.S.; Rudd, A.G.; Wolfe, C.D. Long-Term Effects of Secondary Prevention on Cognitive Function in Stroke Patients. Circulation 2013, 128, 1341–1348. [Google Scholar] [CrossRef]
- Skoog, I.; Nilsson, L.; Persson, G.; Lernfelt, B.; Landahl, S.; Palmertz, B.; Andreasson, L.-A.; Odén, A.; Svanborg, A. 15-year longitudinal study of blood pressure and dementia. Lancet 1996, 347, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Nyenhuis, D.L.; Gorelick, P.B.; Freels, S.; Garron, D.C. Cognitive and functional decline in African Americans with VaD, AD, and stroke without dementia. Neurology 2002, 58, 56–61. [Google Scholar] [CrossRef]
- Wang, C.; Yi, X.; Zhang, B.; Liao, D.; Lin, J.; Chi, L. Clopidogrel Plus Aspirin Prevents Early Neurologic Deterioration and Improves 6-Month Outcome in Patients With Acute Large Artery Atherosclerosis Stroke. Clin. Appl. Thromb. 2014, 21, 453–461. [Google Scholar] [CrossRef]
- Bath, P.M.; Woodhouse, L.J.; Appleton, J.P.; Beridze, M.; Christensen, H.; Dineen, R.A.; Flaherty, K.; Duley, L.; England, T.J.; Havard, D.; et al. Triple versus guideline antiplatelet therapy to prevent recurrence after acute ischaemic stroke or transient ischaemic attack: The TARDIS RCT. Health Technol. Assess. 2018, 22, 1–76. [Google Scholar] [CrossRef]
- Hilkens, N.A.; Algra, A.; Kappelle, L.J.; Bath, P.M.; Csiba, L.; Rothwell, P.M.; Greving, J.P. Early time course of major bleeding on antiplatelet therapy after TIA or ischemic stroke. Neurology 2018, 90, e683–e689. [Google Scholar] [CrossRef] [PubMed]
- Shoamanesh, A.; Pearce, L.A.; Bazan, C.; Catanese, L.; McClure, L.A.; Sharma, M.; Marti-Fabregas, J.; Anderson, D.C.; Kase, C.S.; Hart, R.G.; et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Ann. Neurol. 2017, 82, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Bittl, J.A.; Baber, U.; Bradley, S.M.; Wijeysundera, D. Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 68, 1116–1139. [Google Scholar] [CrossRef]
- Melis, R.J.; Haaksma, M.L.; Terrera, G.M. Understanding and predicting the longitudinal course of dementia. Curr. Opin. Psychiatry 2019, 32, 123–129. [Google Scholar] [CrossRef]
| Effect of Aspirin on Cognitive Impairment and Dementia | ||||
|---|---|---|---|---|
| Effect of Aspirin | Trial | Trial Design | Patient Number | Outcome |
| Primary Prevention of Cognitive Impairment and Dementia | ASCEND [19] | Randomised controlled trial | 15,480 | Rates of dementia, cognitive impairment, or confusion were similar to placebo |
| JPAD [20] | Randomised controlled trial | 2121 | No significant difference between aspirin vs placebo in the prevention of dementia | |
| AAA [21] | Randomised controlled trial | 3350 | Did not show any benefit in preserving cognitive function | |
| Aspirin and Cognitive Decline in Alzheimer’s Dementia (AD) | ASPREE [22] | Randomised controlled trial | 19,114 | Did not find any significant difference between the aspirin and placebo groups with regards to dementia |
| AD2000 [23] | Randomised open-label trial | 310 | No association between aspirin and risk of AD | |
| Health in Men [24] | Population-based retrospective cohort study | 3679 | No association between aspirin and risk of AD | |
| Chang CW et al. [25] | Population-based retrospective cohort study | 28,321 | Yes–decreased risk of incident AD | |
| Ferrari C et al. [26] | Cohort study | 160 | Yes–odds of a patient on aspirin having a rapid decline in MMSE over 2 years were lower | |
| Sydney Older Persons Study [27] | Longitudinal study | 647 | Yes–inverse association between aspirin and presence of AD | |
| Alzheimer’s Disease Neuroimaging Initiative [28] | Longitudinal study | 1866 | Yes–associated with slower cognitive decline in AD, but not but in patients with normal cognition or mild cognitive impairment | |
| Aspirin and Cognitive Decline in Vascular Dementia (VD) | Meyer et al. [29] | Randomised controlled trial | 70 | Yes–associated with better cognitive scores and cerebral perfusion values |
| ESTHER [30] | Prospective cohort study | 5258 | Yes–aspirin reduced hazard for VD | |
| UK Biobank [31] | Prospective cohort study | 305,394 | Yes–aspirin reduced hazard for VD | |
| Sydney Older Persons Study [27] | Longitudinal study | 647 | No–did not protect against VD | |
| Cardiovascular Health Cognition Study [32] | Prospective cohort study | 3229 | No–did not protect against VD | |
| Canadian Study of Health and Aging [33] | Cohort study | 8623 | Increased risk of VD | |
| Rotterdam study [34] | Cohort study | 6989 | Increased risk of VD | |
| Aspirin and Cognitive Decline in Cerebral Small Vessel Disease (CSVD) | CHALLENGE trial [35] | Multicentre, double-blind, randomised controlled trial | 256 | No difference in the effect of cilostazol or aspirin compared to the expected progression of white matter change in CSVD |
| Silence study [36] | Longitudinal, randomised, double blind controlled trial | 83 | No difference in cognitive decline | |
| Aspirin and Dementia in Patients with Coronary Heart Disease (CHD) | Meta-analysis [37] of ESTHER [30] and UK Biobank [31] | Subjects with pre-existing CHD benefited strongly from low-dose aspirin | ||
| Aspirin and Dementia in Patients with Late Onset Depression (LOD) | Ya-Hsu Yang et al. [38] | Population-based study | 46,439 | Lower incidence of incident dementia in patients on aspirin |
| Aspirin in Women | JPAD2 [39] | Longitudinal follow up of original JPAD cohort | 2359 | Significant reduction in the risk of dementia was seen in in women but not in men |
| Kern S et al. [40] | Population-based cohort study | 681 | Significantly less dementia in high cardiovascular risk women who were randomised to the aspirin group | |
| Alzheimer’s Disease Neuroimaging Initiative [28] | Longitudinal study | 1866 | Aspirin was associated with slower decline in male AD patients but not female AD patients | |
| Meta-analysis [37] of ESTHER [30] and UK Biobank [31] | Aspirin was associated with decreased risk of all cause dementia and VD in males, but not in females | |||
| Women’s Health Study [41] | Randomised, double blind, placebo-controlled cohort study | 6377 | Women assigned aspirin did not do better than those placebos; some evidence for reduced decline in category (semantic) fluency in the aspirin group; aspirin was associated with less cognitive decline in current smokers and hyperlipidaemia | |
| Kang et al. [42] | Observational study | 13,255 | No association with cognitive decline | |
| High Dose Aspirin | Nilsson SE et al. [43] | Longitudinal study | 702 | High-dose aspirin resulted in lower prevalence of AD and better cognitive function |
| Sydney Older Persons Study [27] | Longitudinal study | 647 | Whilst aspirin reduced risk of development of Alzheimer’s disease, there was no difference in outcome between low and high doses | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thong, E.H.; Lee, E.C.Y.; Yun, C.-Y.; Li, T.Y.W.; Sia, C.-H. Aspirin Therapy, Cognitive Impairment, and Dementia—A Review. Future Pharmacol. 2023, 3, 144-161. https://doi.org/10.3390/futurepharmacol3010011
Thong EH, Lee ECY, Yun C-Y, Li TYW, Sia C-H. Aspirin Therapy, Cognitive Impairment, and Dementia—A Review. Future Pharmacology. 2023; 3(1):144-161. https://doi.org/10.3390/futurepharmacol3010011
Chicago/Turabian StyleThong, Elizabeth H., Edward C. Y. Lee, Choi-Ying Yun, Tony Y. W. Li, and Ching-Hui Sia. 2023. "Aspirin Therapy, Cognitive Impairment, and Dementia—A Review" Future Pharmacology 3, no. 1: 144-161. https://doi.org/10.3390/futurepharmacol3010011
APA StyleThong, E. H., Lee, E. C. Y., Yun, C.-Y., Li, T. Y. W., & Sia, C.-H. (2023). Aspirin Therapy, Cognitive Impairment, and Dementia—A Review. Future Pharmacology, 3(1), 144-161. https://doi.org/10.3390/futurepharmacol3010011







