The Effects of Photodynamic Therapy on Genetically Determined Glioma Syndromes Associated with an Increased Risk of Disease
Abstract
1. Introduction
2. Gene Expression Analysis
2.1. Li–Fraumeni Band Sydrome (LFS)
2.2. Lynch Syndrome (LS)
3. Selected Neurocutaneous Diseases
3.1. Tuberous Sclerosis Syndrome (TSC) and Gliomas in TSC
3.2. Neurofibromatosis Type 1 (NF1) and Gliomas in NF1
3.3. Neurofibromatosis Type 2 (NF2) and Gliomas in NF2
3.4. Von Hippel–Lindau Syndrome (VHL)
4. Glioma Treatment
5. Photodynamic Therapy
6. The Effect of PDT on Gene Expression in Cells
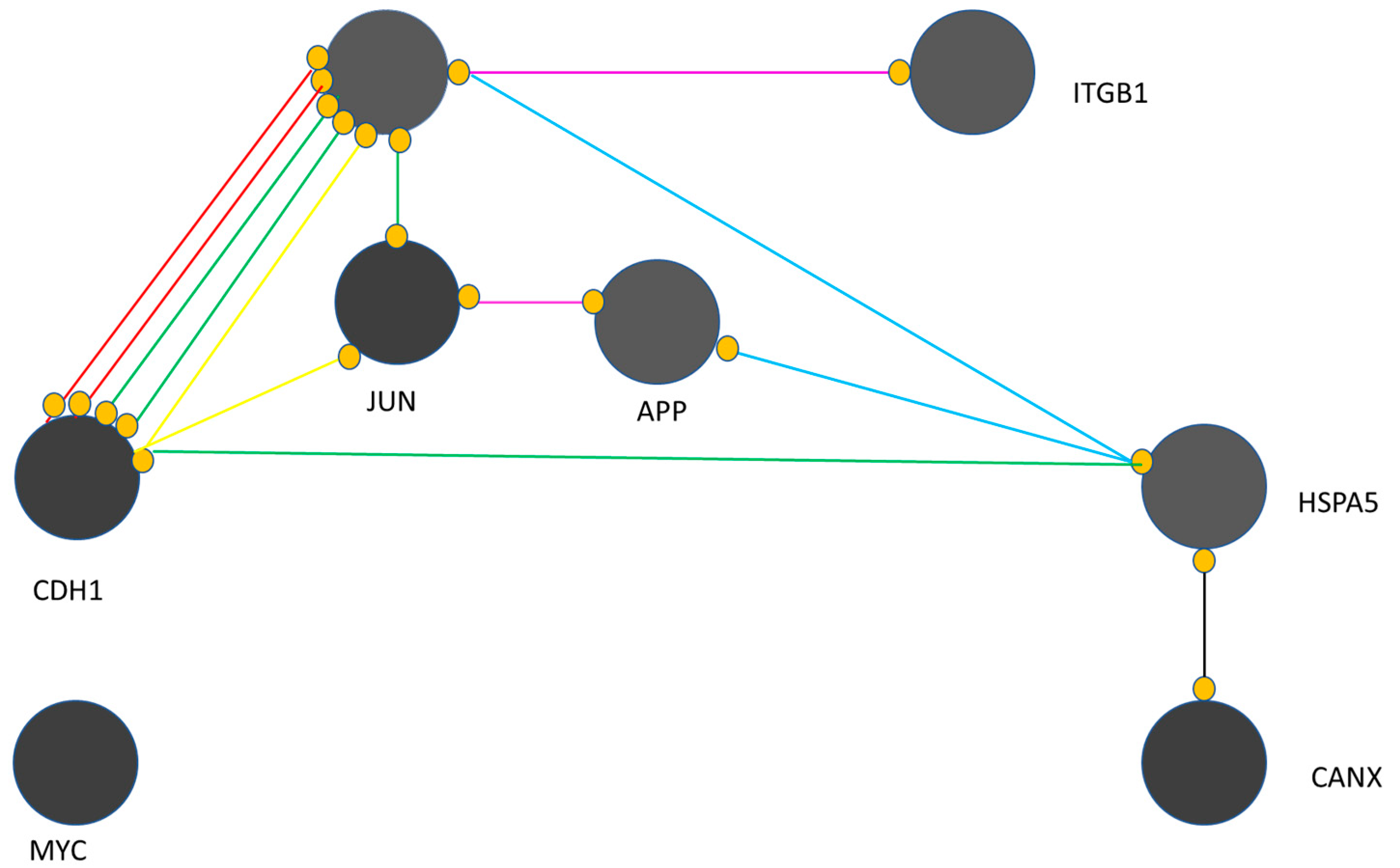
- EGFR is critical in the presented map;
- MYC remains isolated and has no interaction with other genes, i.e., CANX, CDH1, JUN, APP, HSPA5, EGFR and ITGB1;
- CANX is associated with HSPA5 only through reaction activity;
- ITGB1 is linked to EGFR through catalytic activity;
- HSPA5 has binding connections with APP and EGFR;
- HSPA5 activates CDH1;
- APP and JUN have an association with post-translational modifications;
- EGFR and CDH1 have complex connections.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schena, M.; Shalon, D.; Heller, R.; Chai, A.; Brown, P.O.; Davis, R.W. Parallel human genome analysis: Microarray-based expression monitoring of 1000 genes. Proc. Natl. Acad. Sci. USA 1996, 93, 10614–10619. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Powers, S.K.; Witmer, P.; Brown, T. Optimal light dose for interstitial photodynamic therapy in treatment for malignant brain tumors. Lasers Surg. Med. 2000, 27, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Shete, S.; Hosking, F.J.; Robertson, L.B.; Dobbins, S.E.; Sanson, M.; Malmer, B.; Simon, M.; Yannick, M.; Blandine, B.; Houlston, R.S.; et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat. Genet. 2009, 41, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, M.; Jenkins, R.B.; Chang, J.S.; Yeh, R.-F.; Xiao, Y.; Decker, P.A.; Ballman, K.V.; Berger, M.; Buckner, J.C.; Chang, S.; et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat. Genet. 2009, 41, 905–908. [Google Scholar] [CrossRef]
- Malmer, B.; Feychting, M.; Lönn, S.; Ahlbom, A.; Henriksson, R. p53 genotypes and risk of glioma and meningioma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2220–2223. [Google Scholar] [CrossRef]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef]
- Vizcaino, M.A.; Palsgrove, D.N.; Yuan, M.; Giannini, C.; Cabrera-Aldana, E.E.; Pallavajjala, A.; Burger, P.C.; Rodriguez, F.J. Granular cell astrocytoma: An aggressive IDH-wildtype diffuse glioma with molecular genetic features of primary glioblastoma. Brain Pathol. 2019, 29, 193–204. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Genetic Abnormalities, Clonal Evolution, and Cancer Stem Cells of Brain Tumors. Med. Sci. 2018, 6, 85. [Google Scholar] [CrossRef]
- Kober, P.; Rymuza, J.; Baluszek, S.; Maksymowicz, M.; Nyc, A.; Mossakowska, B.J.; Zieliński, G.; Kunicki, J.; Bujko, M. DNA Methylation Pattern in Somatotroph Pituitary Neuroendocrine Tumors. Neuroendocrinology 2024, 114, 51–63. [Google Scholar] [CrossRef]
- Balss, J.; Meyer, J.; Mueller, W.; Korshunov, A.; Hartmann, C.; von Deimling, A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008, 116, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Hänsel, M.; Hamel, W.; Kunzmann, R.; Hölzel, F. Karyotype analyses of 20 human glioma cell lines. Acta Neurochir. 1994, 126, 17–26. [Google Scholar] [CrossRef]
- Fritz, G.; Tano, K.; Mitra, S.; Kaina, B. Inducibility of the DNA repair gene encoding O6-methylguanine-DNA methyltransferase in mammalian cells by DNA-damaging treatments. Mol. Cell Biol. 1991, 11, 4660–4668. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Francis, S.S.; Barnholtz-Sloan, J.S. Epidemiology of Brain and Other CNS Tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22 (Suppl. S2), iv1–iv96, Erratum in Neuro Oncol. 2022, 24, 1214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leung, S.Y.; Chan, T.L.; Chung, L.P.; Chan, A.S.; Fan, Y.W.; Hung, K.N.; Kwong, W.K.; Ho, J.W.; Yuen, S.T. Microsatellite instability and mutation of DNA mismatch repair genes in gliomas. Am. J. Pathol. 1998, 153, 1181–1188. [Google Scholar] [CrossRef]
- Melin, B.; Dahlin, A.M.; Andersson, U.; Wang, Z.; Henriksson, R.; Hallmans, G.; Bondy, M.L.; Johansen, C.; Feychting, M.; Ahlbom, A.; et al. Known glioma risk loci are associated with glioma with a family history of brain tumours—A case-control gene association study. Int. J. Cancer. 2013, 132, 2464–2468. [Google Scholar] [CrossRef]
- Stahl, J. Mismatch repair proteins and microsatellites hit clinical practice. Adv. Anat. Pathol. 2000, 7, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Panek, P.; Jezela-Stanek, A. Genetyczne i molekularne podłoża rozwoju glejaka. Postępy Biochem. 2023, 69, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Kamiya-Matsuoka, C.; Slopis, J.M.; McCutcheon, I.E.; Majd, N.K. Therapeutic Strategies for Gliomas Associated With Cancer Predisposition Syndromes. JCO Precis. Oncol. 2024, 8, e2300442. [Google Scholar] [CrossRef] [PubMed]
- Wańkowicz, P.; Przemysław, N. Glioblastoma Multiforme–the Progress of Knowledge on the Pathogenesis of C. Pomeranian J. Life Sci. 2014, 60, 40–43. [Google Scholar]
- Gargallo, P.; Yáñez, Y.; Segura, V.; Juan, A.; Torres, B.; Balaguer, J.; Oltra, S.; Castel, V.; Cañete, A. Li-Fraumeni syndrome heterogeneity. Clin. Transl. Oncol. 2020, 22, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Zelley, K.; Nichols, K.E.; Garber, J. Li-Fraumeni Syndrome. In GeneReviews®; 1999 January 19 [updated 2019 November, 21]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1999. [Google Scholar] [PubMed]
- Guidi, M.; Giunti, L.; Lucchesi, M.; Scoccianti, S.; Giglio, S.; Favre, C.; Oliveri, G.; Sardi, I. Brain tumors in Li-Fraumeni syndrome: A commentary and a case of a gliosarcoma patient. Future Oncol. 2017, 13, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Boisselier, B.; Sanson, M.; Crinière, E.; Liva, S.; Marie, Y.; Carpentier, C.; Paris, S.; Laigle-Donadey, F.; Mokhtari, K.; et al. Tumor genomic profiling and TP53 germline mutation analysis of first-degree relative familial gliomas. Cancer Genet. Cytogenet. 2007, 176, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kaneda, M.; Futagawa, M.; Takeshita, M.; Kim, S.; Nakama, M.; Kawashita, N.; Tatsumi-Miyajima, J. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int. J. Clin. Oncol. 2019, 24, 999–1011, Erratum in Int. J. Clin. Oncol. 2019, 24, 1012. [Google Scholar] [CrossRef] [PubMed]
- Büttner, R.; Friedrichs, N. Erblicher Darmkrebs bei Lynch-/HNPCC-Syndrom in Deutschland [Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany]. Pathologe 2019, 40, 584–591. (In German) [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Zub, W.L.; Waliszewska-Prosół, M.; Bladowska, J.; Obara, K.; Ejma, M. Wieloletnie przeżycie chorych z glejakiem wielopostaciowym—Opisy przypadków. Pol. Przegląd Neurol. 2016, 12, 107–115. [Google Scholar]
- Alnahhas, I.; Rayi, A.; Ong, S.; Giglio, P.; Puduvalli, V. Management of gliomas in patients with Lynch syndrome. Neuro Oncol. 2021, 23, 167–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maratt, J.K.; Stoffel, E. Identification of Lynch Syndrome. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Kastrinos, F.; Stoffel, E.M. History, genetics, and strategies for cancer prevention in Lynch syndrome. Clin. Gastroenterol. Hepatol. 2014, 12, 715–727, quiz e41–e43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pellat, A.; Netter, J.; Perkins, G.; Cohen, R.; Coulet, F.; Parc, Y.; Svrcek, M.; Duval, A.; André, T. Syndrome de Lynch: Quoi de neuf? [Lynch syndrome: What is new?]. Bull. Cancer. 2019, 106, 647–655. (In French) [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, C.; Ladelund, S.; Rambech, E.; Persson, A.; Petersen, A.; Nilbert, M. Glioblastomas, astrocytomas and oligodendrogliomas linked to Lynch syndrome. Eur. J. Neurol. 2015, 22, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Peltomäki, P.; Nyström, M.; Mecklin, J.P.; Seppälä, T.T. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology 2023, 164, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; McHugh, T.W. Lynch Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Kampitsi, C.E.; Nordgren, A.; Mogensen, H.; Pontén, E.; Feychting, M.; Tettamanti, G. Neurocutaneous Syndromes, Perinatal Factors, and the Risk of Childhood Cancer in Sweden. JAMA Netw. Open. 2023, 6, e2325482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ullrich, N.J. Neurocutaneous Syndromes and Brain Tumors. J. Child. Neurol. 2016, 31, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.P. Tuberous Sclerosis Complex. Semin. Pediatr. Neurol. 2021, 37, 100875. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int. J. Mol. Sci. 2021, 22, 5850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirbe, A.C.; Gutmann, D.H. Neurofibromatosis type 1: A multidisciplinary approach to care. Lancet Neurol. 2014, 13, 834–843. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwaarde, R.S.; Ahmad, S.; van Nesselrooij, B.; Zandee, W.; Giles, R.H. Von Hippel-Lindau Syndrome. In GeneReviews®; 2000; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2024. [Google Scholar] [PubMed]
- Myong, N.H.; Park, B.J. Malignant glioma arising at the site of an excised cerebellar hemangioblastoma after irradiation in a von Hippel-Lindau disease patient. Yonsei Med. J. 2009, 50, 576–581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaish, M.; Kumar, R.; Mittal, R.D.; Mittal, B. Evaluation of microsatellite instability in tumors of central nervous system: A pilot study. Indian J. Clin. Biochem. 2004, 19, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xu, L.; Geng, M.; Hu, S. Expression and Clinical Significance of Lymphocyte Subpopulations and Peripheral Inflammatory Markers in Glioma. J. Inflamm. Res. 2024, 17, 9423–9451. [Google Scholar] [CrossRef]
- Aldape, K.; Simmons, M.L.; Davis, R.L.; Miike, R.; Wiencke, J.; Barger, G.; Lee, M.; Chen, P.; Wrensch, M. Discrepancies in diagnoses of neuroepithelial neoplasms: The San Francisco Bay Area Adult Glioma Study. Cancer 2000, 88, 2342–2349. [Google Scholar] [CrossRef]
- Karak, A.K.; Singh, R.; Tandon, P.N.; Sarkar, C. A comparative survival evaluation and assessment of interclassification concordance in adult supratentorial astrocytic tumors. Pathol. Oncol. Res. 2000, 6, 46–52. [Google Scholar] [CrossRef]
- Stummer, W.; Stepp, H.; Möller, G.; Ehrhardt, A.; Leonhard, M.; Reulen, H.J. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir. 1998, 140, 995–1000. [Google Scholar] [CrossRef]
- Muller, P.J.; Wilson, B.C. Photodynamic therapy of brain tumors—A work in progress. Lasers Surg. Med. 2006, 38, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Verwanger, T.; Sanovic, R.; Aberger, F.; Frischauf, A.M.; Krammer, B. Gene expression pattern following photodynamic treatment of the carcinoma cell line A-431 analysed by cDNA arrays. Int. J. Oncol. 2002, 21, 1353–1359. [Google Scholar] [CrossRef]
- Wild, P.J.; Krieg, R.C.; Seidl, J.; Stoehr, R.; Reher, K.; Hofmann, C.; Louhelainen, J.; Rosenthal, A.; Hartmann, A.; Pilarsky, C.; et al. RNA expression profiling of normal and tumor cells following photodynamic therapy with 5-aminolevulinic acid-induced protoporphyrin IX in vitro. Mol. Cancer Ther. 2005, 4, 516–528. [Google Scholar] [CrossRef]
- Hill, M.S.; Vande Zande, P.; Wittkopp, P.J. Molecular and evolutionary processes generating variation in gene expression. Nat. Rev. Genet. 2021, 22, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy and the development of metal-based photosensitisers. Met. Based Drugs. 2008, 2008, 276109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer-Betz, F. Untersuchung uber sie biologishe (potodynamische) wirkung des hematophorphyrins and under derivative des blut und gallenharstofs. Dtsch. Arch. Klin. Med. 1913, 112, 476–503. [Google Scholar]
- Javed, Z.; Aziz, H.F.; Shamim, M.S. Photodynamic Therapy in Adult Intra-axial Brain Tumours. J. Pak. Med. Assoc. 2024, 74, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aebisher, D.; Przygórzewska, A.; Myśliwiec, A.; Dynarowicz, K.; Krupka-Olek, M.; Bożek, A.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Current Photodynamic Therapy for Glioma Treatment: An Update. Biomedicines 2024, 12, 375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers 2023, 15, 3427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Hsia, T.; Small, J.L.; Yekula, A.; Batool, S.M.; Escobedo, A.K.; Ekanayake, E.; You, D.G.; Lee, H.; Carter, B.S.; Balaj, L. Systematic Review of Photodynamic Therapy in Gliomas. Cancers 2023, 15, 3918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska-Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors-A Review of the Literature. Molecules 2022, 27, 6847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwartz, C.; Stepp, H.; Rühm, A.; Tonn, J.C.; Kreth, S.; Kreth, F.W. Interstitial photodynamic therapy for de-novo glioblastoma multiforme WHO IV: A feasibility study. In Proceedings of the 66th Annual Meeting of the German Society of Neurosurgery (DGNC), Karlsruhe, Germany, 7–10 June 2015; pp. 7–10. [Google Scholar]
- Walsh, K.M.; Ohgaki, H.; Wrensch, M.R. Epidemiology. Handb. Clin. Neurol. 2016, 134, 3–18. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of gliomas. In Current Understanding and Treatment of Gliomas; Cancer Treatment and Research; Springer: Cham, Switzerland, 2015; Volume 163, pp. 1–14. [Google Scholar] [CrossRef]
- Huang, S.; Sun, C.; Hou, Y.; Tang, Y.; Zhu, Z.; Zhang, Z.; Zhang, Y.; Wang, L.; Zhao, Q.; Chen, M.-G.; et al. A comprehensive bioinformatics analysis on multiple Gene Expression Omnibus datasets of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Sci. Rep. 2018, 8, 7630. [Google Scholar] [CrossRef]
- Cho, S.B. Estimation of gene regulatory networks from cancer transcriptomics data. Processes 2021, 9, 1758. [Google Scholar] [CrossRef]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59 (Suppl. S2), S21–S26. [Google Scholar] [CrossRef]
- Sanovic, R.; Krammer, B.; Grumboeck, S.; Verwanger, T. Time-resolved gene expression profiling of human squamous cell carcinoma cells during the apoptosis process induced by photodynamic treatment with hypericin. Int. J. Oncol. 2009, 35, 921–939. [Google Scholar] [CrossRef]
- Gupta, N.; Martin, P.M.; Miyauchi, S.; Ananth, S.; Herdman, A.V.; Martindale, R.G.; Podolsky, R.; Ganapathy, V. Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer. Biochem. Biophys. Res. Commun. 2006, 343, 571–577. [Google Scholar] [CrossRef]
- Liu, W.; Baer, M.R.; Bowman, M.J.; Pera, P.; Zheng, X.; Morgan, J.; Pandey, R.A.; Oseroff, A.R. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin. Cancer Res. 2007, 13, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Steadman, K.; Polgar, O.; Bates, S.E. ABCG2-mediated transport of photosensitizers: Potential impact on photodynamic therapy. Cancer Biol. Ther. 2005, 4, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Singh, G.; Rainbow, A.J. The role of the p53 tumor suppressor in the response of human cells to photofrin-mediated photodynamic therapy. Photochem. Photobiol. 2000, 71, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, V.A.; Hornung, R.; Fedier, A.; Fehr, M.K.; Walt, H.; Haller, U.; Fink, D. Photodynamic therapy of DNA mismatch repair-deficient and -proficient tumour cells. Br. J. Cancer. 2002, 86, 1130–1135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, M.; He, S.; Chen, P.; Li, Q.; Zhu, M.; Guo, J.; Jiang, L.; Wang, Q.; Peng, X.; Li, S.; et al. Photodynamic therapy in a patient with facial angiofibromas due to tuberous sclerosis complex. Photodiagnosis Photodyn. Ther. 2019, 28, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, Y.; Huang, X.; Zhang, L.; Peng, D.; Zhang, G. The combination of photodynamic therapy and ultrapulse carbon dioxide laser for facial angiofibromas in tuberous sclerosis complex: A case report. Photodiagnosis Photodyn. Ther. 2022, 37, 102725. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, S.; Tommila, P.; Palosaari, T.; Kivelä, T. Photodynamic therapy with verteporfin to induce regression of aggressive retinal astrocytomas. Acta Ophthalmol. 2008, 86, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Quirk, B.; Olasz, E.; Kumar, S.; Basel, D.; Whelan, H. Photodynamic Therapy for Benign Cutaneous Neurofibromas Using Aminolevulinic Acid Topical Application and 633 nm Red Light Illumination. Photobiomodul Photomed. Laser Surg. 2021, 39, 411–417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muir, D.; Perrin, G.; Neubauer, D.; Whelan, H. Photodynamic therapy development using NF1 tumor xenografts. In Proceedings of the Children’s Tumor Foundation Conference, Bonita Springs, FL, USA, 6–10 June 2008. [Google Scholar]
- Di Nicola, M.; Williams, B.K., Jr.; Hua, J.; Bekerman, V.P.; Mashayekhi, A.; Shields, J.A.; Shields, C.L. Photodynamic Therapy for Retinal Hemangioblastoma: Treatment Outcomes of 17 Consecutive Patients. Ophthalmol. Retina. 2022, 6, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Gomer, C. Isolation and initial characterization of mouse tumor cells resistant to porphyrin-mediated photodynamic therapy. Cancer Res. 1991, 51, 4243–4249. [Google Scholar]
- Gomer, C.; Ryter, S.; Ferrario, A.; Rucker, N.; Wong, S.; Fisher, A. Photodynamic Therapy-mediated oxidative stress can induce the expression of heat shock proteins. Cancer Res. 1996, 56, 2355–2360. [Google Scholar] [PubMed]
- Ruhdorfer, S.; Sanovic, R.; Sander, V.; Krammer, B.; Verwanger, T. Gene expression profiling of the human carcinoma cell line A-431 after 5-aminolevulinic acid-based photodynamic treatment. Int. J. Oncol. 2007, 30, 1253–1262. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inglot, J.; Strzelczyk, J.K.; Tylutki, J.; Bartusik-Aebisher, D.; Aebisher, D. The Effects of Photodynamic Therapy on Genetically Determined Glioma Syndromes Associated with an Increased Risk of Disease. Oxygen 2025, 5, 2. https://doi.org/10.3390/oxygen5010002
Inglot J, Strzelczyk JK, Tylutki J, Bartusik-Aebisher D, Aebisher D. The Effects of Photodynamic Therapy on Genetically Determined Glioma Syndromes Associated with an Increased Risk of Disease. Oxygen. 2025; 5(1):2. https://doi.org/10.3390/oxygen5010002
Chicago/Turabian StyleInglot, Jadwiga, Joanna Katarzyna Strzelczyk, Jakub Tylutki, Dorota Bartusik-Aebisher, and David Aebisher. 2025. "The Effects of Photodynamic Therapy on Genetically Determined Glioma Syndromes Associated with an Increased Risk of Disease" Oxygen 5, no. 1: 2. https://doi.org/10.3390/oxygen5010002
APA StyleInglot, J., Strzelczyk, J. K., Tylutki, J., Bartusik-Aebisher, D., & Aebisher, D. (2025). The Effects of Photodynamic Therapy on Genetically Determined Glioma Syndromes Associated with an Increased Risk of Disease. Oxygen, 5(1), 2. https://doi.org/10.3390/oxygen5010002








