Conquering Space with Crops That Produce Ample Oxygen and Antioxidants
Abstract
:1. Introduction
1.1. Molecular Oxygen Plays Unique and Essential Roles for Life
1.2. Reactive Oxygen Can Kill
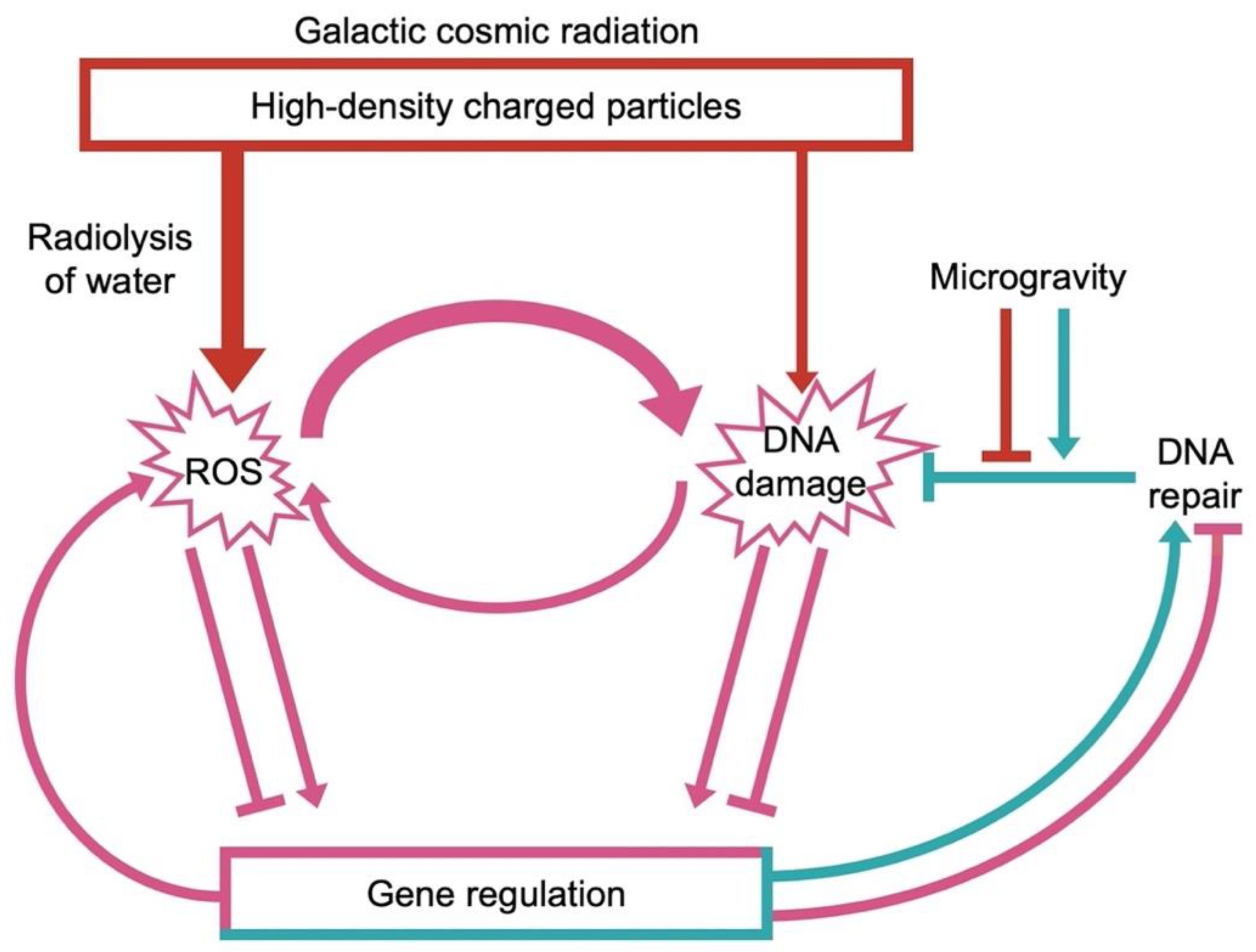
2. The Challenges of Space Environments
ROS and Chronic Inflammation in Astronauts
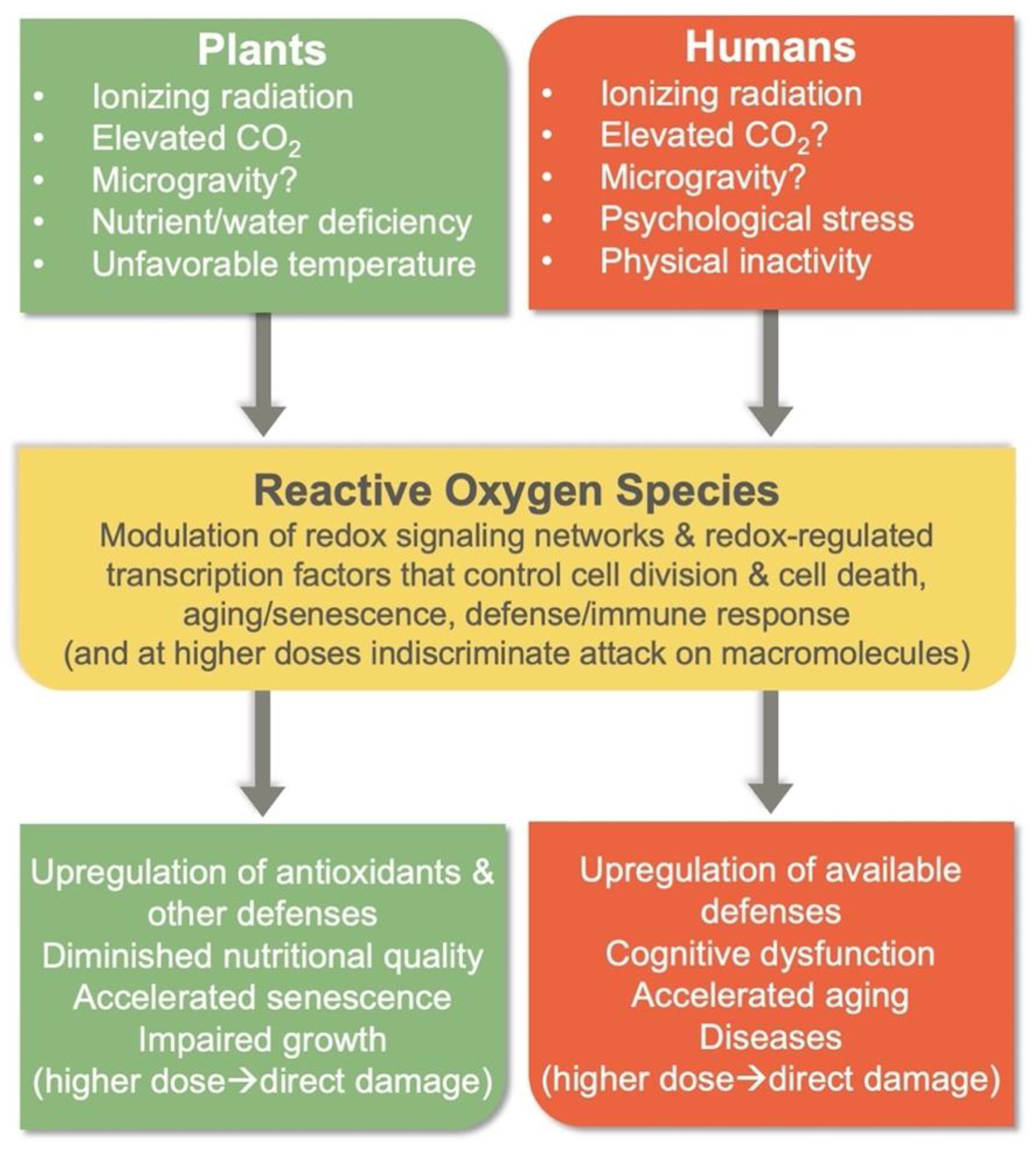
3. The Multi-Hit Hypothesis: Interaction among Different Stresses in a Space Environment
3.1. Specific Plant Responses
3.2. Human Physiology
4. Redox-Based Orchestration of Growth, Development, and Defenses
4.1. Early-Warning Systems for Oxidative Stress
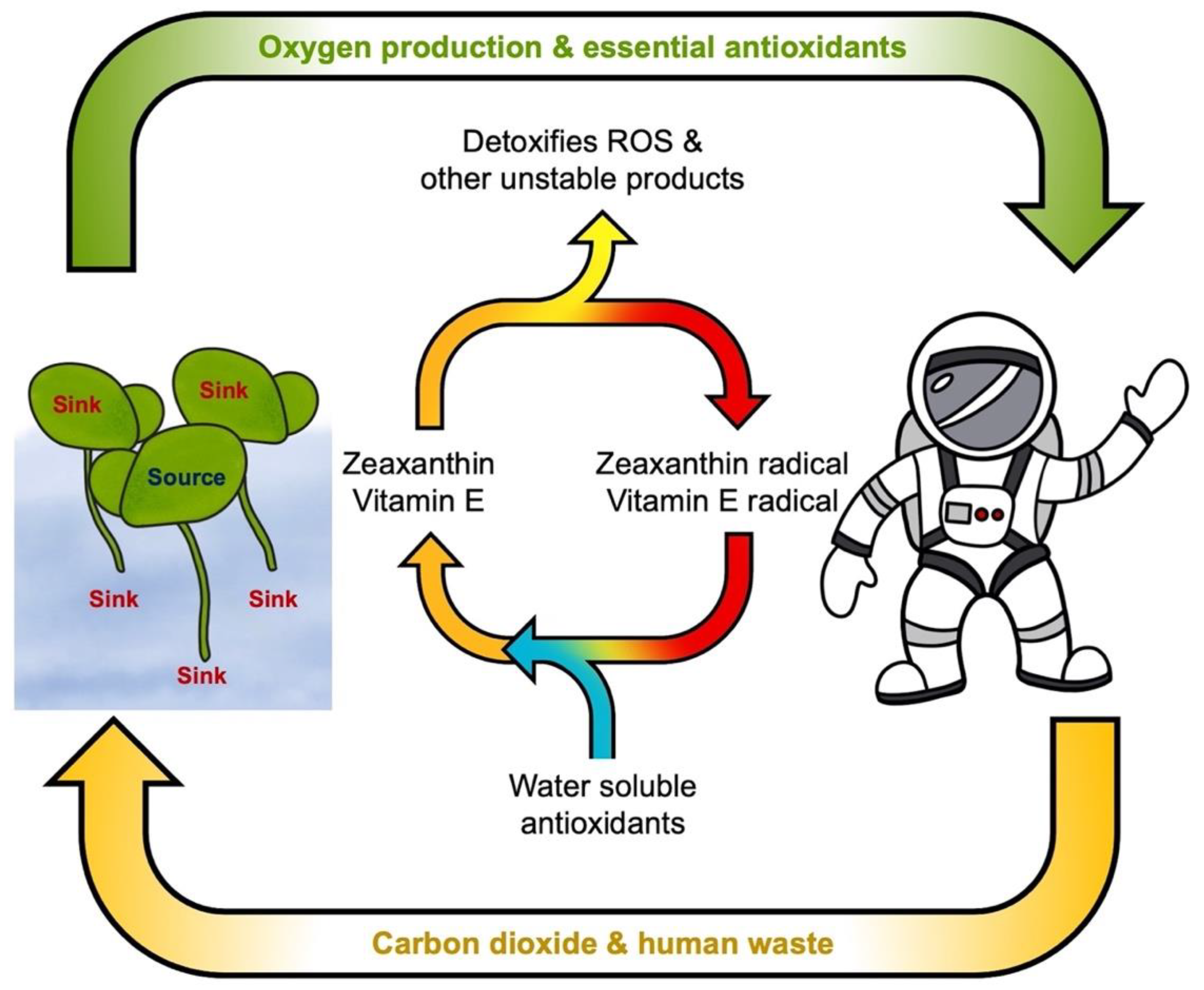
4.2. Gene Regulation by Derivatives of Lipid Peroxidation and the Need for Dietary Antioxidant Metabolites
4.3. Zeaxanthin and Lutein Protect Photosynthesis
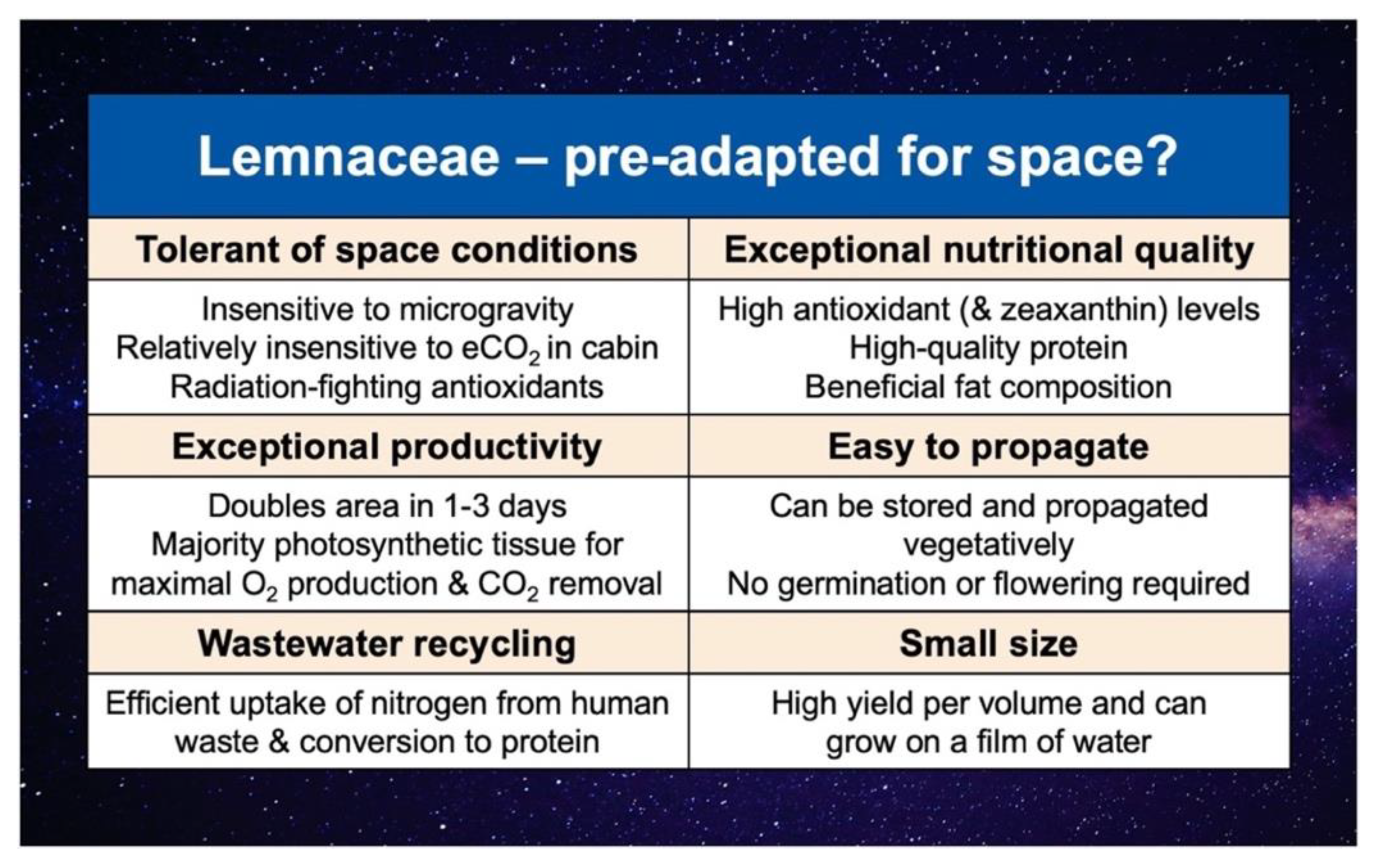
5. The Case for Lemnaceae as Space Crops
5.1. An Unusual Combination of Multiple Attractive Traits
5.2. Can the Aquatic Lifestyle Be Seen as a Pre-Adaptation for Spaceflight Environments
5.3. Exceptional Antioxidant Content
5.4. Performance under Elevated CO2
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berkner, L.V.; Marshall, L.C. On the Origin and Rise of Oxygen Concentration in the Earth’s Atmosphere. J. Atmos. Sci. 1965, 22, 225–261. [Google Scholar] [CrossRef] [Green Version]
- Nursall, J.R. Oxygen as a Prerequisite to the Origin of the Metazoa. Nature 1959, 183, 1170–1172. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The Many Facets of Hypoxia in Plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Srikanth, S.; Lum, S.K.Y.; Chen, Z. Mangrove Root: Adaptations and Ecological Importance. Trees 2016, 30, 451–465. [Google Scholar] [CrossRef]
- Tobias, B.; Garr, J.; Erne, M. International Space Station Water Balance Operations. In Proceedings of the 41st International Conference on Environmental Systems, Portland, OR, USA, 17–21 July 2011; p. 5150. [Google Scholar]
- Ferl, R.; Wheeler, R.; Levine, H.G.; Paul, A.-L. Plants in Space. Curr. Opin. Plant Biol. 2002, 5, 258–263. [Google Scholar] [CrossRef]
- Fu, Y.; Li, L.; Xie, B.; Dong, C.; Wang, M.; Jia, B.; Shao, L.; Dong, Y.; Deng, S.; Liu, H. How to Establish a Bioregenerative Life Support System for Long-Term Crewed Missions to the Moon or Mars. Astrobiology 2016, 16, 925–936. [Google Scholar] [CrossRef]
- Ligrone, R. The Great Oxygenation Event. In Biological Innovations that Built the World; Springer: Cham, Switzerland, 2019; pp. 129–154. [Google Scholar] [CrossRef]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The Rise of Oxygen in Earth’s Early Ocean and Atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Commentary: Oxidative Stress Reconsidered. Genes Nutr. 2009, 4, 161–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edreva, A. Generation and Scavenging of Reactive Oxygen Species in Chloroplasts: A Submolecular Approach. Agric. Ecosyst. Environ. 2005, 106, 119–133. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. Rapid Systemic Signaling during Abiotic and Biotic Stresses: Is the ROS Wave Master of All Trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; Zhao, M.; Liu, X.; Wang, X.; Nie, Y.; Li, P.; Liu, T.; Ge, R.; Han, F. Reduced Expression of Citrate Synthase Leads to Excessive Superoxide Formation and Cell Apoptosis. Biochem. Biophys. Res. Commun. 2017, 485, 388–394. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Gómez, X.; Sanon, S.; Zambrano, K.; Asquel, S.; Bassantes, M.; Morales, J.E.; Otáñez, G.; Pomaquero, C.; Villarroel, S.; Zurita, A.; et al. Key Points for the Development of Antioxidant Cocktails to Prevent Cellular Stress and Damage Caused by Reactive Oxygen Species (ROS) during Manned Space Missions. npj Microgravity 2021, 7, 35. [Google Scholar] [CrossRef]
- Phan, Q.T.; Sipka, T.; Gonzalez, C.; Levraud, J.-P.; Lutfalla, G.; Nguyen-Chi, M. Neutrophils Use Superoxide to Control Bacterial Infection at a Distance. PLoS Pathog. 2018, 14, e1007157. [Google Scholar] [CrossRef] [PubMed]
- Doitsh, G.; Greene, W.C. Dissecting How CD4 T Cells Are Lost during HIV Infection. Cell Host Microbe 2016, 19, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Che, M.; Wang, R.; Li, X.; Wang, H.-Y.; Zheng, X.S. Expanding Roles of Superoxide Dismutases in Cell Regulation and Cancer. Drug Discov. Today 2016, 21, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. Ascorbate Peroxidase–a Hydrogen Peroxide-scavenging Enzyme in Plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Richards, J.T.; Feiveson, A.H.; Richards, S.E.; Neelam, S.; Dreschel, T.W.; Plante, I.; Hada, M.; Wu, H.; Massa, G.D. Response of Arabidopsis thaliana and Mizuna Mustard Seeds to Simulated Space Radiation Exposures. Life 2022, 12, 144. [Google Scholar] [CrossRef]
- Datta, K.; Suman, S.; Kallakury, B.V.S.; Fornace, A.J. Exposure to Heavy Ion Radiation Induces Persistent Oxidative Stress in Mouse Intestine. PLoS ONE 2012, 7, e42224. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; Macaeva, E.; Quintens, R. Space Radiation Effects on Plant and Mammalian Cells. Acta Astronaut. 2014, 104, 419–431. [Google Scholar] [CrossRef]
- Pazhanisamy, S.K.; Li, H.; Wang, Y.; Batinic-Haberle, I.; Zhou, D. NADPH Oxidase Inhibition Attenuates Total Body Irradiation-Induced Haematopoietic Genomic Instability. Mutagenesis 2011, 26, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhang, J.; Walker, S.J.; Dworakowski, R.; Lakatta, E.G.; Shah, A.M. Involvement of NADPH Oxidase in Age-Associated Cardiac Remodeling. J. Mol. Cell. Cardiol. 2010, 48, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Parihar, V.K.; Allen, B.D.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.K.; Chmielewski, N.N.; Giedzinski, E.; Acharya, M.M.; Britten, R.A.; et al. Cosmic Radiation Exposure and Persistent Cognitive Dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef] [Green Version]
- Giovanetti, A.; Tortolici, F.; Rufini, S. Why Do the Cosmic Rays Induce Aging? Front. Physiol. 2020, 11, 955. [Google Scholar] [CrossRef]
- Sridharan, D.M.; Asaithamby, A.; Bailey, S.M.; Costes, S.V.; Doetsch, P.W.; Dynan, W.S.; Kronenberg, A.; Rithidech, K.N.; Saha, J.; Snijders, A.M.; et al. Understanding Cancer Development Processes after HZE-Particle Exposure: Roles of ROS, DNA Damage Repair and Inflammation. Radiat. Res. 2015, 183, 1–26. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R. Spaceflight-Related Ocular Changes: The Potential Role of Genetics, and the Potential of B Vitamins as a Countermeasure. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 481–488. [Google Scholar] [CrossRef]
- Yatagai, F.; Honma, M.; Dohmae, N.; Ishioka, N. Biological Effects of Space Environmental Factors: A Possible Interaction between Space Radiation and Microgravity. Life Sci. Space Res. 2019, 20, 113–123. [Google Scholar] [CrossRef]
- Ikeda, H.; Muratani, M.; Hidema, J.; Hada, M.; Fujiwara, K.; Souda, H.; Yoshida, Y.; Takahashi, A. Expression Profile of Cell Cycle-Related Genes in Human Fibroblasts Exposed Simultaneously to Radiation and Simulated Microgravity. Int. J. Mol. Sci. 2019, 20, 4791. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.M.; Luxton, J.J.; McKenna, M.J.; Taylor, L.E.; George, K.A.; Jhavar, S.G.; Swanson, G.P. Ad Astra—Telomeres in Space! Int. J. Radiat. Biol. 2022, 98, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Luxton, J.J.; Bailey, S.M. Twins, Telomeres, and Aging—In Space! Plast. Reconstr. Surg. 2021, 147, 7S–14S. [Google Scholar] [CrossRef]
- Tan, S.; Pei, W.; Huang, H.; Zhou, G.; Hu, W. Additive Effects of Simulated Microgravity and Ionizing Radiation in Cell Death, Induction of ROS and Expression of RAC2 in Human Bronchial Epithelial Cells. npj Microgravity 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Baselet, B.; Vermeesen, R.; Moreels, M.; Baatout, S.; Rahiman, F.; Miles, X.; Nair, S.; du Plessis, P.; Engelbrecht, M.; et al. Immunological Changes During Space Travel: A Ground-Based Evaluation of the Impact of Neutron Dose Rate on Plasma Cytokine Levels in Human Whole Blood Cultures. Front. Phys. 2020, 8, 568124. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Narumi, I.; Satoh, K.; Funayama, T.; Kikuchi, M.; Kitayama, S.; Watanabe, H. Radiation Response Mechanisms of the Extremely Radioresistant Bacterium Deinococcus radiodurans. Biol. Sci. Space 2004, 18, 134–135. [Google Scholar]
- Moors, K.A.; Ott, E.; Weckwerth, W.; Milojevic, T. Proteomic Response of Deinococcus radiodurans to Short-Term Real Microgravity during Parabolic Flight Reveals Altered Abundance of Proteins Involved in Stress Response and Cell Envelope Functions. Life 2021, 12, 23. [Google Scholar] [CrossRef]
- Barravecchia, I.; De Cesari, C.; Forcato, M.; Scebba, F.; Pyankova, O.V.; Bridger, J.M.; Foster, H.A.; Signore, G.; Borghini, A.; Andreassi, M. Microgravity and Space Radiation Inhibit Autophagy in Human Capillary Endothelial Cells, through Either Opposite or Synergistic Effects on Specific Molecular Pathways. Cell. Mol. Life Sci. 2022, 79, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, Q.; Xu, W.; Li, F.; Li, H.; Lu, J.; Wu, L.; Wu, Y.; Liu, M.; Bian, P. Modulation of Modeled Microgravity on Radiation-Induced Bystander Effects in Arabidopsis thaliana. Mutat. Res. 2015, 773, 27–36. [Google Scholar] [CrossRef]
- Kordyum, E.L. Biology of Plant Cells in Microgravity and under Clinostating. Int. Rev. Cytol. 1997, 171, 1–78. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Polutchko, S.K.; Zenir, M.C.; Fourounjian, P.; Stewart, J.J.; López-Pozo, M.; Adams, W.W., III. Intersections: Photosynthesis, Abiotic Stress, and the Plant Microbiome. Photosynthetica 2022, 60, 59–69. [Google Scholar] [CrossRef]
- Agüera, E.; De la Haba, P. Leaf Senescence in Response to Elevated Atmospheric CO2 Concentration and Low Nitrogen Supply. Biol. Plant. 2018, 62, 401–408. [Google Scholar] [CrossRef]
- Tausz-Posch, S.; Tausz, M.; Bourgault, M. Elevated [CO2] Effects on Crops: Advances in Understanding Acclimation, Nitrogen Dynamics and Interactions with Drought and Other Organisms. Plant Biol. 2020, 22, 38–51. [Google Scholar] [CrossRef]
- Adavi, S.B.; Sathee, L. Elevated CO2 Alters Tissue Balance of Nitrogen Metabolism and Downregulates Nitrogen Assimilation and Signalling Gene Expression in Wheat Seedlings Receiving High Nitrate Supply. Protoplasma 2021, 258, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Adavi, S.B.; Sathee, L. Elevated CO2 Differentially Regulates Root Nitrate Transporter Kinetics in a Genotype and Nitrate Dose-Dependent Manner. Plant Sci. 2021, 305, 110807. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink Regulation of Photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Stewart, J.J.; Demmig-Adams, B. Photosynthetic Modulation in Response to Plant Activity and Environment. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 493–563. [Google Scholar] [CrossRef]
- Adams, W.W., III; Muller, O.; Cohu, C.M.; Demmig-Adams, B. May Photoinhibition Be a Consequence, Rather than a Cause, of Limited Plant Productivity? Photosynth. Res. 2013, 117, 31–44. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W., III. Growth and Nutritional Quality of Lemnaceae Viewed Comparatively in an Ecological and Evolutionary Context. Plants 2022, 11, 145. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef] [PubMed]
- Polutchko, S.K.; Glime, G.N.; Demmig-Adams, B. Synergistic Action of Membrane-Bound and Water-Soluble Antioxidants in Neuroprotection. Molecules 2021, 26, 5385. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Jiang, Y.; Li, X. The Impact of Health Promotion Interventions on Telomere Length: A Systematic Review. Am. J. Health Promot. 2020, 34, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Hou, G.; Li, D.; Yuan, T.-F. A Possible Change Process of Inflammatory Cytokines in the Prolonged Chronic Stress and Its Ultimate Implications for Health. Sci. World J. 2014, 2014, 780616. [Google Scholar] [CrossRef]
- Adams, R.B.; Egbo, K.N.; Demmig-Adams, B. High-Dose Vitamin C Supplements Diminish the Benefits of Exercise in Athletic Training and Disease Prevention. Nutr. Food Sci. 2014, 44, 95–101. [Google Scholar] [CrossRef]
- McGregor, H.R.; Lee, J.K.; Mulder, E.R.; De Dios, Y.E.; Beltran, N.E.; Kofman, I.S.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Brain Connectivity and Behavioral Changes in a Spaceflight Analog Environment with Elevated CO2. NeuroImage 2021, 225, 117450. [Google Scholar] [CrossRef]
- Zwart, S.R.; Gibson, C.R.; Gregory, J.F.; Mader, T.H.; Stover, P.J.; Zeisel, S.H.; Smith, S.M. Astronaut Ophthalmic Syndrome. FASEB J. 2017, 31, 3746–3756. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.J. Free Radicals Generated by Contracting Muscle: By-Products of Metabolism or Key Regulators of Muscle Function? Free Radic. Biol. Med. 2008, 44, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarrone, M.; Nieuwenhuizen, W.F.; Dullens, H.F.; Catani, M.V.; Melino, G.; Veldink, G.A.; Vliegenthart, J.F.; Agrò, A.F. Membrane Modifications in Human Erythroleukemia K562 Cells during Induction of Programmed Cell Death by Transforming Growth Factor Β1 or Cisplatin. Eur. J. Biochem. 1996, 241, 297–302. [Google Scholar] [CrossRef]
- Wojcik, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A. A Review of Natural and Synthetic Antioxidants Important for Health and Longevity. Curr. Med. Chem. 2010, 17, 3262–3288. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Banthiya, S.; Van Leyen, K. Mammalian Lipoxygenases and Their Biological Relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally Diverse Metabolites from Fatty Acid Oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Inflammatory Cytokine Storms. Mo. Med. 2020, 117, 539–542. [Google Scholar] [PubMed]
- Dietz, K.-J.; Hell, R. Thiol Switches in Redox Regulation of Chloroplasts: Balancing Redox State, Metabolism and Oxidative Stress. Biol. Chem. 2015, 396, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-Cleavage-Triggered Chemosensors and Their Biological Applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Trujillo-Hernandez, J.A.; Reichheld, J.-P. Thiol Based Redox Signaling in Plant Nucleus. Front. Plant Sci. 2018, 9, 705. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Artiach, G.; Sarajlic, P.; Bäck, M. Inflammation and Its Resolution in Coronary Artery Disease: A Tightrope Walk between Omega-6 and Omega-3 Polyunsaturated Fatty Acids. Kardiol. Pol. 2020, 78, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-Y.; Ke, D.-S.; Chen, J.-Y. Essential Fatty Acids and Human Brain. Acta Neurol. Taiwan 2009, 18, 231–241. [Google Scholar]
- Masamoto, K.; Tanishita, K. Oxygen Transport in Brain Tissue. J. Biomech. Eng. 2009, 131, 074002. [Google Scholar] [CrossRef]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of Polyunsaturated Fatty Acids in Human Brain Structure and Function across the Lifespan: An Update on Neuroimaging Findings. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Catorce, M.N.; Gevorkian, G. Evaluation of Anti-Inflammatory Nutraceuticals in LPS-Induced Mouse Neuroinflammation Model: An Update. Curr. Neuropharmacol. 2020, 18, 636–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. The Location and Function of Vitamin E in Membranes. Mol. Membr. Biol. 2000, 17, 143–156. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as Modulators of Lipid Membrane Physical Properties. Biochim. Biophys. Acta 2005, 1740, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and Zeaxanthin as Protectors of Lipid Membranes against Oxidative Damage: The Structural Aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Havaux, M.; García-Plazaola, J.I. Beyond Non-Photochemical Fluorescence Quenching: The Overlapping Antioxidant Functions of Zeaxanthin and Tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Polutchko, S.K.; Adams, W.W., III. Structure-Function-Environment Relationship of the Isomers Zeaxanthin and Lutein. Photochem 2022, 2, 308–325. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin Has Enhanced Antioxidant Capacity with Respect to All Other Xanthophylls in Arabidopsis Leaves and Functions Independent of Binding to PSII Antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef] [Green Version]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and Orientation of Xanthophylls in a Lipid Bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Wu, Y.; Zhang, Y.; Wang, Z.; Liu, X. Lutein Suppresses Inflammatory Responses through Nrf2 Activation and NF-ΚB Inactivation in Lipopolysaccharide-Stimulated BV-2 Microglia. Mol. Nutr. Food Res. 2015, 59, 1663–1673. [Google Scholar] [CrossRef]
- Zhou, X.; Gan, T.; Fang, G.; Wang, S.; Mao, Y.; Ying, C. Zeaxanthin Improved Diabetes-Induced Anxiety and Depression through Inhibiting Inflammation in Hippocampus. Metab. Brain Dis. 2018, 33, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids Supplementation and Inflammation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Li, J.; Abdel-Aal, E.-S.M. Dietary Lutein and Cognitive Function in Adults: A Meta-Analysis of Randomized Controlled Trials. Molecules 2021, 26, 5794. [Google Scholar] [CrossRef]
- Nouchi, R.; Suiko, T.; Kimura, E.; Takenaka, H.; Murakoshi, M.; Uchiyama, A.; Aono, M.; Kawashima, R. Effects of Lutein and Astaxanthin Intake on the Improvement of Cognitive Functions among Healthy Adults: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, B.R., Jr.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Tazeddinova, D.; Aljoumaa, K.; Kazhmukhanbetkyzy, Z.A.; Orazov, A.; Toshev, A.D. Carotenoids: Therapeutic Strategy in the Battle against Viral Emerging Diseases, COVID-19: An Overview. Prev. Nutr. Food Sci. 2021, 26, 241. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Renzi-Hammond, L.M.; Bovier, E.R.; Fletcher, L.M.; Miller, L.S.; Mewborn, C.M.; Lindbergh, C.A.; Baxter, J.H.; Hammond, B.R. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients 2017, 9, 1246. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, M.A.; Canas, J.A.; Fanelli-Kuczmarski, M.T.; Maldonado, A.I.; Shaked, D.; Kivimaki, M.; Evans, M.K.; Zonderman, A.B. Association of Antioxidant Vitamins A, C, E and Carotenoids with Cognitive Performance over Time: A Cohort Study of Middle-Aged Adults. Nutrients 2020, 12, 3558. [Google Scholar] [CrossRef]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.; Troen, A.M.; Snodderly, D.M. Cognitive Findings of an Exploratory Trial of Docosahexaenoic Acid and Lutein Supplementation in Older Women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimers Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Yong, L.C.; Petersen, M.R.; Sigurdson, A.J.; Sampson, L.A.; Ward, E.M. High Dietary Antioxidant Intakes Are Associated with Decreased Chromosome Translocation Frequency in Airline Pilots. Am. J. Clin. Nutr. 2009, 90, 1402–1410. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Baseggio, M.; Murray, M.; Magallanes-Lundback, M.; Kaczmar, N.; Chamness, J.; Buckler, E.S.; Smith, M.E.; DellaPenna, D.; Tracy, W.F.; Gore, M.A. Natural Variation for Carotenoids in Fresh Kernels Is Controlled by Uncommon Variants in Sweet Corn. Plant Genome 2020, 13, e20008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K. Hen Egg Carotenoids (Lutein and Zeaxanthin) and Nutritional Impacts on Human Health: A Review. CyTA J. Food 2017, 15, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Phelan, D.; Prado-Cabrero, A.; Nolan, J.M. Analysis of Lutein, Zeaxanthin, and Meso-Zeaxanthin in the Organs of Carotenoid-Supplemented Chickens. Foods 2018, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.A.; Díaz-Gómez, J.; Fuentes-Font, L.; Angulo, E.; Gosálvez, L.F.; Sandmann, G.; Portero-Otin, M.; Capell, T.; Zhu, C.; Christou, P. Poultry Diets Containing (Keto) Carotenoid-Enriched Maize Improve Egg Yolk Color and Maintain Quality. Anim. Feed Sci. Technol. 2020, 260, 114334. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Skřivanová, E.; Bubancová, I. Increase in Lutein and Zeaxanthin Content in the Eggs of Hens Fed Marigold Flower Extract. Czech J. Anim. Sci. 2015, 60, 89–96. [Google Scholar] [CrossRef]
- Asker, D.; Beppu, T.; Ueda, K. Unique Diversity of Carotenoid-Producing Bacteria Isolated from Misasa, a Radioactive Site in Japan. Appl. Microbiol. Biotechnol. 2007, 77, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.A.; Barker, D.H.; Demmig-Adams, B.; Adams, W.W., III. Acclimation of Leaf Carotenoid Composition and Ascorbate Levels to Gradients in the Light Environment within an Australian Rainforest. Plant Cell Environ. 1996, 19, 1083–1090. [Google Scholar] [CrossRef]
- Burke, M.; Edge, R.; Land, E.J.; Truscott, T.G. Characterisation of Carotenoid Radical Cations in Liposomal Environments: Interaction with Vitamin C. J. Photochem. Photobiol. B 2001, 60, 1–6. [Google Scholar] [CrossRef]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free Radical Recycling and Intramembrane Mobility in the Antioxidant Properties of Alpha-Tocopherol and Alpha-Tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [Green Version]
- Tran, E.; Demmig-Adams, B. Vitamins and Minerals: Powerful Medicine or Potent Toxins? Nutr. Food Sci. 2007, 37, 50–60. [Google Scholar] [CrossRef]
- Ward, C.H.; Wilks, S.S.; Craft, H.L. Effects of Prolonged near Weightlessness on Growth and Gas Exchange of Photosynthetic Plants. Dev. Ind. Microbiol. 1970, 11, 276–295. [Google Scholar]
- Escobar, C.M.; Escobar, A.C. Duckweed: A Tiny Aquatic Plant with Enormous Potential for Bioregenerative Life Support Systems. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Yuan, J.; Xu, K. Effects of Simulated Microgravity on the Performance of the Duckweeds Lemna aequinoctialis and Wolffia globosa. Aquat. Bot. 2017, 137, 65–71. [Google Scholar] [CrossRef]
- Romano, L.E.; Aronne, G. The World Smallest Plants (Wolffia sp.) as Potential Species for Bioregenerative Life Support Systems in Space. Plants 2021, 10, 1896. [Google Scholar] [CrossRef]
- Ward, C.H.; Wilks, S.S. Use of Algae and Other Plants in the Development of Life Support Systems. Am. Biol. Teach. 1963, 25, 512–521. [Google Scholar] [CrossRef]
- Kawamata, Y.; Shibui, Y.; Takumi, A.; Seki, T.; Shimada, T.; Hashimoto, M.; Inoue, N.; Kobayashi, H.; Narita, T. Genotoxicity and Repeated-Dose Toxicity Evaluation of Dried Wolffia globosa Mankai. Toxicol. Rep. 2020, 7, 1233–1241. [Google Scholar] [CrossRef]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S. Return of the Lemnaceae: Duckweed as a Model Plant System in the Genomics and Postgenomics Era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Q.; Han, F.; Le, J. Higher Plants in Space: Microgravity Perception, Response, and Adaptation. Microgravity Sci. Technol. 2015, 27, 377–386. [Google Scholar] [CrossRef]
- Anikeeva, I.D.; Kostina, L.N.; Vaulina, E.N. Experiments with Air-Dried Seeds of Arabidopsis thaliana (L.) Heynh. and Crepis capillaris (L.) Wallr., Aboard Salyut 6. Adv. Space Res. 1983, 3, 129–133. [Google Scholar] [CrossRef]
- Escobar, C.M.; Escobar, A.C.; Power, G.J.; Nabity, J.A. µG-LilyPondTM: Preliminary Design of a Floating Plant Pond for Microgravity. In Proceedings of the 50th International Conference on Environmental Systems, Lisbon, Portugal, 12–16 July 2020. [Google Scholar]
- Gale, J.; Smernoff, D.T.; Macler, B.A.; MacElroy, R.D. Carbon Balance and Productivity of Lemna gibba, a Candidate Plant for CELSS. Adv. Space Res. 1989, 9, 43–52. [Google Scholar] [CrossRef]
- Oron, G.; Wildschut, L.; Porath, D. Waste Water Recycling by Duckweed for Protein Production and Effluent Renovation. Water Sci. Technol. 1985, 17, 803–817. [Google Scholar] [CrossRef]
- Wolverton, B.; McDonald, R. Upgrading Facultative Wastewater Lagoons with Vascular Aquatic Plants. J. Water Pollut. Control Fed. 1979, 51, 305–313. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ Toxicity in Higher Plants: A Critical Review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K. Nutritional Value of the Duckweed Species of the Genus Wolffia (Lemnaceae) as Human Food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohedano, R.A.; Costa, R.H.; Tavares, F.A.; Belli Filho, P. High Nutrient Removal Rate from Swine Wastes and Protein Biomass Production by Full-Scale Duckweed Ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and Essential Carotenoid Micronutrients in Lemna gibba as a Function of Growth Light Intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity. Cells 2021, 10, 1481. [Google Scholar] [CrossRef]
- Al-Ahmary, K.M. The Carotenoids of Some Food Stuffs in Saudi Arabia. Int. J. Food Sci. Nutr. 2010, 61, 823–828. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (Lutein, Zeaxanthin) Content in Fruits, Vegetables and Corn and Egg Products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Murillo, E.; Meléndez-Martínez, A.J.; Portugal, F. Screening of Vegetables and Fruits from Panama for Rich Sources of Lutein and Zeaxanthin. Food Chem. 2010, 122, 167–172. [Google Scholar] [CrossRef]
- Sommerburg, O.; Keunen, J.E.; Bird, A.C.; Van Kuijk, F.J. Fruits and Vegetables That Are Sources for Lutein and Zeaxanthin: The Macular Pigment in Human Eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef]
- Kim, J.-S.; An, C.G.; Park, J.-S.; Lim, Y.P.; Kim, S. Carotenoid Profiling from 27 Types of Paprika (Capsicum annuum L.) with Different Colors, Shapes, and Cultivation Methods. Food Chem. 2016, 201, 64–71. [Google Scholar] [CrossRef]
- Hemminge Natesh, N.; Abbey, L.; Asiedu, S.K. An Overview of Nutritional and Antinutritional Factors in Green Leafy Vegetables. Horticult. Int. J. 2017, 1, 00011. [Google Scholar] [CrossRef] [Green Version]
- Diotallevi, C.; Angeli, A.; Vrhovsek, U.; Gobbetti, M.; Shai, I.; Lapidot, M.; Tuohy, K. Measuring Phenolic Compounds in Mankai: A Novel Polyphenol and Amino Rich Plant Protein Source. Proc. Nutr. Soc. 2020, 79, E434. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, Y.; Yi, Z.; Tian, X.; Li, J.; Jin, Y.; He, K.; Liu, P.; Du, A.; Huang, Y. Determining the Nutritional Value and Antioxidant Capacity of Duckweed (Wolffia arrhiza) under Artificial Conditions. LWT 2022, 153, 112477. [Google Scholar] [CrossRef]
- Burgner, S.E.; Nemali, K.; Massa, G.D.; Wheeler, R.M.; Morrow, R.C.; Mitchell, C.A. Growth and Photosynthetic Responses of Chinese Cabbage (Brassica rapa L. cv. Tokyo Bekana) to Continuously Elevated Carbon Dioxide in a Simulated Space Station “Veggie” Crop-Production Environment. Life Sci. Space Res. 2020, 27, 83–88. [Google Scholar] [CrossRef]
- Michael, T.P.; Ernst, E.; Hartwick, N.; Chu, P.; Bryant, D.; Gilbert, S.; Ortleb, S.; Baggs, E.L.; Sree, K.S.; Appenroth, K.J. Genome and Time-of-Day Transcriptome of Wolffia australiana Link Morphological Minimization with Gene Loss and Less Growth Control. Genome Res. 2021, 31, 225–238. [Google Scholar] [CrossRef]
- Padhan, B.K.; Sathee, L.; Meena, H.S.; Adavi, S.B.; Jha, S.K.; Chinnusamy, V. CO2 Elevation Accelerates Phenology and Alters Carbon/Nitrogen Metabolism vis-à-vis ROS Abundance in Bread Wheat. Front. Plant Sci. 2020, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Henriques, R. Sugars and the Speed of Life—Metabolic Signals That Determine Plant Growth, Development and Death. Physiol. Plant. 2022, 174, e13656. [Google Scholar] [CrossRef] [PubMed]
- Hieber, A.D.; Bugos, R.C.; Yamamoto, H.Y. Plant Lipocalins: Violaxanthin de-Epoxidase and Zeaxanthin Epoxidase. Biochim. Biophys. Acta 2000, 1482, 84–91. [Google Scholar] [CrossRef]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and Regulation of the Violaxanthin Cycle: The Role of Antenna Proteins and Membrane Lipids. Biochim. Biophys. Acta 2009, 1787, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethmann, S.; Melzer, M.; Schwarz, N.; Jahns, P. The Zeaxanthin Epoxidase Is Degraded along with the D1 Protein during Photoinhibition of Photosystem II. Plant Direct 2019, 3, e00185. [Google Scholar] [CrossRef] [Green Version]
- Hoang, M.H.; Kim, H.-S.; Zulfugarov, I.S.; Lee, C.-H. Down-Regulation of Zeaxanthin Epoxidation in Vascular Plant Leaves under Normal and Photooxidative Stress Conditions. J. Plant Biol. 2020, 63, 331–336. [Google Scholar] [CrossRef]
- Reinhold, C.; Niczyporuk, S.; Beran, K.C.; Jahns, P. Short-Term down-Regulation of Zeaxanthin Epoxidation in Arabidopsis thaliana in Response to Photo-Oxidative Stress Conditions. Biochim. Biophys. Acta 2008, 1777, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polutchko, S.K.; Adams, W.W., III; Escobar, C.M.; Demmig-Adams, B. Conquering Space with Crops That Produce Ample Oxygen and Antioxidants. Oxygen 2022, 2, 211-226. https://doi.org/10.3390/oxygen2020016
Polutchko SK, Adams WW III, Escobar CM, Demmig-Adams B. Conquering Space with Crops That Produce Ample Oxygen and Antioxidants. Oxygen. 2022; 2(2):211-226. https://doi.org/10.3390/oxygen2020016
Chicago/Turabian StylePolutchko, Stephanie K., William W. Adams, III, Christine M. Escobar, and Barbara Demmig-Adams. 2022. "Conquering Space with Crops That Produce Ample Oxygen and Antioxidants" Oxygen 2, no. 2: 211-226. https://doi.org/10.3390/oxygen2020016







