Abstract
Photosensitized excitation of molecular oxygen generates singlet oxygen, a reactive oxygen species that has been studied in biological systems, synthetic methods and in aquatic ecosystems. The reaction of singlet oxygen with tertiary amines is important because they are widely used as electron donors in photochemical reactions. Herein we studied the reaction of singlet oxygen with multiple tertiary amines including ethylenediamine tetraacetic acid (EDTA), triethanolamine (TEOA) and triethylamine (TEA). Singlet oxygen was generated using the photosensitizers methylene blue or chlorin e6 and red light with output at 660 nm. TEOA and TEA generated more hydrogen peroxide (H2O2), the stable end product, than EDTA at all pH values tested and regardless of the photosensitizer used. Both histidine and imidazole scavenged singlet oxygen and decreased H2O2 yield. The extent of histidine scavenging was pH-dependent for the combination of methylene blue and EDTA but not for TEOA or TEA. The combination of chlorin e6 and EDTA generated less H2O2 because both contain multiple negative charges that limit their interaction. Multiple tertiary amines that are used as biochemical buffers produced similar quantities of H2O2 as EDTA, TEOA and TEA. However, these sulfonic acid-containing tertiary amines did not function as electron donors in a benzoquinone photoreduction assay.
1. Introduction
Singlet oxygen (1O2) that is formed by photoexcitation of paramagnetic triplet molecular oxygen (O2), is diamagnetic, short-lived and reacts readily with a range of organic functional groups [1,2,3]. Photosensitized generation of an excited state of O2 was verified by Foote and Wexler in 1964 [4]. Although 1O2 forms in some light-independent reactions, most 1O2 research focuses on its generation using a photosensitizer and light. Some commonly used chemical photosensitizers include methylene blue (MB), Rose Bengal and phenosafranine, as well as the biological molecules riboflavin and chlorin e6, a chlorophyll metabolite. 1O2 reactivity has been explored in a range of contexts from synthetic methodologies, environmental reactions in aquatic ecosystems and in biological systems [1,5,6].
Chlorophyll is the light-harvesting pigment of photosynthesis and photoexcitation of it by red light initiates electron transfer to plastoquinone, a para-benzoquinone that is embedded in chloroplast membranes of plant cells [7,8]. The ultimate end products of electron transfer in photosynthesis are glucose and O2. Although photoreduction is the predominant outcome, when light is excessive, the photoexcited state of chlorophyll initiates 1O2 formation [9]. As a result, plants contain multiple antioxidants that scavenge 1O2 to limit cellular damage [10].
In another biological context, the combination of photosensitizers, O2 and light is used to generate 1O2 in photodynamic therapy (PDT) [11,12,13]. PDT is used to initiate cell death in dermatology, oncology and ophthalmology [11]. 1O2 reacts with a wide range of biological molecules including DNA, protein amino acids and lipids [5,14]. These reactions often result in the formation of additional, more persistent reactive oxygen species (ROS) including superoxide anion (O2−•) and hydrogen peroxide (H2O2) [15].
In our studies of benzoquinone and naphthoquinone photoreduction by MB and select chlorophyll metabolites, we routinely use tertiary amines as electron donors [16,17,18]. Ethylenediamine tetraacetic acid (EDTA), triethanolamine (TEOA) and triethylamine (TEA) are widely used electron donors in photoreduction processes because they are inexpensive, and their decomposition products are well-characterized and inert [19]. In one key experiment, we showed that the catechol neurotransmitter, dopamine, could be photo-oxidized by 1O2 to its ortho-quinone and then photoreduced in situ using a combination of MB or a chlorophyll metabolite and a tertiary amine electron donor [17].
Our interest in the dual nature of photosensitizers as both mediators of photoreduction and photo-oxidation prompted this work. Under ambient O2 conditions when photoreduction was first initiated, the reaction of 1O2 with the tertiary amine electron donors produced some H2O2. Because the only reported rate constant for EDTA and 1O2 was ~105 M−1 sec−1, we dismissed this reaction as negligible because photoreduction dominated [20]. However, the ratio of quinone photoreduction to 1O2-mediated H2O2 formation from the tertiary amines appeared to be dependent on the photosensitizer employed [16].
The competing reaction of 1O2 with histidine, a well-characterized scavenger, is also of interest because we showed that histidine enhanced rates of photoreduction and decreased H2O2 formation [16,18,21]. Histidine was superior to azide, also an 1O2 scavenger, because it reacted with 1O2 in a 4 + 2 cycloaddition reaction to effectively remove O2 from solution. This differs from azide, where O2 is regenerated and additional azide-based radicals form [22,23]. Rate constants on the order of 107 M−1 sec−1 in aqueous solution have been reported for the reaction of histidine and 1O2 though there is some pH dependence with faster rates at higher pH [24,25,26,27].
Further, the reaction of 1O2 with tertiary amines is worthy of further investigation because many common biochemical buffers are tertiary amines, including HEPES, PIPES, MES and MOPS. Only limited research has examined the reaction of tertiary amines with 1O2 and, to our knowledge, not across a range of pH values using different photosensitizers [6,15,20]. Herein, we employed the straightforward detection of H2O2, the end product of 1O2-mediated oxidation of tertiary amines, to explore this reaction in more detail. Our objectives were three-fold: (1) to examine the effects of pH and photosensitizer on 1O2 reactions with select tertiary amines; (2) to assess histidine and imidazole scavenging of 1O2 during reactions with tertiary amines; and (3) to predict reaction outcomes of 1O2 with tertiary amines and histidine or imidazole using published rate constants as a guide.
2. Materials and Methods
Materials: All chemicals were from Fisher Scientific (Waltham, MA, USA) or Sigma (St. Louis, MO, USA) and were of the highest purity available. Chlorin e6 and all benzoquinone stock solutions were prepared in DMF and used immediately or stored at −20 °C for up to a month. All other solutions were prepared in water or 10 mM PB pH 7.4. Tertiary amine stock solutions in water (100 or 200 mM) were adjusted to pH 7.4 with NaOH. All phosphate buffers (PB) were equilibrated to room temperature (20–22 °C) to ensure no variation in dissolved O2. Histidine and imidazole solutions in water were prepared fresh daily.
Red light specifications: A 36-watt red light composed of eighteen 2-watt LEDs was used for all photochemical experiments. Samples were placed under the lamp on an aluminum-lined surface in a shallow box (11 cm from light source to surface). The maximum wavelength of emitted light was 660 nm. The intensity was quantitated in lux, and intensity (as a function of the distance from the light source to the samples) was measured regularly to ensure consistent exposure, as described in [17]. Red light irradiation times were optimized to limit photobleaching of MB or chlorin e6 and did not exceed 7.5 min.
Detection of H2O2 with TMB and HRP: For reactions containing only photosensitizers and tertiary amines, TMB and HRP were used to detect H2O2. Aliquots (20 μL) of photochemical reactions were combined with 0.5 mM TMB and 0.5 μM HRP in 100 μL. After blue color development, 100 μL 1 M HCl was added. Absorbance was read at 450 nm in a 96-well plate. A standard curve from 0 to 40 μM H2O2 was used to calculate H2O2 concentrations in the photo-oxidation reactions (Supplemental Figure S1).
Detection of histidine and imidazole: Histidine or imidazole were reacted with diazotized sulfanilic acid under alkaline conditions to form an azo dye based on published methods [28,29]. Briefly, 1% sulfanilic acid in 1 M HCl and 5% NaNO3 were combined 1:1 to generate diazotized sulfanilic acid. Dark and light-exposed samples containing histidine or imidazole (10 μL aliquots) were combined with 30 μL of diazotized sulfanilic acid solution in a 96-well plate. Na2CO3 solution (60 μL, 10%) was added and the resulting red-orange coupling product was detected at 490 nm. Standard curves from 0 to 150 μM histidine or imidazole were used to determine concentrations in photochemical reactions. (Supplemental Figures S2 and S3)
Photoreduction of CoQ0: Solutions (100 μL total) of CoQ0 (1 mM), 5 μM MB and 5 mM of each tertiary amine in 10 mM PB 7.4 were aliquoted into a 96-well plate. Absorbance was measured at 405 nm prior to light exposure and at time intervals of 1, 2, 5 and 7 min. Controls with only CoQ0 and MB (no tertiary amine) were also performed.
Photoreduction of CoQ0, methyl and methoxyBQ rate enhancement by histidine Benzoquinones (0.6 mM) were combined with 2 μM MB and 2 mM EDTA (1 mL) in a semimicro cuvette in 10 mM PB pH 7.4. Absorbance scans from 250 to 750 nm were collected prior to light exposure and at time intervals of 1, 3 and 5 min. Histidine or imidazole (up to 1 mM) were added prior to light exposure. The cuvette was inverted three times to normalize O2 concentration. HRP was added after irradiation and the resulting increase in oxidized benzoquinone absorbance was used to calculate H2O2 concentration. For CoQ0, absorbance at 405 nm was used to calculate concentration (740 M−1 cm−1 in 10 mM PB pH 7.4) [16]. For methoxyBQ, absorbance at 366 nm was used. Oxidized methylBQ absorbs weakly at 320-330 nm; therefore, absorbance of reduced methylBQ at 286 nm was used to determine concentration. Sodium ascorbate or NaBH4 were added after dark scans or after the quinones had been photoreduced to test for complete photoreduction (~5 equivalents).
Data analysis: For each experiment, at least three independent experiments were performed in duplicate or triplicate. Mean values were calculated for each independent experiment (mean ± error). For figures showing error bars, mean values were averaged and error calculated. Details for each are stated in Figure Legends.
3. Results
3.1. Generation of Singlet Oxygen
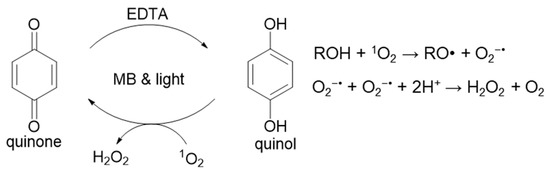
Singlet oxygen is generated by the combination of visible light, a photosensitizer (PS) and O2. Its initial reaction with tertiary amines (R3N:) produces superoxide anion and an amine radical cation, as shown in Scheme 1. Two superoxide anions disproportionate to yield H2O2 and regenerate O2. We employ tertiary amines as sacrificial electron donors in our photoreduction studies and know that photo-oxidation processes mediated by 1O2 occur concurrently when O2 is present [16,18,21]. Only one report determined a rate constant in water for the reaction of 1O2 with EDTA, a commonly used tertiary amine, and some metal-EDTA complexes [20]. For these reasons, we sought to study the reaction of 1O2 with commonly used tertiary amines in more detail.
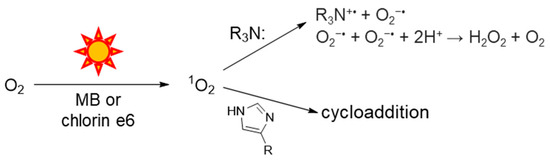
Scheme 1.
Photochemical generation of 1O2; scavenging by histidine or imidazole.
3.2. Detection of H2O2
To detect H2O2 generated by the reaction of 1O2 with tertiary amines, we used horseradish peroxidase (HRP) and a colorimetric co-substrate, TMB, that generates a stable yellow product under acidic conditions [30,31,32]. If catalase, an enzyme that scavenges H2O2 (Equation (1)), was added prior to HRP and TMB, no yellow color was detected.
2H2O2 → 2H2O + O2
To react with 1O2 as shown in Scheme 1, tertiary amines must be deprotonated. Therefore, we examined the pH dependence of H2O2 formation using the tertiary amines EDTA, triethanolamine (TEOA) and triethylamine (TEA) (Scheme 2). We routinely perform photoreduction studies with methylene blue (MB) as the photosensitizer and a red light with maximum output at 660 nm. The combination of MB and 36-watt red light also generates 1O2 efficiently; therefore, it was used herein. We use phosphate buffers (PBs) when studying reactions that generate 1O2 and other ROS because phosphate is already oxidized and inert.
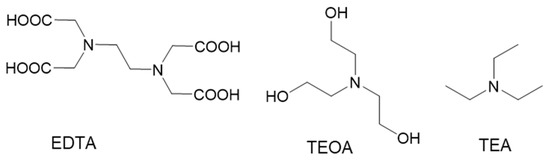
Scheme 2.
Structures of tertiary amines.
3.3. Effect of pH on H2O2 Yield
Figure 1 shows that for the three tertiary amines tested, the H2O2 concentration increased as buffer pH increased. In the absence of red light or MB, no H2O2 was detected. Two 1O2 are required to generate one H2O2 but, because one O2 is regenerated, the stoichiometry is one O2 consumed per H2O2. The concentration of dissolved O2 is ~300 μM in 10 mM PB at 20–22 °C. The H2O2 concentrations in Figure 1 are all below 300 μM, confirming that dissolved O2 was not depleted after 5 min of photo-oxidation.
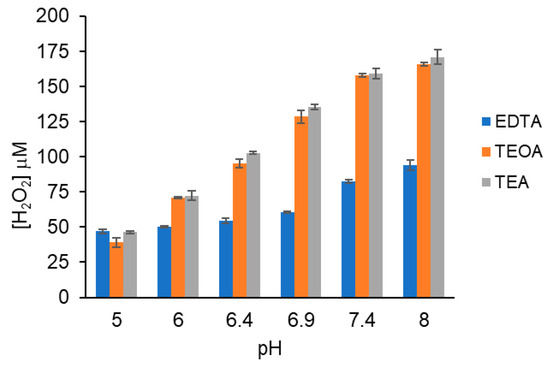
Figure 1.
pH-dependent generation of H2O2 by MB, red light and tertiary amines. Reactions (100 μL) contained 5 μM MB and 5 mM tertiary amine in 10 mM PB of varying pH and were irradiated for 5 min. Aliquots (20 μL) were combined with 10 mM PB pH 7.4, 0.5 mM TMB and 0.5 μM HRP (100 μL total). Reactions were quenched with 100 μL 1 M HCl and absorbance measured at 450 nm. [H2O2] was determined from a H2O2 standard curve. These data are the average of three independent experiments performed in duplicate.
For EDTA, the H2O2 concentration increased two-fold as pH increased from 5 to 8. For TEOA and TEA, H2O2 concentrations increased four-fold from pH 5-8 and their yields were equivalent at all pH values tested, despite a substantial difference in amine pKa values. TEOA and TEA have pKa value of 7.78 and 10.75, respectively. EDTA has two amine groups with pKa values of 6.13 and 10.37; however, despite its lower pKa value and two amines/mole, EDTA generated less H2O2 than TEOA or TEA at all pH values tested.
3.4. Singlet Oxygen Scavenging by Histidine
One goal of this work was to examine relative rate constants for the reaction of 1O2 with tertiary amines and the amino acid histidine and imidazole, the R group of histidine (Scheme 1). Histidine is a well-characterized 1O2 scavenger that we have employed in our photoreduction studies [24,26]. We reported that histidine enhanced rates of benzoquinone and naphthoquinone photoreduction by limiting the reaction of newly reduced quinols with 1O2 that was generated concurrently under ambient oxygen conditions [16,18,21]. Further histidine scavenges 1O2 to form an oxygenated species, oxo-histidine, thereby depleting O2 from solution.
To ensure that histidine did not interfere with H2O2 detection, up to 1.5 mM histidine was combined with HRP, TMB and known amounts of H2O2. Absorbance readings for oxidized TMB with histidine or imidazole were identical to those of controls that did not contain either. We also performed control experiments where histidine and MB were irradiated (no tertiary amine) in 10 mM PB pH 7.4 for 5 min. Under these conditions, no H2O2 was generated, which is consistent with the mode of 1O2 scavenging by histidine.
Figure 2A shows that the addition of histidine decreased H2O2 formation by EDTA, TEOA and TEA in 10 mM PB at pH 7.4 in a dose-dependent manner. The concentration of each amine was 5 mM and the highest histidine concentration was 1.5 mM. For TEOA and TEA, H2O2 yield decreased by 75%, with only 0.5 mM histidine and at 1.5 mM histidine, the decrease was 90%. By contrast, for EDTA, with 1.5 mM histidine, H2O2 decreased by 60% from 83 μM to 34 μM.
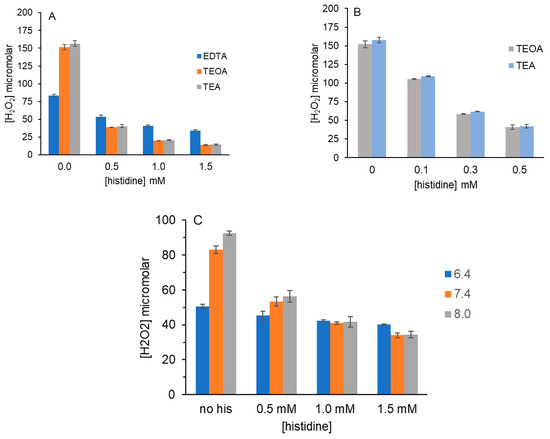
Figure 2.
Effect of histidine on H2O2 yield from MB, red light and tertiary amines. (A) Reactions (100 μL) containing 5 μM MB, 5 mM tertiary amine and 0-1.5 mM histidine in 10 mM PB pH 7.4 were irradiated for 5 min. (B) Reactions containing 5 μM MB, 5 mM TEOA or TEA and 0-0.5 mM histidine in 10 mM PB pH 7.4 were irradiated for 5 min. (C) Reactions (100 μL) containing 5 μM MB, 5 mM EDTA and 0-1.5 mM histidine in 10 mM PB pH 6.4, 7.4 or 8.0 were irradiated for 5 min. H2O2 was detected as described in Figure 1. Data in each graph are the average of three independent experiments performed in duplicate.
To find a more linear range of histidine concentrations required to decrease H2O2 yield with TEOA and TEA, we tested 0.1 to 0.5 mM histidine (Figure 2B). This lower range of histidine concentrations produced the desired dose–response. Again, TEOA and TEA generated equivalent H2O2 concentrations despite differences in their amine pKa values.
Based on these results, competition assays to determine relative rate constants for the reaction of 1O2 with tertiary amines and histidine are not straightforward. Figure 1 indicates that the structure of the tertiary amine influences its reaction of 1O2 with much higher H2O2 yields for the neutral amines, TEOA and TEA. With its multiple negative charges, EDTA may be less reactive because 1O2 cannot approach it as readily. Given that O2 is hydrophobic, it may not approach positively charged MB that is associated with EDTA. Further, histidine’s imidazole ring has a pKa of ~7 and it too will have a partial position charge at pH 7.4.
To address this, 1O2 scavenging by histidine with EDTA as the tertiary amine was examined in 10 mM PB at pH 6.4, 7.4 and 8.0. As in Figure 1, H2O2 yield from EDTA (no histidine) was pH dependent, with increased H2O2 as pH increased. For all pH values tested, we observed dose-dependent decreases in H2O2 with histidine; however, the decrease was greater at pH 8.0 than at pH 7.4 and much lower at pH 6.4. With 5 mM EDTA and 1.5 mM histidine at pH 6.4, H2O2 yield decreased by only 20% vs. 60% at pH 7.4 and by 65% at pH 8.0. The striking change between pH 6.4 and 7.4 implicates the protonation of histidine’s imidazole ring for optimal photo-oxygenation by 1O2. Because the imidazole ring undergoes a 4 + 2 cycloaddition with 1O2, its protonation may limit reactivity. Alternatively, the protonated imidazole ring of histidine at lower pH may associate with the negative charges on EDTA in a manner that limits its exposure to 1O2.
For TEOA and TEA, we did not observe pH-dependent differences in histidine scavenging of 1O2 as reflected in H2O2 concentration endpoints. As in Figure 1, there was pH dependence for H2O2 yield by TEOA and TEA but at pH 6.4, 7.4 and 8.0, H2O2 yield consistently decreased by 70–75% with only 0.5 mM histidine present. While this argues against a dependence on imidazole protonation, both TEOA and TEA are neutral and would not interact with the positively charged imidazole ring of histidine.
3.5. Singlet Oxygen Scavenging by Imidazole
When imidazole rather than the amino acid histidine was employed as the 1O2 scavenger in competition studies, we also observed a decrease in H2O2 yield from EDTA (Figure 3A) as imidazole increased. H2O2 yield decreased by 36% from 83 μM (no imidazole) to 53 μM with 1.5 mM imidazole. This differs from Figure 2A, where 1.5 mM histidine decreased H2O2 yield by 60% to 34 μM.
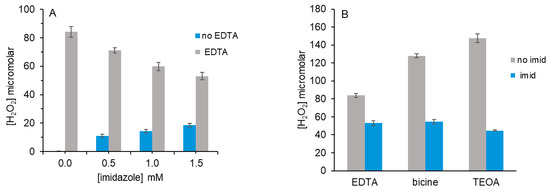
Figure 3.
Effect of imidazole on H2O2 yield from MB, red light and tertiary amines. (A) Reactions (100 μL) contained 5 μM MB, 0–1.5 mM imidazole and 5 mM EDTA or 5 μM MB and imidazole (0–1.5 mM) in 10 mM PB pH 7.4. Samples were irradiated for 5 min. (B) Reactions containing 5 μM MB, 5 mM EDTA, bicine or TEOA and 1.5 mM imidazole in 10 mM PB pH 7.4 were irradiated for 5 min. H2O2 was detected as described in Figure 1. Data in each graph are the average of two independent experiments performed in triplicate.
In the absence of any tertiary amine, the combination of MB, 1.5 mM imidazole and red light produced 18 μM H2O2 (Figure 3A). This was not observed for histidine. The addition of catalase prevented a color change in our HRP assay, confirming that H2O2 formed. If the H2O2 yield from 1.5 mM imidazole alone (no EDTA) were subtracted from the total H2O2 yield of 53 μM (from both imidazole and 5 mM EDTA), it would match that of 1.5 mM histidine and 5 mM EDTA in Figure 2A. There is no method to discern if H2O2 was produced from the reaction of 1O2 with EDTA or with imidazole.
When additional tertiary amines were assayed with 1.5 mM imidazole, we observed decreased H2O2 yield (Figure 3B). Because TEOA and TEA results were identical in Figure 1 and Figure 2, only TEOA is shown in Figure 3B. In addition to TEOA and EDTA, we assayed bicine, which is a hybrid of TEOA and EDTA that has only one negative charge but an amine pKa value of 8.3 (vs. 7.8 for TEOA).
H2O2 yield from the reaction of bicine with 1O2 was greater than EDTA but less than that of TEOA, as predicted from its hybrid structure. The percent decrease in H2O2 yield with 1.5 mM imidazole followed the same trend. For bicine and imidazole, H2O2 decreased by 57% vs. 70% for TEOA and by 36% for EDTA and imidazole. As in Figure 2A, some portion of the H2O2 detected for amine plus imidazole may be attributed to the reaction of imidazole with 1O2.
3.6. Chlorin e6 as Photosensitizer for Singlet Oxygen Production
All data presented in Figure 1, Figure 2 and Figure 3 used 5 μM MB as the photosensitizer. Chlorin e6 is a chlorophyll derivative with three carboxyl groups that will be deprotonated at neutral pH (Scheme 3). It is an effective photosensitizer used in photodynamic therapy to target a wide range of cancer cells [11,12,33]. With its negatively charged carboxylates, it is different from MB, which has one positive charge at neutral pH.
Scheme 3.
Structures of MB and chlorin e6.
In Figure 4A, we tested the effect of the photosensitizer on the reaction of 1O2 with EDTA, TEOA and TEA at multiple pH values. Figure 4A shows that H2O2 yield from the reaction of 1O2 with EDTA did not increase as pH increased from 5 to 8 but rather remained constant (blue bars). This is in stark contrast with Figure 1, where H2O2 from 1O2 and EDTA increased two-fold in that pH range. Further, the absolute H2O2 values are also considerably lower in Figure 4A (15 μM) than in Figure 1 (83 μM) even though we used 15 μM chlorin e6 vs. 5 μM MB in Figure 1, Figure 2 and Figure 3.
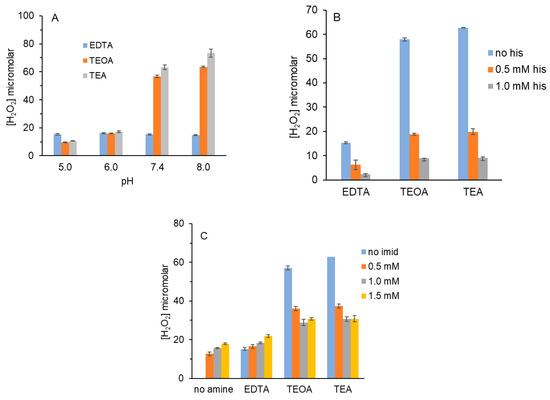
Figure 4.
H2O2 yield by chlorin e6, red light and tertiary amines: pH dependence and effects of histidine and imidazole. (A) Reactions (100 μL) containing 15 μM chlorin e6 and 5 mM tertiary amines in 10 mM PB of varying pH were irradiated for 5 min. (B) Reactions (100 μL) containing 15 μM chlorin e6, 5 mM tertiary amine and 0-1.0 mM histidine in 10 mM PB pH 7.4 were irradiated for 5 min. (C) Reactions (100 μL) containing 15 μM chlorin e6, 5 mM tertiary amine and 0-1.5 mM imidazole in 10 mM PB pH 7.4 were irradiated for 5 min. For all, H2O2 was detected as described in Figure 1. Data in each graph are the average of at least two independent experiments performed in triplicate.
For TEOA and TEA, H2O2 yield increased 6-fold and 7-fold, respectively, as pH increased from 5 to 8. At pH 8, H2O2 yield with 5 mM TEOA, 15 μM chlorin e6 and 5 min of red light was 65 μM vs. 170 μM with 5 μM MB in Figure 1. One report stated that chlorin e6 can form aggregates at or below pH 5 [34].While this may explain the pH-dependent yesincreases for TEOA and TEA above pH 5, it does not explain the lack of pH dependence for EDTA. We attribute the low H2O2 yield to the charge repulsion between EDTA with its four negative charges and chlorin e6 with three negative charges at all pHs tested. For this reason, chlorin e6 is unlikely to generate 1O2 in close proximity to EDTA.
Figure 4B shows that histidine competed with EDTA, TEOA and TEA for 1O2 when chlorin e6 was the photosensitizer, as evidenced by decreased H2O2 yield. The % decrease for TEOA and TEA with 0.5 and 1.0 mM histidine was comparable to that seen in Figure 2A, where MB was the photosensitizer. Because the H2O2 values for EDTA were so low in Figure 4B, 1.0 mM histidine was sufficient to block nearly all H2O2 formation.
By contrast, when imidazole was used as the 1O2 scavenger in competition with EDTA, TEOA and TEA, H2O2 concentrations did not increase to the same extent as with histidine (Figure 4C). Indeed, for EDTA and increasing imidazole, H2O2 yields increased. Figure 4C shows that, in the absence of amine, some H2O2 from imidazole was detected, in agreement with Figure 3A, where MB was the photosensitizer.
Clearly, both histidine and imidazole function as 1O2 scavengers to reduce H2O2 yield in Figure 2 and Figure 3. As a result, they will have been oxidized. To confirm this, we performed a colorimetric assay in which histidine or imidazole reacted with diazotized sulfanilic acid under alkaline conditions to form an azo dye product [28,29]. If the aromaticity of the imidazole ring is disrupted via an oxidation process, no colored product will form. Numerous reports show that both are oxidized to form 2-oxo-histidine and 2-oxo-imidazole.
Samples containing MB and histidine or imidazole with or without EDTA were irradiated and the amount of azo dye product was determined. The yield of colored product from identical dark samples was also measured so that concentrations consumed could be calculated. Supplemental Figures S4 and S5 show that more histidine or imidazole was consumed in the absence of EDTA, as expected. The concentrations of histidine and imidazole that were oxidized are higher than the H2O2 concentrations measured under identical conditions. To disrupt imidazole aromaticity, only one 1O2 is required per imidazole ring [29]. However, to generate one H2O2, two 1O2 are required, according to Scheme 1.
3.7. Tertiary Amine Buffers Generate Hydrogen Peroxide
Multiple common biochemical buffers that are tertiary amines including HEPES, PIPES, MOPS and MES were assayed for their reactivity with 1O2, using MB as the photosensitizer. Their structures are shown in Supplemental Figure S6. H2O2 yields for each in 10 mM PB at pH 7.4 are summarized in Figure 5A and compared with EDTA, bicine and TEOA. All generated H2O2 with nearly identical yields for the two piperazines, HEPES and PIPES. H2O2 yields for MOPS and MES that contain a morpholine ring were the lowest of those tested. HEPES, PIPES, MOPS and MES all contain sulfonic acids that will be deprotonated at neutral pH.
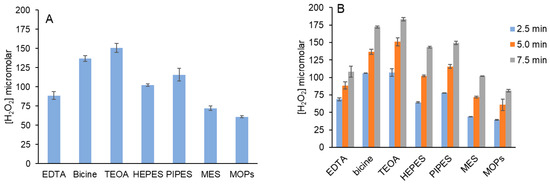
Figure 5.
H2O2 yield by MB, red light and tertiary amine buffers. (A) Reactions (100 μL) contained 5 μM MB and 5 mM tertiary amine in 10 mM PB pH 7.4. Samples were irradiated for 5 min. These data are the average of three independent experiments performed in duplicate. (B) Reactions were prepared as in (A). Irradiation times were 2.5, 5 and 7.5 min. H2O2 was detected as described in Figure 1. Data in each graph are the average of at least two independent experiments performed in triplicate.
Time courses for H2O2 production for all tertiary amines were also performed to assess any variability (Figure 5B). Because the reaction of 1O2 with tertiary amines is dependent on dissolved O2 concentration, H2O2 formation in the first 2.5 min was often greatest, with only modest increases over time for EDTA, bicine and TEOA in Figure 5A. Time courses for HEPES and PIPES are similar, as were those for MES and MOPS.
3.8. Select Tertiary Amines Function as Electron Donors in Photoreduction
Equal concentrations of each tertiary amine in Figure 5A,B were tested as electron donors in a benzoquinone photoreduction assay using MB as the photosensitizer. CoQ0, a more water-soluble benzoquinone that is an analog of Coenzyme Q, was used because we routinely monitor its reduction at 405 nm [16]. Scheme 4 shows reduction in a para-quinone to the quinol form. The combination of MB and EDTA is very efficient, with 172 turnovers (mol CoQ0 reduced per mol MB) (Table 1). Bicine, and to a lesser extent TEOA, functioned as electron donors under the conditions employed, but the others did not.
Scheme 4.
Photochemical redox cycling of quinones to produce H2O2.

Table 1.
Comparison of H2O2 yield and photoreduction turnovers for tertiary amines.
In our prior work, we tested multiple benzoquinones in photoreduction assays using the combination of MB as photosensitizer and EDTA as the electron donor [16,21]. Under ambient O2, photo-oxidation to generate 1O2 was a competing reaction that likely produced some H2O2 via the reaction of EDTA with 1O2 described herein. However, 1O2 also reacts with newly reduced quinols to regenerate oxidized benzoquinones in a photochemical redox cycle (Scheme 4) [35]. The reoxidation of the quinol also generated H2O2 via hydrogen atom abstraction to form RO• and a peroxyl radical that deprotonates to superoxide anion.
As in Scheme 1, superoxide anion disproportionation produces H2O2. When histidine was included, the rate of photoreduction increased, and less H2O2 was generated because 1O2 was scavenged by histidine. In support of photochemical redox cycling as the predominant source of H2O2, we determined that more H2O2 formed during photoreduction reactions with MB, EDTA and benzoquinone than in the absence of benzoquinone, where EDTA was the sole source of H2O2. We speculated that we could use rate enhancements by histidine due to 1O2 scavenging during photoreduction to evaluate which quinols were more susceptible to reoxidation by 1O2 [35,36].
3.9. Histidine Enhances Benzoquinone Photoreduction Rates
Given that this work explores competition between tertiary amines and histidine for 1O2, we examined photoreduction rate enhancement in more detail. Figure 6 shows rates of photoreduction for three benzoquinones alone and with three concentrations of histidine. For all three, as histidine increased, the photoreduction rate increased. Rate enhancement was modest for CoQ0 and methoxyBQ, using 1.0 mM histidine with increases of 24% and 14%, respectively. With methylBQ, the rate enhancement was greater than 100%. These data show that of the three benzoquinones, methylBQ is the easiest to photoreduce because its rate in the absence of histidine was greatest. Further, its quinol is most reactive with 1O2 because of the dramatically increased rate when histidine limited reoxidation by 1O2 [35,36,37].
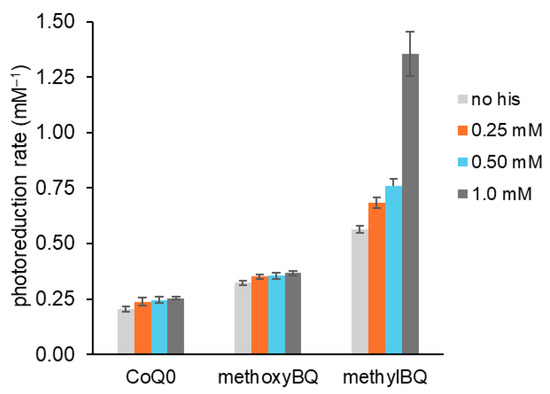
Figure 6.
Effect of histidine on rates of benzoquinone photoreduction Benzoquinones (0.6 mM) were combined with 2 μM MB and 2 mM EDTA (1 mL) in a semimicro cuvette in 10 mM PB pH 7.4. Absorbance scans from 250 to 750 nm were performed prior to light exposure and at time intervals. Histidine or imidazole (up to 1 mM) were added prior to light exposure. First-order rate constants (mM−1) were calculated from plots of ln[quinone] vs. time.
Further, as histidine increased, the yield of H2O2 during photoreduction for all three benzoquinones decreased, which is consistent with 1O2 scavenging. In these assays, we add HRP after photoreduction and use the resulting increase in oxidized benzoquinone to calculate H2O2 concentrations. Reduced quinols are HRP co-substrates. When imidazole rather than histidine was used as the 1O2 scavenger, we observed equivalent increases in photoreduction rates and similar decreases in H2O2.
Although only 2 μM MB and 2 mM EDTA were used for photoreduction (5 min max), H2O2 concentrations were as high as 250 μM, whereas with 5 μM MB and 5 mM EDTA, H2O2 never exceeded 83 μM after 5 min of irradiation (Figure 1). This supports reoxidation of the newly reduced quinol as the major source of H2O2 during photochemical redox cycling, not EDTA oxidation by 1O2.
4. Discussion
The results herein show that the tertiary amines we employ as electron donors in quinone photoreduction experiments produce H2O2 in a pH-dependent manner when they react with 1O2. The pH dependence for EDTA, TEOA and TEA in Figure 1 using MB as a photosensitizer is likely dependent on their chemical structure, polarity and to some extent, on their amine pKa values. The absolute amount of H2O2 formed is higher for the neutral tertiary amines, TEOA and TEA, vs. EDTA, which has four negatively charged carboxylates at all pHs tested. Although MB has a positive charge that would interact with EDTA, electrostatics do not appear to be advantageous because EDTA produced less H2O2 than TEOA or TEA.
H2O2 yields for TEOA and TEA were identical at all pH values tested, despite amine pKa values that differ by three orders of magnitude (Table 1). If amine pKa were the only criterion for reactivity with 1O2, one would expect much lower H2O2 yields for TEA with its pKa of 10.75. However, even at pH 5, a small fraction of deprotonated TEA would be present and once it reacted, equilibrium would shift to generate more.
These data suggest that the interaction between the tertiary amine and 1O2 is also critical to the formation of H2O2. Both O2 and 1O2 are nonpolar and therefore would be expected to have greater affinity for nonpolar TEA rather than TEOA with its three hydroxyl groups. The enhanced interaction between TEA and 1O2 due to their hydrophobicity may compensate for the difference in TEOA and TEA amine pKa values.
When chlorin e6 was used as the photosensitizer in Figure 4, TEOA and TEA were superior to EDTA at pH 7.4 and 8.0 but not at the lower pHs tested. We attribute this to chlorin e6 aggregation at low pH which limits its ability to generate 1O2 regardless of the tertiary amine used [34,38]. 1O2 clearly forms with chlorin e6 at pH 7.4 and 8.0, because we observed that H2O2 formed with TEOA and TEA in Figure 4.
The lack of pH dependence for EDTA with chlorin e6 in Figure 4 vs. MB in Figure 1 is likely due to charge repulsion between EDTA and chlorin e6, because both contain multiple negative charges at all pHs tested in Figure 4. While an attractive interaction between MB and EDTA was deemed inconsequential in Figure 1, the negative charge repulsion between EDTA and chlorin e6 likely plays a role in Figure 4. 1O2 formation requires direct energy transfer from the excited photosensitizer, PS* to O2, followed by rapid reaction of 1O2 with the tertiary amine (Scheme 1).
In Figure 1, we used 5 μM MB vs. 15 μM chlorin e6 in Figure 4 (tertiary amines at 5 mM) because chlorin e6 was less reactive in our photoreduction experiments. We were hesitant to increase chlorin e6 concentrations because chlorophyll-derived photosensitizers can dimerize and require some organic solvent to ensure full solubility and reactivity [16,38].
The ability of histidine to scavenge 1O2 and decrease H2O2 yields is evident in Figure 2A,B. In these competition assays, up to 1.5 mM histidine competed with 5 mM tertiary amine for 1O2. The reported rate constant for histidine and 1O2 is ~107 M−1 s−1, which is two orders of magnitude greater than that of 1O2 and EDTA [20,24,26,27]. For this reason, we expected as little as 0.015 mM histidine to compete with EDTA for 1O2 but it did not. Under our competitive conditions, the relative rate constants must be comparable at pH 7.4 when EDTA is the tertiary amine. With 5 mM TEOA or TEA, only 0.1-0.5 mM histidine competed effectively, suggesting that the rate constants differed by one order of magnitude (Figure 2B).
Our work herein is important, because it shows that the rate constants for 1O2 and different tertiary amines will vary and that the identity of the photosensitizer likely plays a role. The EDTA and 1O2 rate constant that was reported by Hessler et al. used Rose Bengal as the photosensitizer [20]. In that work, rate constants for EDTA and its metal complexes were determined from the quenching of the 1O2 phosphorescence at 1270 nm. Also, in some kinetic studies, reactions are performed in D2O because it extends the half-life of 1O2 [39]. By comparison, we are using the endpoint of H2O2 in H2O to assess competing 1O2 reactions.
Figure 2C shows that the ability of histidine to scavenge 1O2 was pH-dependent when EDTA was the tertiary amine, but not with TEOA and TEA. Our data showing greater histidine reactivity with 1O2 at pH 8.0 vs. 7.4 or 6.4 is consistent with the range of published rate constants for histidine and 1O2. Matheson and Lee reported that the rate constant for histidine and 1O2 varied with pH and identified a pKa of 6.9 [24]. This may be attributed to the protonation state of the imidazole ring of histidine, which reacts with 1O2 via 4 + 2 cycloaddition. Indeed, we saw the greatest increase in histidine scavenging between pH 6.4 and 7.4 (Figure 2C).
Figure 3A showed that imidazole was also effective as a 1O2 scavenger; however, in the absence of tertiary amine, low concentrations of H2O2 formed, which differed from histidine that was assayed at the same concentrations. We observed the same low H2O2 yields with two different commercial sources of imidazole (99% pure); however, the reasons for this remain unclear at this time.
When the contribution of H2O2 from the direct reaction of imidazole with 1O2 was subtracted, the H2O2 decrease with imidazole was comparable to the histidine results in Figure 2A. In Figure 3B, imidazole scavenging was better with bicine and TEOA than with EDTA, which is also consistent with Figure 2B.
As in Figure 3, imidazole results in Figure 4C, where chlorin e6 was used as the photosensitizer, are complicated by the generation of H2O2 in the absence of any tertiary amine. Absolute H2O2 values in Figure 4C are low due to the detrimental combination of chlorin e6 and EDTA. As a result, imidazole did not appear to scavenge H2O2. For this reason, we do not recommend imidazole as a 1O2 scavenger when H2O2 yield is the measured outcome.
The generation of H2O2 by the combination of MB, light and multiple tertiary amines that are frequently used in biochemistry is noteworthy (Figure 5A). Undesired photo-oxidation of HEPEs in cell culture experiments that also contained riboflavin has been reported [40,41]. Sun et al. also reported that HEPEs and several other tertiary amines reacted with 1O2 to produce H2O2 [15]. Their interest in this reaction focused on photodynamic therapies to target antibiotic-resistant bacteria.
To our knowledge, the reactivities of the additional tertiary amines in Figure 5 toward 1O2 using MB as the photosensitizer has not been explored. From a structural perspective, the piperazine-based HEPES and PIPES were more reactive than EDTA and the morpholines, MES and MOPS. None of them functioned as electron donors in a quinone photoreduction assay where EDTA and bicine, and to a lesser extent TEOA, afforded high turnover numbers (moles quinone reduced per mole MB in Table 1). Like EDTA and bicine, they have at least one negative charge at pH 7.4 due to their sulfonic acid groups. Their lack of reactivity in photoreduction is puzzling, because we believe that electrostatic interactions between MB and negatively charged electron donors like EDTA and bicine facilitate electron transfer during photoreduction.
Data in Figure 6 summarizing the effects of histidine on benzoquinone photoreduction provides further insights into the generation of H2O2 during photochemical redox cycling. Consistently with our prior work, we observed more photo-generated H2O2 in a mixture of MB, EDTA and quinone vs. MB and EDTA alone. More importantly, Figure 6 shows that each benzoquinone photoreduction rate is different, with the more easily reduced methylBQ showing the highest rate (in the absence of histidine). The rate of methylBQ photoreduction was also more sensitive to histidine. According to Scheme 4, the quinol of methylBQ must react with 1O2 more readily than the quinols of the other benzoquinones tested. This is consistent with published rate constants for 1O2 and other reduced benzoquinones and naphthoquinones [35,37,42].
Lastly, this work suggests a photochemical method to generate H2O2. Herein, we generated H2O2 using MB, multiple common tertiary amines, mild red light and ambient O2 in neutral aqueous solution. While dissolved O2 will become limiting, this can be rectified by bubbling solutions with air or O2 gas. The current H2O2 industrial process relies on a Pd catalyst, H2 and O2 gases, anthraquinone as a redox cycler and a mixture of organic solvents [43,44]. These tertiary amines and MB are very water soluble and nontoxic, with no organic solvents required. Thus, our research on the photochemical reactions of chlorophyll metabolites and methylene blue may be of value for development of a green method for H2O2 synthesis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/oxygen5040022/s1, Figure S1: H2O2 standard curve with TMB and HRP; Figure S2: histidine reactivity with diazotized sulfanilic acid under alkaline conditions; Figure S3: imidazole reactivity with diazotized sulfanilic acid under alkaline conditions; Figure S4: Photo-oxidation of histidine +/− 5 mM EDTA; Figure S5: Photo-oxidation of imidazole +/− 5 mM EDTA; Figure S6: Structures of tertiary amines used.
Author Contributions
Conceptualization, L.M.L.; methodology, G.S., E.L. and L.M.L.; formal analysis, G.S. and L.M.L.; investigation, G.S., E.L. and L.M.L.; writing—original draft preparation, L.M.L.; writing—review and editing, L.M.L.; supervision, L.M.L.; project administration, L.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Raw data, detailed procedures and all Excel files are available upon request.
Acknowledgments
The authors acknowledge the William & Mary Green Fee for internal support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Bicine | Bis(2-hydroxyethyl)aminoacetic acid |
| CoQ0 | 2,3-dimethoxy-5-methyl-p-benzoquinone |
| DMF | dimethylformamide |
| EDTA | ethylenediaminetetraacetic acid |
| HEPES | 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid |
| His | histidine |
| H2O2 | hydrogen peroxide |
| HRP | horseradish peroxidase |
| imid | imidazole |
| MB | methylene blue |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| methoxyBQ | methoxy benzoquinone |
| methylBQ | methyl benzoquinone |
| NaBH4 | sodium borohydride |
| PB | phosphate buffer |
| PIPES | piperazine-N,N′-bis(2-ethanesulfonic acid) |
| TEA | triethylamine |
| TEOA | triethanolamine |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
References
- Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A.; Pace, A. Photochemically Produced Singlet Oxygen: Applications and Perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet Oxygen: There Is Indeed Something New Under the Sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Foote, C.S.; Wexler, S. Singlet Oxygen. A Probable Intermediate in Photosensitized Autoxidations. J. Am. Chem. Soc. 1964, 86, 3880–3881. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Ferroud, C.; Rool, P.; Santamaria, J. Singlet Oxygen Mediated Alkaloid Tertiary Amines Oxidation by Single Electron Transfer. Tetrahedron Lett. 1998, 39, 9423–9426. [Google Scholar] [CrossRef]
- Scheer, H. Chlorophyll Breakdown in Aquatic Ecosystems. Proc. Natl. Acad. Sci. USA 2012, 109, 17311–17312. [Google Scholar] [CrossRef]
- Havaux, M. Plastoquinone in and Beyond Photosynthesis. Trends Plant Sci. 2020, 25, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A. Singlet Oxygen Production in Photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Annamuradov, B.; Ateyeh, M.; Street, C.; Whipple, H.; Er, A.O. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem 2024, 4, 434–461. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Chapter Nine—Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. In Inorganic Reaction Mechanisms; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 70, pp. 343–394. ISBN 0898-8838. [Google Scholar]
- Kessel, D. Clinical Medicine Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Sun, J.; Peng, W.; Fan, B.; Gan, D.; Li, L.; Liu, P.; Shen, J. Tertiary Amines Convert 1O2 to H2O2 with Enhanced Photodynamic Antibacterial Efficiency. J. Hazard. Mater. 2022, 435, 128948. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Lessard, E.E.; Reed, J.A.; Warsen, M.G.; Zimmer, S.; Landino, L.M. Concurrent Photooxidation and Photoreduction of Catechols and Para-Quinones by Chlorophyll Metabolites. Photochem 2024, 4, 346–360. [Google Scholar] [CrossRef]
- Landino, L.M.; Shuckrow, Z.T.; Mooney, A.S.; Lauderback, C.O.; Lorenzi, K.E. Photo-Oxidation and Photoreduction of Catechols by Chlorophyll Metabolites and Methylene Blue. Chem. Res. Toxicol. 2022, 35, 1851–1862. [Google Scholar] [CrossRef]
- Landino, L.M.; Reed, J.A. Photochemical Redox Cycling of Naphthoquinones Mediated by Methylene Blue and Pheophorbide A. Molecules 2025, 30, 1351. [Google Scholar] [CrossRef]
- Pellegrin, Y.; Odobel, F. Les Donneurs d’électron Sacrificiels Pour La Production de Combustible Solaire. Comptes Rendus Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef]
- Hessler, D.P.; Frimmel, F.H.; Oliveros, E.; Braun, A.M. Solvent Isotope Effect on the Rate Constants of Singlet—Oxygen Quenching by Edta and Its Metal Complexes. Helv. Chim. Acta 1994, 77, 859–868. [Google Scholar] [CrossRef]
- Warsen, M.G.; Zimmer, S.; Phan, K.; Landino, L.M. Photochemical Redox Reactions of 2,6-Dichlorophenolindophenol and Its Use to Detect Photoreduced Quinones. Photochem 2025, 5, 19. [Google Scholar] [CrossRef]
- Hasty, N.; Merkel, P.B.; Radlick, P.; Kearns, D.R. Role of Azide in Singlet Oxygen Reactions: Reaction of Azide with Singlet Oxygen. Tetrahedron Lett. 1972, 13, 49–52. [Google Scholar] [CrossRef]
- Harbour, J.R.; Issler, S.L. Involvement of the Azide Radical in the Quenching of Singlet Oxygen by Azide Anion in Water. J. Am. Chem. Soc. 1982, 104, 903–905. [Google Scholar] [CrossRef]
- Matheson, I.B.C.; Lee, J. Chemical reaction rates of amino acids with singlet oxygen. Photochem. Photobiol. 1979, 29, 879–881. [Google Scholar] [CrossRef]
- Matheson, I.B.C.; Etheridge, R.D.; Kratowich, N.R.; Lee, J. The quenching of singlet oxygen by amino acids and proteins. Photochem. Photobiol. 1975, 21, 165–171. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet Oxygen-Mediated Damage to Proteins and Its Consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Davies, M.J. Reactive Species Formed on Proteins Exposed to Singlet Oxygen. Photochem. Photobiol. Sci. 2004, 3, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Van Balgooy, J.N.A.; Roberts, E. Methods for Study of Imidazole Compounds and Application to Brain and Cancer Cells. Biochem. Pharmacol. 1973, 22, 1405–1415. [Google Scholar] [CrossRef]
- Bernal, F.A.; Orduz-Diaz, L.L.; Guerrero-Perilla, C.; Coy-Barrera, E.D. Diazo Coupling Reaction of Catechins and Alkylresorcinols with Diazotized Sulfanilic Acid for Quantitative Purposes in Edible Sources: Method Development and Validation. Food Anal. Methods 2016, 9, 411–418. [Google Scholar] [CrossRef]
- Lopes, G.R.; Pinto, D.C.G.A.; Silva, A.M.S. Horseradish Peroxidase (HRP) as a Tool in Green Chemistry. RSC Adv. 2014, 4, 37244–37265. [Google Scholar] [CrossRef]
- Conyers, S.M.; Kidwell, D.A. Chromogenic Substrates for Horseradish Peroxidase. Anal. Biochem. 1991, 192, 207–211. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish Peroxidase: A Modern View of a Classic Enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin E6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Čunderlíková, B.; Gangeskar, L.; Moan, J. Acid–Base Properties of Chlorin E6: Relation to Cellular Uptake. J. Photochem. Photobiol. B Biol. 1999, 53, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Morimoto, H.; Kikuchi, S.; Nagaoka, S. Kinetic Study of Free-Radical-Scavenging Action of Biological Hydroquinones (Reduced Forms of Ubiquinone, Vitamin K and Tocopherol Quinone) in Solution. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1993, 1157, 313–317. [Google Scholar] [CrossRef]
- Schwan, S.; Schröder, D.; Wegner, H.A.; Janek, J.; Mollenhauer, D. Substituent Pattern Effects on the Redox Potentials of Quinone-Based Active Materials for Aqueous Redox Flow Batteries. ChemSusChem 2020, 13, 5480–5488. [Google Scholar] [CrossRef]
- Huynh, M.T.; Anson, C.W.; Cavell, A.C.; Stahl, S.S.; Hammes-Schiffer, S. Quinone 1 e− and 2 e−/2 H+ Reduction Potentials: Identification and Analysis of Deviations from Systematic Scaling Relationships. J. Am. Chem. Soc. 2016, 138, 15903–15910. [Google Scholar] [CrossRef]
- Eichwurzel, I.; Stiel, H.; Röder, B. Photophysical Studies of the Pheophorbide a Dimer. J. Photochem. Photobiol. B Biol. 2000, 54, 194–200. [Google Scholar] [CrossRef]
- Merkel, P.B.; Nilsson, R.; Kearns, D.R. Deuterium Effects on Singlet Oxygen Lifetimes in Solutions. New Test of Singlet Oxygen Reactions. J. Am. Chem. Soc. 1972, 94, 1030–1031. [Google Scholar] [CrossRef]
- Zigler, J.S.; Lepe-Zuniga, J.L.; Vistica, B.; Gery, I. Analysis of the Cytotoxic Effects of Light-Exposed Hepes-Containing Culture Medium. In Vitro Cell. Dev. Biol. 1985, 21, 282–287. [Google Scholar] [CrossRef]
- Lepe-Zuniga, J.L.; Zigler, J.S.; Gery, I. Toxicity of Light-Exposed Hepes Media. J. Immunol. Methods 1987, 103, 145. [Google Scholar] [CrossRef]
- Elhajj, S.; Gozem, S. First and Second Reductions in an Aprotic Solvent: Comparing Computational and Experimental One-Electron Reduction Potentials for 345 Quinones. J. Chem. Theory Comput. 2024, 20, 6227–6240. [Google Scholar] [CrossRef] [PubMed]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zeng, X.; Zhang, X. Production of Hydrogen Peroxide by Photocatalytic Processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).