Abstract
Mitochondrial dysfunction significantly contributes to the pathogenesis and progression of chronic obstructive pulmonary disease (COPD). To treat mitochondrial dysfunction in COPD, novel drug delivery systems (DDS) are needed. In this review, we provide a brief overview of the current understanding of the factors in COPD and highlight the trends in novel nanocarriers/nanoparticles for targeting mitochondrial dysfunction. These drug-encapsulated nanoparticles are still in the early stages of clinical application but represent the most promising system for COPD therapy.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common lung disease and a general term for progressive diseases of the respiratory system that comprise chronic bronchitis and emphysema. COPD is characterized by chronic inflammation, progressive airway constriction, and alveolar destruction, resulting in symptoms such as persistent cough, shortness of breath, and increased mucus production [1]. The symptoms of this incurable lung disease are thought to be mainly caused by inflammation of bronchial/airway epithelial cells, impaired lung fibroblasts, and alveolar destruction [2]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has recently proposed a new definition of COPD as a “heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration, exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction” [2]. While cigarette smoking is the primary cause of COPD, prolonged exposure to harmful environmental pollutants, including dust, fumes, chemicals, air pollution (such as PM2.5 and toxic gases), and indoor smoke from coal or biomass fuels can also contribute to the onset of the disease [1]. The World Health Organization reported over 320 million COPD patients in 2020, and the disease is also attributed to over 3 million annual deaths, the third leading cause of death globally [3].
Despite the worldwide prevalence of COPD, the molecular processes underlying the disease still remain elusive [4]. Therefore, current treatment methods for COPD are limited to symptomatic therapy. Since COPD is progressive, the treatment approach depends on severity. Bronchodilators (β2 agonists, anticholinergics, and theophylline) are commonly used for stable COPD patients. For more severe cases, combination therapy of long-acting β2 agonists (LABA) or long-acting muscarinic antagonists (LAMA) with inhaled corticosteroids (ICS) is the standard treatment for COPD. Triple therapy (LABA/LAMA/ICS) might also be considered an option for certain patients. These medications are generally administrated by inhalation [5].
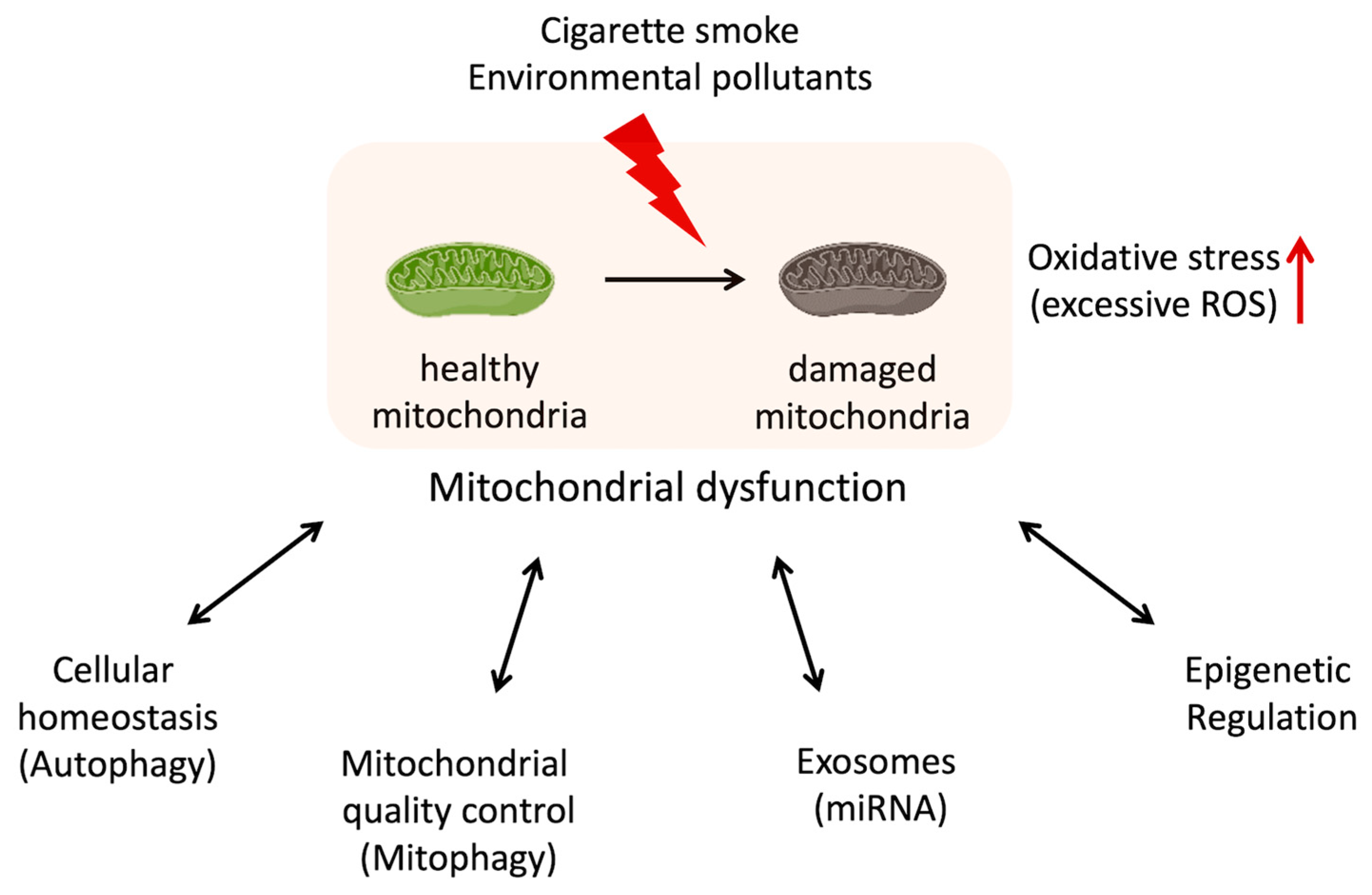
Mitochondria are known as “the powerhouse of the cell” because of their main role in the energy production of ATP synthesis via oxidative phosphorylation. But mitochondria are not just the powerhouse of the cell. In addition to energy production, mitochondria execute many other crucial functions such as regulation of cellular metabolism, nucleotide-synthesizing, calcium-buffering, and control of cell cycle and cell death [6]. The decline or dysregulation of these functions of mitochondria is leading to the overproduction of ROS, loss of ATP and metabolite production, disruption of calcium homeostasis, and senescence induction [6]. The accumulation of dysfunctional mitochondria in cells is associated with metabolic disorders [7,8], signal transduction abnormalities [9], inflammation [10], and malfunction not only at the cellular level but also in tissues, organs, and the entire organism. In recent years, the relationship between mitochondrial dysfunction and many diseases, such as cancer, diabetes, and neurodegenerative diseases, has been well-recognized [6,11,12]. Although the exact mechanisms are still being studied, recent studies suggest that mitochondrial dysfunction contributes to the development and progression of COPD [13,14]. Oxidative stress, inflammation, apoptosis, cellular senescence, skeletal muscle dysfunction, and autophagy/mitophagy dysfunction might be related to both mitochondrial dysfunction and COPD (Table 1). These connections are being explored, but the relationship is complex and not fully understood [15]. Researchers continue to investigate the molecular pathways and mechanisms underlying this relationship in order to better understand how mitochondrial dysfunction contributes to COPD pathogenesis (Figure 1).

Table 1.
Potential relationship between mitochondrial dysfunction and COPD.
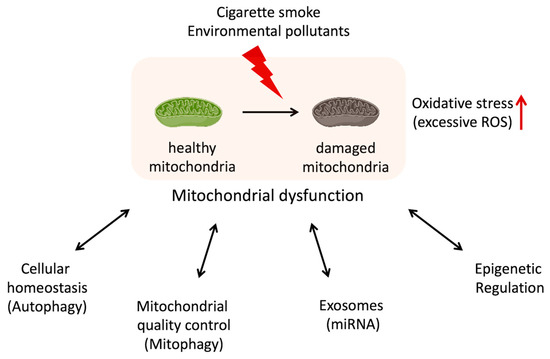
Figure 1.
Potential relationship between mitochondrial dysfunction and dysregulation in the cellular function with COPD.
Here, we provide a current overview of how mitochondrial dysfunctions impinge on COPD. Additionally, this review highlights a nanoparticle-based drug delivery system (DDS) for COPD therapy.
2. Mitochondrial Dysfunction in COPD
2.1. Oxidative Stress/Reactive Oxygen Species (ROS)
Oxidative stress has been recognized as a major risk factor for COPD pathogenesis and a target for COPD treatments. Exogenous ROS, such as cigarette smoke or harmful stimuli, and endogenous ROS from activated inflammatory cells or dysfunctional mitochondria may affect signaling pathways and antioxidant defenses, leading to damage and death of epithelial cells in the airway and lung, which may contribute to the development and progression of COPD [16].
Currently, there is accumulating evidence of mitochondrial dysfunction and overproduction of mitochondrial ROS in the lung/pulmonary epithelial cells of COPD patients [17,18,30]. Cigarette smoke contains 1015–1017 ROS/puff [31], responsible for mitochondrial DNA (mtDNA) damage and mitochondrial ROS generation. Oxidative stress-induced mitochondrial ROS activate the immune response in lung tissue, resulting in the production of inflammatory cytokines and secretion of pro-inflammatory interleukins [19]. Long-term cigarette smoke exposure leads to changes in mitochondrial structure and function in bronchial epithelial cells [32], resulting in extensive apoptosis [20,21] and cellular senescence [22,23].
Mitochondrial ROS and dysfunction of mitochondria contribute to the reduction in skeletal muscle strength and exercise endurance in COPD patients [24]. Skeletal muscle cells in COPD patients are characterized by increased mitochondrial ROS, decreased mitochondrial biogenesis and ATP production, and impaired mitochondrial respiratory chain complexes, possibly associated with mitochondrial apoptosis [25].
2.2. Autophagy and Mitophagy
Autophagy and mitophagy are cellular processes for maintaining cellular health and functions and preventing the accumulation of dysfunctional cellular components. Autophagy degrades damaged or abnormal cellular components to maintain cellular homeostasis. Mitophagy is a specific form of autophagy that selectivity removes impaired mitochondria.
Accumulating evidence has demonstrated the activation of autophagy in the lung tissue of human COPD patients and model mice [26]. For example, cigarette smoke extract-exposed mice lung tissues showed a remarkable elevation of the LC3-II/LC3-I ratio [33]. Autophagy activation protects the lung by decreasing cellular senescence [27]. However, autophagy exerts both protective and destructive effects in COPD [28,29]. Activated autophagy increases inflammatory cytokines such as TNF-α, IL-6, and IL-8, leading to aggravating pathological processes of COPD and affecting prognosis [33]. Therefore, whether autophagy activation protects the lung or aggravates COPD needs further elucidation.
Mitophagy is generally thought to control the quality of mitochondria by eliminating dysfunctional mitochondria [34]. However, in COPD patients, cigarette smoke extract impairs Parkin-dependent mitophagy by the accumulation of cytoplasmic p53, which inhibits Parkin recruitment to damaged mitochondria [35]. Impaired mitophagy and accumulation of dysfunctional mitochondria affect the activity of epithelial cells in the lung and promote COPD. In addition, cigarette smoke exposure-induced mitophagy regulates RIP1/RIP3 kinase-depended necroptosis, a programmed cell death in necrosis. In the lungs of COPD patients, the expression of mitophagy-associated molecule PINK1 and necroptosis-associated molecule RIP3 is enhanced compared to that of healthy individuals, suggesting that mitophagy is associated with necroptosis induction involved in the pathogenesis of COPD [36].
Understanding these mechanisms could potentially lead to the development of targeted therapies to restore or enhance autophagy and mitophagy processes. However, further research is needed to fully elucidate the precise roles and interactions of autophagy/mitophagy in COPD.
3. Mitochondrial Transfer
Transfer of healthy mitochondria can restore damaged cells and maintain tissue homeostasis. For example, intercellular mitochondrial transfer from mesenchymal stem cells (MSCs) to lung structural cells reduces mitochondrial damage due to oxidative stress [37]. Therefore, intercellular mitochondrial transfer and artificial mitochondrial transfer have been expected for novel therapeutic approaches for COPD [38,39].
Agrawal et al. demonstrated that levels of mitochondrial Rho-GTPase (Miro1) in MSCs regulate intercellular mitochondrial transfer. Mitochondrial dysfunction in bronchial epithelial cells treated with a pro-inflammatory supernatant of IL-13-induced macrophages was reversed by the donation of mitochondria from Miro1-overexpressed MSCs. This result suggests that amplification of mitochondrial transfer may increase the efficacy of MSCs to repair cell functions of damaged cells in COPD [40].
A recent report demonstrates that airway smooth muscle cells (ASMCs) also exchange mitochondria by extracellular vesicles (EVs) under the stress of cigarette smoke. The authors also found autophagy-related proteins in the EVs, suggesting that the transferred damaged mitochondria were delivered for outsourced degradation referred to as “transmitophagy” owing to regulation bioenergetics and cellular function in airways. The results are particularly intriguing since they suggest novel homeostatic mechanisms in lung stromal cells and a new potential treatment modality for COPD [41].
4. Relationship between Epigenetic Dysregulation and Mitochondrial Dysfunction
Epigenetics refers to the regulation of gene expression without any alterations in DNA sequence but rather by changes to DNA methylation (methylation of cytosines in the promotor region of genes) or histone modification (acetylation or methylation of lysine in histone tails). These epigenetic modifications influence how genes turn-on or turn-off, thereby affecting the activity of specific genes and corresponding proteins [42].
Several studies have suggested a connection between epigenetic changes and the development and progression of COPD. For example, cigarette smoke and air pollution can induce epigenetic changes [43,44]. Liu et al. reported that chronic exposure of cigarette smoke condensate to human small airway epithelial cells and bronchial epithelial cells induces histone alterations (increasing of H3K27Me3 and decreasing of H4K16Ac and H4K20Me3) together with a reduction in DNA methyltransferase-1 (DNMT1) and an increase in DNMT3b expression, resulting in hypomethylation of repetitive DNA sequences (D4Z4, NBL2, and LINE-1) and hypermethylation of tumor suppressor genes such as RASSF1A and RAR-β [45]. These changes can alter the expression/silencing of the target genes, increasing the risk of developing COPD. The DNA methylation patterns of human lung fibroblasts of different COPD stages were recently analyzed [46]. The high-resolution multi-omic analysis demonstrated that significant changes, particularly in genome regulatory regions, occur at an early stage. They identified that the regions contain binding sites for transcription factors TCF21 and FOSL2/FRA2 as novel regulators of the key fibroblast processes in COPD [46].
In patients with COPD, the epigenome of lung cells has significantly altered and regulated changes in cellular programming. The suppression and prevention of the development of COPD are aided by the dysregulation of histone deacetylase (HDAC) activity caused by certain variables and miRNAs. Additionally, COPD treatments are suggested that target HDAC2 and miRNA. However, it is still unknown which epigenetic alterations directly affect lung fibroblasts or how these changes activate aberrant signaling pathways inducing fibroblast dysfunction [44].
Epigenetic modifications can also influence mitochondrial metabolism and biogenesis. For example, changes in mitochondrial DNA can influence the regulation of genes encoding proteins involved in oxidative phosphorylation. The increased production of ROS from damaged mitochondria can lead to DNA damage and modify epigenetic marks [47]. Conversely, these epigenetic changes can affect gene expression and contribute to the development of COPD. In summary, epigenetic dysregulation and mitochondrial dysfunction are intricately linked processes that can influence each other bidirectionally.
Understanding the intricate relationship between epigenetics and mitochondrial dysfunction in COPD could provide insights into disease mechanisms and the discovery of novel biomarkers or potential therapeutic targets. In addition, epigenetic modifications are reversible in some cases, which raises the possibility of developing interventions to mitigate the symptoms of COPD or slow progression by targeting these epigenetic changes [48,49]. However, further research is needed to fully elucidate the specific epigenetic mechanisms underlying COPD and their potential clinical applications.
5. Nanocarrier-Based Treatments for COPD
Oxidative stress is an effective target for COPD treatment, but conventional antioxidants face obstructions such as airway immune response, mucous hypersecretion, inflammation, and low bioavailability, necessitating an efficient DDS [50].
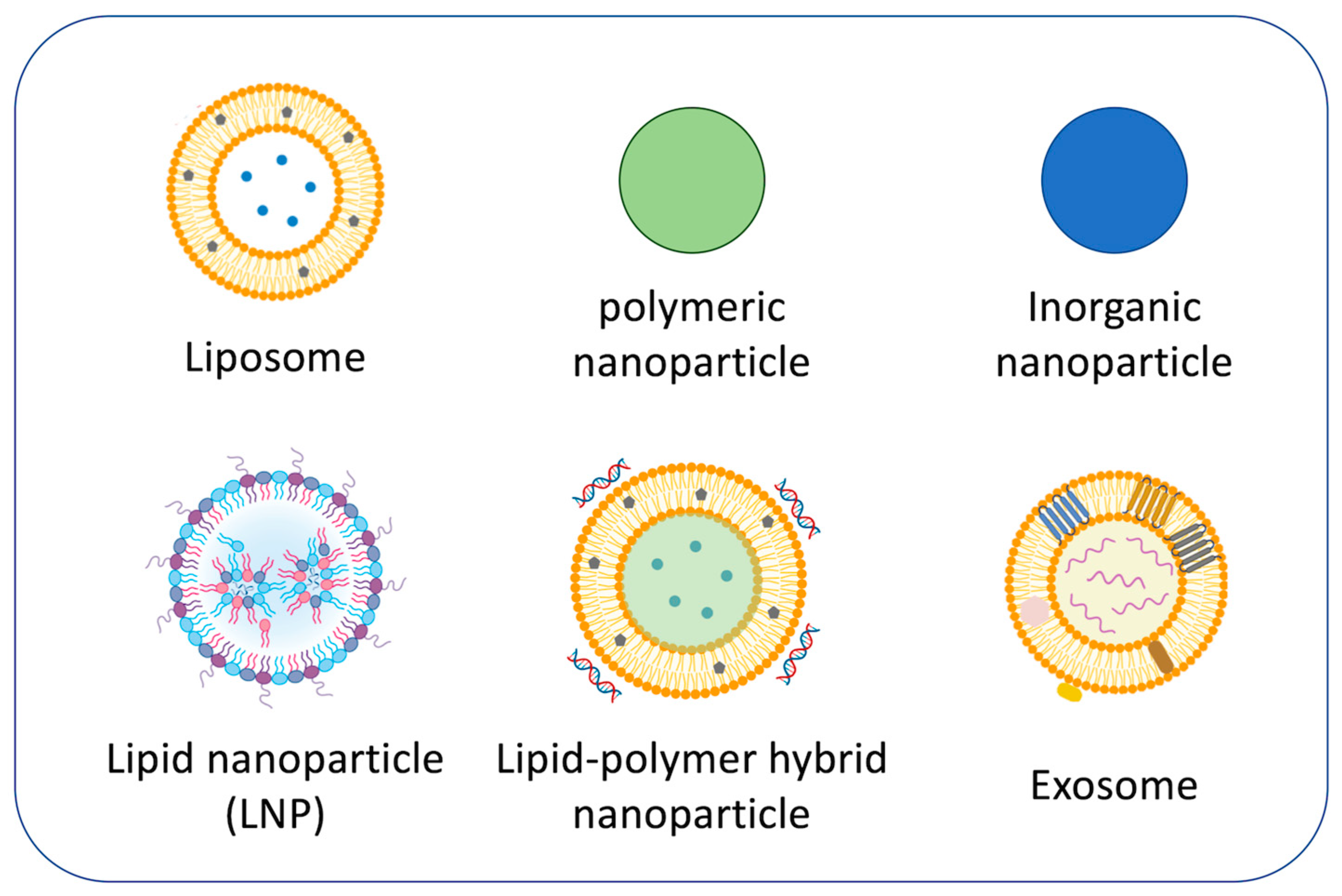
The most successful drug delivery platform for COPD currently under development is nanoparticles used as carriers (Figure 2) because nanoparticles provide various advantages [51,52,53,54]. For instance, the use of nanoparticles allows for better penetration into alveolar epithelial tissue than conventional treatments, minimizes drug dosage by attachment of targeting function, and can transport poorly soluble drugs, leading to enhanced treatment efficacy together with reducing toxicity [55]. Nanoparticle-based drug carriers can also be modified to effectively deliver drugs to target organelles such as mitochondria [56]. However, current challenges in nanoparticle-based respiratory disease treatment are airway defense, mucus hypersecretion, and severe inflammation. Surface modification and size control of nanoparticles are important to enhance biodistribution [57,58].
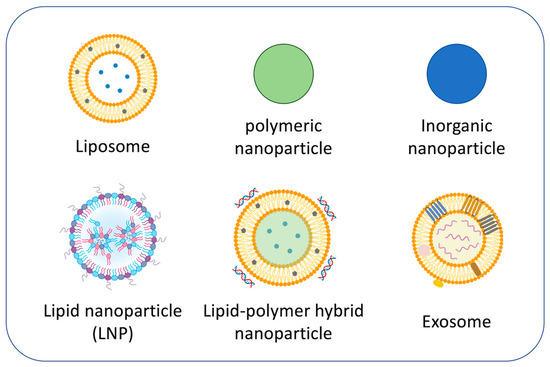
Figure 2.
Representative nanoparticles for COPD treatments.
5.1. Liposomes
Liposomes are a promising platform for nanoparticle-based DDS and the most common type of FDA/EMA-approved nanoparticles. Liposomes can transport both hydrophilic and hydrophobic/lipophilic drugs, making them suitable for combination therapy in COPD. In addition, liposomes often modify their surface to enhance the delivery of encapsulated drugs [59,60,61]. For example, curcumin-loaded liposomes coated with chitosan or hyaluronic acid were prepared to improve the antioxidant effect [62]. Both examples of polymer-coated liposomes greatly improved the stability, biocompatibility, and antioxidant ability of curcumin to protect A549 cells from mitochondrial oxidative stress. In particular, hyaluronic acid-coated liposomes showed enhancement of proliferation and metabolic activity [62].
A major challenge in transferring liposome-based treatments from research to clinical stages exists in the administration method of liposomes. Although inhaled therapy is a fundamental key in COPD treatments, inhalation of liposomes has limitations due to the instability of liposomes during dehydration, storage, and nebulization, leading to leakage of drugs. To be effective, liposomes must maintain their physical properties at pre-/post-inhalation, necessitating approaches for increased stability. Currently, a number of nebulizable liposome formulations have entered clinical trials [63].
5.2. Polymeric Nanoparticles
Biodegradable polymer-based nanoparticles have great potential as DDS and various polymeric nanoparticles have been reported for COPD treatments.
PLA nanoparticles are FDA-approved carriers and have been applied as drug carriers for the treatment of COPD. Buhecha et al. prepared poly(lactic acid) nanoparticles (PLA NPs) co-encapsulated theophylline (hydrophilic bronchodilator) and budesonide (lipophilic corticosteroid) by a double emulsification solvent diffusion method for combination therapy of COPD [64]. The PLA NPs showed sustained release of the two drugs into a 1:1 mixture of simulated lung fluid and MeOH, which is expected to extend the dose intervals and reduce the incidence of side effects.
Neutrophils have emerged as a novel target in COPD treatments. Neutrophils usually help defend the lungs against the risk of infections, but migration and accumulation of activated neutrophils at inflammatory tissues lead to further damage to the lungs in COPD patients. However, drug delivery to neutrophils remains a significant challenge. Surface modification with specific ligands can improve targeting and enhance drug delivery. Vij et al. demonstrated that a PEGylated PLGA-nanoparticle conjugated with anti-NIMP-R14 antibodies selectively delivered ibuprofen into neutrophils to inhibit neutrophilic inflammation in COPD model mice [65].
In recent years, the study of RNA-based COPD treatments has increased. Direct delivery of RNA is optimal for minimizing adverse reactions, but the elevation of RNA stability during delivery remains the next challenge to realize RNA therapies [66]. Mohamed et al. demonstrate the potential of polymeric nanoparticles as a miRNA delivery vehicle. They used a mixture of poly(glycerol adipate-co-ω-pentadecalactone) (PGA-co-PDL) and cationic lipid (DOTAP) for the carrier of miR-146a. The miRNA-loaded nanocomposite particles effectively delivered miR-146a into lung epithelial cells and reduced the expression of the target gene and the level of the IRAK1 protein [67]. Then, the authors applied the miR-146a/PGA-co-PDL nanoparticles for dry powder inhalation. L-leucine and mannitol were used as dispersion enhancers and protective excipients to formulate micro-sized composite particles by spray-drying [68].
5.3. Lipid-Polymer Hybrid Nanoparticles
Lipid-polymer nanoparticles are hybrid-type nanoparticles with a biodegradable polymer core and lipid shell. These lipid-enveloped polymeric nanoparticles have features of polymeric nanoparticles and liposomes.
Craparo et al. prepared alveolar macrophage-targeted lipid-polymer hybrid nanoparticles as a potential carrier for the delivery of Roflumilast, a PDE4 inhibitor, to treat COPD because the overexpression of PDE4 in alveolar macrophages implicated the inflammatory pathogen [69]. Roflumilast-loaded polymeric nanoparticles and liposomal vesicles of DPPC and DSPE-PEG2000-mannose phospholipids were combined by high-pressure homogenization. The obtained core-shell type lipid-polymer hybrid nanoparticles slowly released the drug which was taken up by macrophages via a mannose-mediated process through binding to mannose receptors overexpressed on the surface of alveolar macrophages in the lungs of COPD patients. The hybrid nanoparticles were successfully encapsulated in poly(vinyl alcohol)/leucine-based microparticles into an inhalable formulation by spray drying.
Excessive ROS production by mitochondrial dysfunction leads to a decrease in histone deacetylase 2 (HDAC2) that causes glucocorticoid resistance in COPD patients in an epigenetic manner [43,70]. To improve glucocorticoid resistance, Chikuma et al. prepared lipid-polymer hybrid nanoparticles composed of a Mn-porphyrin dimer (MnPD)-encapsulated PLGA core coated with a HDAC2-encoding plasmid DNA (pHDAC2)-bound cationic lipid (DOTAP) shell. Co-delivery of MnPD and pHDAC2 exhibited effective elimination of mitochondrial ROS and significant enhancement of HDAC2 expression with a decrease in IL-8 expression that indicates improvement of glucocorticoid resistance in COPD model cells [71].
5.4. Lipid Nanoparticles
Lipid nanoparticles (LNPs) are now widely used for nucleic acid delivery vehicles, which generally consist of four lipids: ionizable lipids necessary to form complexes with nucleic acids and promote endosomal escape, phospholipids for particle formation, cholesterol for stability and membrane fusion, and PEGylated lipids for improved circulation [72].
Recently, powdered LNPs compatible with dry powder inhaler devices have been investigated. Powdered LNPs prepared under proper manufacturing conditions not only recapitulate drug efficacy but also improve the stability of nanoparticles and encapsulated drugs. Zimmermann et al. tested the spray-drying process of siRNA-encapsulated LNP powders. The spray dried LNPs, prepared using 5% lactose solution, effectively penetrated into the artificial mucus layer and showed efficient gene silencing (>90% protein downregulation) in H1299 cells with good cellular compatibility. The spray dried LNPs also efficiently encapsulated up to 50% silenced GAPDH genes in ex vivo human lung tissues [73].
5.5. Inorganic Nanoparticles
Metallic nanoparticles such as Au and TiO2 or inorganic materials such as calcium phosphate have been suggested as potential nanocarriers for COPD treatment [74,75].
Wang et al. fabricated mitochondria-targeting porous Se@SiO2 antioxidant nanoparticles, which showed evaluated protective effects on airway epithelial cells and an acute lung injury model mouse through reducing mitochondrial ROS. Pretreatment with the Se@SiO2 nanoparticles significantly increased the resistance to oxidative injury and suppressed LPS-induced gene expression changes (downregulation of mitochondrial fusion proteins such as OPA1 and MFN2) by maintaining mitochondrial dynamics, suggesting therapeutic potential for acute lung injury [76].
Although intercellular mitochondrial transfer protects the injured cells, it limits transfer efficiency and selectivity for therapeutic utilization. Huang et al. report a potential method of using iron oxide nanoparticles that induce the transfer of healthy mitochondria from hMSCs to damaged cells to repair mitochondrial dysfunctions by enhancement of connexin43-containing intercellular gap junctions through the JNK pathway. The nanoparticle-treated MSCs significantly reduced fibrosis progression in a pulmonary fibrosis model mouse without serious safety issues [77].
Li et al. successfully prepared drug-controlled-release chitosan/black phosphorus quantum dot nanoparticles (PEG@CS/BPQDs-AM NPs) by ion crosslinking and PEG modification [78]. The nanoparticles effectively penetrated into the pulmonary mucus barrier and adhered to mucosal epithelial cells. Then, BPQDs were rapidly degraded and released amikacin, an antibacterial drug for gram-negative bacilli lung infections intercurrent with COPD. At the same time, the protonated chitosan effectively avoided biofilm formation by the antibacterial effect. In vivo experiments in mice demonstrated a significant improvement of airway obstruction and inhibition effects on pseudomonas aeruginosa, leading to a significant reduction in the toxicity and side effects.
On the other hand, it has been noted that inorganic nanoparticles induce potential toxicity through the accumulation of ROS, mitochondrial damage, inflammation, apoptosis, DNA damage, cell cycle, and epigenetic regulation linked with mitochondrial dysfunction [79,80].
5.6. Exosomes
Exosomes and EVs are small vesicles released from various cells for transporting bioactive molecules and signaling molecules such as proteins and nucleic acids for intercellular communication. Recently, they have gained significant attention due to their roles in physiological and pathological processes, including their potential involvement in COPD [81,82]. Several studies reported increased secretion of endothelial cell-derived exosomes in COPD patients, suggesting that the exosomes are related to the exacerbation of COPD [83]. The promotion of COPD also affects the profile of miRNA in exosomes. For instance, cigarette smoke exposure alters exosomes released from bronchial epithelial cells and significantly increases miR-210. The exosomes are taken up by lung fibroblasts, and the delivered miR-210 downregulates autophagy and induces differentiation into myofibroblasts. Accumulation of this degeneration potentially leads to exacerbated airflow obstruction [84].
Preclinical studies suggest that MSC-based cell therapy is a novel treatment modality for COPD due to its differentiation potential into alveolar epithelial cells and immunomodulatory properties [85]. Although the MSC-mediated effects are the paracrine action of their exosomes, most of the study of COPD treatments using MSC-derived exosomes is still limited to animal models [86].
Maremanda et al. investigated the combination treatment of MSCs and MSC-derived exosomes in cigarette smoke-exposed COPD model mice. Although cigarette smoke exposure led to a significant increase in mitochondrial fission protein DRP1 and damage-associated molecular pattern mediators S100A4/AB, HMGB1, RAGE, and AGE, the combination treatment increased the expression of mitochondrial fusion genes (mfn1, mfn2, and opa1) and mitochondrial transport/mitophagy facilitating genes (rhot1), indicating the protection effects against lung inflammation caused by mitochondrial dysfunction [87].
While the role of exosomes and EVs in COPD is an active area of research, our understanding of their precise contributions to the disease is still evolving. Further studies are needed to elucidate the influence of COPD progression and to explore their therapeutic targets. In addition, the heterogeneity of exosomes might limit the extensive application in COPD patients [86].
6. Concluding Remarks
In this concise review, we have overviewed current knowledge and efforts about treatments for COPD focused on mitochondrial dysfunction and nanoparticle-based therapy. As tackled against the intricate and multi-faceted connections between mitochondrial dysfunction and COPD, nanoparticle-based DDS offers a promising avenue to enhance therapeutic efficacy. Alongside exploring advanced drug delivery methods, further efforts to elucidate the molecular pathways linking mitochondrial dysfunction and COPD are crucial for developing more efficient targeted therapeutic strategies. By leveraging knowledge and drug-delivery systems, we can enhance the precision and effectiveness of COPD therapies, potentially improving the quality of life for patients with COPD.
Author Contributions
Conceptualization, K.S. and H.K.; writing—original draft preparation, K.S.; writing—review and editing, K.S. and H.K.; supervision, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Tokyo Metropolitan Government Infectious Disease Research Project and the Tokyo Metropolitan Government Advanced Research, grant number R4-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Ahmadian Heris, J.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.A.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheets of COPD, World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 31 July 2023).
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Ydoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. 2020, 5, 248. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Roversi, S.; Beghé, B. Triple therapy for symptomatic patients with COPD. Lancet 2017, 389, 1864–1865. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Moos, W.H.; Faller, D.V.; Glavas, I.P.; Harpp, D.N.; Kamperi, N.; Kanara, I.; Kodukula, K.; Mavrakis, A.N.; Pernokas, J.; Pernokas, M.; et al. Pathogenic mitochondrial dysfunction and metabolic abnormalities. Biochem. Pharmacol. 2021, 193, 114809. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 2014, 12, 34. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Zhunina, O.A.; Yabbarov, N.G.; Grechko, A.V.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. The Role of Mitochondrial Dysfunction in Vascular Disease, Tumorigenesis, and Diabetes. Front. Mol. Biosci. 2021, 8, 671908. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial dysfunction in neurological disorders: Exploring mitochondrial transplantation. NPJ Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Lerner, C.A.; Sundar, I.K.; Rahman, I. Mitochondrial redox system, dynamics, and dysfunction in lung inflammaging and COPD. Int. J. Biochem. Cell Biol. 2016, 81 Pt B, 294–306. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Xiao, H.; Wei, X. Mitochondrial dysfunction and chronic lung disease. Cell Biol. Toxicol. 2019, 35, 493–502. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Paudel, K.R.; Tan, N.W.; Cheong, K.S.; Khoo, S.S.Q.; Seow, S.M.; Chellian, J.; Candasamy, M.; Patel, V.K.; Arora, P.; et al. Targeting the mitochondria in chronic respiratory diseases. Mitochondrion 2022, 67, 15–37. [Google Scholar] [CrossRef]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxidative Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.A.; Lopes-Pacheco, M.; Rocco, P.R.M. Oxidative Stress-Derived Mitochondrial Dysfunction in Chronic Obstructive Pulmonary Disease: A Concise Review. Oxidative Med. Cell. Longev. 2021, 2021, 6644002. [Google Scholar] [CrossRef] [PubMed]
- Mumby, S.; Adcock, I.M. Recent evidence from omic analysis for redox signalling and mitochondrial oxidative stress in COPD. J. Inflamm. 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef] [PubMed]
- Plataki, M.; Tzortzaki, E.; Rytila, P.; Demosthenes, M.; Koutsopoulos, A.; Siafakas, N.M. Apoptotic mechanisms in the pathogenesis of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 161–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef]
- Rivas, M.; Gupta, G.; Costanzo, L.; Ahmed, H.; Wyman, A.E.; Geraghty, P. Senescence: Pathogenic Driver in Chronic Obstructive Pulmonary Disease. Medicina 2022, 58, 817. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Kuwano, K. Cellular senescence-an aging hallmark in chronic obstructive pulmonary disease pathogenesis. Respir. Investig. 2022, 60, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J.; et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Zoll, J.; Charles, A.L.; Charloux, A.; de Blay, F.; Diemunsch, P.; Sibilia, J.; Piquard, F.; Geny, B. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: Central actor and therapeutic target. Exp. Physiol. 2013, 98, 1063–1078. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef]
- Kuwano, K.; Araya, J.; Hara, H.; Minagawa, S.; Takasaka, N.; Ito, S.; Kobayashi, K.; Nakayama, K. Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). Respir. Investig. 2016, 54, 397–406. [Google Scholar] [CrossRef]
- Albano, G.D.; Montalbano, A.M.; Gagliardo, R.; Profita, M. Autophagy/Mitophagy in Airway Diseases: Impact of Oxidative Stress on Epithelial Cells. Biomolecules 2023, 13, 1217. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, J.; Mohammadtursun, N.; Hu, Z.; Li, Q.; Zhao, Z.; Zhang, H.; Dong, J. Dual role of autophagy/mitophagy in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2019, 56, 116–125. [Google Scholar] [CrossRef]
- Manevski, M.; Muthumalage, T.; Devadoss, D.; Sundar, I.K.; Wang, Q.; Singh, K.P.; Unwalla, H.J.; Chand, H.S.; Rahman, I. Cellular stress responses and dysfunctional mitochondrial-cellular senescence, and therapeutics in chronic respiratory diseases. Redox Biol. 2020, 33, 101443. [Google Scholar] [CrossRef]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef]
- Hoffmann, R.F.; Zarrintan, S.; Brandenburg, S.M.; Kol, A.; de Bruin, H.G.; Jafari, S.; Dijk, F.; Kalicharan, D.; Kelders, M.; Gosker, H.R.; et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 2013, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, J.; Wang, T.; Zhang, X.; Liu, L.; Wang, H.; Wu, Y.; Xu, D.; Wen, F. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells. Sci. Rep. 2016, 6, 37751. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Araya, J.; Ito, S.; Kobayashi, K.; Takasaka, N.; Yoshii, Y.; Wakui, H.; Kojima, J.; Shimizu, K.; Numata, T.; et al. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L737–L746. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Sundar, I.K.; Lerner, C.A.; Gerloff, J.; Tormos, A.M.; Yao, H.; Rahman, I. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: Implications for chronic obstructive pulmonary disease. FASEB J. 2015, 29, 2912–2929. [Google Scholar] [CrossRef]
- Mizumura, K.; Cloonan, S.M.; Nakahira, K.; Bhashyam, A.R.; Cervo, M.; Kitada, T.; Glass, K.; Owen, C.A.; Mahmood, A.; Washko, G.R.; et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investig. 2014, 124, 3987–4003. [Google Scholar] [CrossRef]
- Murray, L.M.A.; Krasnodembskaya, A.D. Concise Review: Intercellular Communication via Organelle Transfer in the Biology and Therapeutic Applications of Stem Cells. Stem Cells 2019, 37, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 65. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Qi, Z.; Cao, L.; Ding, S. Mitochondrial transfer/transplantation: An emerging therapeutic approach for multiple diseases. Cell Biosci. 2022, 12, 66. [Google Scholar] [CrossRef]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Kumar, M.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar]
- Frankenberg Garcia, J.; Rogers, A.V.; Mak, J.C.W.; Halayko, A.J.; Hui, C.K.M.; Xu, B.; Chung, K.F.; Rodriguez, T.; Michaeloudes, C.; Bhavsar, P.K. Mitochondrial Transfer Regulates Bioenergetics in Healthy and Chronic Obstructive Pulmonary Disease Airway Smooth Muscle. Am. J. Respir. Cell Mol. Biol. 2022, 67, 471–481. [Google Scholar] [CrossRef]
- Schamberger, A.C.; Mise, N.; Meiners, S.; Eickelberg, O. Epigenetic mechanisms in COPD: Implications for pathogenesis and drug discovery. Expert Opin. Drug Discov. 2014, 9, 609–628. [Google Scholar] [CrossRef]
- Zhang, L.; Valizadeh, H.; Alipourfard, I.; Bidares, R.; Aghebati-Maleki, L.; Ahmadi, M. Epigenetic Modifications and Therapy in Chronic Obstructive Pulmonary Disease (COPD): An Update Review. COPD 2020, 17, 333–342. [Google Scholar] [CrossRef]
- Wu, D.D.; Song, J.; Bartel, S.; Krauss-Etschmann, S.; Rots, M.G.; Hylkema, M.N. The potential for targeted rewriting of epigenetic marks in COPD as a new therapeutic approach. Pharmacol. Ther. 2018, 182, 1–14. [Google Scholar] [CrossRef]
- Liu, F.; Killian, J.K.; Yang, M.; Walker, R.L.; Hong, J.A.; Zhang, M.; Davis, S.; Zhang, Y.; Hussain, M.; Xi, S.; et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene 2010, 29, 3650–3664. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, U.; Llamazares Prada, M.; Pohl, S.T.; Richter, M.; Tamas, R.; Schuler, M.; Keller, C.; Mijosek, V.; Muley, T.; Schneider, M.A.; et al. High-resolution transcriptomic and epigenetic profiling identifies novel regulators of COPD. EMBO J. 2023, 42, e111272. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, T.; Pawar, J.; Rathod, R.; Patel, M.; Fernandes, V.; Kumar, R.; Singh, S.B.; Khatri, D.K. Mitochondrial quality control: Epigenetic signatures and therapeutic strategies. Neurochem. Int. 2021, 148, 105095. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Ito, K.; Barnes, P.J. Accelerated ageing of the lung in COPD: New concepts. Thorax 2015, 70, 482–489. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, W.; Zhang, J.R.; Li, C.Y.; Zhang, J.; Lv, X.J. Roles of sirtuin family members in chronic obstructive pulmonary disease. Respir. Res. 2022, 23, 66. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Song, L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: A review. J. Nanobiotechnol. 2020, 18, 145. [Google Scholar] [CrossRef]
- de Menezes, B.R.C.; Rodrigues, K.F.; Schatkoski, V.M.; Pereira, R.M.; Ribas, R.G.; Montanheiro, T.L.D.A.; Thim, G.P. Current advances in drug delivery of nanoparticles for respiratory disease treatment. J. Mater. Chem. B 2021, 9, 1745–1761. [Google Scholar] [CrossRef]
- Virmani, T.; Kumar, G.; Virmani, R.; Sharma, A.; Pathak, K. Nanocarrier-based approaches to combat chronic obstructive pulmonary disease. Nanomedicine 2022, 17, 1833–1854. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Long, M.M.; Gao, L.L.; Chen, Y.J.; Li, F.; Shi, Y.; Gu, N. Nanomedicines Targeting Respiratory Injuries for Pulmonary Disease Management. Adv. Funct. Mater. 2022, 32, 2112258. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Qayoomian, M.; Beigoli, S.; Boskabady, M.H. Recent advances in nanoparticle applications in respiratory disorders: A review. Front. Pharmacol. 2023, 14, 1059343. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, X.; Zeng, Y.; Lin, D.; Wu, J. Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 2021, 14, 2067–2089. [Google Scholar] [CrossRef]
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Sánchez, L.Á.; Gámez-Méndez, A.; Martínez-Ruiz, M.; Nájera-Martínez, E.F.; Morales-Flores, B.A.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Iqbal, H.M.N. Nanostructures for drug delivery in respiratory diseases therapeutics: Revision of current trends and its comparative analysis. J. Drug Deliv. Sci. Technol. 2022, 70, 103219. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Nano-delivery to the lung—By inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Elhissi, A. Liposomes for Pulmonary Drug Delivery: The Role of Formulation and Inhalation Device Design. Curr. Pharm. Des. 2017, 23, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Buhecha, M.D.; Lansley, A.B.; Somavarapu, S.; Pannala, A.S. Development and characterization of PLA nanoparticles for pulmonary drug delivery: Co-encapsulation of theophylline and budesonide, a hydrophilic and lipophilic drug. J. Drug Deliv. Sci. Technol. 2019, 53, 101128. [Google Scholar] [CrossRef]
- Vij, N.; Min, T.; Bodas, M.; Gorde, A.; Roy, I. Neutrophil targeted nano-drug delivery system for chronic obstructive lung diseases. Nanomedicine 2016, 12, 2415–2427. [Google Scholar] [CrossRef]
- Mei, D.; Tan, W.S.D.; Tay, Y.; Mukhopadhyay, A.; Wong, W.S.F. Therapeutic RNA Strategies for Chronic Obstructive Pulmonary Disease. Trends Pharmacol. Sci. 2020, 41, 475–486. [Google Scholar] [CrossRef]
- Mohamed, A.; Kunda, N.K.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Polymeric nanoparticles for the delivery of miRNA to treat Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Pharm. Biopharm. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Mohamed, A.; Pekoz, A.Y.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Pulmonary delivery of Nanocomposite Microparticles (NCMPs) incorporating miR-146a for treatment of COPD. Int. J. Pharm. 2019, 569, 118524. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cabibbo, M.; Scialabba, C.; Giammona, G.; Cavallaro, G. Inhalable Formulation Based on Lipid-Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast. Biomacromolecules 2022, 23, 3439–3451. [Google Scholar] [CrossRef]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Chikuma, K.; Arima, K.; Asaba, Y.; Kubota, R.; Asayama, S.; Sato, K.; Kawakami, H. The potential of lipid-polymer nanoparticles as epigenetic and ROS control approaches for COPD. Free Radic. Res. 2020, 54, 829–840. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.M.; Baldassi, D.; Chan, K.; Adams, N.B.P.; Neumann, A.; Porras-Gonzalez, D.L.; Wei, X.; Kneidinger, N.; Stoleriu, M.G.; Burgstaller, G.; et al. Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery. J. Control. Release 2022, 351, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M.; Quaile, O.; Wenk, A.; Wigge, C.; Eigeldinger-Berthou, S.; Hirn, S.; Schäffler, M.; Schleh, C.; Möller, W.; Mall, M.A.; et al. Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Part. Fibre Toxicol. 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Roulet, A.; Armand, L.; Dagouassat, M.; Rogerieux, F.; Simon-Deckers, A.; Belade, E.; Van Nhieu, J.T.; Lanone, S.; Pairon, J.C.; Lacroix, G.; et al. Intratracheally administered titanium dioxide or carbon black nanoparticles do not aggravate elastase-induced pulmonary emphysema in rats. BMC Pulm. Med. 2012, 12, 38. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Deng, G.; Liu, X.; Wu, X.; Hu, H.; Zhang, Y.; Gao, W.; Li, Q. Mitochondria-Modulating Porous Se@SiO2 Nanoparticles Provide Resistance to Oxidative Injury in Airway Epithelial Cells: Implications for Acute Lung Injury. Int. J. Nanomedicine 2020, 15, 2287–2302. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, T.; Jiang, X.; Li, A.; Su, Y.; Bian, Q.; Wu, H.; Lin, R.; Li, N.; Cao, H.; et al. Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy. Sci. Adv. 2021, 7, eabj0534. [Google Scholar] [CrossRef]
- Li, Z.; Luo, G.; Hu, W.P.; Hua, J.L.; Geng, S.; Chu, P.K.; Zhang, J.; Wang, H.; Yu, X.F. Mediated Drug Release from Nanovehicles by Black Phosphorus Quantum Dots for Efficient Therapy of Chronic Obstructive Pulmonary Disease. Angew. Chem. Int. Ed. Engl. 2020, 59, 20568–20576. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Gomez, N.; James, V.; Onion, D.; Fairclough, L.C. Extracellular vesicles and chronic obstructive pulmonary disease (COPD): A systematic review. Respir. Res. 2022, 23, 82. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Q.; Du, T.; Gabriel, A.N.A.; Wang, X.; Sun, L.; Li, X.; Xu, K.; Jiang, X.; Zhang, Y. The Potential Roles of Exosomes in Chronic Obstructive Pulmonary Disease. Front. Med. 2021, 7, 618506. [Google Scholar] [CrossRef] [PubMed]
- Hough, K.P.; Chanda, D.; Duncan, S.R.; Thannickal, V.J.; Deshane, J.S. Exosomes in immunoregulation of chronic lung diseases. Allergy 2017, 72, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Araya, J.; Ito, S.; Kobayashi, K.; Kosaka, N.; Yoshioka, Y.; Kadota, T.; Hara, H.; Kuwano, K.; Ochiya, T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J. Extracell. Vesicles 2015, 4, 28388. [Google Scholar] [CrossRef] [PubMed]
- Broekman, W.; Khedoe, P.P.S.J.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef]
- Rajabi, H.; Konyalilar, N.; Erkan, S.; Mortazavi, D.; Korkunc, S.K.; Kayalar, O.; Bayram, H.; Rahbarghazi, R. Emerging role of exosomes in the pathology of chronic obstructive pulmonary diseases; destructive and therapeutic properties. Stem Cell Res. Ther. 2022, 13, 144. [Google Scholar] [CrossRef]
- Maremanda, K.P.; Sundar, I.K.; Rahman, I. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol. Appl. Pharmacol. 2019, 385, 114788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).