Preventing Microbial Infections with Natural Phenolic Compounds
Abstract
1. Introduction
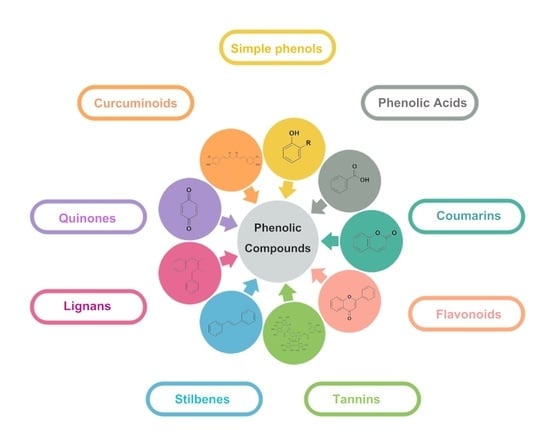
2. Natural Phenolic Compounds against Microbes
2.1. Simple Phenols and Phenolic Acids
| Secondary Metabolite Class | Subclasses | Compound | Source | Microorganism | Positive Control | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | Gram-Positive Bacteria | Gram- Negative Bacteria | Fungi | |||||
| Phenolic acids | Benzoic acid derivatives | Benzoic acid | Neurospora crassa (Microorganism) | SAa (587) | ECa (274), PA a (302) | CAa (347), AN a (570) | Streptomycin SA a (44), EC a (210), PA a (210) | Ketoconazole CA a (200), AN a (200) | [39] | |
| Gallic acid | Caesalpinia mimosoides Lamk (Plant) | SA (1250) | Streptomycin SA (0.16) | [49] | ||||||
| Diospyros virginiana L. (Plant) | LM (40), SA (10), BC c (25) | EC (40), ST (10), PA (25) | AN (30), AV (10), AF (25) | Streptomycin LM (150), SA (250), BC c (50), | Streptomycin EC (100), ST (50), PA (50) | Ketoconazole AN (200), AV (200), AF (200) | [50] | |||
| Mezoneuron benthamianum (Plant) | SAc (100) | ECc (25), PA c (100) | - | - | [51] | |||||
| 4- Hydroxybenzoic acid | Ganoderma lucidum (Plant) | LM (30), SA (3), BC (3) | EC (30), ST (3), PA (3) | AN (30), AV (3), AF c (120) | Streptomycin LM (170), SA (40), BC (90) | Streptomycin EC (170), ST (170), PA (170) | Ketoconazole AN (200), AV (200), AF c (200) | [52] | ||
| 4-(2′R, 4′-dihydroxybutoxy) benzoic acid | Penicillium sp. of Nerium indicum (Microorganism) | EC (125), PA (125) | Streptomycin sulfate EC (7.81), PA (7.81) | [53] | ||||||
| Vanillic acid | Stenoloma chusanum (Plant) | CA (50), AN (100), TR (50) | - | [54] | ||||||
| Cinnamic acid derivatives | Cinnamic acid | Ganoderma lucidum (Plant) | LM (7), SA (1.5), BC (1.5) | EC (7), ST (1.5), PA (0.7) | AN (30), AV (7), AF (7) | Streptomycin LM (170), SA (40), BC (90) | Streptomycin EC (170), ST (170), PA (170) | Ketoconazole AN (200), AV (200), AF (200) | [52] | |
| Caffeic acid | Nauclea latifolia leaf (Plant) | SA (5000) | EC (625), PA (2500) | Streptomycin SA (125) | Streptomycin EC (125), PA (500) | [55] | ||||
| p-Coumaric acid | Stereospermum zenkeri (Plant) | SAa (37.50) | Ampicillin SA a (0.80) | [56] | ||||||
| trans-o-coumaric acid | Distichochlamys benenica (Plant) | SA (249.5) | EC (1001.4), PA (1001.4) | Ciprofloxacin SA (0.215) | Ciprofloxacin EC (0.013), PA (0.013) | [57] | ||||
| Coumarins | Simple coumarins | Umbelliferone | Loeselia Mexicana (Plant) | CA (50), TR (25) | Nystatin CA (8), TR (-) Miconazole CA (-), TR (4) | [58] | ||||
| Ferulago Species (Plant) | SA (250) | EC (500), PA (250) | CA (125) | Streptomycin SA (6.25) | Streptomycin EC (25), PA (25) | Ketoconazole CA (25) Miconazole CA (3) | [59] | |||
| Osthol | Magydaris tomentosa (Plant) | SA (64), SE (32) | EC (256), PA (128) | Cefotaxime SA (2), SE (0.1) | Cefotaxime EC (0.1), PA (1.6) | [60] | ||||
| Prangos hulusii (Plant) | SA (125), MRSA c (16) | Cefotaxime SA (2), MRSA c (16) | [61] | |||||||
| Prangos pabularia (Plant) | MRSA (31.25) | PA (31.25) | - | - | [62] | |||||
| Ferulago Species (Plant) | SA (500) | EC (500), PA (250) | CA (500) | Streptomycin SA (6.25) | Streptomycin EC (25), PA (25) | Ketoconazole CA (25) Miconazole CA (3) | [59] | |||
| Novobiocin | Nocardiopsis gilva (Microorganism) | SA (64) | Kanamycin SA (4) | [63] | ||||||
| Streptomyces strain (Microorganism) | MRSA (0.25) | - | [64] | |||||||
| Ulopterol | Toddalia asiatica (L.) Lam. (Plant) | SA (125), MRSA c (250), SE (15.6) | ECc (62.5–250), ST (125), SF (62.5), PA (125) | CA (250), AF (15.6), TR (250) | Streptomycin SA (6.25), MRSA c (6.25), SE (25) | Streptomycin EC c (25), ST (30), SF (6.25), PA (25) | Ketoconazole CA (25), AF (<12.5), TR (<12.5) | [65] | ||
| Ferulago Species (Plant) | SA (500) | EC (500), PA (500) | CA (250) | Streptomycin SA (6.25) | Streptomycin EC (25), PA (25) | Ketoconazole CA (25) Miconazole CA (3) | [59] | |||
| Furanocoumarins | Peucedanin | Peucedanum luxurians (Plant) | SA (1500), SE (1750) | EC (2750), PA (1400) | Netilmicin SA (4), SE (4) | Netilmicin EC (10), PA (88) | [66] | |||
| Oxypeucedanin hydrate | Angelica pancicii Vandas (Apiaceae) (Plant) | LM (1000), SA (1000) | EC (1000), ST (1000), PA (1000) | Streptomycin LM (170), SA (40) | Streptomycin EC (170), ST (170), PA (170) | [67] | ||||
| Angelica lucida (Plant) | SA (650), SE (600) | EC (650), PA (810) | Netilmicin SA (4), SE (4) | Netilmicin EC (10), PA (3) | [68] | |||||
| (R)-(+) oxypeucedanin hydrate | Ficus exasperata (Plant) | MRSAc (78.12), BC c (9.76) | ECb,c (39.06), PA b,c (156.25) | CAc (39.06) | Gentamicin MRSA c (4.88), BC c (4.88) | Gentamicin EC b,c (4.88), PA b,c (9.76) | Nystatin CA c (19.53) | [69] | ||
| Imperatorin | Heracleum mantegazzianum Sommier and Levier (Apiaceae) (Plant) | SA (250–1000), BC (500), SE (1000) | EC (1000), ST (1000), PA (1000) | CA (250) | - | - | - | [70] | ||
| Magydaris tomentosa (Plant) | SA (32), SE (32) | EC (32), PA (64) | Cefotaxime SA (2), SE (0.1) | Cefotaxime EC (0.1), PA (1.6) | [60] | |||||
| Angelica lucida (Plant) | SA (45), SE (35) | EC (25), PA (70) | Netilmicin SA (4), SE (4) | Netilmicin EC (10), PA (3) | [68] | |||||
| Prangos pabularia (Plant) | MRSA (62.5) | PA (65.5) | - | - | [62] | |||||
| 5-methoxy-3-(3-methyl-2,3- Dihydroxybutyl) psoralen | Dorstenia turbinata (Plant) | MRSAc (39.06) | EC b,c (78.12), PA b,c (39.06) | CA c (19.53), CG c (39.06), TR c (9.76) | Gentamycin MRSA c (9.76) | Gentamycin EC b,c (4.88), PA b,c (9.76) | Nystatin CA c (19.53) | [71] | ||
| Pyrano coumarins | Agasyllin | Ferulago campestris (Plant) | SAa,c (64) | PA a,c (125) | - | Cefotaxime SA a,c (resistant) | Cefotaxime PA a,c (32) | [72] | ||
| Zosima absinthifolia (Plant) | SA (5000) | EC (5000) | Gentamycin SA (8) | Gentamycin EC (8) | [73] | |||||
| Bi-coumarin (Dicoumarin) | Daphnoretin | Loeselia mexicana (Plant) | CA (50), TR (25), AN (100) | Nystatin CA (8), TR (-) AN (-) Miconazole CA (-), TR (4) AN (8) | [58] | |||||
| Flavonoids | Flavonols | Myricetin | Diospyros virginiana L. (Plant) | LM (10), SA (5), BC c (2.5) | EC (15), ST (15), PA (150) | AN (5), AV (2.5), AF (2.5) | Streptomycin LM (150), SA (250), BC c (50) | Streptomycin EC (100), ST (50), PA (50) | Ketoconazole AN (200), AV (200), AF (200) | [50] |
| Quercetin | Diospyros virginiana L. (Plant) | LM (10), SA (1), BC c (2.5) | EC (15), ST (15), PA (200) | AN (5), AV (2.5), AF (2.5) | Streptomycin LM (150), SA (250), BC c (50) | Streptomycin EC (100), ST (50), PA (50) | Ketoconazole AN (200), AV (200), AF (200) | [50] | ||
| Nauclea latifolia (Plant) | SA (156) | EC (2500), PA (1250) | Streptomycin SA (125) | Streptomycin EC (125), PA (500) | [55] | |||||
| Euphorbia schimperiana (Plant) | LM (450), SA (420), BC (430) | EC (430), PA (420) | - | - | [74] | |||||
| Macaranga conglomerate (Plant) | SA (500) | EC (500), PA (500) | Ciprofloxacin SA (15.6) | Ciprofloxacin EC (1.0), PA (15.6) | [75] | |||||
| Monanthotaxis littoralis (Plant) | SA (16) | EC (16), PA (16) | CA (16), CN (8) | Vancomycin SA (0.5) | Vancomycin EC (32), PA (16) | Fluconazole CA (1.0), CN (2.0) | [76] | |||
| Flavones | 6,7,4′-trimethyl flavone | Wulfenia amherstiana (Plant) | SA (127.06–128.94) | PA (510.98–513.02) | CA (127.37–128.63), CG (255.18–256.82), FS (511.02–512.98) | - | - | - | [77] | |
| Luteolin | Diospyros virginiana L. (Plant) | LM (1.5), SA (1.5), BC c (2.5) | EC (15), ST (20), PA (200) | AN (10), AV (5), AF (2.5) | Streptomycin LM (150), SA (250), BC c (50) | Streptomycin EC (100), ST (50), PA (50) | Ketoconazole AN (200), AV (200), AF (200) | [50] | ||
| Flavanols (Flavan-3-ols) | (+)-Catechin-3′-O-rhamnopyranoside | Neocarya macrophylla (Sabine) Prance (Chrysobalanaceae) (Plant) | SA c (25) | PA c (25), EC c (25) | CA c (6.25) | - | - | - | [78] | |
| (−)-Catechin | Prunus avium L. (Plant) | LM (100), SA a (100), BC a (100) | EC (100) | - | - | [79] | ||||
| Isoflavones | Myrsininone A | Ficus auriculata (Plant) | BC (2.03), SE (0.51) | EC (2.03), PA (4.06) | Streptomycin sulfate BC (0.23), SE (0.23) | Streptomycin sulfate EC (0.45), PA (0.45) | [80] | |||
| Daidzein | Spatholobus parviflorus (Plant) | BC (64) | PA (128) | Vancomycin BC (0.25) | Gentamycin PA (1.0) | [81] | ||||
| Lupalbigenin | Maclura cochinchinensis (Lour.) Corner (Plant) | SA (1), MRSA (1) | CA (4) | Vancomycin SA (0.5), MRSA (1.0) | Ampicillin CA (0.25) | [82] | ||||
| Flavanones | Lupinifolin | Derris reticulata Craib (Plant) | SA (12.5), BC (12.5), SE (25) | Penicillin G SA (0.05), BC (ND), SE (0.05) | [83] | |||||
| 7-O-(2,2-dimethylallyl)-aromadendrin | Maclura cochinchinensis (Lour.) Corner (Plant) | SA (32), MRSA (32) | CA (64) | Vancomycin SA (0.5), MRSA (1.0) | Ampicillin CA (0.25) | [82] | ||||
| Tannins | Gallotannins | Penta-O-galloylglucose | Rhus trichocarpa Miquel (Plant) | SA (64–128), MRSA (64–128), BC (32), SE (32) | CA (64) | Vancomycin SA (0.25–1), MRSA (0.25–1), BC (>64), SE (1) | Vancomycin CA (32) | [84] | ||
| Ellagitannins | Punicalagin | Punica granatum L. (Plant) | SA (0.6), SE (0.6) | EC (1.2), PA (0.6) | CA (1.2) | - | - | - | [85] | |
| 3,3′-di-O-methylellagic acid | Euphorbia schimperiana (Plant) | LM (450), SA (450), BC (450) | EC (450), PA (430) | - | - | [74] | ||||
| Isorugosins B | Liquidambar formosana (Plant) | MRSA (32.46–63.96) | Oxacillin (128.05–256.1) | [86] | ||||||
| Vescalagin | Cork (Plant) | SA (500), MRSA (125) | PA (1000) | - | - | [87] | ||||
| Castalagin | - | - | ||||||||
| Condensed tannins | A type-proanthocyanidin | Quercus ilex (Plant) | LM (100.72), SA (100.72), BC c (100.72) | EC (100.72), ST (100.72), PA (100.72) | AN (100.72), AF (100.72), AV (100.72) | Streptomycin LM (150.04), SA (100.03), BC c (25.01) | Streptomycin EC (100.03), ST (100.03), PA (100.03) | Ketoconazole AN (201.94), AF (201.94), AV (201.94) | [88] | |
| Phlorotannins | Fucofuroeckol-A | Eisenia bicyclis (Marine algae) | CA b,c (512) | Fluconazole CA b,c (512–8197) | [89] | |||||
| Dieckol | Ecklonia stolonifera (Marine algae) | MRSAa,c (64–128) | EC (256), ST (256), SF (256) | Ampicillin MRSA a,c (128–512) | Vancomycin EC (512), ST (512), SF (256) | [90] | ||||
| Stilbenes | Stilbene Monomers | Resveratrol | Mezoneuron benthamianum (Plant) | SAc (25) | ECc (25), PA c (25), PA (200) | - | - | [51] | ||
| Nauclea pobeguiinii (Plant) | ECb,c (32–128), PA a,c (256) | Chloramphenicol EC b,c (64), PA a,c (256) | [91] | |||||||
| Gnetum gnemon L. (Plant) | EC (>3000) | SC (2000) | - | - | [92] | |||||
| Bacillus sp. N strain (Microorganism) | SA (32) | EC (32), PA (64) | CA (64) | Ciprofloxacin SA (5) Cefotaxime SA (250) | Ciprofloxacin EC (5), PA (10) Cefotaxime EC (100), PA (500) | Amphotericin B CA (50) | [93] | |||
| Piceatannol | Mezoneuron benthamianum (Plant) | SAc (25) | EC c (25), PA c (300), | - | - | [51] | ||||
| Spirotropis longifolia (Plant) | CAc (2), CG c (4), TR c (8) | Fluconazole CA c (>64), CG c (8), TR c (2) | [94] | |||||||
| Pterostilbene | Commercial Product | LM (64), SA (4), BC (16) | EC (512), PA (512) | Chlorhexidine LM (8), SA (32), BC (8) | Chlorhexidine EC (32), PA (32) | [95] | ||||
| Oxyresveratrol | Spirotropis longifolia (Plant) | CAc (>64), CG c (8), TR c (16) | Fluconazole CA c (>64), CG c (8), TR c (2) | [94] | ||||||
| Morus alba L. (Plant) | TR (500) | Miconazole nitrat TR (1) | [96] | |||||||
| Chiricanine A | Arachis hypogaea (Plant) | MRSA (12.5) | - | [97] | ||||||
| 3,5-Dihydroxy-4-isopropylstilbene | Bacillus sp. N strain (Microorganism) | SA (8) | EC (>1000), PA (>1000) | CA (24) | Ciprofloxacin SA (5) Cefotaxime SA (250) | Ciprofloxacin EC (5), PA (10) Cefotaxime EC (100), PA (500) | Amphotericin B CA (50) | [93] | ||
| Photorhabdus luminescens (Microorganism) | CN (12), AF (12) | - | [98] | |||||||
| Stilbene Dimers | Monalittorin | Monanthotaxis littoralis (Plant) | SA (64) | EC (65), PA (64) | CA (16), CN (16), | Vancomycin SA (0.5) | Vancomycin EC (32), PA (16) | Fluconazole CA (1.0), CN (2.0) | [76] | |
| Gnetin D | Spirotropis longifolia (Plant) | CAc (64), CG c (32), TR c (8) | Fluconazole CA c (>64), CG c (8), TR c (2) | [94] | ||||||
| Gnetin C | Gnetum gnemon L. (Plant) | EC (1000) | SC (500) | - | - | [92] | ||||
| Longistylin A | Cajanus cajan (Plant) | SA (1.56), BC (25), MRSA (1.56) | EC (>100) | Vancomycin SA (1.56), BC (50), MRSA (0.78) | Vancomycin EC (50) | [99] | ||||
| Monalittorin | Monanthotaxis littoralis (Plant) | SA (64) | EC (64), PA (64) | CA (16), CN (16) | Vancomycin SA (0.5) | Vancomycin EC (32), PA (16) | Fluconazole CA (1.0), CN (2.0) | [76] | ||
| Stilbene Oligomers | Rockiol A and Rockiol B | Paeonia rockii (Plant) | SA (25) | EC (200), PA (200) | Penicillin G SA (10) | Penicillin G EC (20), PA (10) | [100] | |||
| Upunaphenol D | Dryobalanops lanceolata (Plant) | SA (45.3), SE (22.7) | EC (>906.9), ST (>906.9), SF (453.4) | Chloramphenicol SA (0.008), SE (0.008) | Chloramphenicol EC (323.132), ST (323.132), SF (0.010) | [101] | ||||
| Heyneanol A | Vitis thunbergii var. taiwaniana (Plant) | SA (2), MRSA (2) | Vancomycin SA (1), MRSA (1) Oxacillin SA (2), MRSA (64–128) | [102] | ||||||
| Lignans | Tetrahydrofuran Lignans | Matairesinol | Centaurea scabiosa (Plant) | SA (10), MRSA (1000), SE (10) | EC (10), PA (10) | Ciprofloxacin SA (2.5 × 10−4), MRSA (2.5 × 10−4), SE (2.5 × 10−5) | Ciprofloxacin EC (2.5 × 10−4), PA (0.0025) | [103] | ||
| Centaurea raphanina ssp. Mixta (Plant) | AN (100), AV (100) | Miconazole AN (1.5), AV (2) | [104] | |||||||
| Lariciresinol | Rubia philippinensis (Plant) | SA (125) | EC (250) | - | - | [105] | ||||
| Sambucus williamsii (Plant) | CA (25) | Amphotericin B CA (6.25) | [106] | |||||||
| Iso-hydroxymatairesinol | Punica granatum L. (Plant) | SA (1500), SE (190) | EC (560), PA (1500) | - | - | [107] | ||||
| Punicatannin C | SA (1500), SE (750) | EC (1120) | - | - | ||||||
| Furofuran Lignans | Sesamin | Zanthoxylum paracanthum Kokwaro (Plant) | SA (500) | Omacilin (0.49) | [108] | |||||
| Phillyrigeninside B | Forsythia suspensa (Plant) | SA (10) | EC (20) | CA (20) | Gentamicin SA (4) | Gentamicin EC (4) | Gentamicin CA (4) | [109] | ||
| Pinoresinol | Cinnamomum Camphora (Plant) | SA (15.60) | EC (31.25), PA (7.80) | - | - | [110] | ||||
| Sambucus williamsii (Plant) | CA (12.5) | Amphotericin B CA (6.5) | [111] | |||||||
| Arylnaphthalene Lignan | 2,3-dimethyl-4-(4′-hydroxy-3′,5′-dimethoxyphenyl)-6-hydroxy-7-methoxy-naphthalene | Ganoderma lipsiense (Microorganism) | SA (1.25), SE (>10) | EC (10) | CA (>10) | Ciprofloxacin SA (0.156), SE (0.156) | Ciprofloxacin EC (0.156) | Ciprofloxacin CA (0.156) | [112] | |
| Arylnaphthalenelactone Lignan | Justicidin B | Nocardia sp. (Microorganism) | SA (1), BC (2.5) | EC (0.5), PA (0.2) | CA (4.5), CN (0.5), AN (0.2) | - | - | - | [113] | |
| Dibenzocyclooctadiene Lignan | Manglisin B | Manglietiastrum sinicum (Plant) | SA (0.025), MRSA (0.025) | Vancomycin hydrochloride SA (1.63 × 10−3), MRSA (8.02 × 10−4) | [114] | |||||
| Quinones | Benzoquinones | Oncocalyxone A | Auxemma oncocalyx (Allem) Taub (Plant) | LM (37.75), SA (18.87), MRSA (18.87–37.75), SE (9.43–37.75) | EC (>151), PA (>151) | CA (>151), CN (>151), AF (>151) | Vancomycin LM (<2.0), SA (1.0), MRSA (1.0), SE (2.0) | Meropenem EC (<0.1), PA (<0.39) | Itraconazole CA (0.25), CN (0.06), AF (0.125) | [115] |
| 2-methyl-6-(-3-methyl-2-butenyl)benzo-1,4-quinone | Gunnera perpensa (Plant) | SA (39), BC (18), SE (9.8) | EC (>6250) | CA (130), CN (70) | Ciproflaxin SA (0.31), BC (2.5), SE (1.25) | Ciproflaxin EC (0.63) | Amphotericin B CA (1.25), CN (2.5) | [116] | ||
| 3,5- dimethoxy-2- methylthio)cyclohexa-2,5 diene-1,4-dione | Diplocentrus melici (Animal) | SA (4) | Ampicillin SA (0.5) | [117] | ||||||
| 2,6-Dimethoxy-1,4-Benzoquinone | Wood tar (Plant) | SA (32) | EC (64), ST (32) | Chloramphenicol SA (32) | Chloramphenicol EC (32), ST (32) | [118] | ||||
| Naphthoquinones | Plumbagin | Diospyros bipindensis (Plant) | SA (20) | Ampicillin SA (0.7) | [119] | |||||
| Plumbago zeylanica L. (Plant) | MRSA (4–8) | [120] | ||||||||
| Diospyros crassiflora (Plant) | CAc (0.78), CG c (3.12), CN c (1.56), AN c (0.78) | Ketoconazole CA c (0.25), CG c (5), CN c (0.25), AN c (0.25) | [121] | |||||||
| Plumbago zeylanica (Plant) | SA (0.5) | EC (8), PA (8) | CA (2) | Ciprofloxacin SA (1.0) Amoxicillin SA (0.5) | Ciprofloxacin EC (0.5), PA (0.5) Amoxicillin EC (4), PA (128) | Ketoconazole CA (256) | [122] | |||
| Plumbago indica (Plant) | SA (3.12), SE (0.018) | Tetracycline HCl SA (0.38), SE (0.048) | [123] | |||||||
| 2-methyl-1,4-naphthoquinone (vitamin K3) | Pulsatilla koreana (Plant) | SA (2.6–4) | PA (4) | CA (32–96), CG (8) | Tetracycline SA (0.5) | Tetracycline PA (0.22–0.38) | Ketoconazole CA (10.6–16), CG (8–13.4) | [124] | ||
| 2-Methoxy-1,4-naphthoquinone | Impatiens balsamina L. (Plant) | SA (16), BC (64) | CAc (0.62–2.50), CA a,c (0.62–1.25), AF c (0.31) | Chloramphenicol SA (8), BC (8) | Amphotericin B CA c (1.1), CA a,c (90), AF c (1.1) | [125] | ||||
| Bluemomycin | Streptomyces sp. (Microorganism) | SA (NA), MRSA c (10.6–39.4), SE (35.6–64.4) | ECa,c (8.9–39.4), ST (8.9–16.1), SF (5.3–19.7), PA (5.3–19.7) | CAc (46.4–53.6), TR (NA) | Streptomycin SA (2.65–9.85), MRSA c (6.25–20.65), SE (17.8–32.2) | Streptomycin EC a,c (10.6–39.4), ST (17.8–32.2), SF (2.65–9.85), PA (10.6–39.4) | Ketoconazole CA c (10.6–39.4), TR (<26.9) | [126] | ||
| 5-hydroxy-3,6-dimethoxy-7-methyl-1,4-naphthalenedione | Xanthium sibiricum (Plant) | SA (2.78), BC (22.2) | EC (5.55) | Ciprofloxacin SA (1.39), BC (5.55) | Ciprofloxacin EC (0.69) | [127] | ||||
| Anthraquinones | Zenkequinone A | Stereospermum zenkeri (Plant) | ECa (37.50), PA a (18.75) | Ampicillin EC a (0.40), PA a (0.80) | [56] | |||||
| Emodin | Rumex abyssinicus (Plant) | SA (8), MRSA (32) | SF (8), PA (16) | CA (8), CN (8) | Ciprofloxacin SA (0.5), MRSA (4) | Ciprofloxacin SF (8), PA (0.5) | Fluconazole CA (1), CN (2) | [128] | ||
| Cassia occidentalis (Plant) | SA (3.9) | EC (>50) | Neomycin SA (6.3) | Neomycin EC (1.6) | [129] | |||||
| Physcion | Rumex abyssinicus (Plant) | SA (8), MRSA (16) | SF (8), PA (8) | CA (8), CN (8) | Ciprofloxacin SA (0.5), MRSA (4) | Ciprofloxacin SF (8), PA (0.5) | Fluconazole CA (1), CN (2) | [128] | ||
| Isoversicolorin C | Aspergillus nidulans (Microorganism) | EC (32) | Chloramphenicol EC (1) | [130] | ||||||
| 2,3-dihydroxy-9,10-anthraquinone | Streptomyces galbus (Microorganism) | SA (>100), MRSA c (12.5), SE (>100) | ECc (50), ST (12.5), SF (25), PA (12.5) | CA (50) | Streptomycin SA (6.25), MRSA c (6.25), SE (12.5) | Streptomycin EC c (25), ST (6.25), SF (6.25), PA (25) | Ketoconazole CA (25) | [131] | ||
| 5-Hydroxy ericamycin | Actinoplanes sp. (Microorganism) | SAb,c (<0.06) MRSA (0.016) MRSA c (<0.06), SE b,c (<0.06) | EC (4), EC b,c (16), PA (16) | Vancomycin SA b,c (1.0–8.0), MRSA (2.0), MRSA c (1.0), SEb,c (2.0) | Vancomycin EC (>64), EC b,c (>64), PA (ND) | [132] | ||||
| Curcuminoids | Curcumin | Zingiber spectabile (Plant) | SA (500), BC (125) | EC (NA) | Tetracycline SA (3.91), BC (1.95) | Tetracycline EC (NA) | [133] | |||
| Curcuma longa Linné | MRSA (125–250), MRSA c (125–250) | Oxacillin MRSA (500–1000, >1000), MRSA c (500–1000) Ciprofloxacin MRSA (7.8–250.0), MRSA c (1.95–15.6) | [134] | |||||||
| Commercial product | SA (25) | PA (50) | - | [135] | ||||||
| Commercial product from Curcuma longa L. (Plant) | SA (125–500), MRSA (>4500), SE (500–2000) | EC (2000), EC c (1500), PA (62.5–5000) | CA (1000–5000), SC (5000) | - | - | - | [136] | |||
| Commercial product | SA (450) | PA (500) | - | - | [137] | |||||
| Commercial product from Curcuma longa | CA (1000), CG (125) | Ketoconazole CA (62.5), CG (1.95) | [138] | |||||||
| Commercial product | SA (0.03), BC (0.05) | EC (0.225) | - | - | [139] | |||||
| Demethoxycurcumin | Zingiber spectabile (Plant) | SA (125), BC (125) | EC (500) | Tetracycline SA (3.91), BC (1.95) | Tetracycline EC (NA) | [133] | ||||
2.2. Coumarin
2.3. Flavonoids
2.4. Tannin
2.5. Stilbenes
2.6. Lignans
2.7. Quinones
2.8. Curcuminoids
3. Limitations in the Therapeutic Usage of Natural Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Yoshikawa, T.T. Antimicrobial Resistance and Aging: Beginning of the End of the Antibiotic Era? J. Am. Geriatr. Soc. 2002, 50, 226–229. [Google Scholar] [CrossRef]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic Resistance Breakers: Current Approaches and Future Directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef]
- Brown, D. Antibiotic Resistance Breakers: Can Repurposed Drugs Fill the Antibiotic Discovery Void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity—A Pharmaco-Toxicological Screening. Antibiotics 2021, 10, 401. [Google Scholar] [CrossRef]
- McKeny, P.T.; Nessel, T.A.; Zito, P.M. Antifungal Antibiotics; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez-del Valle, M.G.; Hernández-López, M.; Ait Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a Potential Natural Compound to Control Pre and Postharvest Diseases of Horticultural Commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Chew, J.; Peh, S.-C.; Sin Yeang, T. Non-Microbial Natural Products That Inhibit Drug-Resistant Staphylococcus Aureus. In Staphylococcus Aureus; Hemeg, H., Ozbak, H., Afrin, F., Eds.; IntechOpen: London, UK, 2019; pp. 1–30. ISBN 978-1-78984-592-1. [Google Scholar]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic Building Blocks for the Assembly of Functional Materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef]
- Oliver, S.; Vittorio, O.; Cirillo, G.; Boyer, C. Enhancing the Therapeutic Effects of Polyphenols with Macromolecules. Polym. Chem. 2016, 7, 1529–1544. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol Uses in Biomaterials Engineering. Biomaterials 2018, 167, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive Compounds and Beneficial Functions of Sprouted Grains. In Sprouted Grains; Elsevier: Duxford, UK, 2019; pp. 191–246. ISBN 978-0-12-811525-1. [Google Scholar]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, Antiviral and Antimicrobial Activities of Alkaloids, Flavonoids, and Phenolic Acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural Products—Antifungal Agents Derived from Plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Jiang, F.; Dusting, G.J. Natural Phenolic Compounds as Cardiovascular Therapeutics: Potential Role of Their Antiinflammatory Effects. Curr. Vasc. Pharmacol. 2003, 1, 135–156. [Google Scholar] [CrossRef]
- Medeiros, K.C.P.; Figueiredo, C.A.V.; Figueredo, T.B.; Freire, K.R.L.; Santos, F.A.R.; Alcantara-Neves, N.M.; Silva, T.M.S.; Piuvezam, M.R. Anti-Allergic Effect of Bee Pollen Phenolic Extract and Myricetin in Ovalbumin-Sensitized Mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef]
- Hahn, D.; Bae, J.-S. Recent Progress in the Discovery of Bioactive Components from Edible Natural Sources with Antithrombotic Activity. J. Med. Food 2018, 22, 109–120. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Gutiérrez-Larraínzar, M.; Rúa, J.; Caro, I.; de Castro, C.; de Arriaga, D.; García-Armesto, M.R.; del Valle, P. Evaluation of Antimicrobial and Antioxidant Activities of Natural Phenolic Compounds against Foodborne Pathogens and Spoilage Bacteria. Food Control 2012, 26, 555–563. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of Natural Products with Hepatoprotective Effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Kougan, G.B.; Tabopda, T.; Kuete, V.; Verpoorte, R. Simple Phenols, Phenolic Acids, and Related Esters from the Medicinal Plants of Africa. In Medicinal Plant Research in Africa; Elsevier: London, UK, 2013; pp. 225–249. ISBN 978-0-12-405927-6. [Google Scholar]
- Stich, H.F.; Rosin, M.P.; Wu, C.H.; Powrie, W.D. The Action of Transition Metals on the Genotoxicity of Simple Phenols, Phenolic Acids and Cinnamic Acids. Cancer Lett. 1981, 14, 251–260. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Muller, A.G.; Sarker, S.D.; Saleem, I.Y.; Hutcheon, G.A. Delivery of Natural Phenolic Compounds for the Potential Treatment of Lung Cancer. Daru 2019, 27, 433–449. [Google Scholar] [CrossRef]
- Liwa, A.C.; Barton, E.N.; Cole, W.C.; Nwokocha, C.R. Chapter 15—Bioactive Plant Molecules, Sources and Mechanism of Action in the Treatment of Cardiovascular Disease. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 315–336. ISBN 978-0-12-802104-0. [Google Scholar]
- Ablikim, G.; Bobakulov, K.; Li, J.; Yadikar, N.; Aisa, H.A. Two New Glucoside Derivatives of Truxinic and Cinnamic Acids from Lavandula angustifolia mill. Nat. Prod. Res. 2021, 35, 2526–2534. [Google Scholar] [CrossRef]
- Buxton, T.; Takahashi, S.; Eddy Doh, A.-M.; Baffoe-Ansah, J.; Owusu, E.O.; Kim, C.-S. Insecticidal Activities of Cinnamic Acid Esters Isolated from Ocimum gratissimum L. and Vitellaria paradoxa Gaertn Leaves against Tribolium castaneum Hebst (Coleoptera: Tenebrionidae). Pest Manag. Sci. 2020, 76, 257–267. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Bioactive Natural Products (Part I); Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 257–312. [Google Scholar]
- Deng, Z.; Li, C.; Luo, D.; Teng, P.; Guo, Z.; Tu, X. A New Cinnamic Acid Derivative from Plant-Derived Endophytic Fungus Pyronema sp. Nat. Prod. Res. 2017, 31, 2413–2419. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Chandrika, M.; Yashavantha Rao, H.C.; Kamalraj, S.; Jayabaskaran, C.; Pugazhendhi, A. Molecular Profiling of Marine Endophytic Fungi from Green Algae: Assessment of Antibacterial and Anticancer Activities. Process Biochem. 2020, 96, 11–20. [Google Scholar] [CrossRef]
- Ramalingam, V.; Narendra Kumar, N.; Harshavardhan, M.; Sampath Kumar, H.M.; Tiwari, A.K.; Suresh Babu, K.; Mudiam, M.K.R. Chemical Profiling of Marine Seaweed Halimeda gracilis Using UPLC-ESI-Q-TOF-MSE and Evaluation of Anticancer Activity Targeting PI3K/AKT and Intrinsic Apoptosis Signaling Pathway. Food Res. Int. 2022, 157, 111394. [Google Scholar] [CrossRef]
- de Siqueira, K.A.; Liotti, R.G.; de Sousa, J.R.; Vendruscullo, S.J.; de Souza, G.B.; de Vasconcelos, L.G.; Januário, A.H.; de Oliveira Mendes, T.A.; Soares, M.A. Streptomyces griseocarneus R132 Expresses Antimicrobial Genes and Produces Metabolites That Modulate Galleria mellonella Immune System. 3 Biotech 2021, 11, 396. [Google Scholar] [CrossRef]
- Keman, D.; Soyer, F. Antibiotic-Resistant Staphylococcus Aureus Does Not Develop Resistance to Vanillic Acid and 2-Hydroxycinnamic Acid after Continuous Exposure in Vitro. ACS Omega 2019, 4, 15393–15400. [Google Scholar] [CrossRef] [PubMed]
- El-Zawawy, N.A.; Ali, S.S.; Khalil, M.A.; Sun, J.; Nouh, H.S. Exploring the Potential of Benzoic Acid Derived from the Endophytic Fungus Strain Neurospora crassa SSN01 as a Promising Antimicrobial Agent in Wound Healing. Microbiol. Res. 2022, 262, 127108. [Google Scholar] [CrossRef] [PubMed]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, e7413504. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–Function Relationships of the Antibacterial Activity of Phenolic Acids and Their Metabolism by Lactic Acid Bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.S.S.; Hogg, T.A. Cell Membrane Damage Induced by Phenolic Acids on Wine Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric Acid Kills Bacteria through Dual Damage Mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Wen, A.; Delaquis, P.; Stanich, K.; Toivonen, P. Antilisterial Activity of Selected Phenolic Acids. Food Microbiol. 2003, 20, 305–311. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial Activity of Phenolic Acids against Commensal, Probiotic and Pathogenic Bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-Tuning of the Hydrophobicity of Caffeic Acid: Studies on the Antimicrobial Activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Araújo, M.O.; Freire Pessoa, H.L.; Lira, A.B.; Castillo, Y.P.; de Sousa, D.P. Synthesis, Antibacterial Evaluation, and QSAR of Caffeic Acid Derivatives. J. Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Antimicrobial Gallic Acid from Caesalpinia mimosoides Lamk. Food Chem. 2007, 100, 1044–1048. [Google Scholar] [CrossRef]
- Rashed, K.; Ćirić, A.; Glamočlija, J.; Soković, M. Antibacterial and Antifungal Activities of Methanol Extract and Phenolic Compounds from Diospyros virginiana L. Ind. Crops Prod. 2014, 59, 210–215. [Google Scholar] [CrossRef]
- Osamudiamen, P.M.; Oluremi, B.B.; Oderinlo, O.O.; Aiyelaagbe, O.O. Trans-Resveratrol, Piceatannol and Gallic Acid: Potent Polyphenols Isolated from Mezoneuron benthamianum Effective as Anticaries, Antioxidant and Cytotoxic Agents. Sci. Afr. 2020, 7, e00244. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.F.R.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R.P. Antimicrobial and Demelanizing Activity of Ganoderma lucidum Extract, p-Hydroxybenzoic and Cinnamic Acids and Their Synthetic Acetylated Glucuronide Methyl Esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- Ma, Y.-M.; Qiao, K.; Kong, Y.; Guo, L.-X.; Li, M.-Y.; Fan, C. A New P-Hydroxybenzoic Acid Derivative from an Endophytic Fungus Penicillium sp. of Nerium indicum. J. Asian Nat. Prod. Res. 2017, 19, 1245–1251. [Google Scholar] [CrossRef]
- Ren, B.; Xia, B.; Li, W.; Wu, J.; Zhang, H. Two Novel Phenolic Compounds from Stenoloma chusanum and Their Antifungal Activity. Chem. Nat. Compd. 2009, 45, 182–186. [Google Scholar] [CrossRef]
- Ajayi, O.S.; Aderogba, M.A.; Akinkunmi, E.O.; Obuotor, E.M.; Majinda, R.R.T. Bioactive Compounds from Nauclea latifolia Leaf Extracts. J. King Saud Univ.-Sci. 2020, 32, 2419–2425. [Google Scholar] [CrossRef]
- Lenta, B.N.; Weniger, B.; Antheaume, C.; Noungoue, D.T.; Ngouela, S.; Assob, J.C.N.; Vonthron-Sénécheau, C.; Fokou, P.A.; Devkota, K.P.; Tsamo, E.; et al. Anthraquinones from the Stem Bark of Stereospermum zenkeri with Antimicrobial Activity. Phytochemistry 2007, 68, 1595–1599. [Google Scholar] [CrossRef]
- Pham, T.V.; Hoang, H.N.T.; Nguyen, H.T.; Nguyen, H.M.; Huynh, C.T.; Vu, T.Y.; Do, A.T.; Nguyen, N.H.; Do, B.H. Anti-Inflammatory and Antimicrobial Activities of Compounds Isolated from Distichochlamys benenica. BioMed Res. Int. 2021, 2021, e6624347. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, V.M.; Rojas, G.; Avilés, M.; Fuentes, M.; Zepeda, G. In Vitro Antifungal Activity of Coumarin Extracted from Loeselia mexicana Brand. Mycoses 2011, 54, e569–e571. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Şimşek, D.; Özbek, H.; Güvenalp, Z.; Altanlar, N.; Kazaz, C.; Kılıc, C. Antimicrobial Activities of Extracts and Isolated Coumarins from the Roots of Four Ferulago Species Growing in Turkey. Iran. J. Pharm. Res. 2019, 18, 1516–1529. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.; Bellone, G.; Formisano, C.; Basile, A.; Cicala, C.; Alfieri, A.; Mascolo, N.; Bruno, M. Antibacterial and Anticoagulant Activities of Coumarins Isolated from the Flowers of Magydaris tomentosa. Planta Med. 2007, 73, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Shikishima, Y.; Takaishi, Y.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; et al. Coumarins and γ-Pyrone Derivatives from Prangos pabularia: Antibacterial Activity and Inhibition of Cytokine Release. Phytochemistry 2002, 59, 649–654. [Google Scholar] [CrossRef]
- Tian, S.-Z.; Pu, X.; Luo, G.; Zhao, L.-X.; Xu, L.-H.; Li, W.-J.; Luo, Y. Isolation and Characterization of New P-Terphenyls with Antifungal, Antibacterial, and Antioxidant Activities from Halophilic Actinomycete Nocardiopsis gilva YIM 90087. J. Agric. Food Chem. 2013, 61, 3006–3012. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Williams, D.E.; Wang, X.L.; Centko, R.; Chen, J.; Andersen, R.J. Marine Sediment-Derived Streptomyces Bacteria from British Columbia, Canada Are a Promising Microbiota Resource for the Discovery of Antimicrobial Natural Products. PLoS ONE 2013, 8, e77078. [Google Scholar] [CrossRef]
- Karunai Raj, M.; Balachandran, C.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. Antimicrobial Activity of Ulopterol Isolated from Toddalia asiatica (L.) Lam.: A Traditional Medicinal Plant. J. Ethnopharmacol. 2012, 140, 161–165. [Google Scholar] [CrossRef]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcourt, L.; Wolfender, J.-L.; Skalicka-Wozniak, K. Isolation and Antimicrobial Activity of Coumarin Derivatives from Fruits of Peucedanum luxurians tamamsch. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef]
- Mileski, K.S.; Trifunović, S.S.; Ćirić, A.D.; Šakić, Ž.M.; Ristić, M.S.; Todorović, N.M.; Matevski, V.S.; Marin, P.D.; Tešević, V.V.; Džamić, A.M. Research on Chemical Composition and Biological Properties Including Antiquorum Sensing Activity of Angelica Pancicii vandas Aerial Parts and Roots. J. Agric. Food Chem. 2017, 65, 10933–10949. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Popova, M.; Graikou, K.; Glowniak, K.; Chinou, I. Coumarins from Angelica lucida L.—Antibacterial Activities. Molecules 2009, 14, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Dongfack, M.D.J.; Lallemand, M.-C.; Kuete, V.; Mbazoa, C.D.; Wansi, J.-D.; Trinh-van-Dufat, H.; Michel, S.; Wandji, J. A New Sphingolipid and Furanocoumarins with Antimicrobial Activity from Ficus exasperata. Chem. Pharm. Bull. 2012, 60, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Walasek, M.; Grzegorczyk, A.; Malm, A.; Skalicka-Woźniak, K. Bioactivity-Guided Isolation of Antimicrobial Coumarins from Heracleum mantegazzianum Sommier & Levier (Apiaceae) Fruits by High-Performance Counter-Current Chromatography. Food Chem. 2015, 186, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ngameni, B.; Kuete, V.; Simo, I.K.; Mbaveng, A.T.; Awoussong, P.K.; Patnam, R.; Roy, R.; Ngadjui, B.T. Antibacterial and Antifungal Activities of the Crude Extract and Compounds from Dorstenia turbinata (Moraceae). S. Afr. J. Bot. 2009, 75, 256–261. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant Activities of Coumarins from the Roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Razavi, S.M.; Imanzadeh, G.; Jahed, F.S.; Zarrini, G. Pyranocoumarins from Zosima absinthifolia (Vent) Link Roots. Russ. J. Bioorg. Chem. 2013, 39, 215–217. [Google Scholar] [CrossRef]
- Aljubiri, S.M.; Mahmoud, K.; Mahgoub, S.A.; Almansour, A.I.; Shaker, K.H. Bioactive Compounds from Euphorbia schimperiana with Cytotoxic and Antibacterial Activities. S. Afr. J. Bot. 2021, 141, 357–366. [Google Scholar] [CrossRef]
- Hashim, I.; Onyari, J.M.; Omosa, L.K.; Maru, S.M.; Nchiozem-Ngnitedem, V.-A.; Karpoormath, R. Conglomeratin: A New Antibacterial Flavonol Derivative from Macaranga conglomerata Brenan (Euphorbiaceae). Nat. Prod. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Dongmo, A.J.N.; Ekom, S.E.; Tamokou, J.-D.; Tagousop, C.N.; Harakat, D.; Voutquenne-Nazabadioko, L.; Ngnokam, D. A New Stilbene Dimer and Other Chemical Constituents from Monanthotaxis littoralis with Their Antimicrobial Activities. Nat. Prod. Sci. 2020, 26, 9. [Google Scholar]
- Kakar, M.; Amin, M.U.; Alghamdi, S.; Sahibzada, M.U.K.; Ahmad, N.; Ullah, N. Antimicrobial, Cytotoxic, and Antioxidant Potential of a Novel Flavone “6,7,4′-Trimethyl Flavone” Isolated from Wulfenia amherstiana. Evid.-Based Complement. Altern. Med. 2020, 2020, e3903682. [Google Scholar] [CrossRef]
- AJ, Y.; MI, A.; AM, M.; AK, H.A. Bioactive (+)-Catechin-3′-O-Rhamnopyranoside from Neocarya macrophylla (Sabine) Prance (Chrysobalanaceae). Egypt. J. Basic Appl. Sci. 2019, 6, 124–136. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Cobo, A.; Ortega-Morente, E.; Gálvez, A.; Alejo-Armijo, A.; Salido, S.; Altarejos, J. Antimicrobial and Antioxidant Activities of Flavonoids Isolated from Wood of Sweet Cherry Tree (Prunus avium L.). J. Wood Chem. Technol. 2021, 41, 104–117. [Google Scholar] [CrossRef]
- Shao, T.-M.; Liao, H.-X.; Li, X.-B.; Chen, G.-Y.; Song, X.-P.; Han, C.-R. A New Isoflavone from the Fruits of Ficus auriculata and Its Antibacterial Activity. Nat. Prod. Res. 2022, 36, 1191–1196. [Google Scholar] [CrossRef]
- Promchai, T.; Janhom, P.; Maneerat, W.; Rattanajak, R.; Kamchonwongpaisan, S.; Pyne, S.G.; Limtharakul, T. Antibacterial and Cytotoxic Activities of Phenolic Constituents from the Stem Extracts of Spatholobus parviflorus. Nat. Prod. Res. 2020, 34, 1394–1398. [Google Scholar] [CrossRef]
- Polbuppha, I.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Limtharakul, T.; Cheenpracha, S.; Pyne, S.G.; Laphookhieo, S. Macluracochinones A-E, Antimicrobial Flavonoids from Maclura cochinchinensis (Lour.) Corner. Phytochemistry 2021, 187, 112773. [Google Scholar] [CrossRef]
- Issarachot, P.; Sangkaew, W.; Sianglum, W.; Saeloh, D.; Limsuwan, S.; Voravuthikunchai, S.P.; Joycharat, N. α-Glucosidase Inhibitory, Antibacterial, and Antioxidant Activities of Natural Substances from the Wood of Derris reticulata Craib. Nat. Prod. Res. 2021, 35, 2858–2865. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Sohn, M.-J.; Lee, J.; Kim, W.-G. Isolation and Identification of Pentagalloylglucose with Broad-Spectrum Antibacterial Activity from Rhus Trichocarpa miquel. Food Chem. 2010, 123, 501–506. [Google Scholar] [CrossRef]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of Punicalagin as the Bioactive Compound behind the Antimicrobial Activity of Pomegranate (Punica granatum L.) Peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Shimozu, Y.; Kuroda, T.; Tsuchiya, T.; Hatano, T. Structures and Antibacterial Properties of Isorugosins H–J, Oligomeric Ellagitannins from Liquidambar formosana with Characteristic Bridging Groups between Sugar Moieties. J. Nat. Prod. 2017, 80, 2723–2733. [Google Scholar] [CrossRef]
- Araújo, A.R.; Araújo, A.C.; Reis, R.L.; Pires, R.A. Vescalagin and Castalagin Present Bactericidal Activity toward Methicillin-Resistant Bacteria. ACS Biomater. Sci. Eng. 2021, 7, 1022–1030. [Google Scholar] [CrossRef]
- Karioti, A.; Sokovic, M.; Ciric, A.; Koukoulitsa, C.; Bilia, A.R.; Skaltsa, H. Antimicrobial Properties of Quercus ilex L. Proanthocyanidin Dimers and Simple Phenolics: Evaluation of Their Synergistic Activity with Conventional Antimicrobials and Prediction of Their Pharmacokinetic Profile. J. Agric. Food Chem. 2011, 59, 6412–6422. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yu, D.; Eom, S.-H.; Kim, H.-J.; Kim, D.-H.; Song, H.-S.; Kim, D.-M.; Kim, Y.-M. Fucofuroeckol-A from Edible Marine Alga Eisenia bicyclis to Restore Antifungal Activity of Fluconazole against Fluconazole-Resistant Candida albicans. J. Appl. Phycol. 2018, 30, 605–609. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kang, M.-S.; Hwang, H.-J.; Eom, S.-H.; Yang, J.-Y.; Lee, M.-S.; Lee, W.-J.; Jeon, Y.-J.; Choi, J.-S.; Kim, Y.-M. Synergistic Effect between Dieckol from Ecklonia stolonifera and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Biotechnol. Bioproc. E 2008, 13, 758–764. [Google Scholar] [CrossRef]
- Seukep, J.A.; Sandjo, L.P.; Ngadjui, B.T.; Kuete, V. Antibacterial and Antibiotic-Resistance Modifying Activity of the Extracts and Compounds from Nauclea pobeguinii against Gram-Negative Multi-Drug Resistant Phenotypes. BMC Complement Altern. Med. 2016, 16, 193. [Google Scholar] [CrossRef]
- Kato, E.; Tokunaga, Y.; Sakan, F. Stilbenoids Isolated from the Seeds of Melinjo (Gnetum gnemon L.) and Their Biological Activity. J. Agric. Food Chem. 2009, 57, 2544–2549. [Google Scholar] [CrossRef]
- Kumar, S.n.; Siji, J.v.; Rajasekharan, K.n.; Nambisan, B.; Mohandas, C. Bioactive Stilbenes from a Bacillus sp. N Strain Associated with a Novel Rhabditid Entomopathogenic Nematode. Lett. Appl. Microbiol. 2012, 54, 410–417. [Google Scholar] [CrossRef]
- Basset, C.; Rodrigues, A.M.S.; Eparvier, V.; Silva, M.R.R.; Lopes, N.P.; Sabatier, D.; Fonty, E.; Espindola, L.S.; Stien, D. Secondary Metabolites from Spirotropis longifolia (DC) Baill and Their Antifungal Activity against Human Pathogenic Fungi. Phytochemistry 2012, 74, 166–172. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial Activity of Resveratrol-Derived Monomers and Dimers against Foodborne Pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef]
- Lu, H.-P.; Jia, Y.-N.; Peng, Y.-L.; Yu, Y.; Sun, S.-L.; Yue, M.-T.; Pan, M.-H.; Zeng, L.-S.; Xu, L. Oxyresveratrol, a Stilbene Compound from Morus alba L. Twig Extract Active Against Trichophyton rubrum. Phytother. Res. 2017, 31, 1842–1848. [Google Scholar] [CrossRef]
- de Bruijn, W.J.C.; Araya-Cloutier, C.; Bijlsma, J.; de Swart, A.; Sanders, M.G.; de Waard, P.; Gruppen, H.; Vincken, J.-P. Antibacterial Prenylated Stilbenoids from Peanut (Arachis hypogaea). Phytochem. Lett. 2018, 28, 13–18. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Wu, H.; Webster, J.M. Identification of Two Pigments and a Hydroxystilbene Antibiotic from Photorhabdus luminescens. Appl. Environ. Microbiol. 1995, 61, 4329–4333. [Google Scholar] [CrossRef]
- Wu, J.; Li, B.; Xiao, W.; Hu, J.; Xie, J.; Yuan, J.; Wang, L. Longistylin A, a Natural Stilbene Isolated from the Leaves of Cajanus cajan, Exhibits Significant Anti-MRSA Activity. Int. J. Antimicrob. Agents 2020, 55, 105821. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.-F.; Gao, J.-Y.; Liu, Y.; Hou, X.-W.; Yin, W.-P.; Deng, R.-X. Two New Resveratrol Trimers with Antibacterial Activities from Seed Cake of Paeonia rockii. Chem. Nat. Compd. 2017, 53, 51–55. [Google Scholar] [CrossRef]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Low, A.L.M.; Mohamad, S.A.S.; Khong, H.Y.; Sufian, A.S.; Manshoor, N.; Takayama, H. Malaysianol B, an Oligostilbenoid Derivative from Dryobalanops lanceolata. Fitoterapia 2012, 83, 1569–1575. [Google Scholar] [CrossRef]
- Peng, S.-C.; Cheng, C.-Y.; Sheu, F.; Su, C.-H. The Antimicrobial Activity of Heyneanol A Extracted from the Root of Taiwanese Wild Grape. J. Appl. Microbiol. 2008, 105, 485–491. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Nahar, L.; Cox, P.J.; Dinan, L.N.; Ferguson, C.A.; Finnie, D.A.; Jaspars, M.; Sarker, S.D. Biological Activity of Lignans from the Seeds of Centaurea scabiosa. Pharm. Biol. 2003, 41, 203–206. [Google Scholar] [CrossRef]
- Panagouleas, C.; Skaltsa, H.; Lazari, D.; Skaltsounis, A.-L.; Sokovic, M. Antifungal Activity of Secondary Metabolites of Centaurea raphanina ssp. Mixta, Growing Wild in Greece. Pharm. Biol. 2003, 41, 266–270. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Paek, W.K.; Lim, J.; Kumar, P.; Kumar, P.; Na, M. Efficacy of (+)-Lariciresinol to Control Bacterial Growth of Staphylococcus aureus and Escherichia coli O157:H7. Front. Microbiol. 2017, 8, 804. [Google Scholar] [CrossRef]
- Hwang, B.; Cho, J.; Hwang, I.; Jin, H.-G.; Woo, E.-R.; Lee, D.G. Antifungal Activity of Lariciresinol Derived from Sambucus williamsii and Their Membrane-Active Mechanisms in Candida Albicans. Biochem. Biophys. Res. Commun. 2011, 410, 489–493. [Google Scholar] [CrossRef]
- Nazeam, J.A.; AL-Shareef, W.A.; Helmy, M.W.; El-Haddad, A.E. Bioassay-Guided Isolation of Potential Bioactive Constituents from Pomegranate Agrifood by-Product. Food Chem. 2020, 326, 126993. [Google Scholar] [CrossRef] [PubMed]
- Kaigongi, M.M.; Lukhoba, C.W.; Yaouba, S.; Makunga, N.P.; Githiomi, J.; Yenesew, A. In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae). Plants 2020, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Zhou, S.; Yang, X.; Zhao, S.; Liu, P. Two New Furofuran Lignan Glycosides from Forsythia suspensa Leaves. Phytochem. Lett. 2021, 41, 34–37. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, J.; Li, Z. Antibacterial Activity and Mechanism of Pinoresinol from Cinnamomum camphora Leaves against Food-Related Bacteria. Food Control 2017, 79, 192–199. [Google Scholar] [CrossRef]
- Hwang, B.; Lee, J.; Liu, Q.-H.; Woo, E.-R.; Lee, D.G. Antifungal Effect of (+)-Pinoresinol Isolated from Sambucus williamsii. Molecules 2010, 15, 3507–3516. [Google Scholar] [CrossRef]
- Bai, M.; Wu, S.-Y.; Zhang, W.-F.; Song, X.-P.; Han, C.-R.; Zheng, C.-J.; Chen, G.-Y. One New Lignan Derivative from the Fruiting Bodies of Ganoderma lipsiense. Nat. Prod. Res. 2019, 33, 2784–2788. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.; Hawas, U.W.; Jaspars, M. Novel Bioactive Metabolites from a Marine Derived Bacterium Nocardia sp. ALAA 2000. J. Antibiot. 2008, 61, 379–386. [Google Scholar] [CrossRef]
- Ding, J.-Y.; Yuan, C.-M.; Cao, M.-M.; Liu, W.-W.; Yu, C.; Zhang, H.-Y.; Zhang, Y.; Di, Y.-T.; He, H.-P.; Li, S.-L.; et al. Antimicrobial Constituents of the Mature Carpels of Manglietiastrum sinicum. J. Nat. Prod. 2014, 77, 1800–1805. [Google Scholar] [CrossRef]
- da Silva, R.E.; de Oliveira Silva Ribeiro, F.; de Carvalho, A.M.A.; Daboit, T.C.; Marinho-Filho, J.D.B.; Matos, T.S.; Pessoa, O.D.L.; de Souza de Almeida Leite, J.R.; de Araújo, A.R.; dos Santos Soares, M.J. Antimicrobial and Antibiofilm Activity of the Benzoquinone Oncocalyxone A. Microb. Pathog. 2020, 149, 104513. [Google Scholar] [CrossRef]
- Drewes, S.E.; Khan, F.; van Vuuren, S.F.; Viljoen, A.M. Simple 1,4-Benzoquinones with Antibacterial Activity from Stems and Leaves of Gunnera perpensa. Phytochemistry 2005, 66, 1812–1816. [Google Scholar] [CrossRef]
- Carcamo-Noriega, E.N.; Sathyamoorthi, S.; Banerjee, S.; Gnanamani, E.; Mendoza-Trujillo, M.; Mata-Espinosa, D.; Hernández-Pando, R.; Veytia-Bucheli, J.I.; Possani, L.D.; Zare, R.N. 1,4-Benzoquinone Antimicrobial Agents against Staphylococcus aureus and Mycobacterium tuberculosis Derived from Scorpion Venom. Proc. Natl. Acad. Sci. USA 2019, 116, 12642–12647. [Google Scholar] [CrossRef] [PubMed]
- Lana, E.J.L.; Carazza, F.; Takahashi, J.A. Antibacterial Evaluation of 1,4-Benzoquinone Derivatives. J. Agric. Food Chem. 2006, 54, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Cesari, I.; Hoerlé, M.; Simoes-Pires, C.; Grisoli, P.; Queiroz, E.F.; Dacarro, C.; Marcourt, L.; Moundipa, P.F.; Carrupt, P.A.; Cuendet, M.; et al. Anti-Inflammatory, Antimicrobial and Antioxidant Activities of Diospyros bipindensis (Gürke) Extracts and Its Main Constituents. J. Ethnopharmacol. 2013, 146, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, H.; Iswarya, S.; Pavithra, N.; Senthilnathan, S.; Gnanamani, A. In Vitro Antibacterial Activity of Plumbagin Isolated from Plumbago zeylanica L. against Methicillin-Resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2019, 69, 41–49. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Tangmouo, J.G.; Lontsi, D.; Etoa, F.X.; Lohoue, P.J. In Vitro Antifungal Activity of Extract and Plumbagin from the Stem Bark of Diospyros Crassiflora Hiern (Ebenaceae). Phytother. Res. 2007, 21, 671–674. [Google Scholar] [CrossRef]
- Adusei, E.B.A.; Adosraku, R.K.; Oppong-Kyekyeku, J.; Amengor, C.D.K.; Jibira, Y. Resistance Modulation Action, Time-Kill Kinetics Assay, and Inhibition of Biofilm Formation Effects of Plumbagin from Plumbago Zeylanica Linn. J. Trop. Med. 2019, 2019, e1250645. [Google Scholar] [CrossRef]
- Kaewbumrung, S.; Panichayupakaranant, P. Isolation of Three Antibacterial Naphthoquinones from Plumbago indica Roots and Development of a Validated Quantitative HPLC Analytical Method. Nat. Prod. Res. 2012, 26, 2020–2023. [Google Scholar] [CrossRef]
- Cho, S.-C.; Sultan, M.Z.; Moon, S.-S. Anti-Acne Activities of Pulsaquinone, Hydropulsaquinone, and Structurally Related 1, 4-Quinone Derivatives. Arch. Pharm. Res. 2009, 32, 489–494. [Google Scholar] [CrossRef]
- Yang, X.; Summerhurst, D.K.; Koval, S.F.; Ficker, C.; Smith, M.L.; Bernards, M.A. Isolation of an Antimicrobial Compound from Impatiens Balsamina L. Using Bioassay-Guided Fractionation. Phytother. Res. 2001, 15, 676–680. [Google Scholar] [CrossRef]
- Balachandran, C.; Al-Dhabi, N.A.; Duraipandiyan, V.; Ignacimuthu, S. Bluemomycin, a New Naphthoquinone Derivative from Streptomyces sp. with Antimicrobial and Cytotoxic Properties. Biotechnol. Lett. 2021, 43, 1005–1018. [Google Scholar] [CrossRef]
- Chen, W.-H.; Liu, W.-J.; Wang, Y.; Song, X.-P.; Chen, G.-Y. A New Naphthoquinone and Other Antibacterial Constituents from the Roots of Xanthium sibiricum. Nat. Prod. Res. 2015, 29, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kengne, I.C.; Feugap, L.D.T.; Njouendou, A.J.; Ngnokam, C.D.J.; Djamalladine, M.D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Tamokou, J.-D.-D. Antibacterial, Antifungal and Antioxidant Activities of Whole Plant Chemical Constituents of Rumex abyssinicus. BMC Complement. Med. Ther. 2021, 21, 164. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Coombes, P.H.; Mulholland, D.A.; van Staden, J. Emodin, an Antibacterial Anthraquinone from the Roots of Cassia occidentalis. S. Afr. J. Bot. 2006, 72, 295–297. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Li, X.-M.; Xu, G.-M.; Li, X.; An, C.-Y.; Wang, B.-G. Antibacterial Anthraquinone Derivatives Isolated from a Mangrove-Derived Endophytic Fungus Aspergillus nidulans by Ethanol Stress Strategy. J. Antibiot. 2018, 71, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Arun, Y.; Duraipandiyan, V.; Ignacimuthu, S.; Balakrishna, K.; Al-Dhabi, N.A. Antimicrobial and Cytotoxicity Properties of 2,3-Dihydroxy-9,10-Anthraquinone Isolated from Streptomyces galbus (ERINLG-127). Appl. Biochem. Biotechnol. 2014, 172, 3513–3528. [Google Scholar] [CrossRef]
- Rhea, J.; Craig Hopp, D.; Rabenstein, J.; Smith, C.; Lucas, S.; Romari, K.; Clarke, M.; Francis, L.; Irigoyen, M.; Luche, M.; et al. 5-Hydroxy Ericamycin, a New Anthraquinone with Potent Antimicrobial Activity. J. Antibiot. 2012, 65, 623–625. [Google Scholar] [CrossRef]
- Sivasothy, Y.; Sulaiman, S.F.; Ooi, K.L.; Ibrahim, H.; Awang, K. Antioxidant and Antibacterial Activities of Flavonoids and Curcuminoids from Zingiber spectabile Griff. Food Control 2013, 30, 714–720. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic Antibacterial Effect of Curcumin against Methicillin-Resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Targhi, A.A.; Moammeri, A.; Jamshidifar, E.; Abbaspour, K.; Sadeghi, S.; Lamakani, L.; Akbarzadeh, I. Synergistic Effect of Curcumin-Cu and Curcumin-Ag Nanoparticle Loaded Niosome: Enhanced Antibacterial and Anti-Biofilm Activities. Bioorganic Chem. 2021, 115, 105116. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Aslam, Z.; Roome, T.; Razzak, A.; Aslam, S.M.; Zaidi, M.B.; Kanwal, T.; Sikandar, B.; Bertino, M.F.; Rehman, K.; Shah, M.R. Investigation of Wound Healing Potential of Photo-Active Curcumin-ZnO-Nanoconjugates in Excisional Wound Model. Photodiagnosis Photodyn. Ther. 2022, 39, 102956. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.T.; Fantini de Figueiredo, G.; Cruz, L.F.; Eliza de Morais, S.; Souza, C.D.F.; Pinto, F.C.H.; Ferreira, J.M.S.; de Araújo, M.G.F. Efficacy of Curcumin in the Treatment of Experimental Vulvovaginal candidiasis. Rev. Iberoam. Micol. 2019, 36, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, Antimutagenic and Antibacterial Activities of Curcumin-β-Diglucoside. Food Chem. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Hoult, J.R.S.; Payá, M. Pharmacological and Biochemical Actions of Simple Coumarins: Natural Products with Therapeutic Potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and Pharmacological Profile. J. Appl. Pharm. Sci. 2012, 236–240. [Google Scholar] [CrossRef]
- Costa, T.M.; Tavares, L.B.B.; de Oliveira, D. Fungi as a Source of Natural Coumarins Production. Appl. Microbiol. Biotechnol. 2016, 100, 6571–6584. [Google Scholar] [CrossRef]
- Bor, T.; Aljaloud, S.O.; Gyawali, R.; Ibrahim, S.A. Chapter 26—Antimicrobials from Herbs, Spices, and Plants. In Fruits, Vegetables, and Herbs; Watson, R.R., Preedy, V.R., Eds.; Academic Press: London, UK, 2016; pp. 551–578. ISBN 978-0-12-802972-5. [Google Scholar]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending Topics on Coumarin and Its Derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 2720. [Google Scholar]
- Lacy, A. Studies on Coumarins and Coumarin-Related Compounds to Determine Their Therapeutic Role in the Treatment of Cancer. CPD 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Steffensky, M.; Mühlenweg, A.; Wang, Z.-X.; Li, S.-M.; Heide, L. Identification of the Novobiocin Biosynthetic Gene Cluster of Streptomyces spheroides NCIB 11891. Antimicrob. Agents Chemother. 2000, 44, 1214–1222. [Google Scholar] [CrossRef]
- Eustáquio, A.S.; Gust, B.; Luft, T.; Li, S.-M.; Chater, K.F.; Heide, L. Clorobiocin Biosynthesis in Streptomyces: Identification of the Halogenase and Generation of Structural Analogs. Chem. Biol. 2003, 10, 279–288. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Li, S.-M.; Heide, L. Identification of the Coumermycin A1 Biosynthetic Gene Cluster of Streptomyces rishiriensis DSM 40489. Antimicrob. Agents Chemother. 2000, 44, 3040–3048. [Google Scholar] [CrossRef]
- Fournier, B.; Hooper, D.C. Mutations in Topoisomerase IV and DNA Gyrase of Staphylococcus aureus: Novel Pleiotropic Effects on Quinolone and Coumarin Activity. Antimicrob. Agents Chemother. 1998, 42, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Confreres, A.; Maxwell, A. GyrB Mutations Which Confer Coumarin Resistance Also Affect DNA Supercoiling and ATP Hydrolysis by Escherichia coli DNA Gyrase. Mol. Microbiol. 1992, 6, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A. The Interaction between Coumarin Drugs and DNA Gyrase. Mol. Microbiol. 1993, 9, 681–686. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Finn, G.J.; Creaven, B.; Egan, D.A. Study of the in Vitro Cytotoxic Potential of Natural and Synthetic Coumarin Derivatives Using Human Normal and Neoplastic Skin Cell Lines. Melanoma Res. 2001, 11, 461–467. [Google Scholar] [CrossRef]
- KhanYusufzai, S.; Osman, H.; Khan, M.S.; Mohamad, S.; Sulaiman, O.; Parumasivam, T.; Gansau, J.A.; Johansah, N. Noviany Design, Characterization, in Vitro Antibacterial, Antitubercular Evaluation and Structure–Activity Relationships of New Hydrazinyl Thiazolyl Coumarin Derivatives. Med. Chem. Res. 2017, 26, 1139–1148. [Google Scholar] [CrossRef]
- Qin, H.-L.; Zhang, Z.-W.; Ravindar, L.; Rakesh, K.P. Antibacterial Activities with the Structure-Activity Relationship of Coumarin Derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Ranjan Sahoo, C.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Kumar Sahu, P.; Dehury, B.; Nath Padhy, R.; Kumar Paidesetty, S. Coumarin Derivatives as Promising Antibacterial Agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Znati, M.; Zardi-Bergaoui, A.; Daami-Remadi, M.; Ben Jannet, H. Semi-Synthesis, Antibacterial, Anticholinesterase Activities, and Drug Likeness Properties of New Analogues of Coumarins Isolated from Ferula lutea (Poir.) Maire. Chem. Afr. 2020, 3, 635–645. [Google Scholar] [CrossRef]
- Mamidala, S.; Peddi, S.R.; Aravilli, R.K.; Jilloju, P.C.; Manga, V.; Vedula, R.R. Microwave Irradiated One Pot, Three Component Synthesis of a New Series of Hybrid Coumarin Based Thiazoles: Antibacterial Evaluation and Molecular Docking Studies. J. Mol. Struct. 2021, 1225, 129114. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-Containing Hybrids and Their Antibacterial Activities. Arch. Der Pharm. 2020, 353, 1900380. [Google Scholar] [CrossRef]
- Song, P.-P.; Zhao, J.; Liu, Z.-L.; Duan, Y.-B.; Hou, Y.-P.; Zhao, C.-Q.; Wu, M.; Wei, M.; Wang, N.-H.; Lv, Y.; et al. Evaluation of Antifungal Activities and Structure–Activity Relationships of Coumarin Derivatives. Pest Manag. Sci. 2017, 73, 94–101. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid Biosynthetic Pathways in Plants: Versatile Targets for Metabolic Engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Miadoková, E. Isoflavonoids—An Overview of Their Biological Activities and Potential Health Benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Khan, F. Chapter 3—Analysis of Polyphenolics. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–197. ISBN 978-0-12-816455-6. [Google Scholar]
- Mukherjee, P.K. Chapter 4—Qualitative Analysis for Evaluation of Herbal Drugs. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–149. ISBN 978-0-12-813374-3. [Google Scholar]
- Yan, S.; Xie, M.; Wang, Y.; Xiao, Q.; Ding, N.; Li, Y. Semi-Synthesis of a Series Natural Flavonoids and Flavonoid Glycosides from Scutellarin. Tetrahedron 2020, 76, 130950. [Google Scholar] [CrossRef]
- Orsat, V.; Routray, W. Chapter 8—Microwave-Assisted Extraction of Flavonoids. In Water Extraction of Bioactive Compounds; Dominguez González, H., González Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 221–244. ISBN 978-0-12-809380-1. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Dixon, R.A.; Steele, C.L. Flavonoids and Isoflavonoids—A Gold Mine for Metabolic Engineering. Trends Plant Sci. 1999, 4, 394–400. [Google Scholar] [CrossRef]
- Wu, S.-C.; Liu, F.; Zhu, K.; Shen, J.-Z. Natural Products That Target Virulence Factors in Antibiotic-Resistant Staphylococcus aureus. J. Agric. Food Chem. 2019, 67, 13195–13211. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Tagousop, C.N.; Tamokou, J.-D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial Activities of Flavonoid Glycosides from Graptophyllum grandulosum and Their Mechanism of Antibacterial Action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Geethalakshmi, R.; Sundaramurthi, J.C.; Sarada, D.V.L. Antibacterial Activity of Flavonoid Isolated from Trianthema decandra against Pseudomonas aeruginosa and Molecular Docking Study of FabZ. Microb. Pathog. 2018, 121, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Mcbain, A.J.; Salgado, A.J.; Simões, M. Combinatorial Activity of Flavonoids with Antibiotics Against Drug-Resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef]
- Sathiya Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined Effect of a Natural Flavonoid Rutin from Citrus Sinensis and Conventional Antibiotic Gentamicin on Pseudomonas aeruginosa Biofilm Formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Dhawale, P.V.; Vineeth, S.K.; Gadhave, R.V.; Mj, J.F.; Supekar, M.V.; Thakur, V.K.; Raghavan, P. Tannin as a Renewable Raw Material for Adhesive Applications: A Review. Mater. Adv. 2022, 3, 3365–3388. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and Biological Significance of Oenothein B and Related Ellagitannin oligomers with Macrocyclic Structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef]
- Khanbabaee, K.; Ree, T. van Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef]
- Rira, M.; Morgavi, D.P.; Popova, M.; Maxin, G.; Doreau, M. Microbial Colonisation of Tannin-Rich Tropical Plants: Interplay between Degradability, Methane Production and Tannin Disappearance in the Rumen. Animal 2022, 16, 100589. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Ito, H. Chapter Seven—High Molecular Weight Plant Poplyphenols (Tannins): Prospective Functions. In Recent Advances in Phytochemistry; Romeo, J.T., Ed.; Chemical Ecology and Phytochemistry of Forest Ecosystems; Elsevier: Amsterdam, The Netherlands, 2005; Volume 39, pp. 163–190. [Google Scholar]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of Tea Tannins and Condensed Tannins with Proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ito, H.; Yoshimura, M.; Miyashita, K.; Hatano, T. C-Glucosidic Ellagitannin Oligomers from Melaleuca Squarrosa Donn Ex Sm., Myrtaceae. Phytochemistry 2008, 69, 3070–3079. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Sidana, J. 5—Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, Cambridge, UK, 2013; pp. 181–204. ISBN 978-0-85709-512-1. [Google Scholar]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin Profile, Antioxidant Properties, and Antimicrobial Activity of Extracts from Two Mediterranean Species of Parasitic Plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, R.; Shaaban, M.; Al-wegaisi, R.; Moharram, F.; El-Rahman, O.A.; El-Messery, S. Antimicrobial Activity and Molecular Docking of Tannins from Pimenta dioica. Lett. Drug Des. Discov. 2018, 15, 508–515. [Google Scholar] [CrossRef]
- Liu, M.; Yang, K.; Wang, J.; Zhang, J.; Qi, Y.; Wei, X.; Fan, M. Young Astringent Persimmon tannin Inhibits Methicillin-Resistant Staphylococcus Aureus Isolated from Pork. LWT 2019, 100, 48–55. [Google Scholar] [CrossRef]
- de Freitas, A.L.D.; Kaplum, V.; Rossi, D.C.P.; da Silva, L.B.R.; Melhem, M.; de Souza Carvalho Melhem, M.; Taborda, C.P.; de Mello, J.C.P.; Nakamura, C.V.; Ishida, K. Proanthocyanidin Polymeric Tannins from Stryphnodendron adstringens Are Effective against Candida spp. Isolates and for Vaginal Candidiasis Treatment. J. Ethnopharmacol. 2018, 216, 184–190. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial Action of Several Tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and Challenges of Tannins as an Alternative to In-Feed Antibiotics for Farm Animal Production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Eom, S.-H.; Lee, D.-S.; Jung, Y.-J.; Park, J.-H.; Choi, J.-I.; Yim, M.-J.; Jeon, J.-M.; Kim, H.-W.; Son, K.-T.; Je, J.-Y.; et al. The Mechanism of Antibacterial Activity of Phlorofucofuroeckol-A against Methicillin-Resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804. [Google Scholar] [CrossRef]
- Liu, M.; Feng, M.; Yang, K.; Cao, Y.; Zhang, J.; Xu, J.; Hernández, S.H.; Wei, X.; Fan, M. Transcriptomic and Metabolomic Analyses Reveal Antibacterial Mechanism of Astringent Persimmon Tannin against Methicillin-Resistant Staphylococcus aureus Isolated from Pork. Food Chem. 2020, 309, 125692. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Ammazzalorso, A.; Amoroso, R.; Giampietro, L. Stilbene Derivatives as New Perspective in Antifungal Medicinal Chemistry. Drug Dev. Res. 2019, 80, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Brachmann, A.O.; Glazer, I.; Lango, L.; Schwär, G.; Clarke, D.J.; Bode, H.B. Bacterial Biosynthesis of a Multipotent Stilbene. Angew. Chem. Int. Ed. 2008, 47, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, G.S.; Baker, B.J.; McClintock, J.B. Isolation and Identification of a Stilbene Derivative from the Antarctic Sponge Kirkpatrickia variolosa. J. Nat. Prod. 1995, 58, 1958–1960. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Sady, S.; Sielicka, M. The Stilbene Profile in Edible Berries. Phytochem. Rev. 2019, 18, 37–67. [Google Scholar] [CrossRef]
- Morabito, G.; Miglio, C.; Peluso, I.; Serafini, M. Chapter 85—Fruit Polyphenols and Postprandial Inflammatory Stress. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 1107–1126. ISBN 978-0-12-398456-2. [Google Scholar]
- Garbicz, D.; Tobiasz, P.; Borys, F.; Pilżys, T.; Marcinkowski, M.; Poterała, M.; Grzesiuk, E.; Krawczyk, H. The Stilbene and Dibenzo[b,f]Oxepine Derivatives as Anticancer Compounds. Biomed. Pharmacother. 2020, 123, 109781. [Google Scholar] [CrossRef]
- Lv, L.; Gu, X.; Tang, J.; Ho, C.-T. Antioxidant Activity of Stilbene Glycoside from Polygonum multiflorum Thunb in Vivo. Food Chem. 2007, 104, 1678–1681. [Google Scholar] [CrossRef]
- Boccellino, M.; Donniacuo, M.; Bruno, F.; Rinaldi, B.; Quagliuolo, L.; Ambruosi, M.; Pace, S.; De Rosa, M.; Olgaç, A.; Banoglu, E.; et al. Protective Effect of Piceatannol and Bioactive Stilbene Derivatives against Hypoxia-Induced Toxicity in H9c2 Cardiomyocytes and Structural Elucidation as 5-LOX Inhibitors. Eur. J. Med. Chem. 2019, 180, 637–647. [Google Scholar] [CrossRef]
- Sham, T.-T.; Li, M.-H.; Chan, C.-O.; Zhang, H.; Chan, S.-W.; Mok, D.K.-W. Cholesterol-Lowering Effects of Piceatannol, a Stilbene from Wine, Using Untargeted Metabolomics. J. Funct. Foods 2017, 28, 127–137. [Google Scholar] [CrossRef]
- Lai, X.; Pei, Q.; Song, X.; Zhou, X.; Yin, Z.; Jia, R.; Zou, Y.; Li, L.; Yue, G.; Liang, X.; et al. The Enhancement of Immune Function and Activation of NF-ΚB by Resveratrol-Treatment in Immunosuppressive Mice. Int. Immunopharmacol. 2016, 33, 42–47. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. Resveratrol Induces Membrane and DNA Disruption via Pro-Oxidant Activity against Salmonella typhimurium. Biochem. Biophys. Res. Commun. 2017, 489, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; An, P.; Zhai, X.; Wang, S.; Kong, Q. The Antibacterial Mechanism of Pterostilbene Derived from Xinjiang Wine Grape: A Novel Apoptosis Inducer in Staphyloccocus aureus and Escherichia coli. LWT 2019, 101, 100–106. [Google Scholar] [CrossRef]
- Joung, D.-K.; Mun, S.-H.; Choi, S.-H.; Kang, O.-H.; Kim, S.-B.; Lee, Y.-S.; Zhou, T.; Kong, R.; Choi, J.-G.; Shin, D.-W.; et al. Antibacterial Activity of Oxyresveratrol against Methicillin-resistant Staphylococcus aureus and Its Mechanism. Exp. Ther. Med. 2016, 12, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial Activity of Resveratrol Structural Analogues: A Mechanistic Evaluation of the Structure-Activity Relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317. [Google Scholar] [CrossRef]
- Yang, D.-S.; Li, Z.-L.; Wang, X.; Yan, H.; Yang, Y.-P.; Luo, H.-R.; Liu, K.-C.; Xiao, W.-L.; Li, X.-L. Denticulatains A and B: Unique Stilbene–Diterpene Heterodimers from Macaranga denticulata. RSC Adv. 2015, 5, 13886–13890. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.-N.; Lou, H.-X. Natural Stilbenes: An Overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Wu, G.-Y.; Zhang, X.; Guo, X.-Y.; Huo, L.-Q.; Liu, H.-X.; Shen, X.-L.; Qiu, S.-X.; Hu, Y.-J.; Tan, H.-B. Prenylated Stilbenes and Flavonoids from the Leaves of Cajanus cajan. Chin. J. Nat. Med. 2019, 17, 381–386. [Google Scholar] [CrossRef]
- Cannatelli, A.; Principato, S.; Colavecchio, O.L.; Pallecchi, L.; Rossolini, G.M. Synergistic Activity of Colistin in Combination with Resveratrol Against Colistin-Resistant Gram-Negative Pathogens. Front. Microbiol. 2018, 9, 1808. [Google Scholar] [CrossRef]
- Singh, D.; Chauhan, N.; Koli, M.; Nayak, S.K.; Subramanian, M. Dimer Stilbene, a Resveratrol Analogue Exhibits Synergy with Antibiotics That Target Protein Synthesis in Eradicating Staphylococcus aureus Infection. Biochimie 2022, 201, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Qu, Y.; Yang, X.; Shi, G.; Wang, X.; Hong, Y.; Drlica, K.; Zhao, X. Resveratrol Antagonizes Antimicrobial Lethality and Stimulates Recovery of Bacterial Mutants. PLoS ONE 2016, 11, e0153023. [Google Scholar] [CrossRef] [PubMed]
- Basri, D.F.; Xian, L.W.; Abdul Shukor, N.I.; Latip, J. Bacteriostatic Antimicrobial Combination: Antagonistic Interaction between Epsilon-Viniferin and Vancomycin against Methicillin-Resistant Staphylococcus aureus. BioMed Res. Int. 2014, 2014, e461756. [Google Scholar] [CrossRef]
- Sheng, J.-Y.; Chen, T.-T.; Tan, X.-J.; Chen, T.; Jia, A.-Q. The Quorum-Sensing Inhibiting Effects of Stilbenoids and Their Potential Structure–Activity Relationship. Bioorganic Med. Chem. Lett. 2015, 25, 5217–5220. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.S.; Bhutle, S.R.; Pedgaonkar, G.S.; Zubaidha, P.K.; Shaikh, R.M.; Rajput, C.G.; Shendarkar, G.S. Synthesis and Biological Evaluation of Some Stilbene-Based Analogues. Med. Chem. Res. 2011, 20, 1158–1163. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Q.; Huang, C.; Yang, M.; Li, J.; Chen, Y.; Yang, B.; Zhao, X. Comparative Cytotoxicity of Halogenated Aromatic DBPs and Implications of the Corresponding Developed QSAR Model to Toxicity Mechanisms of Those DBPs: Binding Interactions between Aromatic DBPs and Catalase Play an Important Role. Water Res. 2020, 170, 115283. [Google Scholar] [CrossRef]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An Update on Bioactive Plant Lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Ward, R.S. Recent Advances in the Chemistry of Lignans. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2000; Volume 24, pp. 739–798. ISBN 978-0-444-50643-6. [Google Scholar]
- Wang, L.-X.; Wang, H.-L.; Huang, J.; Chu, T.-Z.; Peng, C.; Zhang, H.; Chen, H.-L.; Xiong, Y.-A.; Tan, Y.-Z. Review of Lignans from 2019 to 2021: Newly Reported Compounds, Diverse Activities, Structure-Activity Relationships and Clinical Applications. Phytochemistry 2022, 202, 113326. [Google Scholar] [CrossRef]
- Eklund, P.; Raitanen, J.-E. 9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol. Molecules 2019, 24, 220. [Google Scholar] [CrossRef]
- Moss, G.P. Nomenclature of Lignans and Neolignans (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1493–1523. [Google Scholar] [CrossRef]
- Wcislo, G.; Szarlej-Wcislo, K. Chapter 8—Colorectal Cancer Prevention by Wheat Consumption: A Three-Valued Logic—True, False, or Otherwise? In Wheat and Rice in Disease Prevention and Health; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 91–111. ISBN 978-0-12-401716-0. [Google Scholar]
- Aldred, E.M.; Buck, C.; Vall, K. (Eds.) Chapter 21—Phenols. In Pharmacology; Churchill Livingstone: Edinburgh, UK, 2009; pp. 149–166. ISBN 978-0-443-06898-0. [Google Scholar]
- Yoder, S.C.; Lancaster, S.M.; Hullar, M.A.J.; Lampe, J.W. Chapter 7—Gut Microbial Metabolism of Plant Lignans: Influence on Human Health. In Diet-Microbe Interactions in the Gut; Tuohy, K., Del Rio, D., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 103–117. ISBN 978-0-12-407825-3. [Google Scholar]
- Pathak, S.; Kesavan, P.; Banerjee, A.; Banerjee, A.; Celep, G.S.; Bissi, L.; Marotta, F. Chapter 25—Metabolism of Dietary Polyphenols by Human Gut Microbiota and Their Health Benefits. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: London, UK, 2018; pp. 347–359. ISBN 978-0-12-813006-3. [Google Scholar]
- Wang, L.-Q. Mammalian Phytoestrogens: Enterodiol and Enterolactone. J. Chromatogr. B 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Desmawati, D.; Sulastri, D. Phytoestrogens and Their Health Effect. Open Access Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Mahendra Kumar, C.; Singh, S.A. Bioactive Lignans from Sesame (Sesamum indicum L.): Evaluation of Their Antioxidant and Antibacterial Effects for Food Applications. J. Food Sci. Technol. 2015, 52, 2934–2941. [Google Scholar] [CrossRef]
- Mousa, W.; Raizada, M. The Diversity of Anti-Microbial Secondary Metabolites Produced by Fungal Endophytes: An Interdisciplinary Perspective. Front. Microbiol. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.H.; Chang, C.J.; McLaughlin, J.L.; Powell, R.G. Justicidin B, a Bioactive Trace Lignan from the Seeds of Sesbania drummondii. J. Nat. Prod. 1986, 49, 1175–1176. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.-H.; Tzeng, Y.-M. Anti-Inflammatory Activities of Constituents Isolated from Phyllanthus polyphyllus. J. Ethnopharmacol. 2006, 103, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.; Elfahmi; Bos, R.; Kayser, O.; Momekov, G.; Konstantinov, S.; Ionkova, I. Production of Justicidin B, a Cytotoxic Arylnaphthalene Lignan from Genetically Transformed Root Cultures of Linum leonii. J. Nat. Prod. 2006, 69, 1014–1017. [Google Scholar] [CrossRef]
- Chen, C.-C.; Hsin, W.-C.; Ko, F.-N.; Huang, Y.-L.; Ou, J.-C.; Teng, C.-M. Antiplatelet Arylnaphthalide lignans from Justicia procumbens. J. Nat. Prod. 1996, 59, 1149–1150. [Google Scholar] [CrossRef]
- Vargas-Arispuro, I.; Reyes-Báez, R.; Rivera-Castañeda, G.; Martínez-Téllez, M.A.; Rivero-Espejel, I. Antifungal Lignans from the Creosotebush (Larrea Tridentata). Ind. Crops Prod. 2005, 22, 101–107. [Google Scholar] [CrossRef]
- Li, K.-M.; Dong, X.; Ma, Y.-N.; Wu, Z.-H.; Yan, Y.-M.; Cheng, Y.-X. Antifungal Coumarins and Lignans from Artemisia annua. Fitoterapia 2019, 134, 323–328. [Google Scholar] [CrossRef]
- Akiyama, K.; Maruyama, M.; Yamauchi, S.; Nakashima, Y.; Nakato, T.; Tago, R.; Sugahara, T.; Kishida, T.; Koba, Y. Antimicrobiological Activity of Lignan: Effect of Benzylic Oxygen and Stereochemistry of 2,3-Dibenzyl-4-Butanolide and 3,4-Dibenzyltetrahydrofuran Lignans on Activity. Biosci. Biotechnol. Biochem. 2007, 71, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Tago, R.; Yamauchi, S.; Maruyama, M.; Akiyama, K.; Sugahara, T.; Kishida, T.; Koba, Y. Structure-Antibacterial Activity Relationship for 9- O,9′- O -Demethyl (+)-Virgatusin. Biosci. Biotechnol. Biochem. 2008, 72, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Yamauchi, S.; Nakato, T.; Maruyama, M.; Sugahara, T.; Kishida, T. Antifungal Activity of Tetra-Substituted Tetrahydrofuran lignan, (−)-Virgatusin, and Its Structure-Activity Relationship. Biosci. Biotechnol. Biochem. 2007, 71, 1028–1035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ouellette, R.J.; Rawn, J.D. 25 - Aryl Halides, Phenols, and Anilines. In Organic Chemistry, 2nd ed.; Ouellette, R.J., Rawn, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 801–828. ISBN 978-0-12-812838-1. [Google Scholar]
- Narmani, A.; Teponno, R.B.; Arzanlou, M.; Surup, F.; Helaly, S.E.; Wittstein, K.; Praditya, D.F.; Babai-Ahari, A.; Steinmann, E.; Stadler, M. Cytotoxic, Antimicrobial and Antiviral Secondary Metabolites Produced by the Plant Pathogenic Fungus Cytospora sp. CCTU A309. Fitoterapia 2019, 134, 314–322. [Google Scholar] [CrossRef]
- Kumar, R.; Subramani, R.; Aalbersberg, W. Three Bioactive Sesquiterpene Quinones from the Fijian Marine Sponge of the Genus hippospongia. Nat. Prod. Res. 2013, 27, 1488–1491. [Google Scholar] [CrossRef]
- Scott Obach, R.; Kalgutkar, A.S. 1.15—Reactive Electrophiles and Metabolic Activation. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 309–347. ISBN 978-0-08-046884-6. [Google Scholar]
- Alamgir, A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts: Volume 2. In Phytochemistry and Bioactive Compounds; Progress in Drug Research; Springer: Cham, Switzerland, 2018; Volume 74, ISBN 978-3-319-92387-1. [Google Scholar]
- Nishina, A.; Uchibori, T. Antimicrobial Activity of 2,6-Dimethoxy-p-Benzoquinone, Isolated from Thick-Stemmed Bamboo, and Its Analogs. Agric. Biol. Chem. 1991, 55, 2395–2398. [Google Scholar] [CrossRef]
- Sánchez-Duffhues, G.; Calzado, M.A.; de Vinuesa, A.G.; Caballero, F.J.; Ech-Chahad, A.; Appendino, G.; Krohn, K.; Fiebich, B.L.; Muñoz, E. Denbinobin, a Naturally Occurring 1,4-Phenanthrenequinone, Inhibits HIV-1 Replication through an NF-ΚB-Dependent Pathway. Biochem. Pharmacol. 2008, 76, 1240–1250. [Google Scholar] [CrossRef]
- Shao, S.-Y.; Wang, C.; Han, S.-W.; Li, S. Two New Phenanthrenequinones with Cytotoxic Activity from the Tubers of Pleione bulbocodioides. Phytochem. Lett. 2020, 35, 6–9. [Google Scholar] [CrossRef]
- Kazmi, M.H.; Malik, A.; Hameed, S.; Akhtar, N.; Noor Ali, S. An Anthraquinone Derivative from Cassia italica. Phytochemistry 1994, 36, 761–763. [Google Scholar] [CrossRef]
- Tran, T.; Saheba, E.; Arcerio, A.V.; Chavez, V.; Li, Q.; Martinez, L.E.; Primm, T.P. Quinones as Antimycobacterial Agents. Bioorganic Med. Chem. 2004, 12, 4809–4813. [Google Scholar] [CrossRef]
- Bisio, A.; Romussi, G.; Russo, E.; Cafaggi, S.; Schito, A.M.; Repetto, B.; De Tommasi, N. Antimicrobial Activity of the Ornamental Species Salvia corrugata, a Potential New Crop for Extractive Purposes. J. Agric. Food Chem. 2008, 56, 10468–10472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Leong, W.F.; Elias, R.J.; Tikekar, R.V. UV-C Irradiated Gallic Acid Exhibits Enhanced Antimicrobial Activity via Generation of Reactive Oxidative Species and Quinone. Food Chem. 2019, 287, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, P.P.; Novotny, P.; Haubrich, N.; McDonald, L.; Cochran, M.; Serdula, J.; Amin, R.W.; Jeffrey, W.H. Bacterial Growth Response to Photoactive Quinones. Photochem. Photobiol. 2010, 86, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Subramani, P.A.; Panati, K.; Lebaka, V.R.; Reddy, D.D.; Narala, V.R. Chapter 21—Nanostructures for Curcumin Delivery: Possibilities and Challenges. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 393–418. ISBN 978-0-323-52727-9. [Google Scholar]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Gomes, A.C.; Pereira-Wilson, C.; Lima, C.F. Chapter 35—Mechanisms of Action of Curcumin on Aging: Nutritional and Pharmacological Applications. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 491–511. ISBN 978-0-12-801816-3. [Google Scholar]
- Li, L.; Leung, P.S. Chapter 13—Pancreatic Cancer, Pancreatitis, and Oxidative Stress. In Gastrointestinal Tissue; Gracia-Sancho, J., Salvadó, J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 173–186. ISBN 978-0-12-805377-5. [Google Scholar]
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-Inflammatory Properties of Curcumin, a Major Constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An Age-Old Anti-Inflammatory and Anti-Neoplastic Agent. J. Tradit. Complement. Med. 2017, 7, 339–346. [Google Scholar] [CrossRef]
- Vieira, T.M.; dos Santos, I.A.; Silva, T.S.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial Activity of Monoketone Curcuminoids Against Cariogenic Bacteria. Chem. Biodivers. 2018, 15, e1800216. [Google Scholar] [CrossRef]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray Dried Curcumin Loaded Nanoparticles for Antimicrobial Photodynamic Therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef]
- Naeini, M.B.; Momtazi, A.A.; Jaafari, M.R.; Johnston, T.P.; Barreto, G.; Banach, M.; Sahebkar, A. Antitumor Effects of Curcumin: A Lipid Perspective. J. Cell. Physiol. 2019, 234, 14743–14758. [Google Scholar] [CrossRef]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Antitumor Activity of Curcumin by Modulation of Apoptosis and Autophagy in Human Lung Cancer A549 Cells through Inhibiting PI3K/Akt/MTOR Pathway. Oncol. Rep. 2018, 39, 1523–1531. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Chapter Three—Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Teow, S.-Y.; Liew, K.; Ali, S.A.; Khoo, A.S.-B.; Peh, S.-C. Antibacterial Action of Curcumin against Staphylococcus aureus: A Brief Review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Wikene, K.O.; Bruzell, E.; Tønnesen, H.H. Characterization and Antimicrobial Phototoxicity of Curcumin Dissolved in Natural Deep Eutectic Solvents. Eur. J. Pharm. Sci. 2015, 80, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Battista, S.; Maggi, M.A.; Bellio, P.; Galantini, L.; D’Archivio, A.A.; Celenza, G.; Colaiezzi, R.; Giansanti, L. Curcuminoids-Loaded Liposomes: Influence of Lipid Composition on Their Physicochemical Properties and Efficacy as Delivery Systems. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124759. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as Potential Therapeutic Natural Product: A Nanobiotechnological Perspective. J. Pharm. Pharmacol. 2016, 68, 1481–1500. [Google Scholar] [CrossRef]
- Kali, A.; Bhuvaneshwar, D.; Charles, P.M.V.; Seetha, K.S. Antibacterial Synergy of Curcumin with Antibiotics against Biofilm Producing Clinical Bacterial Isolates. J. Basic Clin. Pharm. 2016, 7, 93–96. [Google Scholar] [CrossRef]
- Gupta, A.; Mahajan, S.; Sharma, R. Evaluation of Antimicrobial Activity of Curcuma Longa Rhizome Extract against Staphylococcus aureus. Biotechnol. Rep. 2015, 6, 51–55. [Google Scholar] [CrossRef]
- Morão, L.G.; Polaquini, C.R.; Kopacz, M.; Torrezan, G.S.; Ayusso, G.M.; Dilarri, G.; Cavalca, L.B.; Zielińska, A.; Scheffers, D.-J.; Regasini, L.O.; et al. A Simplified Curcumin Targets the Membrane of Bacillus subtilis. Microbiologyopen 2019, 8, e00683. [Google Scholar] [CrossRef]
- Shlar, I.; Droby, S.; Choudhary, R.; Rodov, V. The Mode of Antimicrobial Action of Curcumin Depends on the Delivery System: Monolithic Nanoparticles vs. Supramolecular Inclusion Complex. RSC Adv. 2017, 7, 42559–42569. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Hegge, A.B.; Andersen, T.; Melvik, J.E.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Formulation and Bacterial Phototoxicity of Curcumin Loaded Alginate Foams for Wound Treatment Applications: Studies on Curcumin and Curcuminoides XLII. J. Pharm. Sci. 2011, 100, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Wikene, K.O.; Hegge, A.B.; Bruzell, E.; Tønnesen, H.H. Formulation and Characterization of Lyophilized Curcumin Solid Dispersions for Antimicrobial Photodynamic Therapy (APDT): Studies on Curcumin and Curcuminoids LII. Drug Dev. Ind. Pharm. 2015, 41, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Hegge, A.B.; Nielsen, T.T.; Larsen, K.L.; Bruzell, E.; Tønnesen, H.H. Impact of Curcumin Supersaturation in Antibacterial Photodynamic Therapy—Effect of Cyclodextrin Type and Amount: Studies on Curcumin and Curcuminoides XLV. J. Pharm. Sci. 2012, 101, 1524–1537. [Google Scholar] [CrossRef]
- Hegge, A.B.; Vukicevic, M.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Solid Dispersions for Preparation of Phototoxic Supersaturated Solutions for Antimicrobial Photodynamic Therapy (APDT): Studies on Curcumin and curcuminoides L. Eur. J. Pharm. Biopharm. 2013, 83, 95–105. [Google Scholar] [CrossRef]
- Hegge, A.B.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Photoinactivation of Staphylococcus Epidermidis Biofilms and Suspensions by the Hydrophobic Photosensitizer Curcumin—Effect of Selected Nanocarrier. Eur. J. Pharm. Sci. 2012, 47, 65–74. [Google Scholar] [CrossRef]
- Bruzell, E.M.; Morisbak, E.; Tønnesen, H.H. Studies on Curcumin and Curcuminoids. XXIX. Photoinduced Cytotoxicity of Curcumin in Selected Aqueous Preparations. Photochem. Photobiol. Sci. 2005, 4, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Condat, M.; Mazeran, P.-E.; Malval, J.-P.; Lalevée, J.; Morlet-Savary, F.; Renard, E.; Langlois, V.; Andalloussi, S.A.; Versace, D.-L. Photoinduced Curcumin Derivative-Coatings with Antibacterial Properties. RSC Adv. 2015, 5, 85214–85224. [Google Scholar] [CrossRef]
- Chai, Z.; Zhang, F.; Liu, B.; Chen, X.; Meng, X. Antibacterial Mechanism and Preservation Effect of Curcumin-Based Photodynamic Extends the Shelf Life of Fresh-Cut Pears. LWT 2021, 142, 110941. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; Li, H.; Zeng, Q.-H.; Wang, J.J.; Liu, H.; Pan, Y.; Zhao, Y. Enhanced Antibacterial and Antibiofilm Functions of the Curcumin-Mediated Photodynamic Inactivation against Listeria Monocytogenes. Food Control 2020, 108, 106886. [Google Scholar] [CrossRef]
- Garcia-Gomes, A.S.; Curvelo, J.A.R.; Soares, R.M.A.; Ferreira-Pereira, A. Curcumin Acts Synergistically with Fluconazole to Sensitize a Clinical Isolate of Candida Albicans Showing a MDR Phenotype. Med. Mycol. 2012, 50, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Manoharlal, R.; Negi, A.S.; Prasad, R. Synergistic Anticandidal Activity of Pure Polyphenol Curcumin I in Combination with Azoles and Polyenes Generates Reactive Oxygen Species Leading to Apoptosis. FEMS Yeast Res. 2010, 10, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Batista de Andrade Neto, J.; Pessoa de Farias Cabral, V.; Brito Nogueira, L.F.; Rocha da Silva, C.; Gurgel do Amaral Valente Sá, L.; Ramos da Silva, A.; Barbosa da Silva, W.M.; Silva, J.; Marinho, E.S.; Cavalcanti, B.C.; et al. Anti-MRSA Activity of Curcumin in Planktonic Cells and Biofilms and Determination of Possible Action Mechanisms. Microb. Pathog. 2021, 155, 104892. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Wang, Y.; Sharma, G.; Shen, J.; Velkov, T.; Xiao, X. Polymyxins–Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment. Antioxidants 2020, 9, 506. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Ali, S. Synergistic Antibacterial Activity of Curcumin with Antibiotics against Staphylococcus aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar]
- Li, Y.; Xu, Y.; Liao, Q.; Xie, M.; Tao, H.; Wang, H.-L. Synergistic Effect of Hypocrellin B and Curcumin on Photodynamic Inactivation of Staphylococcus aureus. Microb. Biotechnol. 2021, 14, 692–707. [Google Scholar] [CrossRef]
- Itzia Azucena, R.-C.; José Roberto, C.-L.; Martin, Z.-R.; Rafael, C.-Z.; Leonardo, H.-H.; Gabriela, T.-P.; Araceli, C.-R. Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli. Antibiotics 2019, 8, 43. [Google Scholar] [CrossRef]
- Gülen, D.; Şafak, B.; Erdal, B.; Günaydın, B. Curcumin-Meropenem Synergy in Carbapenem Resistant Klebsiella Pneumoniae Curcumin-Meropenem Synergy. Iran J. Microbiol. 2021, 13, 345–351. [Google Scholar] [CrossRef]
- Marathe, S.A.; Kumar, R.; Ajitkumar, P.; Nagaraja, V.; Chakravortty, D. Curcumin Reduces the Antimicrobial Activity of Ciprofloxacin against Salmonella Typhimurium and Salmonella typhi. J. Antimicrob. Chemother. 2013, 68, 139–152. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.V.; Rana, A.; Bhagat, K.; Gulati, H.K.; Kumar, R.; Salwan, R.; Bhagat, K.; Kaur, G.; Singh, N.; et al. Monocarbonyl Curcumin-Based Molecular Hybrids as Potent Antibacterial Agents. ACS Omega 2019, 4, 11673–11684. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-Assisted Ionic Liquid Extraction of Bioactive Compound from Turmeric (Curcuma longa L.): Isolation, Purification and Analysis of Curcumin. Ind. Crops Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Mo, H.; Zhao, Y.; Li, H.; Zhang, H.; Hu, L.; Zhou, X. Thymol Mediates Bactericidal Activity against Staphylococcus aureus by Targeting an Aldo–Keto Reductase and Consequent Depletion of NADPH. J. Agric. Food Chem. 2019, 67, 8382–8392. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kaur, M.; Bansal, M. New Insights of Structural Activity Relationship of Curcumin and Correlating Their Efficacy in Anticancer Studies with Some Other Similar Molecules. Am. J. Cancer Res. 2021, 11, 3755–3765. [Google Scholar] [PubMed]
- Yang, H.; Du, Z.; Wang, W.; Song, M.; Sanidad, K.; Sukamtoh, E.; Zheng, J.; Tian, L.; Xiao, H.; Liu, Z.; et al. Structure–Activity Relationship of Curcumin: Role of the Methoxy Group in Anti-Inflammatory and Anticolitis Effects of Curcumin. J. Agric. Food Chem. 2017, 65, 4509–4515. [Google Scholar] [CrossRef]
- Ahsan, H.; Parveen, N.; Khan, N.U.; Hadi, S.M. Pro-Oxidant, Anti-Oxidant and Cleavage Activities on DNA of Curcumin and Its Derivatives Demethoxycurcumin and Bisdemethoxycurcumin. Chem.-Biol. Interact. 1999, 121, 161–175. [Google Scholar] [CrossRef]
- Hatamipour, M.; Ramezani, M.; Tabassi, S.A.S.; Johnston, T.P.; Sahebkar, A. Demethoxycurcumin: A Naturally Occurring Curcumin Analogue for Treating Non-Cancerous Diseases. J. Cell. Physiol. 2019, 234, 19320–19330. [Google Scholar] [CrossRef]
- Harvey, A. Strategies for Discovering Drugs from Previously Unexplored Natural Products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The Evolving Role of Natural Products in Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural Products in Drug Discovery. In Pharmacognosy—Medicinal Plants; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Montaser, R.; Luesch, H. Marine Natural Products: A New Wave of Drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Negi, P.S. Plant Extracts for the Control of Bacterial Growth: Efficacy, Stability and Safety Issues for Food Application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Wu, S.; Chappell, J. Metabolic Engineering of Natural Products in Plants; Tools of the Trade and Challenges for the Future. Curr. Opin. Biotechnol. 2008, 19, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Deharo, E. A Call for Using Natural Compounds in the Development of New Antimalarial Treatments—An Introduction. Malar. J. 2011, 10, S1. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. CRISPR/Cas Genome Editing to Optimize Pharmacologically Active Plant Natural Products. Pharmacol. Res. 2021, 164, 105359. [Google Scholar] [CrossRef] [PubMed]
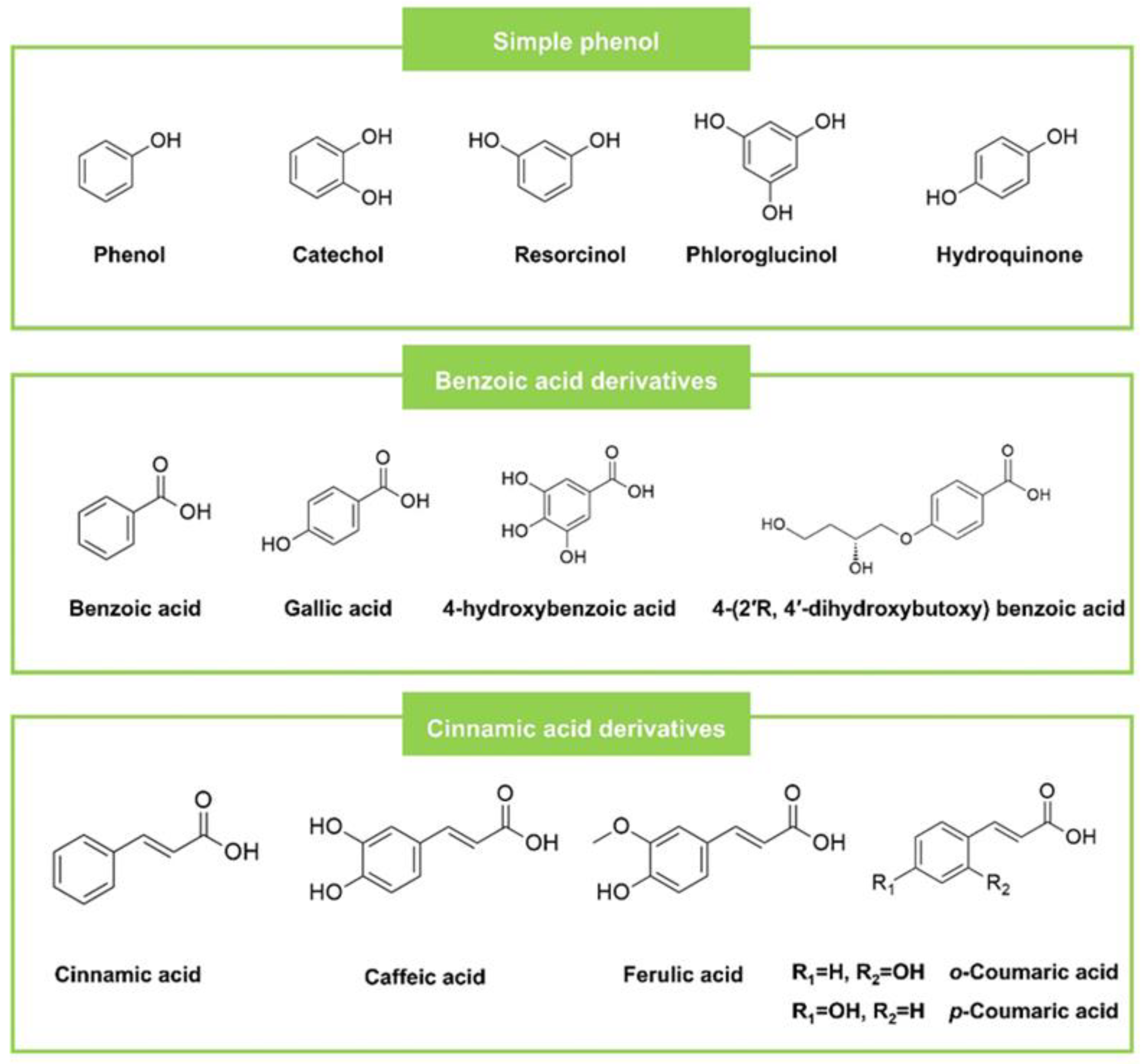
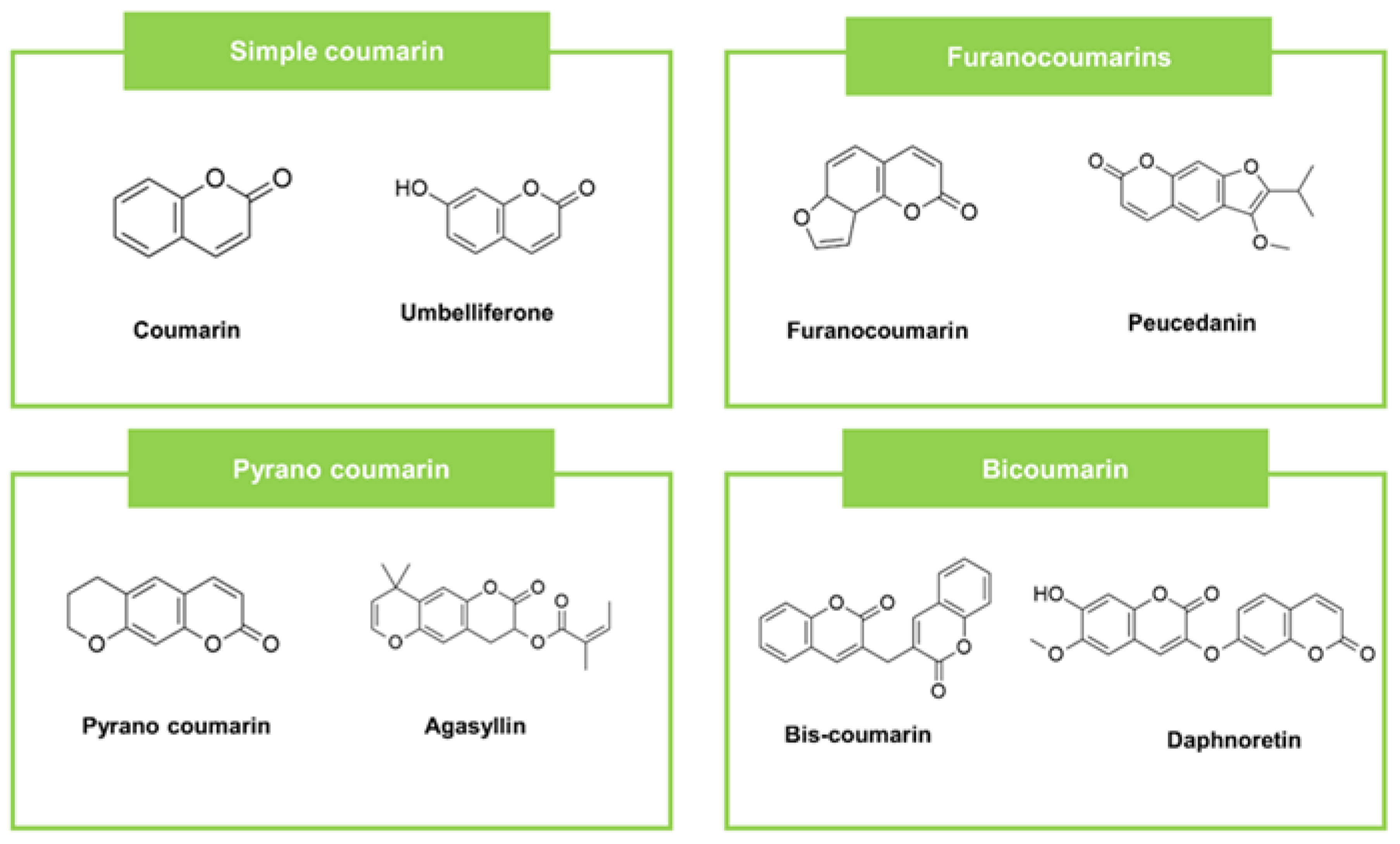
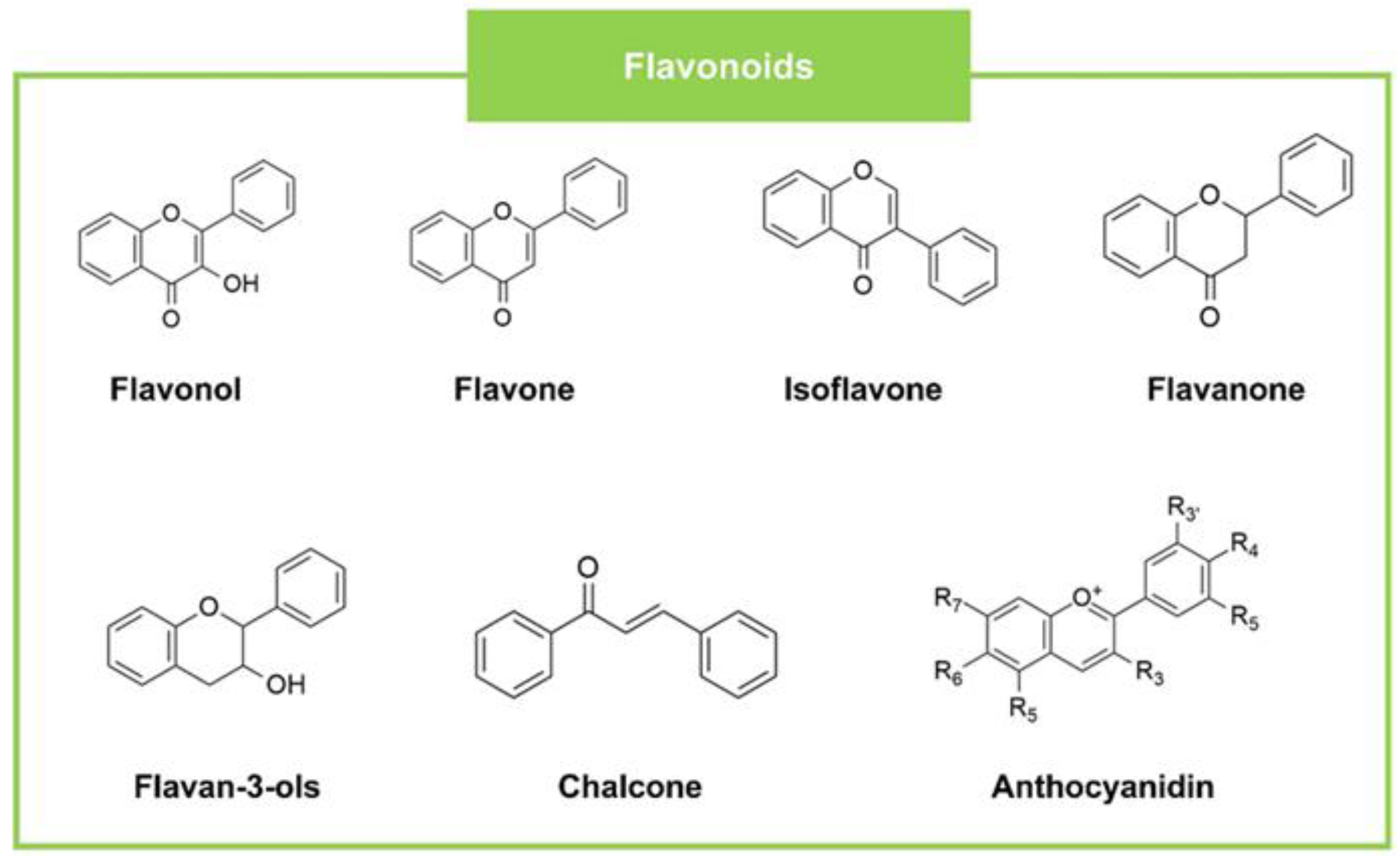
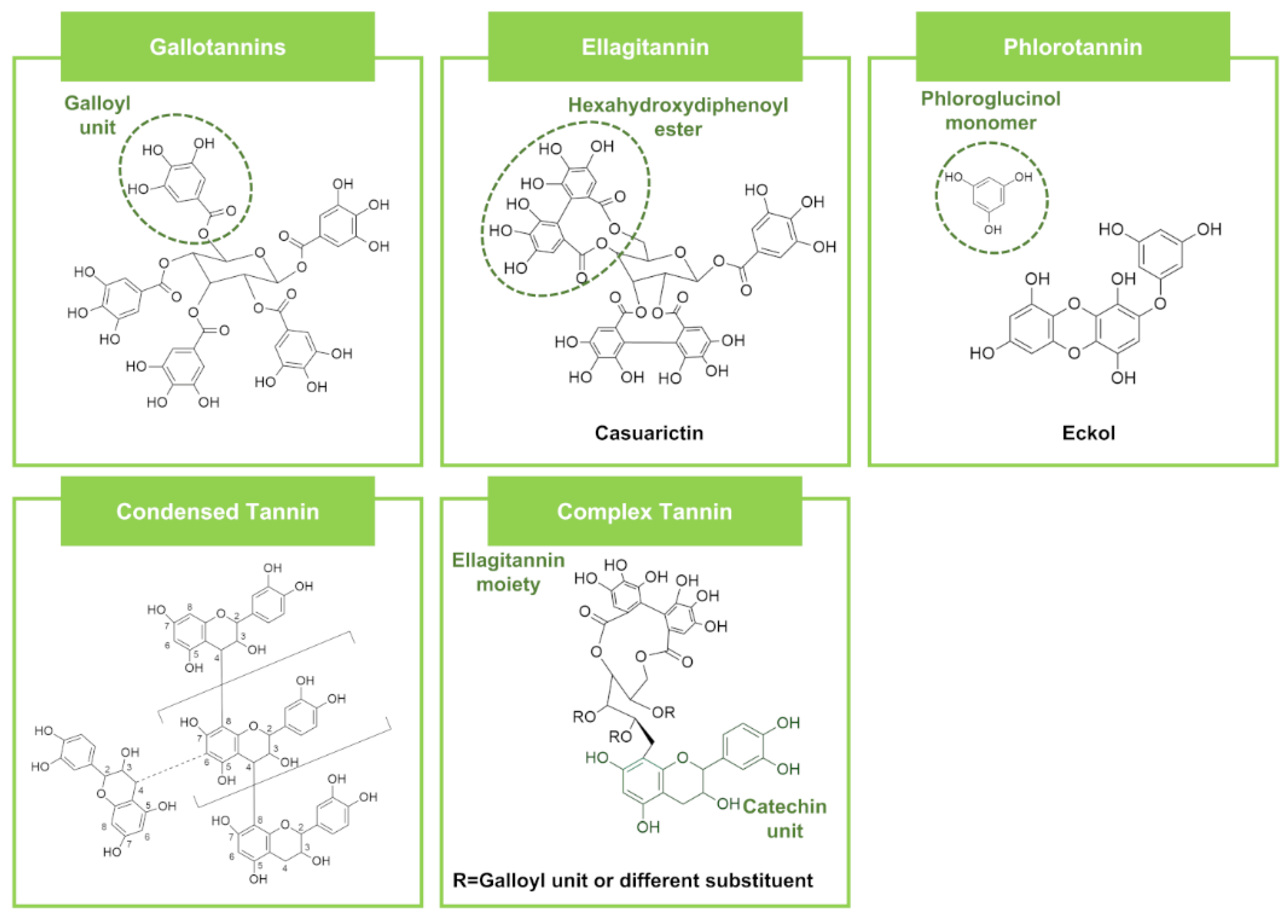
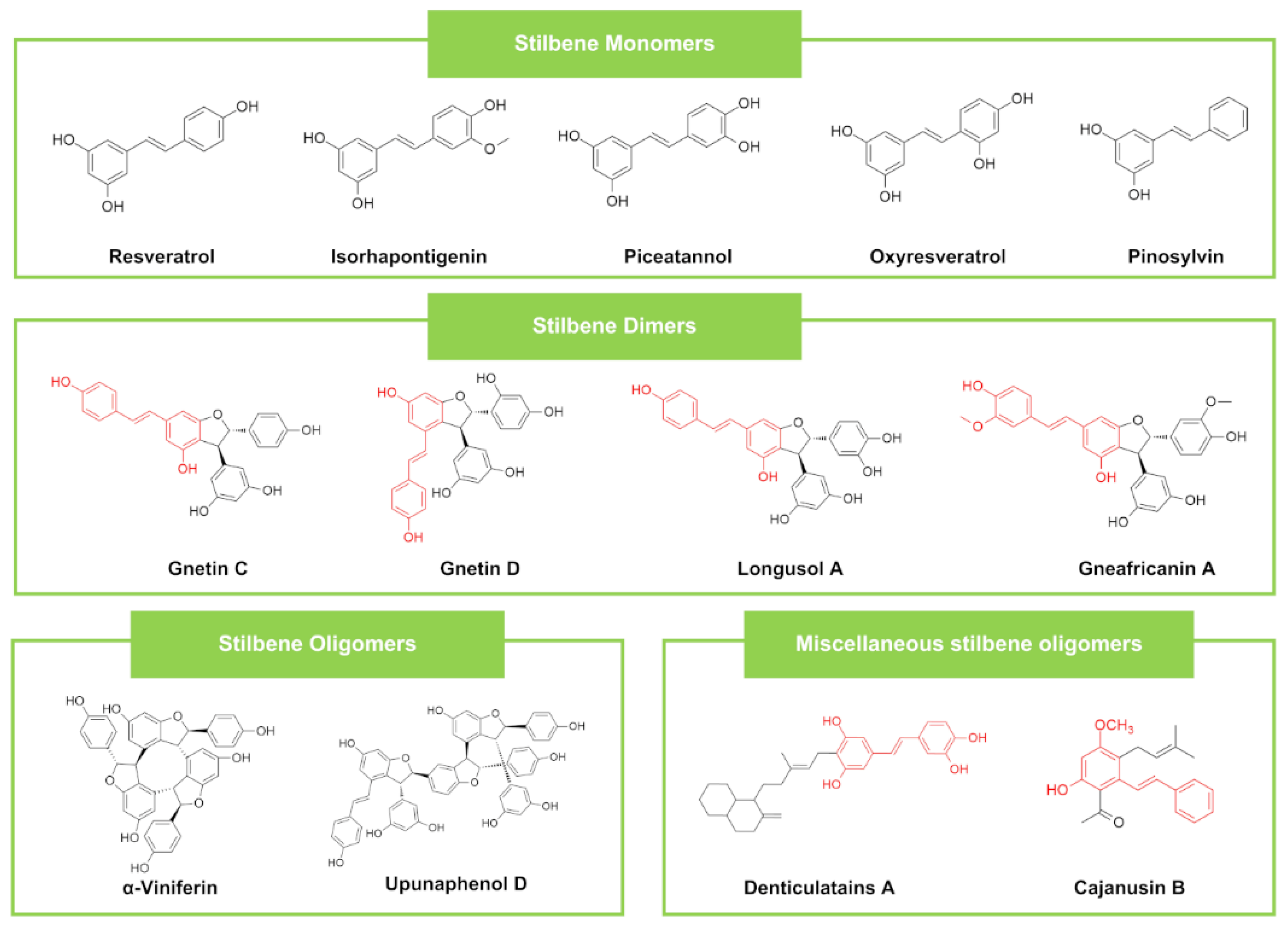
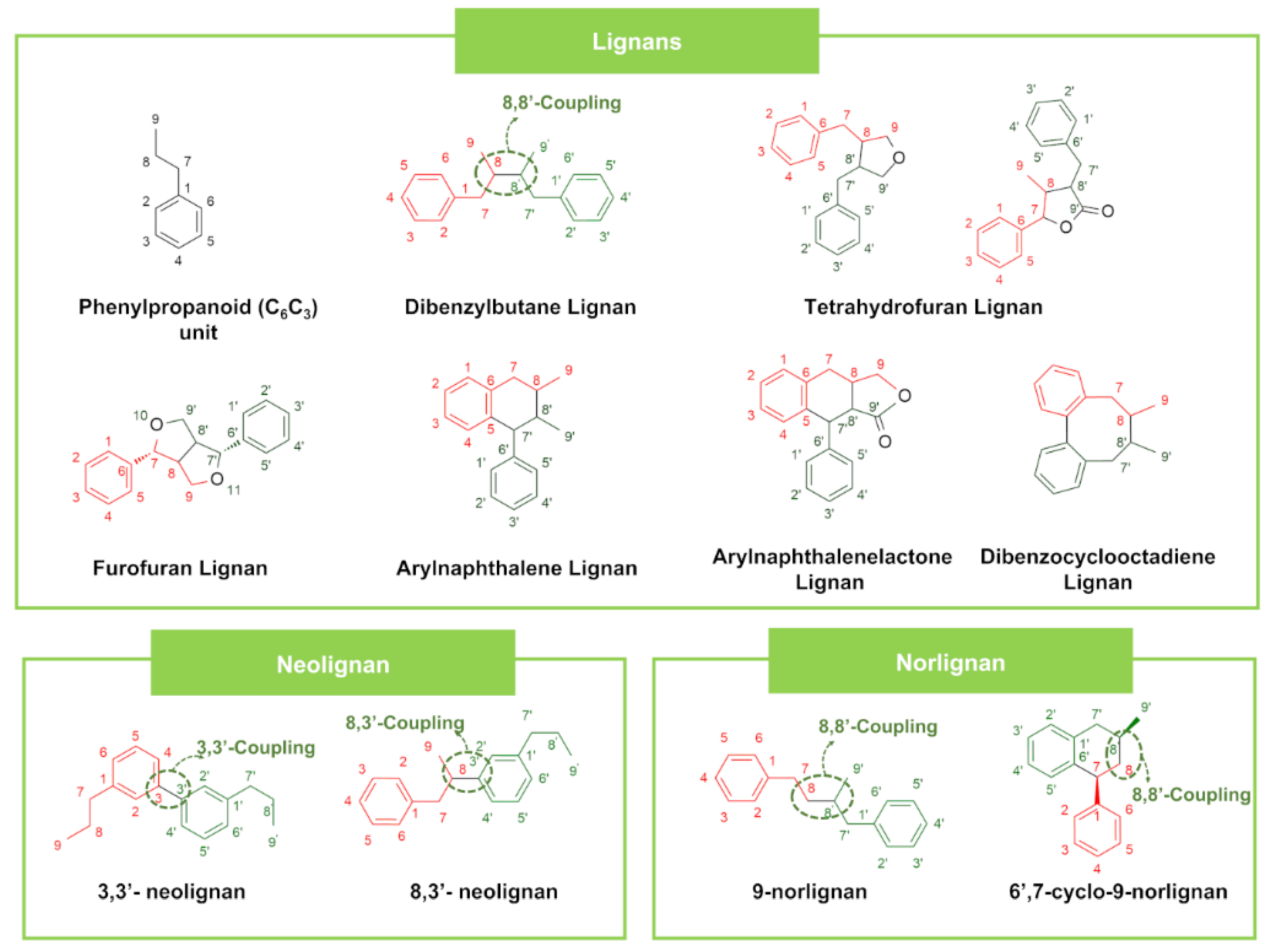
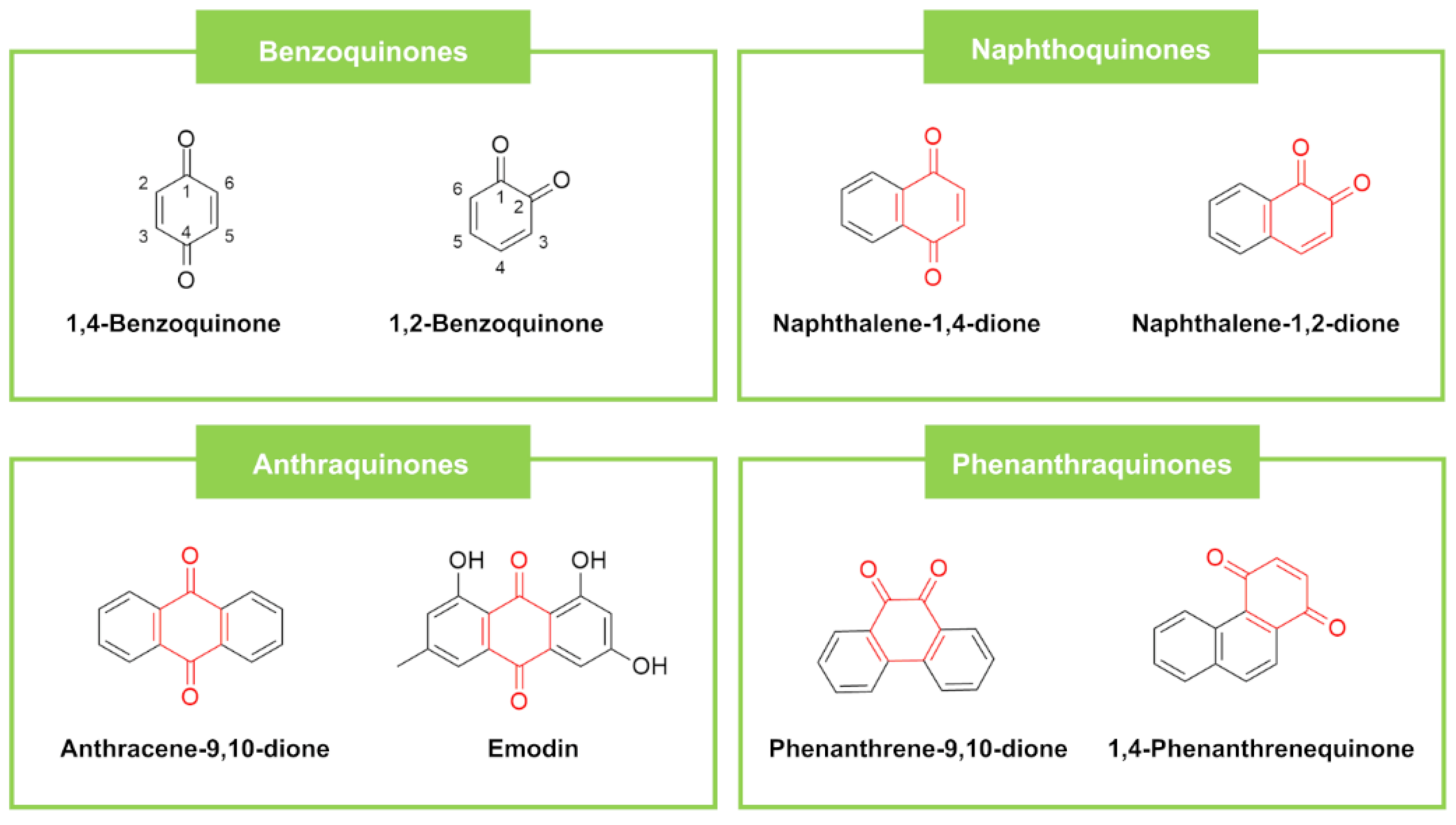
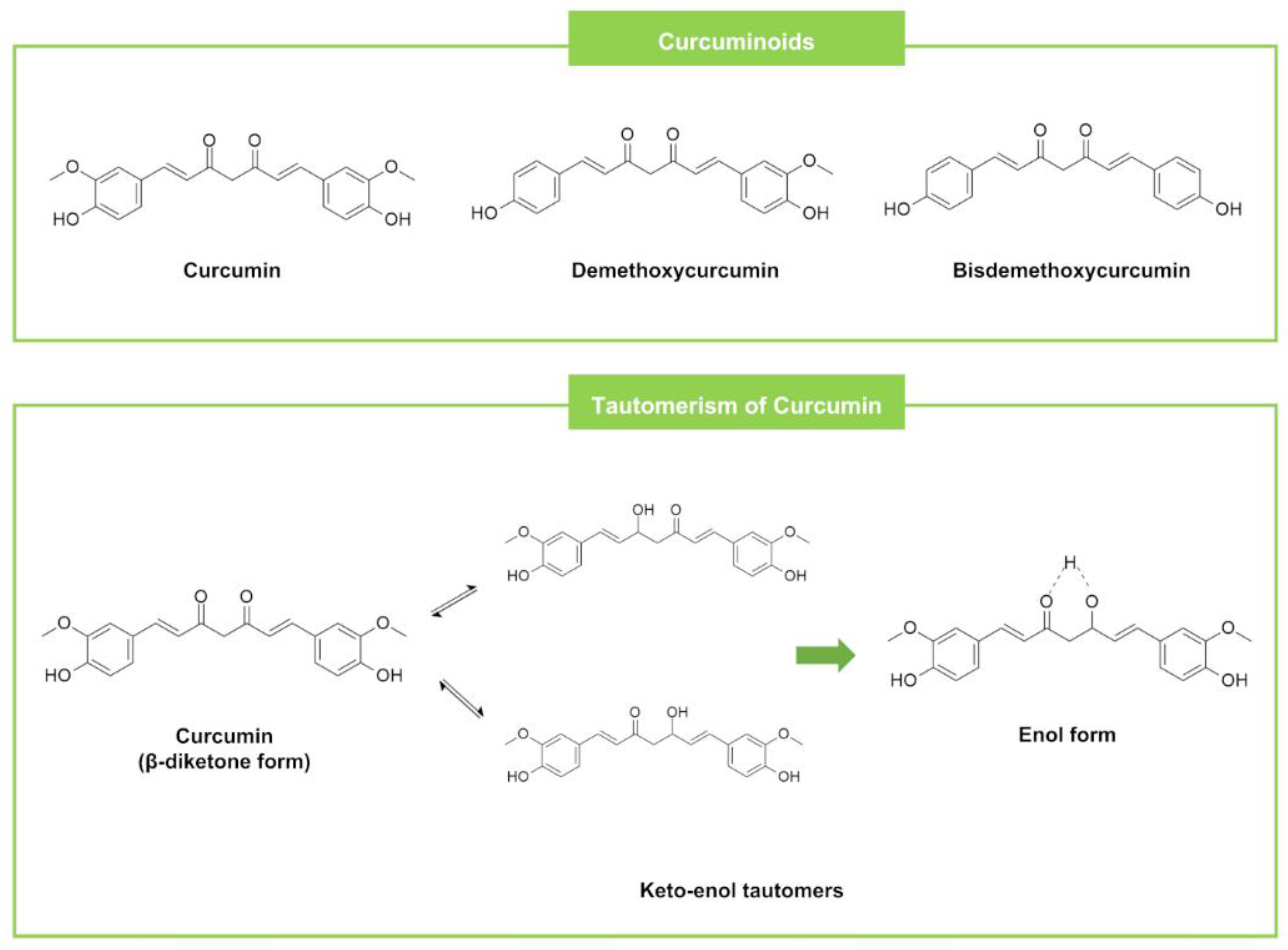
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460-498. https://doi.org/10.3390/futurepharmacol2040030
Ecevit K, Barros AA, Silva JM, Reis RL. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacology. 2022; 2(4):460-498. https://doi.org/10.3390/futurepharmacol2040030
Chicago/Turabian StyleEcevit, Kardelen, Alexandre A. Barros, Joana M. Silva, and Rui L. Reis. 2022. "Preventing Microbial Infections with Natural Phenolic Compounds" Future Pharmacology 2, no. 4: 460-498. https://doi.org/10.3390/futurepharmacol2040030
APA StyleEcevit, K., Barros, A. A., Silva, J. M., & Reis, R. L. (2022). Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacology, 2(4), 460-498. https://doi.org/10.3390/futurepharmacol2040030