Use of eDNA to Determine Source Locations of Deadly Jellyfish (Cubozoa) in an Open Coastal System
Abstract
1. Introduction
2. Materials and Methods
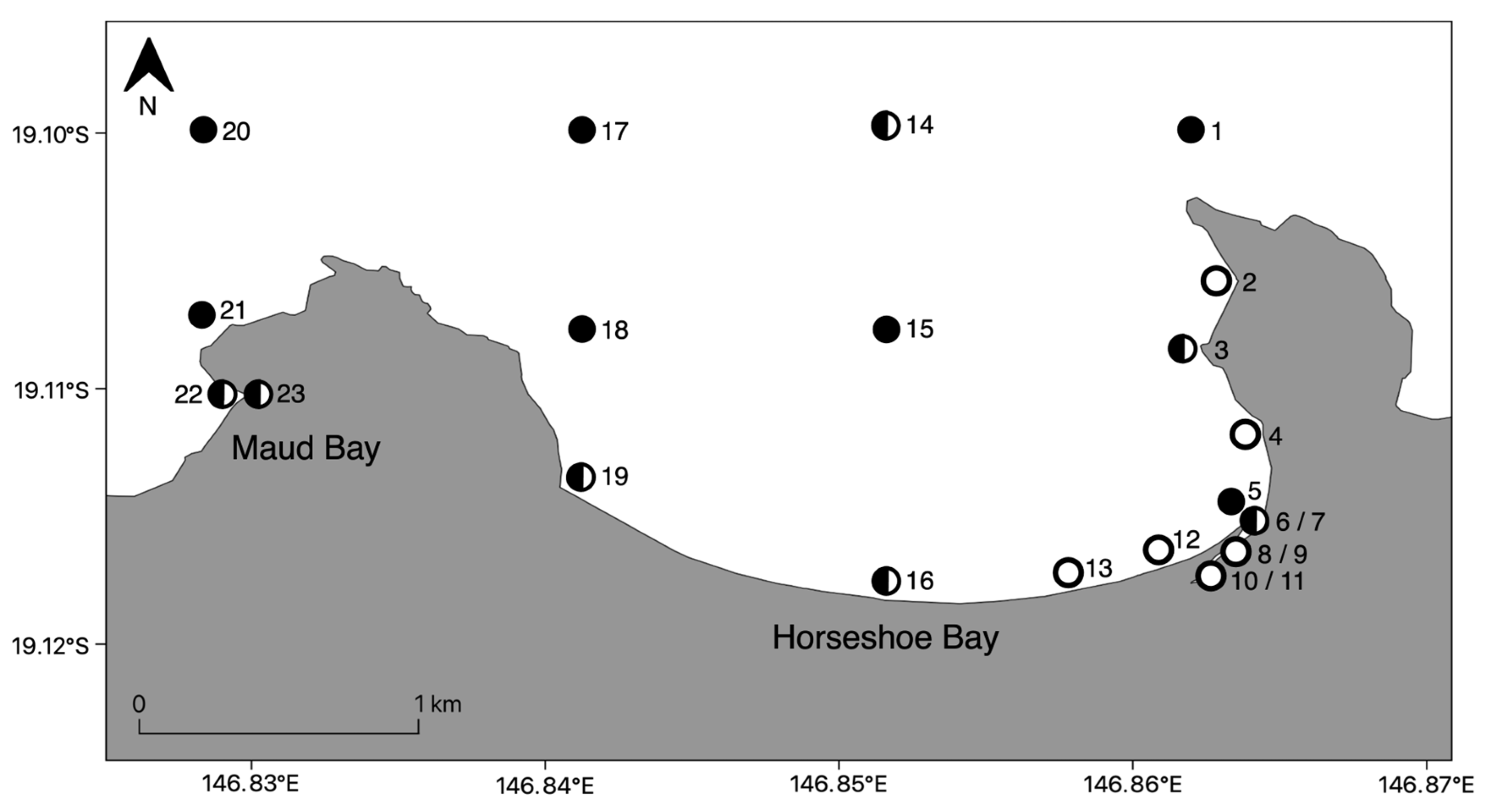
2.1. Study Area
2.2. Field Sampling
2.3. eDNA Extraction and Purification
2.4. Quantitative PCR
2.5. Statistical Analysis
3. Results
3.1. Seasonality of Chironex fleckeri Medusae within Horseshoe Bay
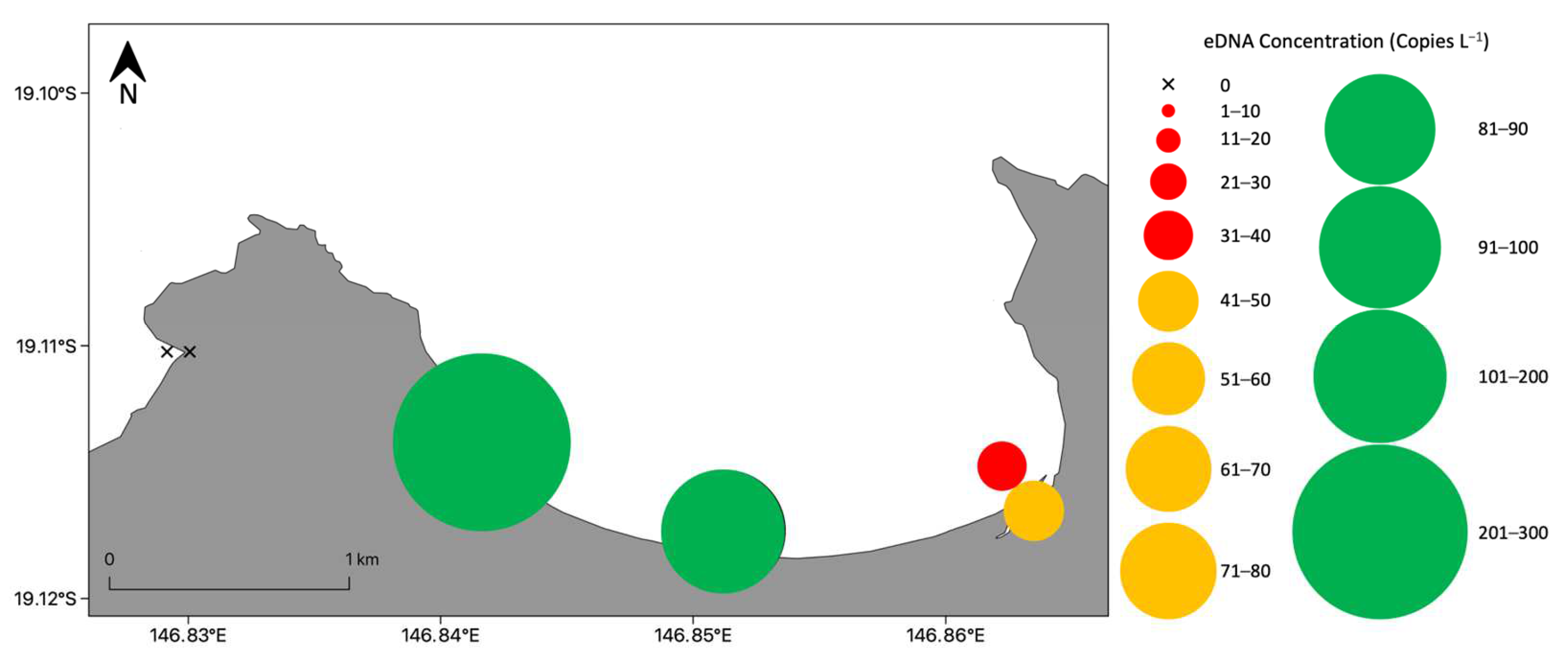
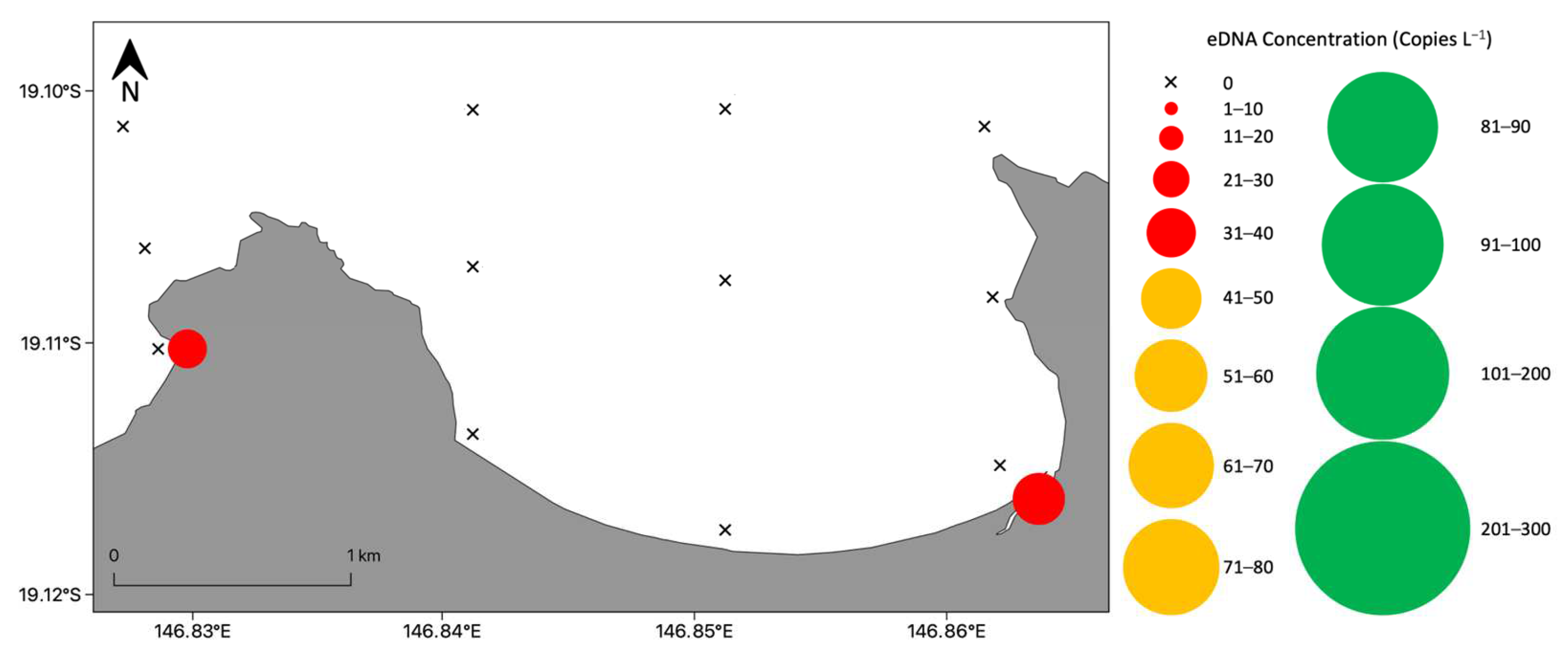
3.2. Detection and Distribution of Chironex fleckeri Medusae
3.2.1. Nearshore Detection of Chironex fleckeri Medusae
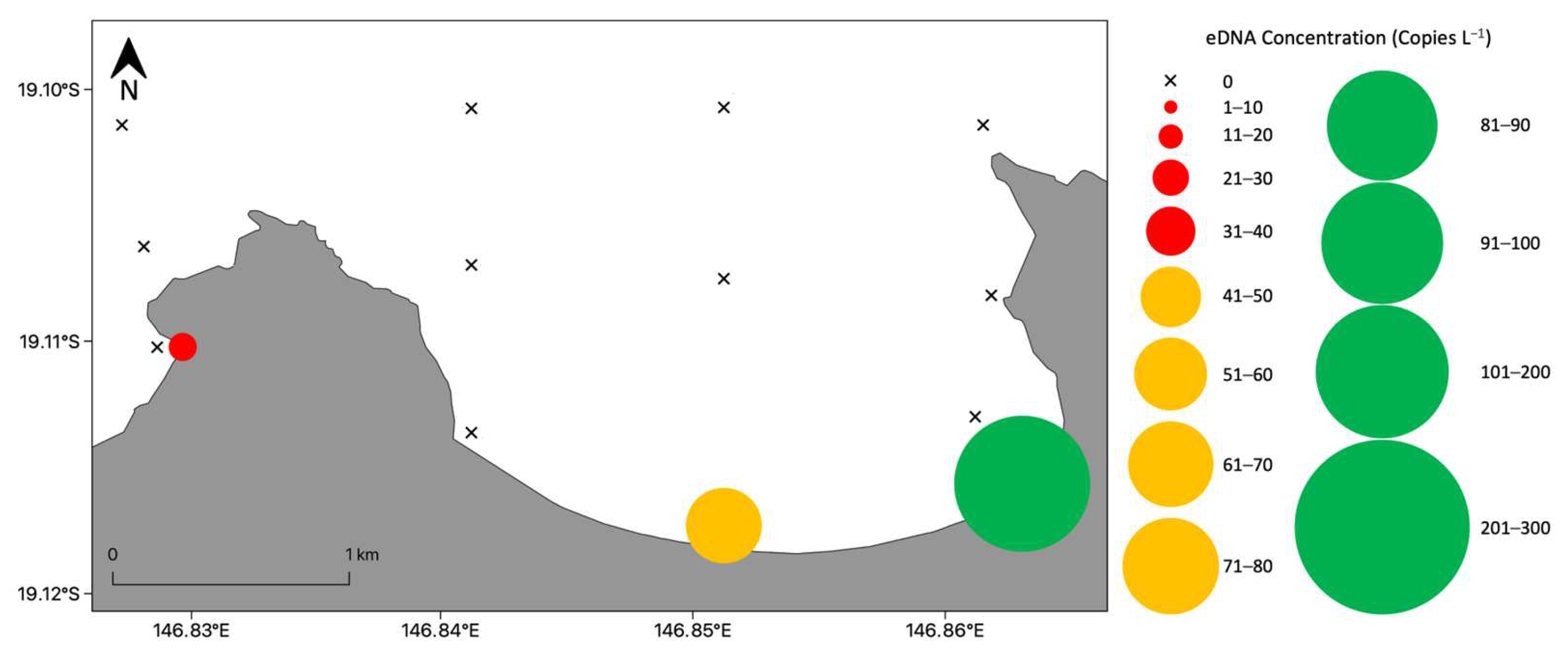
3.2.2. Bay Wide Sampling Design for Chironex fleckeri Medusae
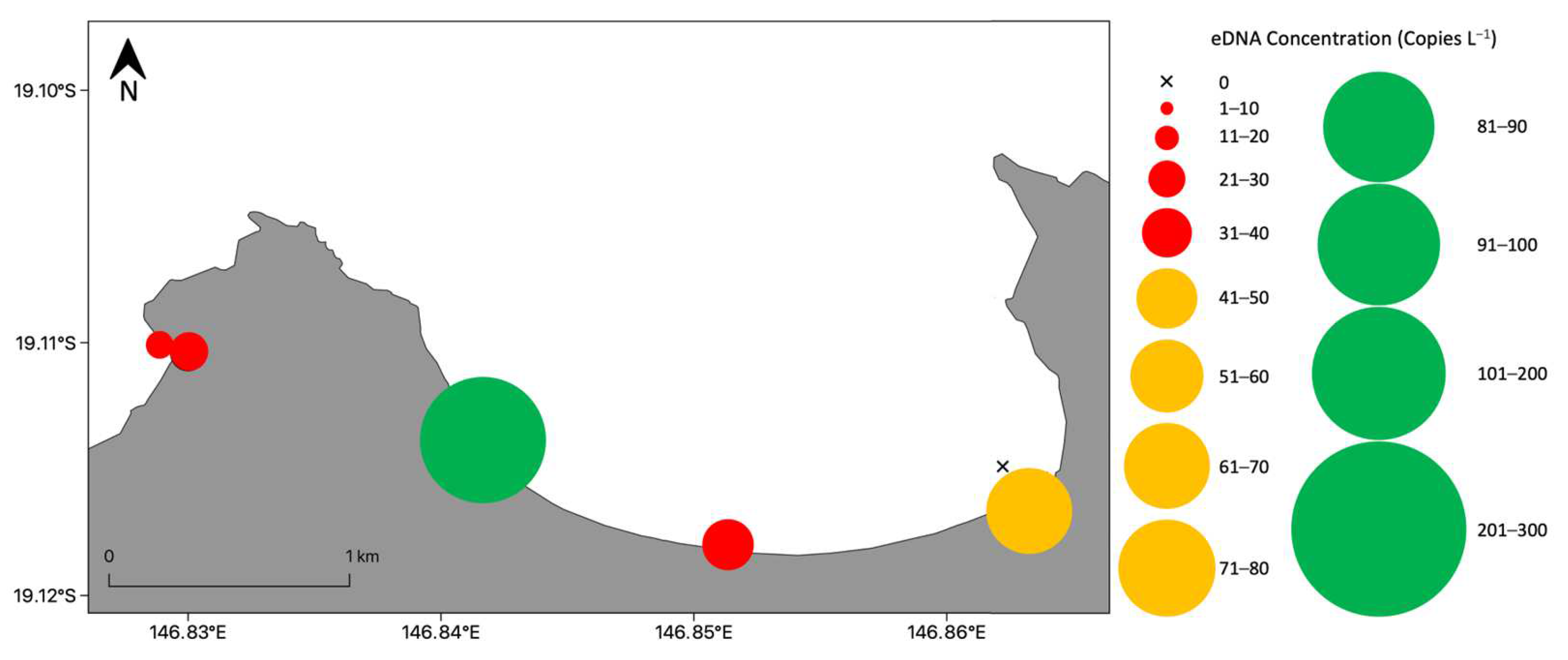
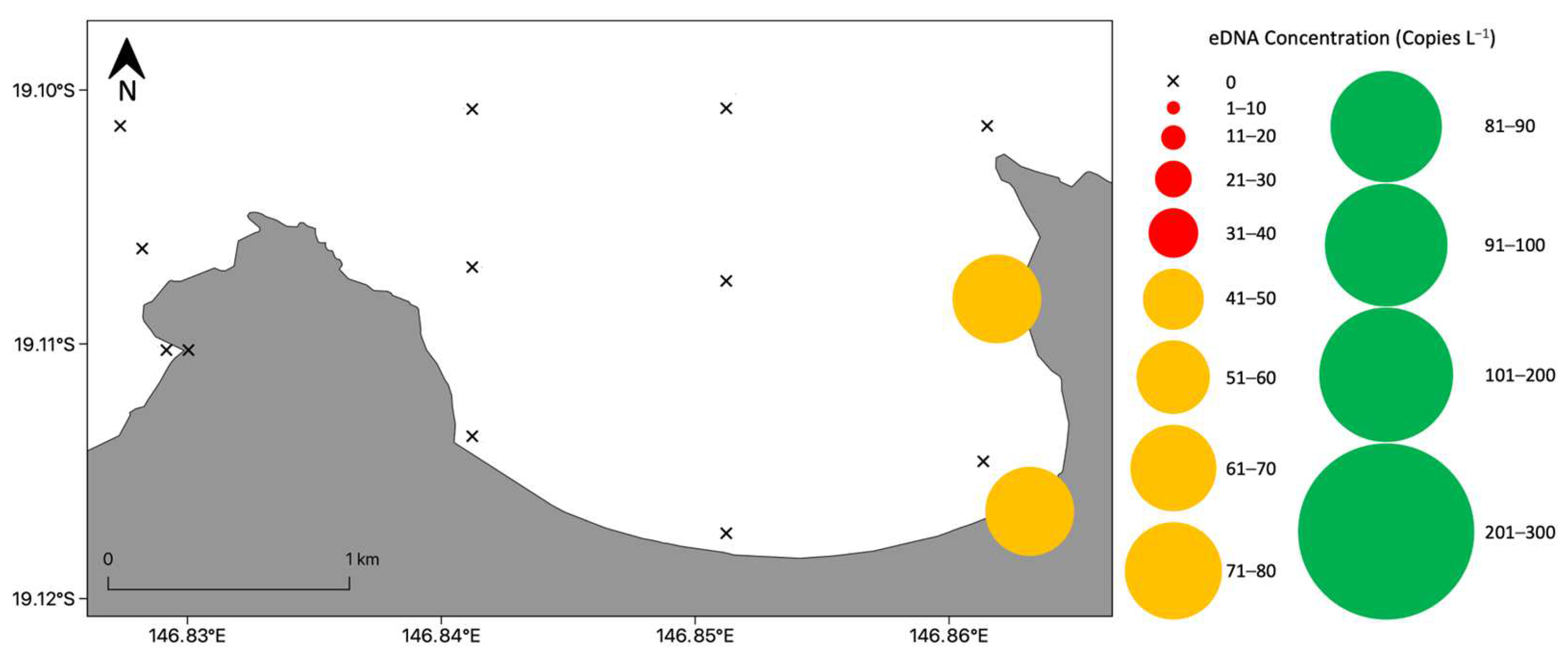
3.3. Detection and Distribution of Chironex fleckeri Polyps
3.3.1. Bay Wide Sampling Design for Chironex fleckeri Polyps
3.3.2. Targeted Sampling to Determine Chironex fleckeri Polyp Hotspots
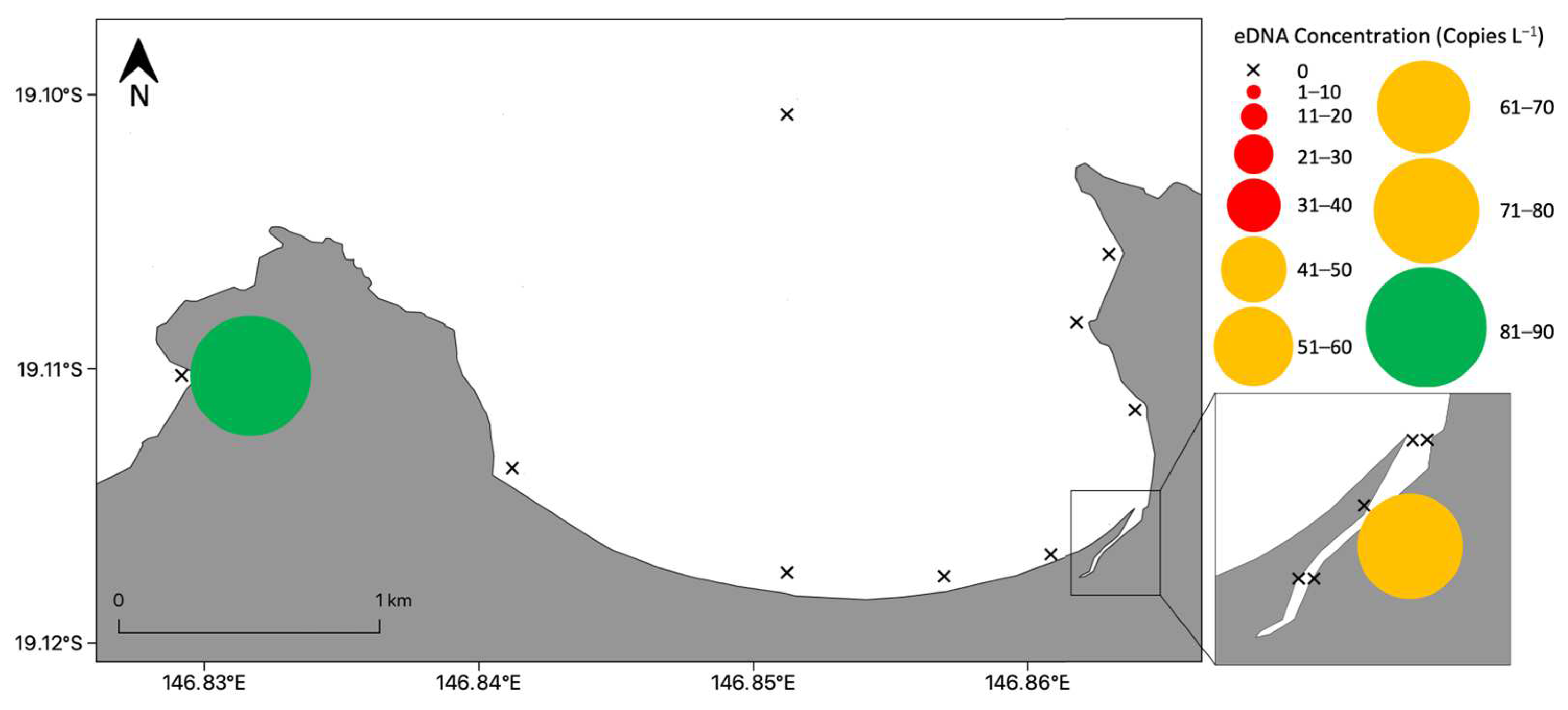
3.4. Detection of Chironex fleckeri near Shore at All Times
4. Discussion
4.1. Distribution of Chironex fleckeri Medusae
4.2. Detection of Chironex fleckeri Polyps
4.3. Evaluating Distributions of Chironex fleckeri Medusae and Polyps for Informed Stock Boundary Assessment and the Generality of eDNA for This Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kingsford, M.J.; Becken, S.; Bordehore, C.; Fuentes, V.L.; Pitt, K.A.; Yangihara, A.A. Empowering stakeholders to manage stinging jellyfish: A perspective. Coast. Manag. 2018, 46, 1–18. [Google Scholar] [CrossRef]
- Graham, W.M.; Gelcich, S.; Robinson, K.L.; Duarte, C.M.; Brotz, L.; Purcell, J.E.; Madin, L.P.; Mianzan, H.; Sutherland, K.R.; Uye, S.-I.; et al. Linking human well-being and jellyfish: Ecosystem services, impacts, and societal responses. Front. Ecol. Environ. 2014, 12, 515–523. [Google Scholar] [CrossRef]
- Lucas, C.H.; Gelcich, S.; Uye, S.-I. Living with Jellyfish: Management and Adaptation Strategies; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Gershwin, L.-A.; De Nardi, M.; Winkel, K.D.; Fenner, P.J. Marine stingers: Review of an under-recognized global coastal management issue. Coast. Manag. 2010, 38, 22–41. [Google Scholar] [CrossRef]
- De Donno, A.; Idolo, A.; Bagordo, F.; Grassi, T.; Leomanni, A.; Serio, F.; Guido, M.; Canitano, M.; Zampardi, S.; Boero, F. Impact of stinging jellyfish proliferations along south Italian coasts: Human health hazards, treatment and social costs. Int. J. Environ. Res. Public Health 2014, 11, 2488–2503. [Google Scholar] [CrossRef]
- Cegolon, L.; Heymann, W.C.; Lange, J.H.; Mastrangelo, G. Jellyfish stings and their management: A review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Belmar, M.; Milisenda, G.; Basso, L.; Doyle, T.K.; Leone, A.; Piraino, S. Jellyfish impacts on marine aquaculture and fisheries. Rev. Fish. Sci. Aquac. 2020, 29, 242–259. [Google Scholar] [CrossRef]
- Bordehore, C.; Alonso, C.; Sánchez-Fernández, L.; Canepa, A.; Acevedo, M.; Nogué, S.; Fuentes, V.L. Lifeguard assistance at Spanish Mediterranean beaches: Jellyfish prevail and proposals for improving risk management. Ocean. Coast. Manag. 2016, 131, 45–52. [Google Scholar] [CrossRef]
- Rodrigues, T.; Domínguez-Pérez, D.; Almeida, D.; Matos, A.; Antunes, A. Medusozoans reported in Portugal and its ecological and economical relevance. Reg. Stud. Mar. Sci. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Kennerley, A.; Wood, L.; Luisetti, T.; Ferrini, S.; Lorenzoni, I. Economic impacts of jellyfish blooms on coastal recreation in a UK coastal town and potential management options. Ocean. Coast. Manag. 2022, 227, 106284. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Mooney, C.J. The Ecology of Box Jellyfishes (Cubozoa). In Jellyfish Blooms; Springer: Berlin/Heidelberg, Germany, 2014; pp. 267–302. [Google Scholar]
- Fenner, P.J.; Harrison, S.L. Irukandji and Chironex fleckeri jellyfish envenomation in tropical Australia. Wilderness Environ. Med. 2000, 11, 233–240. [Google Scholar] [CrossRef]
- Currie, B.J.; Jacups, S.P. Prospective study of Chironex fleckeri and other box jellyfish stings in the “Top End” of Australia’s Northern Territory. Med. J. Aust. 2005, 183, 631–636. [Google Scholar] [CrossRef]
- Gershwin, L.-a.; Richardson, A.J.; Winkel, K.D.; Fenner, P.J.; Lippmann, J.; Hore, R.; Avila-Soria, G.; Brewer, D.; Kloser, R.J.; Steven, A. Biology and Ecology of Irukandji Jellyfish (Cnidaria: Cubozoa). In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 66, pp. 1–85. [Google Scholar]
- Crowley-Cyr, L.; Gershwin, L.-A. Protecting the Public from Hazardous Jellyfish: A Wicked Problem for Regulators and Operators? In The Cnidaria: Only a Problem or also a Resource? Nova Science Publishers: Hauppauge, NY, USA, 2021. [Google Scholar]
- Gershwin, L.-A.; Crowley-Cyr, L. Forecasting Hazardous Jellyfish: Shifting Perceptions from Black Swans Events to White. In The Cnidaria: Only a Problem or also a Resource? Nova Science Publishers: Hauppauge, NY, USA, 2021. [Google Scholar]
- Kingsford, M.J.; Schlaefer, J.A.; Morrissey, S.J. Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish. Diversity 2021, 13, 174. [Google Scholar] [CrossRef]
- Courtney, R.; Browning, S.; Seymour, J. Early Life History of the ‘Irukandji’ Jellyfish Carukia barnesi. PLoS ONE 2016, 11, e0151197. [Google Scholar] [CrossRef]
- Arai, M.N. A Functional Biology of Scyphozoa; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Courtney, R.; Seymour, J. Seasonality in Polyps of a Tropical Cubozoan: A latina nr mordens. PLoS ONE 2013, 8, e69369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Collins, A.G.; Jarms, G. WoRMS Cubozoa: World List of Cubozoa (Version 2018-04-01). In Species 2000 & ITIS Catalogue of Life, 2018 Annual Checklist; Roskov, Y., Abucay, L., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., et al., Eds.; Species; Naturalis: Leiden, The Netherlands, 2000; Available online: www.catalogueoflife.org/annual-checklist/2018 (accessed on 2 February 2021).
- Mooney, C.J.; Kingsford, M.J. Discriminating populations of medusae (Chironex fleckeri, Cubozoa) using statolith microchemistry. Mar. Freshw. Res. 2017, 68, 1144–1152. [Google Scholar] [CrossRef]
- Mooney, C.J.; Kingsford, M.J. Statolith morphometrics as a tool to distinguish among populations of three cubozoan species. Hydrobiologia 2017, 787, 111–121. [Google Scholar] [CrossRef]
- Schlaefer, J.A.; Wolanski, E.; Lambrechts, J.; Kingsford, M.J. Behavioural and oceanographic isolation of an island-based jellyfish (Copula sivickisi, Class Cubozoa) population. Sci. Rep. 2021, 11, 10280. [Google Scholar] [CrossRef] [PubMed]
- Schlaefer, J.A.; Wolanski, E.; Yadav, S.; Kingsford, M.J. Behavioural maintenance of highly localised jellyfish (Copula sivickisi, class Cubozoa) populations. Mar. Biol. 2020, 167, 40. [Google Scholar] [CrossRef]
- Schlaefer, J.A.; Wolanski, E.; Kingsford, M.J. Swimming behaviour can maintain localised jellyfish (Chironex fleckeri: Cubozoa) populations. Mar. Ecol. Prog. Ser. 2018, 591, 287–302. [Google Scholar] [CrossRef]
- Bolte, B.; Goldsbury, J.; Huerlimann, R.; Jerry, D.; Kingsford, M. Validation of eDNA as a viable method of detection for dangerous cubozoan jellyfish. Environ. DNA 2021, 3, 769–779. [Google Scholar] [CrossRef]
- Morrissey, S.J.; Jerry, D.; Kingsford, M. Use of eDNA to test hypotheses on the ecology of Chironex fleckeri (Cubozoa). Mar. Ecol. Prog. Ser. 2024, 728, 25–41. [Google Scholar] [CrossRef]
- Morrissey, S.J.; Jerry, D.R.; Kingsford, M.J. Genetic Detection and a Method to Study the Ecology of Deadly Cubozoan Jellyfish. Diversity 2022, 14, 1139. [Google Scholar] [CrossRef]
- Anzama, Y.; Oka, S.; Teruya, M.; Toshino, S.; Tanimoto, M.; Hanahara, N.; Kuba, Y.; Miyagi, A.; Fukuchi, Y. Real-time PCR assay for detecting environmental DNA of box jellyfish. J. Hyg. Anim. Health 2023, 74, 13–20. [Google Scholar]
- Sathirapongsasuti, N.; Khonchom, K.; Poonsawat, T.; Pransilpa, M.; Ongsara, S.; Detsri, U.; Bungbai, S.; Lawanangkoon, S.-A.; Pattanaporkrattana, W.; Trakulsrichai, S. Rapid and Accurate Species-Specific PCR for the Identification of Lethal Chironex Box Jellyfish in Thailand. Int. J. Environ. Res. Public Health 2021, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.W. Chironex Fleckeri: Distribution and Movements Around Magnetic Island; TW Brown: North Queensland, Australia, 1973. [Google Scholar]
- Kingsford, M.; Seymour, J.; O’Callaghan, M. Abundance Patterns of Cubozoans on and near the Great Barrier Reef. In Jellyfish Blooms IV; Springer: Berlin/Heidelberg, Germany, 2012; pp. 257–268. [Google Scholar]
- Hartwick, R. Distributional Ecology and Behaviour of the Early Life Stages of the Box-Jellyfish Chironex Fleckeri. In Coelenterate Biology: Recent Research on Cnidaria and Ctenophora; Springer: Berlin/Heidelberg, Germany, 1991; pp. 181–188. [Google Scholar]
- Cutress, C.; Studebaker, J. Development of the Cubome-Dusae, Carybdea Marsupials. Proc. Assoc. Isl. Mar. Lab. Caribb. 1973, 9, 25. [Google Scholar]
- Gordon, M.; Seymour, J. Growth, development and temporal variation in the onset of six Chironex fleckeri medusae seasons: A contribution to understanding jellyfish ecology. PLoS ONE 2012, 7, e31277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mooney, C.; Kingsford, M. Sources and Movements of Chironex Fleckeri Medusae Using Statolith Elemental Chemistry. In Jellyfish Blooms IV; Springer: Berlin/Heidelberg, Germany, 2012; pp. 269–277. [Google Scholar]
- Jeunen, G.J.; Lamare, M.D.; Knapp, M.; Spencer, H.G.; Taylor, H.R.; Stat, M.; Bunce, M.; Gemmell, N.J. Water stratification in the marine biome restricts vertical environmental DNA (eDNA) signal dispersal. Environ. DNA 2020, 2, 99–111. [Google Scholar] [CrossRef]
- Gray, C.A.; Kingsford, M.J. Variability in thermocline depth and strength, and relationships with vertical distributions of fish larvae and mesozooplankton in dynamic coastal waters. Mar. Ecol. Prog. Ser. 2003, 247, 211–224. [Google Scholar] [CrossRef][Green Version]
- Edmunds, R.C.; Burrows, D. Got Glycogen?: Development and Multispecies Validation of the Novel Preserve, Precipitate, Lyse, Precipitate, Purify (PPLPP) Workflow for Environmental DNA Extraction from Longmire’s Preserved Water Samples. J. Biomol. Tech. JBT 2020, 31, 125. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.K.; Huerlimann, R.; Edmunds, R.C.; Budd, A.M.; Le Port, A.; Kyne, P.M.; Jerry, D.R.; Simpfendorfer, C.A. Improved detection sensitivity using an optimal eDNA preservation and extraction workflow and its application to threatened sawfishes. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2021, 31, 2131–2148. [Google Scholar] [CrossRef]
- Budd, A.M.; Cooper, M.K.; Le Port, A.; Schils, T.; Mills, M.S.; Deinhart, M.E.; Huerlimann, R.; Strugnell, J.M. First detection of critically endangered scalloped hammerhead sharks (Sphyrna lewini) in Guam, Micronesia, in five decades using environmental DNA. Ecol. Indic. 2021, 127, 107649. [Google Scholar] [CrossRef]
- Trujillo-González, A.; Edmunds, R.; Becker, J.; Hutson, K. Parasite detection in the ornamental fish trade using environmental DNA. Sci. Rep. 2019, 9, 5173. [Google Scholar] [CrossRef] [PubMed]
- Bessell, T.J.; Appleyard, S.A.; Stuart-Smith, R.D.; Johnson, O.J.; Ling, S.D.; Heather, F.J.; Lynch, T.P.; Barrett, N.S.; Stuart-Smith, J. Using eDNA and SCUBA surveys for detection and monitoring of a threatened marine cryptic fish. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2023, 33, 431–442. [Google Scholar] [CrossRef]
- Villacorta-Rath, C.; Espinoza, T.; Cockayne, B.; Schaffer, J.; Burrows, D. Environmental DNA analysis confirms extant populations of the cryptic Irwin’s turtle within its historical range. BMC Ecol. Evol. 2022, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Lahoz-Monfort, J.J.; Guillera-Arroita, G.; Tingley, R. Statistical approaches to account for false-positive errors in environmental DNA samples. Mol. Ecol. Resour. 2016, 16, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, C.S.; Sepulveda, A.; Ray, A.; Baumgardt, J.; Waits, L.P. Environmental DNA as a new method for early detection of New Zealand mudsnails (Potamopyrgus antipodarum). Freshw. Sci. 2013, 32, 792–800. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Kielgast, J.; Iversen, L.L.; Møller, P.R.; Rasmussen, M.; Willerslev, E. Detection of a diverse marine fish fauna using environmental DNA from seawater samples. PLoS ONE 2012, 7, e41732. [Google Scholar] [CrossRef]
- Congram, M.; Torres Vilaça, S.; Wilson, C.C.; Kyle, C.J.; Lesbarrères, D.; Wikston, M.J.; Beaty, L.; Murray, D.L. Tracking the prevalence of a fungal pathogen, Batrachochytrium dendrobatidis (chytrid fungus), using environmental DNA. Environ. DNA 2022, 4, 687–699. [Google Scholar] [CrossRef]
- Harrison, J.B.; Sunday, J.M.; Rogers, S.M. Predicting the fate of eDNA in the environment and implications for studying biodiversity. Proc. R. Soc. B 2019, 286, 20191409. [Google Scholar] [CrossRef]
- Shogren, A.J.; Tank, J.L.; Egan, S.P.; Bolster, D.; Riis, T. Riverine distribution of mussel environmental DNA reflects a balance among density, transport, and removal processes. Freshw. Biol. 2019, 64, 1467–1479. [Google Scholar] [CrossRef]
- Stoeckle, B.C.; Beggel, S.; Kuehn, R.; Geist, J. Influence of stream characteristics and population size on downstream transport of freshwater mollusk environmental DNA. Freshw. Sci. 2021, 40, 191–201. [Google Scholar] [CrossRef]
- Shogren, A.J.; Tank, J.L.; Egan, S.P.; August, O.; Rosi, E.J.; Hanrahan, B.R.; Renshaw, M.A.; Gantz, C.A.; Bolster, D. Water flow and biofilm cover influence environmental DNA detection in recirculating streams. Environ. Sci. Technol. 2018, 52, 8530–8537. [Google Scholar] [CrossRef]
- Pont, D.; Rocle, M.; Valentini, A.; Civade, R.; Jean, P.; Maire, A.; Roset, N.; Schabuss, M.; Zornig, H.; Dejean, T. Environmental DNA reveals quantitative patterns of fish biodiversity in large rivers despite its downstream transportation. Sci. Rep. 2018, 8, 10361. [Google Scholar] [CrossRef]
- Ellis, M.R.; Clark, Z.S.; Treml, E.A.; Brown, M.S.; Matthews, T.G.; Pocklington, J.B.; Stafford-Bell, R.E.; Bott, N.J.; Nai, Y.H.; Miller, A.D. Detecting marine pests using environmental DNA and biophysical models. Sci. Total Environ. 2022, 816, 151666. [Google Scholar] [CrossRef]
- Andruszkiewicz, E.A.; Koseff, J.R.; Fringer, O.B.; Ouellette, N.T.; Lowe, A.B.; Edwards, C.A.; Boehm, A.B. Modeling environmental DNA transport in the coastal ocean using Lagrangian particle tracking. Front. Mar. Sci. 2019, 6, 477. [Google Scholar] [CrossRef]
- West, K.; Travers, M.J.; Stat, M.; Harvey, E.S.; Richards, Z.T.; DiBattista, J.D.; Newman, S.J.; Harry, A.; Skepper, C.L.; Heydenrych, M. Large-scale eDNA metabarcoding survey reveals marine biogeographic break and transitions over tropical north-western Australia. Divers. Distrib. 2021, 27, 1942–1957. [Google Scholar] [CrossRef]
- Albonetti, L.; Maiello, G.; Cariani, A.; Carpentieri, P.; Ferrari, A.; Sbrana, A.; Shum, P.; Talarico, L.; Russo, T.; Mariani, S. DNA metabarcoding of trawling bycatch reveals diversity and distribution patterns of sharks and rays in the central Tyrrhenian Sea. ICES J. Mar. Sci. 2023, 80, 664–674. [Google Scholar] [CrossRef]
- Fukumoto, S.; Ushimaru, A.; Minamoto, T. A basin-scale application of environmental DNA assessment for rare endemic species and closely related exotic species in rivers: A case study of giant salamanders in Japan. J. Appl. Ecol. 2015, 52, 358–365. [Google Scholar] [CrossRef]
- Morrissey, S.J.; Yanagihara, A.A.; Kingsford, M.J. Utility of statolith elemental chemistry as a proxy for temperature to elucidate the movements of the Irukandji jellyfish species Alatina alata. Mar. Biol. 2020, 167, 134. [Google Scholar] [CrossRef]
- Morrissey, S.J.; Schlaefer, J.A.; Kingsford, M.J. Experimental validation of the relationships between cubozoan statolith elemental chemistry and salinity and temperature. J. Exp. Mar. Biol. Ecol. 2020, 527, 151375. [Google Scholar] [CrossRef]
- Gordon, M.; Seymour, J. Quantifying movement of the tropical Australian cubozoan Chironex fleckeri using acoustic telemetry. Hydrobiologia 2009, 616, 87–97. [Google Scholar] [CrossRef]
- Carrette, T.; Alderslade, P.; Seymour, J. Nematocyst ratio and prey in two Australian cubomedusans, Chironex fleckeri and Chiropsalmus sp. Toxicon 2002, 40, 1547–1551. [Google Scholar] [CrossRef]
- Robertson, A.; Duke, N. Mangroves as nursery sites: Comparisons of the abundance and species composition of fish and crustaceans in mangroves and other nearshore habitats in tropical Australia. Mar. Biol. 1987, 96, 193–205. [Google Scholar] [CrossRef]
- Mooney, C.J.; Kingsford, M.J. The influence of salinity on box jellyfish (Chironex fleckeri, Cubozoa) statolith elemental chemistry. Mar. Biol. 2016, 163, 103. [Google Scholar] [CrossRef]
- Colin, S.P.; Kremer, P. Population maintenance of the scyphozoan Cyanea sp. settled planulae and the distribution of medusae in the Niantic River, Connecticut, USA. Estuaries 2002, 25, 70–75. [Google Scholar] [CrossRef][Green Version]
- Toyokawa, M.; Aoki, K.; Yamada, S.; Yasuda, A.; Murata, Y.; Kikuchi, T. Distribution of ephyrae and polyps of jellyfish Aurelia aurita (Linnaeus 1758) sensu lato in Mikawa Bay, Japan. J. Oceanogr. 2011, 67, 209–218. [Google Scholar] [CrossRef]
- Yates, M.C.; Derry, A.M.; Cristescu, M.E. Environmental RNA: A revolution in ecological resolution? Trends Ecol. Evol. 2021, 36, 601–609. [Google Scholar] [CrossRef]
- Parsley, M.B.; Goldberg, C.S. Environmental RNA can distinguish life stages in amphibian populations. Mol. Ecol. Resour. 2023. [Google Scholar] [CrossRef]
- Shahrestani, S.; Bi, H. Settlement and survival of Chrysaora chesapeakei polyps: Implications for adult abundance. Mar. Ecol. Prog. Ser. 2018, 601, 139–151. [Google Scholar] [CrossRef]
- Siebert, S.; Juliano, C.E. Sex, polyps, and medusae: Determination and maintenance of sex in cnidarians. Mol. Reprod. Dev. 2017, 84, 105–119. [Google Scholar] [CrossRef]
- Yamaguchi, M. Early life history of the sea wasp, Chironex fleckeri (Class Cubozoa). Dev. Cell. Biol. Coelenterates 1980, 11–16. [Google Scholar]
- Adams, C.I.; Knapp, M.; Gemmell, N.J.; Jeunen, G.-J.; Bunce, M.; Lamare, M.D.; Taylor, H.R. Beyond biodiversity: Can environmental DNA (eDNA) cut it as a population genetics tool? Genes 2019, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Andres, K.J.; Lodge, D.M.; Sethi, S.A.; Andrés, J. Detecting and analysing intraspecific genetic variation with eDNA: From population genetics to species abundance. Mol. Ecol. 2023, 32, 4118–4132. [Google Scholar] [CrossRef] [PubMed]
- Zanovello, L.; Girardi, M.; Marchesini, A.; Galla, G.; Casari, S.; Micheletti, D.; Endrizzi, S.; Fedrigotti, C.; Pedrini, P.; Bertorelle, G. A validated protocol for eDNA-based monitoring of within-species genetic diversity in a pond-breeding amphibian. Sci. Rep. 2023, 13, 4346. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrissey, S.J.; Jerry, D.R.; Kingsford, M.J. Use of eDNA to Determine Source Locations of Deadly Jellyfish (Cubozoa) in an Open Coastal System. Coasts 2024, 4, 198-212. https://doi.org/10.3390/coasts4010011
Morrissey SJ, Jerry DR, Kingsford MJ. Use of eDNA to Determine Source Locations of Deadly Jellyfish (Cubozoa) in an Open Coastal System. Coasts. 2024; 4(1):198-212. https://doi.org/10.3390/coasts4010011
Chicago/Turabian StyleMorrissey, Scott J., Dean R. Jerry, and Michael J. Kingsford. 2024. "Use of eDNA to Determine Source Locations of Deadly Jellyfish (Cubozoa) in an Open Coastal System" Coasts 4, no. 1: 198-212. https://doi.org/10.3390/coasts4010011
APA StyleMorrissey, S. J., Jerry, D. R., & Kingsford, M. J. (2024). Use of eDNA to Determine Source Locations of Deadly Jellyfish (Cubozoa) in an Open Coastal System. Coasts, 4(1), 198-212. https://doi.org/10.3390/coasts4010011






