Structural Effects on the Reaction of Singlet Oxygen with Tertiary Amines in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results
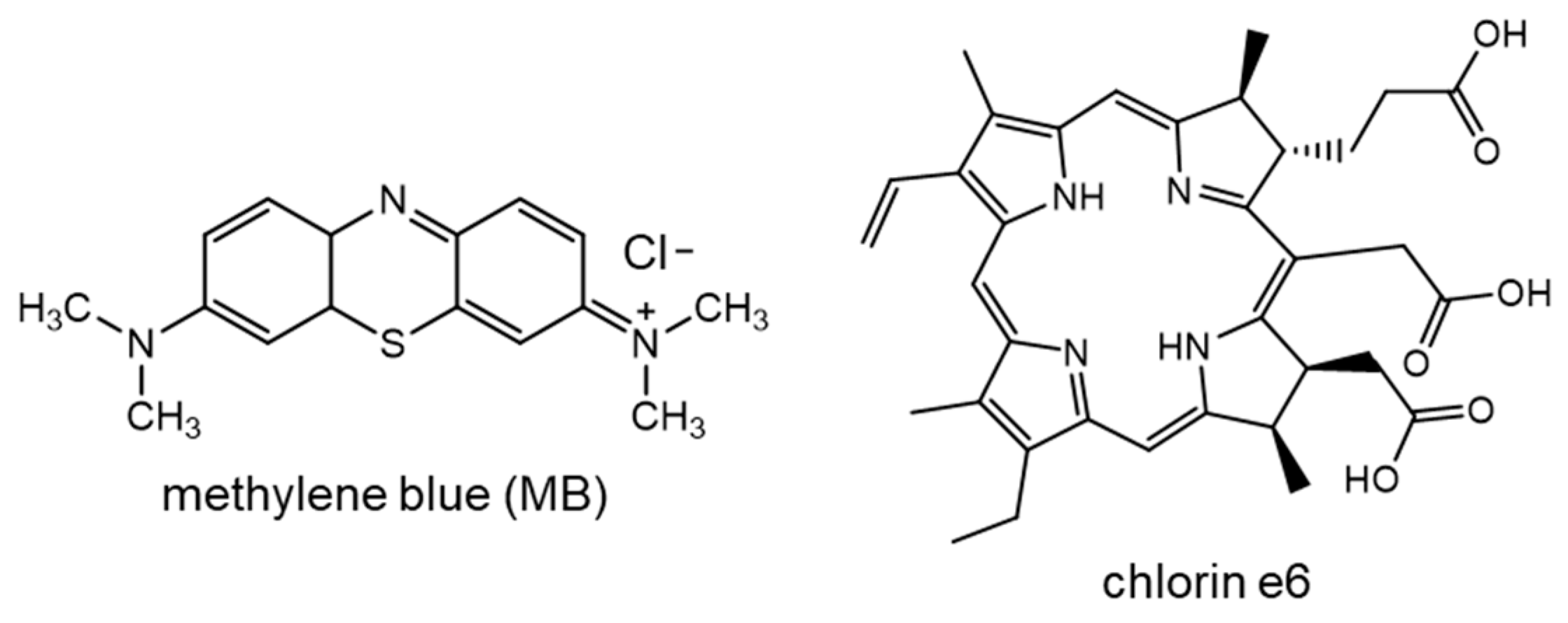
3.1. Generation of Singlet Oxygen
3.2. Detection of H2O2
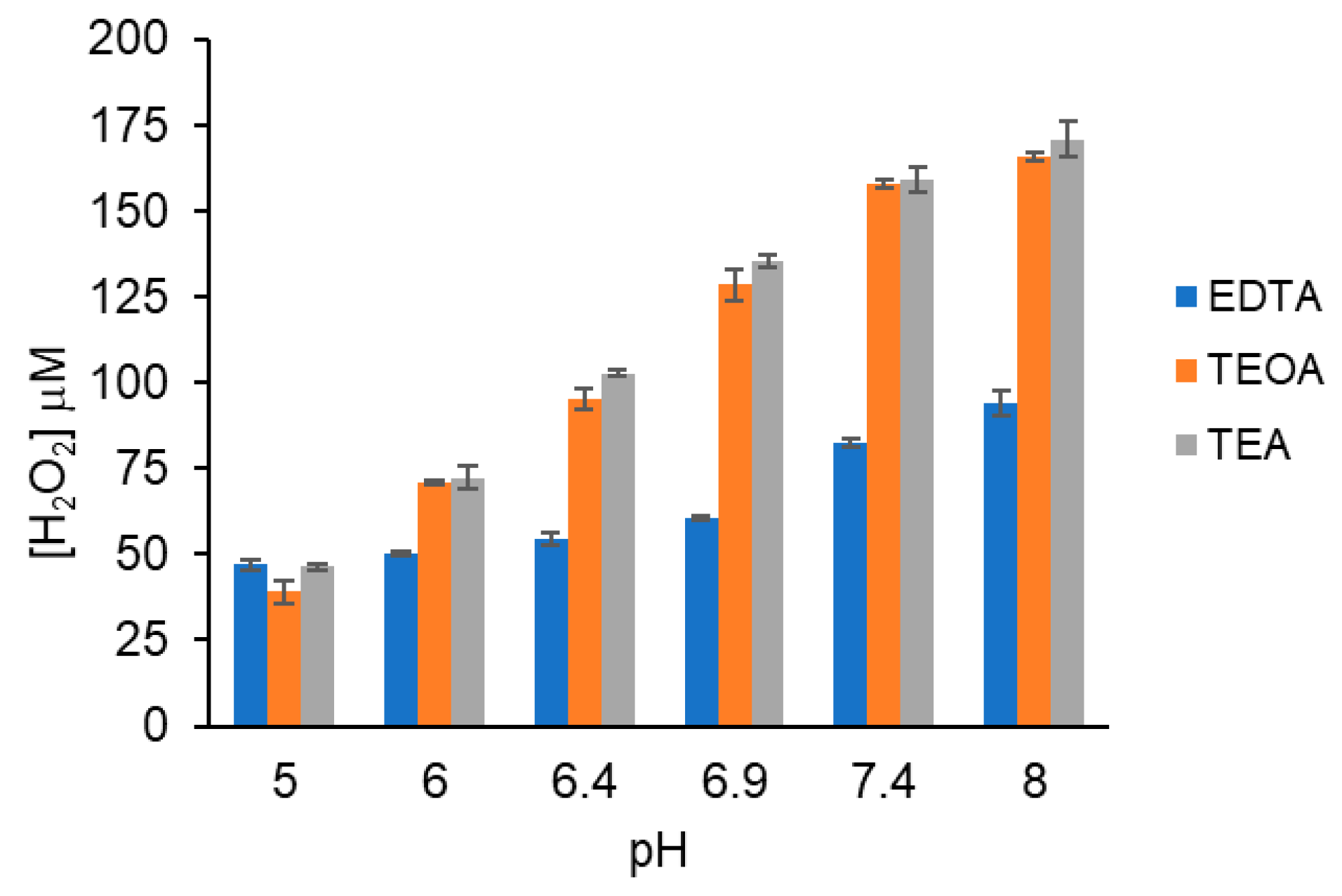
3.3. Effect of pH on H2O2 Yield
3.4. Singlet Oxygen Scavenging by Histidine
3.5. Singlet Oxygen Scavenging by Imidazole
3.6. Chlorin e6 as Photosensitizer for Singlet Oxygen Production
3.7. Tertiary Amine Buffers Generate Hydrogen Peroxide
3.8. Select Tertiary Amines Function as Electron Donors in Photoreduction
3.9. Histidine Enhances Benzoquinone Photoreduction Rates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bicine | Bis(2-hydroxyethyl)aminoacetic acid |
| CoQ0 | 2,3-dimethoxy-5-methyl-p-benzoquinone |
| DMF | dimethylformamide |
| EDTA | ethylenediaminetetraacetic acid |
| HEPES | 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid |
| His | histidine |
| H2O2 | hydrogen peroxide |
| HRP | horseradish peroxidase |
| imid | imidazole |
| MB | methylene blue |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| methoxyBQ | methoxy benzoquinone |
| methylBQ | methyl benzoquinone |
| NaBH4 | sodium borohydride |
| PB | phosphate buffer |
| PIPES | piperazine-N,N′-bis(2-ethanesulfonic acid) |
| TEA | triethylamine |
| TEOA | triethanolamine |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
References
- Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A.; Pace, A. Photochemically Produced Singlet Oxygen: Applications and Perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet Oxygen: There Is Indeed Something New Under the Sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Foote, C.S.; Wexler, S. Singlet Oxygen. A Probable Intermediate in Photosensitized Autoxidations. J. Am. Chem. Soc. 1964, 86, 3880–3881. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Ferroud, C.; Rool, P.; Santamaria, J. Singlet Oxygen Mediated Alkaloid Tertiary Amines Oxidation by Single Electron Transfer. Tetrahedron Lett. 1998, 39, 9423–9426. [Google Scholar] [CrossRef]
- Scheer, H. Chlorophyll Breakdown in Aquatic Ecosystems. Proc. Natl. Acad. Sci. USA 2012, 109, 17311–17312. [Google Scholar] [CrossRef]
- Havaux, M. Plastoquinone in and Beyond Photosynthesis. Trends Plant Sci. 2020, 25, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A. Singlet Oxygen Production in Photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Annamuradov, B.; Ateyeh, M.; Street, C.; Whipple, H.; Er, A.O. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem 2024, 4, 434–461. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Chapter Nine—Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Inorganic Reaction Mechanisms; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 70, pp. 343–394. ISBN 0898-8838. [Google Scholar]
- Kessel, D. Clinical Medicine Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Sun, J.; Peng, W.; Fan, B.; Gan, D.; Li, L.; Liu, P.; Shen, J. Tertiary Amines Convert 1O2 to H2O2 with Enhanced Photodynamic Antibacterial Efficiency. J. Hazard. Mater. 2022, 435, 128948. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Lessard, E.E.; Reed, J.A.; Warsen, M.G.; Zimmer, S.; Landino, L.M. Concurrent Photooxidation and Photoreduction of Catechols and Para-Quinones by Chlorophyll Metabolites. Photochem 2024, 4, 346–360. [Google Scholar] [CrossRef]
- Landino, L.M.; Shuckrow, Z.T.; Mooney, A.S.; Lauderback, C.O.; Lorenzi, K.E. Photo-Oxidation and Photoreduction of Catechols by Chlorophyll Metabolites and Methylene Blue. Chem. Res. Toxicol. 2022, 35, 1851–1862. [Google Scholar] [CrossRef]
- Landino, L.M.; Reed, J.A. Photochemical Redox Cycling of Naphthoquinones Mediated by Methylene Blue and Pheophorbide A. Molecules 2025, 30, 1351. [Google Scholar] [CrossRef]
- Pellegrin, Y.; Odobel, F. Les Donneurs d’électron Sacrificiels Pour La Production de Combustible Solaire. Comptes Rendus Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef]
- Hessler, D.P.; Frimmel, F.H.; Oliveros, E.; Braun, A.M. Solvent Isotope Effect on the Rate Constants of Singlet—Oxygen Quenching by Edta and Its Metal Complexes. Helv. Chim. Acta 1994, 77, 859–868. [Google Scholar] [CrossRef]
- Warsen, M.G.; Zimmer, S.; Phan, K.; Landino, L.M. Photochemical Redox Reactions of 2,6-Dichlorophenolindophenol and Its Use to Detect Photoreduced Quinones. Photochem 2025, 5, 19. [Google Scholar] [CrossRef]
- Hasty, N.; Merkel, P.B.; Radlick, P.; Kearns, D.R. Role of Azide in Singlet Oxygen Reactions: Reaction of Azide with Singlet Oxygen. Tetrahedron Lett. 1972, 13, 49–52. [Google Scholar] [CrossRef]
- Harbour, J.R.; Issler, S.L. Involvement of the Azide Radical in the Quenching of Singlet Oxygen by Azide Anion in Water. J. Am. Chem. Soc. 1982, 104, 903–905. [Google Scholar] [CrossRef]
- Matheson, I.B.C.; Lee, J. Chemical reaction rates of amino acids with singlet oxygen. Photochem. Photobiol. 1979, 29, 879–881. [Google Scholar] [CrossRef]
- Matheson, I.B.C.; Etheridge, R.D.; Kratowich, N.R.; Lee, J. The quenching of singlet oxygen by amino acids and proteins. Photochem. Photobiol. 1975, 21, 165–171. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet Oxygen-Mediated Damage to Proteins and Its Consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Davies, M.J. Reactive Species Formed on Proteins Exposed to Singlet Oxygen. Photochem. Photobiol. Sci. 2004, 3, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Van Balgooy, J.N.A.; Roberts, E. Methods for Study of Imidazole Compounds and Application to Brain and Cancer Cells. Biochem. Pharmacol. 1973, 22, 1405–1415. [Google Scholar] [CrossRef]
- Bernal, F.A.; Orduz-Diaz, L.L.; Guerrero-Perilla, C.; Coy-Barrera, E.D. Diazo Coupling Reaction of Catechins and Alkylresorcinols with Diazotized Sulfanilic Acid for Quantitative Purposes in Edible Sources: Method Development and Validation. Food Anal. Methods 2016, 9, 411–418. [Google Scholar] [CrossRef]
- Lopes, G.R.; Pinto, D.C.G.A.; Silva, A.M.S. Horseradish Peroxidase (HRP) as a Tool in Green Chemistry. RSC Adv. 2014, 4, 37244–37265. [Google Scholar] [CrossRef]
- Conyers, S.M.; Kidwell, D.A. Chromogenic Substrates for Horseradish Peroxidase. Anal. Biochem. 1991, 192, 207–211. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish Peroxidase: A Modern View of a Classic Enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin E6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Čunderlíková, B.; Gangeskar, L.; Moan, J. Acid–Base Properties of Chlorin E6: Relation to Cellular Uptake. J. Photochem. Photobiol. B Biol. 1999, 53, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Morimoto, H.; Kikuchi, S.; Nagaoka, S. Kinetic Study of Free-Radical-Scavenging Action of Biological Hydroquinones (Reduced Forms of Ubiquinone, Vitamin K and Tocopherol Quinone) in Solution. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1993, 1157, 313–317. [Google Scholar] [CrossRef]
- Schwan, S.; Schröder, D.; Wegner, H.A.; Janek, J.; Mollenhauer, D. Substituent Pattern Effects on the Redox Potentials of Quinone-Based Active Materials for Aqueous Redox Flow Batteries. ChemSusChem 2020, 13, 5480–5488. [Google Scholar] [CrossRef]
- Huynh, M.T.; Anson, C.W.; Cavell, A.C.; Stahl, S.S.; Hammes-Schiffer, S. Quinone 1 e− and 2 e−/2 H+ Reduction Potentials: Identification and Analysis of Deviations from Systematic Scaling Relationships. J. Am. Chem. Soc. 2016, 138, 15903–15910. [Google Scholar] [CrossRef]
- Eichwurzel, I.; Stiel, H.; Röder, B. Photophysical Studies of the Pheophorbide a Dimer. J. Photochem. Photobiol. B Biol. 2000, 54, 194–200. [Google Scholar] [CrossRef]
- Merkel, P.B.; Nilsson, R.; Kearns, D.R. Deuterium Effects on Singlet Oxygen Lifetimes in Solutions. New Test of Singlet Oxygen Reactions. J. Am. Chem. Soc. 1972, 94, 1030–1031. [Google Scholar] [CrossRef]
- Zigler, J.S.; Lepe-Zuniga, J.L.; Vistica, B.; Gery, I. Analysis of the Cytotoxic Effects of Light-Exposed Hepes-Containing Culture Medium. In Vitro Cell. Dev. Biol. 1985, 21, 282–287. [Google Scholar] [CrossRef]
- Lepe-Zuniga, J.L.; Zigler, J.S.; Gery, I. Toxicity of Light-Exposed Hepes Media. J. Immunol. Methods 1987, 103, 145. [Google Scholar] [CrossRef]
- Elhajj, S.; Gozem, S. First and Second Reductions in an Aprotic Solvent: Comparing Computational and Experimental One-Electron Reduction Potentials for 345 Quinones. J. Chem. Theory Comput. 2024, 20, 6227–6240. [Google Scholar] [CrossRef] [PubMed]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zeng, X.; Zhang, X. Production of Hydrogen Peroxide by Photocatalytic Processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef] [PubMed]








| Tertiary Amine | Amine pKa | [H2O2] μM | Mol CoQ0 Red/mol MB |
|---|---|---|---|
| EDTA | 6.13, 10.37 * | 108 ± 8 | 170 ± 2 |
| Bicine | 8.35 | 172 ± 2 | 142 ± 6 |
| TEOA | 7.78 | 183 ± 2 | 63 ± 2 |
| TEA | 10.75 | 187 ± 6 | ND |
| HEPES | 7.48 | 143 ± 1 | ND |
| PIPES | 6.76 | 150 ± 2 | ND |
| MES | 6.15 | 102 ± 1 | ND |
| MOPS | 7.15 | 81 ± 2 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sliwinski, G.; Lessard, E.; Landino, L.M. Structural Effects on the Reaction of Singlet Oxygen with Tertiary Amines in Aqueous Solution. Oxygen 2025, 5, 22. https://doi.org/10.3390/oxygen5040022
Sliwinski G, Lessard E, Landino LM. Structural Effects on the Reaction of Singlet Oxygen with Tertiary Amines in Aqueous Solution. Oxygen. 2025; 5(4):22. https://doi.org/10.3390/oxygen5040022
Chicago/Turabian StyleSliwinski, Grace, Emily Lessard, and Lisa M. Landino. 2025. "Structural Effects on the Reaction of Singlet Oxygen with Tertiary Amines in Aqueous Solution" Oxygen 5, no. 4: 22. https://doi.org/10.3390/oxygen5040022
APA StyleSliwinski, G., Lessard, E., & Landino, L. M. (2025). Structural Effects on the Reaction of Singlet Oxygen with Tertiary Amines in Aqueous Solution. Oxygen, 5(4), 22. https://doi.org/10.3390/oxygen5040022






