Ecosystem Services Provided by Kelp Forests of the Humboldt Current System: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
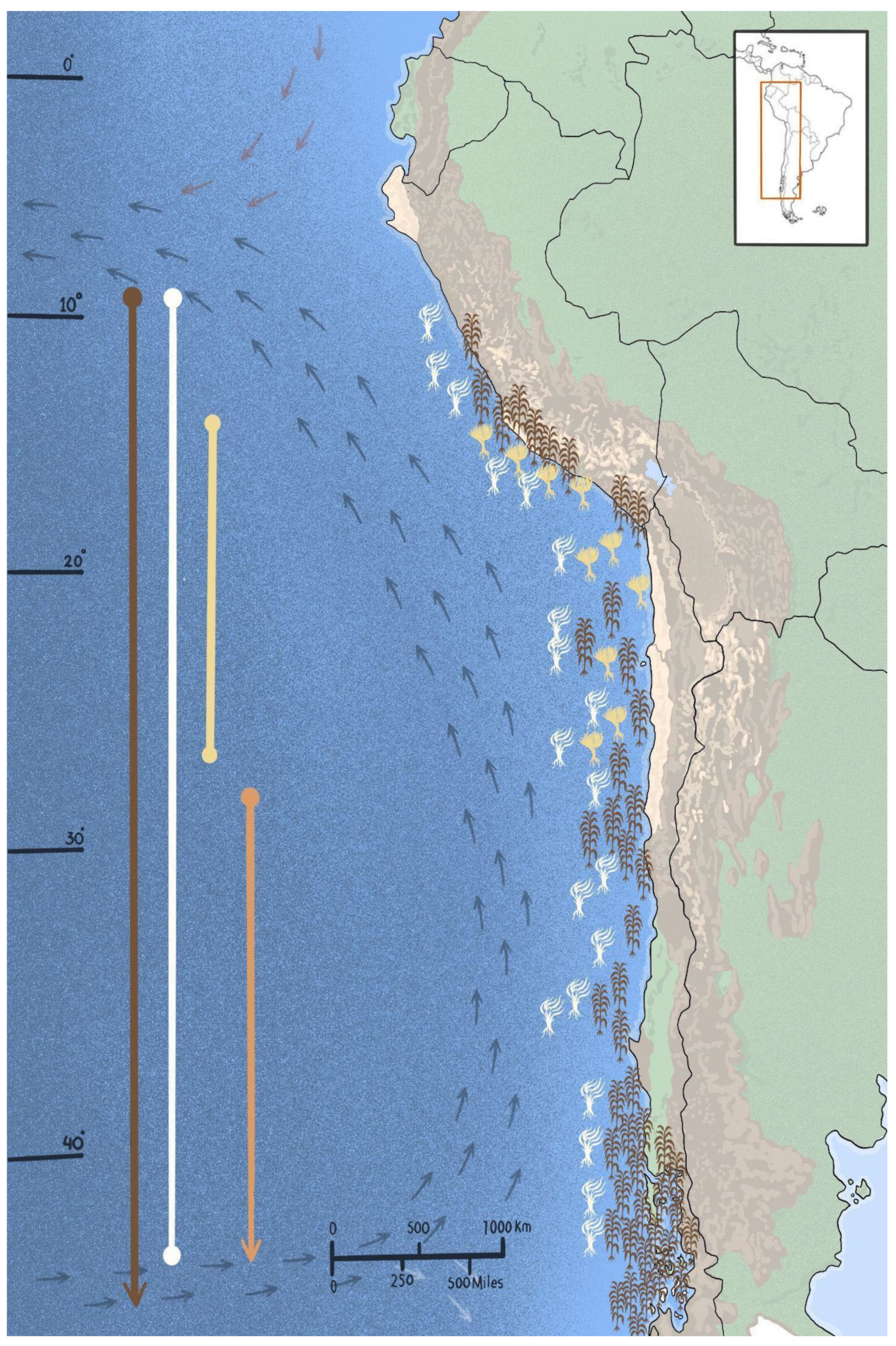
3.1. The Forest-Forming Species of the Humboldt Current System
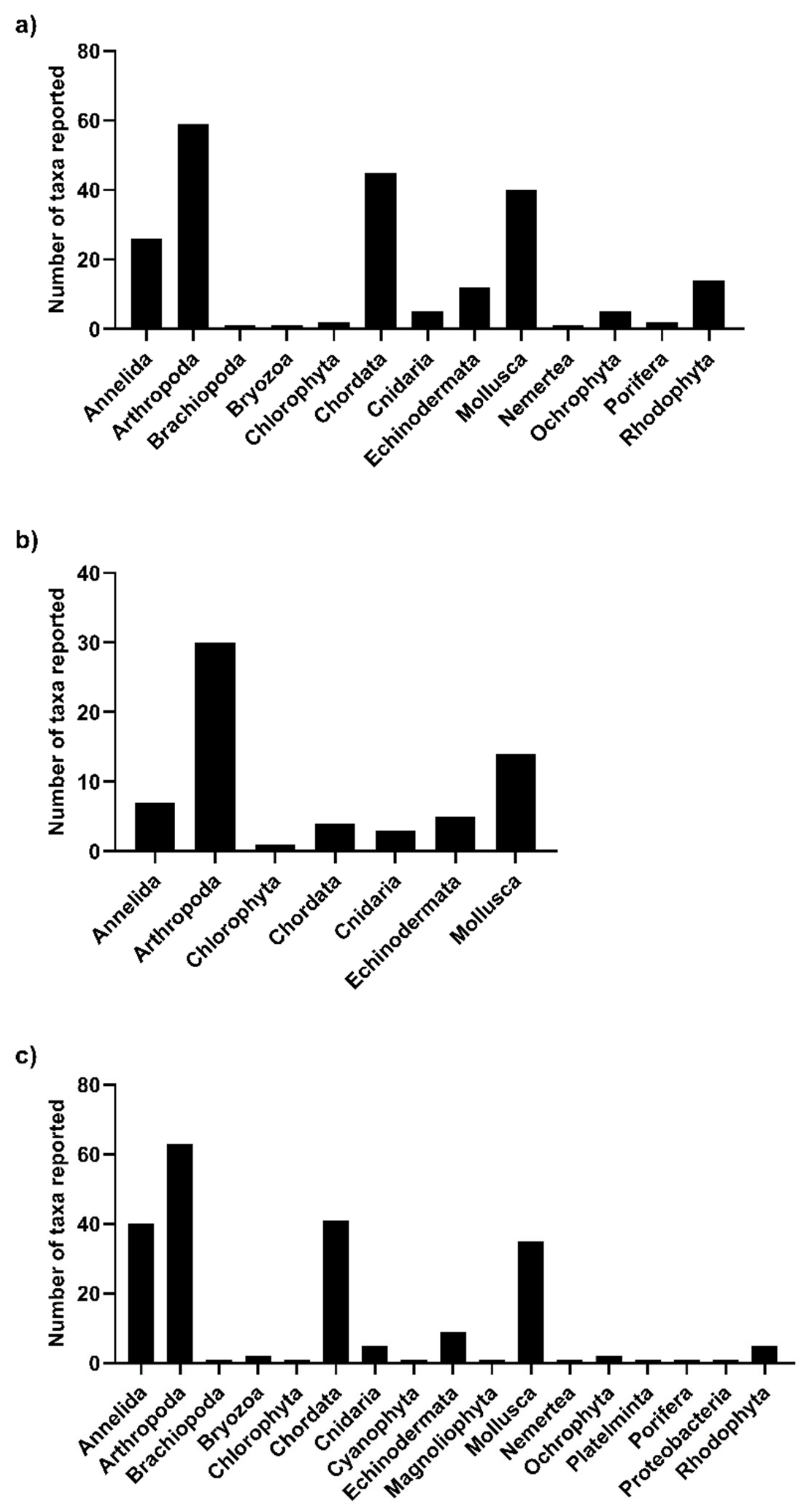
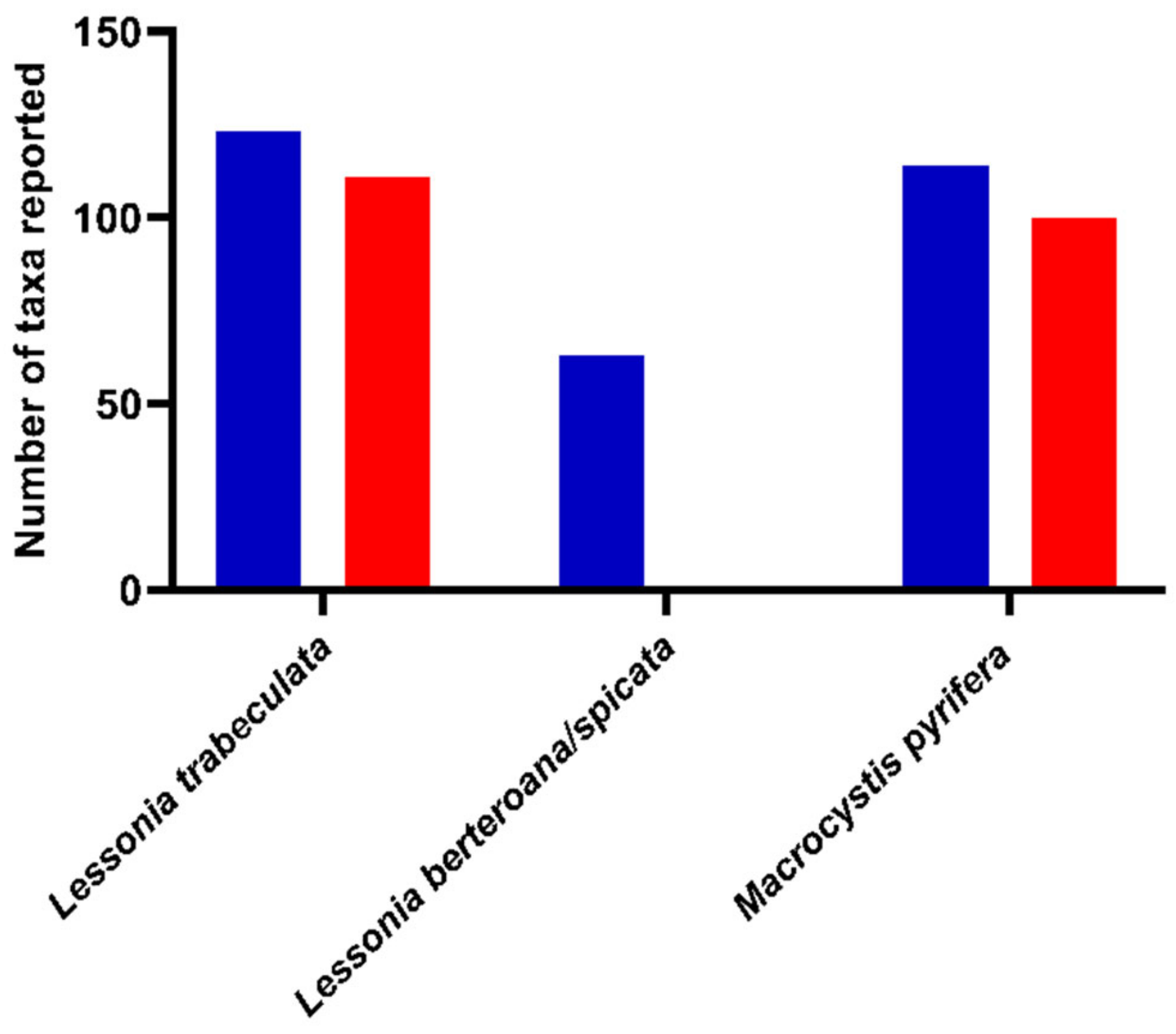
3.2. Overall Search
3.3. General Ecosystem Services of Humboldtian Kelp Forests
3.4. Supporting and Regulating Services
3.5. Provisioning Services and Economical Benefits
3.6. Cultural Services
3.7. Threats and Gaps
4. Conclusions
Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Wetlands and Water; World Resources Institute: Washington, DC, USA, 2005; Available online: https://www.millenniumassessment.org/documents/document.358.aspx.pdf (accessed on 23 July 2022).
- Danley, B.; Widmark, C. Evaluating conceptual definitions of ecosystem services and their implications. Ecol. Econ. 2016, 126, 132–138. [Google Scholar] [CrossRef]
- De la Barrera, F.; Bachmann-Vargas, P.; Tironi, A. La investigación de servicios ecosistémicos en Chile: Una revisión sistemática. Investig. Geogr. 2015, 50, 3–18. [Google Scholar] [CrossRef]
- MINAM. Sexto Informe Nacional Sobre Diversidad Biológica: La Biodiversidad en Cifras; Ministerio del Ambiente: Lima, Peru, 2019. Available online: https://cdn.www.gob.pe/uploads/document/file/360831/La_Biodiversidad_en_Cifras_final.pdf (accessed on 23 July 2022).
- Phelan, A.; Ruhanen, L.; Mair, J. Ecosystem services approach for community-based ecotourism: Towards an equitable and sustainable blue economy. J. Sustain. Tour. 2020, 28, 1665–1685. [Google Scholar] [CrossRef]
- Rakovic, J.; Futter, M.N.; Kyllmar, K.; Rankinen, K.; Stutter, M.I.; Vermaat, J.; Collentine, D. Nordic Bioeconomy Pathways: Future narratives for assessment of water-related ecosystem services in agricultural and forest management. Ambio 2020, 49, 1710–1721. [Google Scholar] [CrossRef]
- D’Armato, D.; Gaio, M.; Semenzin, E. A review of LCA assessments of forest-based bioeconomy products and processes under an ecosystem services perspective. Sci. Total Environ. 2020, 706, 135859. [Google Scholar]
- Deng, C.; Liu, J.; Nie, X.; Li, Z.; Liu, Y.; Xiao, H.; Hu, X.; Wang, L.; Zhang, Y.; Zhang, G.; et al. How trade-offs between ecological construction and urbanization expansion affect ecosystem services. Ecol. Indic. 2021, 122, 107253. [Google Scholar] [CrossRef]
- Eddy, T.D.; Lam, V.W.Y.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Greer, K.; Palomares, M.L.D.; Bruno, J.F.; Ota, Y.; Cheung, W.W.L. Global Decline in Capacity of Coral Reefs to Provide Ecosystem Services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]
- Del Río-Mena, T.; Willemen, L.; Tesfamariam, G.T.; Beukes, O.; Nelson, A. Remote sensing for mapping ecosystem services to support evaluation of ecological restoration interventions in an arid landscape. Ecol. Indic. 2020, 113, 106182. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Hernandez, M.E. Ecosystem services of wetlands. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 1–4. [Google Scholar] [CrossRef]
- Mtwana Nordlund, L.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass ecosystem services and their variability across genera and geographical regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Woodhead, A.J.; Hicks, C.C.; Norström, A.V.; Williams, G.J.; Graham, N.A. Coral reef ecosystem services in the Anthropocene. Funct. Ecol. 2019, 33, 1023–1034. [Google Scholar] [CrossRef]
- De Paula, J.C.; Lopes-Filho, E.A.P.; De Carvalho, W.F.; De Souza, C.A.C.; Yoneshigue-Valentin, Y. Long-term changes in macroalgae assemblages reveal a gradual biodiversity loss over the last 200 years in the hypereutrophic Guanabara Bay. Mar. Environ. Res. 2020, 162, 105153. [Google Scholar] [CrossRef]
- Fulton, C.J.; Abesamis, R.A.; Berkström, C.; Depczynski, M.; Graham, N.A.J.; Holmes, T.H.; Kulbicki, M.; Noble, M.M.; Radford, B.T.; Tano, S.; et al. Form and function of tropical macroalgal reefs in the Anthropocene. Funct. Ecol. 2019, 33, 989–999. [Google Scholar] [CrossRef]
- Smale, D.A.; Burrows, M.T.; Moore, P.; O’Connor, N.; Hawkins, S.J. Threats and knowledge gaps for ecosystem services provided by kelp forests: A northeast Atlantic perspective. Ecol. Evol. 2013, 3, 4016–4038. [Google Scholar] [CrossRef]
- D’Agata, S. Ecosystems services at risk. Nat. Clim. Change 2022, 12, 13–14. [Google Scholar] [CrossRef]
- Constanza, R. La economía ecológica de la sostenibilidad: Invertir en capital natural. In Medio Ambiente y Desarrollo Sostenible: Más allá del Informe Brundtland; Editorial Trotta: Madrid, Spain, 1997; pp. 103–114. [Google Scholar]
- Pendleton, L.H.; Thébaud, O.; Mongruel, R.C.; Levrel, H. Has the value of global marine and coastal ecosystem services changed? Mar. Policy 2016, 64, 156–158. [Google Scholar] [CrossRef]
- Berrios, F.; Campbell, D.E.; Ortiz, M. Emergy evaluation of benthic ecosystems influenced by upwelling in northern Chile: Contributions of the ecosystems to the regional economy. Ecol. Modell. 2017, 359, 146–164. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Zuñiga, S.; Tala, F.; Piaget, N.; Rodríguez, D.C.; Vega, J.M. Economic valuation of kelp forests in northern Chile: Values of goods and services of the ecosystem. J. Appl. Phycol. 2014, 26, 1081–1088. [Google Scholar] [CrossRef]
- Fraser, C.I. Is bull-kelp kelp? The role of common names in science. N. Z. J. Mar. Freshwater Res. 2012, 46, 279–284. [Google Scholar] [CrossRef]
- Graham, M.H.; Vásquez, J.A.; Buschmann, A.H. Global ecology of the giant kelp Macrocystis: From ecotypes to ecosystems. Oceanogr. Mar. Biol. 2007, 45, 39–88. [Google Scholar]
- Kilinç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2013; pp. 735–748. [Google Scholar]
- Hasselström, L.; Thomas, J.B.; Nordström, J.; Cervin, G.; Nylund, G.M.; Pavia, H.; Gröndahl, F. Socio Economic prospects of a seaweed bioeconomy in Sweden. Sci. Rep. 2020, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Afonso, N.C.; Catarino, M.D.; Silva, A.; Cardoso, S.M. Brown Macroalgae as valuable food ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Murie, K.A.; Bourdeau, P.E. Fragmented kelp forest canopies retain their ability to alter local seawater chemistry. Sci. Rep. 2020, 10, 11939. [Google Scholar] [CrossRef] [PubMed]
- Bayley, D.; Brickle, P.; Brewin, P.; Golding, N.; Pelembe, T. Valuation of kelp forest ecosystem services in the Falkland Islands: A case study integrating blue carbon sequestration potential. One Ecosyst. 2021, 6, e62811. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Substantial blue carbon in overlooked Australian kelp forests. Sci. Rep. 2020, 10, 1–6. [Google Scholar]
- Rosman, J.H.; Koseff, J.R.; Monismith, S.G.; Grover, J. A field investigation into the effects of a kelp forest (Macrocystis pyrifera) on coastal hydrodynamics and transport. J. Geophys. Res. Oceans 2007, 112, C2. [Google Scholar] [CrossRef]
- Miller, R.J.; Lafferty, K.D.; Lamy, T.; Kui, L.; Rassweiler, A.; Reed, D.C. Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc. Royal Soc. B 2018, 285, 20172571. [Google Scholar] [CrossRef]
- Ospina-Alvarez, A.; De Juan, S.; Davis, K.J.; González, C.; Fernández, M.; Navarrete, S.A. Integration of biophysical connectivity in the spatial optimization of coastal ecosystem services. Sci. Total Environ. 2020, 733, 139367. [Google Scholar] [CrossRef]
- Eckman, J.E.; Duggins, D.O.; Sewell, A.T. Ecology of understory kelp environments. I. Effects of kelps on flow and particle transport near the bottom. J. Exp. Mar. Biol. Ecol. 1989, 129, 173–187. [Google Scholar] [CrossRef]
- Santos, I.R.; Burdige, D.J.; Jennerjahn, T.C.; Bouillon, S.; Cabral, A.; Serrano, O.; Wernberg, T.; Filbee-Dexter, K.; Guimond, J.A.; Tamborski, J.J. The renaissance of Odum’s outwelling hypothesis in ‘Blue Carbon’ science. Estuar. Coast. Shelf Sci. 2021, 255, 107361. [Google Scholar] [CrossRef]
- Ortiz, M.; Campos, L.; Berrios, F.; Rodriguez, F.; Hermosillo, B.; González, J. Network properties and keystoneness assessment in different intertidal communities dominated by two ecosystem engineer species (SE Pacific coast): A comparative analysis. Ecol. Modell. 2013, 250, 307–318. [Google Scholar] [CrossRef]
- Pérez-Matus, A.; Carrasco, S.A.; Ospina-Alvarez, A. Relaciones de longitud-peso para 25 peces costeros asociados a macroalgas pardas del centro y norte de Chile. Rev. Biol. Mar. Oceanogr. 2014, 49, 141–145. [Google Scholar]
- Uribe, R.A.; Ortiz, M.; Macaya, E.C.; Pacheco, A.S. Successional patterns of hard-bottom macrobenthic communities at kelp bed (Lessonia trabeculata) and barren ground sublittoral systems. J. Exp. Mar. Biol. Ecol. 2015, 472, 180–188. [Google Scholar] [CrossRef]
- Trujillo, J.E.; Pardo, L.M.; Vargas-Chacoff, L.; Valdivia, N. Sharks in the forest: Relationships between kelp physical-complexity attributes and egg deposition sites of the red-spotted catshark. Mar. Ecol. Prog. Ser. 2019, 610, 125–135. [Google Scholar] [CrossRef]
- Dayton, P.K.; Tegner, M.J.; Parnell, P.E.; Edwards, P.B. Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecol. Monogr. 1992, 62, 421–445. [Google Scholar] [CrossRef]
- Edwards, M.S. Estimating scale-dependency in disturbance impacts: El Niño and giant kelp forests in the northeast Pacific. Oecologia 2004, 138, 436–447. [Google Scholar] [CrossRef]
- Scheibling, R.E.; Feehan, C.J.; Lauzon-Guay, J.S. Climate change, disease and the dynamics of a kelp-bed ecosystem in Nova Scotia. In Climate Change Perspectives from the Atlantic: Past, Present and Future; Fernández-Palacios, J.M., De Nascimiento, L., Hernández, J.C., Clemente, S., Gonzáles, A., Díaz-Gonzáles, J.P., Eds.; Servicio de Publicaciones; Universidad de La Laguna: San Cristobal de La Laguna, Spain, 2013; pp. 361–387. [Google Scholar]
- Wernberg, T.; Kendrick, G.A.; Toohey, B.D. Modification of the physical environment by an Ecklonia radiata (Laminariales) canopy and implications for associated foliose algae. Aquat. Ecol. 2005, 39, 419–430. [Google Scholar] [CrossRef]
- Uribe, R.A.; Ortiz, M.; Pacheco, A.S.; Araya, R. Early succession of micro-periphyton communities in kelp bed and barren ground ecological systems. Mar. Ecol. 2015, 36, 1415–1427. [Google Scholar] [CrossRef]
- Carbajal, P.; Arakaki, N.; Perez-Araneda, K.; Tellier, F. Morphological, Genetic, and ecological differences among the low-latitude kelps Eisenia cokeri and E. gracilis. 12th Int. Phycol. Congr. Phycol. 2021, 60 (Suppl. 1), 27. [Google Scholar]
- Cronin, P.; Ryan, F.; Coughlan, M. Undertaking a literature review: A step-by-step approach. Br. J. Nurs. 2008, 17, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Mengist, W.; Soromessa, T.; Legese, G. Method for conducting systematic literature review and meta-analysis for environmental science research. MethodsX 2020, 7, 100777. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Costanza, R.; Fisher, B.; Mulder, K.; Liu, S.; Christopher, T. Biodiversity and ecosystem services: A multi-scale empirical study of the relationship between species richness and net primary production. Ecol. Econ. 2007, 61, 478–491. [Google Scholar] [CrossRef]
- Bastian, O. The role of biodiversity in supporting ecosystem services in Natura 2000 sites. Ecol. Indic. 2013, 24, 12–22. [Google Scholar] [CrossRef]
- Boyd, J.; Banzhaf, S. What are ecosystem services? The need for standardized environmental accounting units. Ecol. Econ. 2007, 63, 616–626. [Google Scholar] [CrossRef]
- Hynes, S.; Chen, W.; Vondolia, K.; Armstrong, C.; O’Connor, E. Valuing the Ecosystem Service Benefits from Kelp Forest Restoration: A Choice Experiment from Norway. Ecol. Econ. 2021, 179, 106833. [Google Scholar] [CrossRef]
- Balvanera, P.; Quijas, S.; Karp, D.S.; Ash, N.; Bennett, E.M.; Boumans, R.; Brown, C.; Chan, K.M.A.; Chaplin-Kramer, R.; Halpern, B.S.; et al. Ecosystem services. In The GEO Handbook on Biodiversity Observation Networks; Walters, M., Scholes, R.J., Eds.; Springer: Cham, Switzeland, 2017; pp. 39–78. [Google Scholar]
- Mayer, A.; Kaufmann, L.; Kalt, G.; Matej, S.; Theurl, M.C.; Morais, T.G.; Leip, A.; Erb, K.H. Applying the Human Appropriation of Net Primary Production framework to map provisioning ecosystem services and their relation to ecosystem functioning across the European Union. Ecosyst. Ser. 2021, 51, 101344. [Google Scholar] [CrossRef]
- Macaya, E.C.; Zuccarello, G.C. Genetic structure of the giant kelp Macrocystis pyrifera along the southeastern Pacific. Mar. Ecol. Prog. Ser. 2010, 420, 103–112. [Google Scholar] [CrossRef]
- Huovinen, P.; Gómez, I. Cold-Temperate Seaweed Communities of the Southern Hemisphere. In Seaweed Biology. Ecological Studies; Wiencke, C., Bischof, K., Eds.; Springer: Heidelberg, Germany, 2012; pp. 293–313. [Google Scholar]
- Ávila-Peltroche, J.; Padilla-Vallejos, J. The seaweed resources of Peru. Bot. Mar. 2020, 63, 381–394. [Google Scholar] [CrossRef]
- Rothäusler, E.; Reinwald, H.; López, B.A.; Tala, F.; Thiel, M. High acclimation potential in floating Macrocystis pyrifera to abiotic conditions even under grazing pressure–a field study. J. Phycol. 2018, 54, 368–379. [Google Scholar] [CrossRef]
- Mogollón, R.; Calil, P.H.R. On the Effects of ENSO on Ocean Biogeochemistry in the Northern Humboldt Current System (NHCS): A Modeling Study. J. Mar. Syst. 2017, 172, 137–159. [Google Scholar] [CrossRef]
- Santelices, B. The discovery of kelp forests in deep-water habitats of tropical regions. Proc. Natl. Acad. Sci. USA 2007, 104, 19163–19164. [Google Scholar] [CrossRef]
- Salavarría, E.; Macaya, E.; Gil-Kodaka, P.; Paul, S.; Troccoli, L. Haplotype diversity of Macrocystis pyrifera (Phaeophyceae: Laminariales) in the central and southern coast of Peru. Pan-Am. J. Aquat. Sci. 2018, 13, 311–319. [Google Scholar]
- Pérez-Araneda, K.; Zevallos, S.; Arakaki, N.; Gamarra, A.; Carbajal, P.; Tellier, F. Lessonia berteroana en Perú: Comprobación de la identidad de la especie y diversidad genética en el borde norte de distribución. Rev. Biol. Mar. Oceanogr. 2020, 55, 270–276. [Google Scholar] [CrossRef]
- Tellier, F.; Tapia, J.; Faugeron, S.; Destombe, C.; Valero, M. The Lessonia nigrescens species complex (Laminariales: Phaeophyceae) shows strict parapatry and complete reproductive isolation in a secondary contact zone. J. Phycol. 2011, 47, 894–903. [Google Scholar] [CrossRef]
- Tellier, F.M.V.; Broitman Rojas, B.O.; Faugeron, S.W. The importance of having two species instead of one in kelp management: The Lessonia nigrescens species complex. Cah. Biol. Mar. 2011, 4, 455–465. [Google Scholar]
- De Juan, S.; Gelcich, S.; Ospina-Alvarez, A.; Perez-Matus, A.; Fernandez, M. Applying an ecosystem service approach to unravel links between ecosystems and society in the coast of central Chile. Sci. Total Environ. 2015, 533, 122–132. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Tala, F.; Vega, A.; Zuñiga, S.; Edding, M.; Piaget, N. Bases Ecológicas y Evaluación de Usos Alternativos Para el Manejo de Praderas de Algas Pardas de la III y IV Regiones; Proyecto FIP: Coquimbo, Chile, 2008; pp. 160–288. [Google Scholar]
- Blamey, L.K.; Bolton, J.J. The economic value of South African kelp forests and temperate reefs: Past, present and future. J. Mar. Syst. 2018, 188, 172–181. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Cáceres, C.W.; Benavides, A.G.; Ojeda, F.P. Ecología trófica del pez herbívoro Aplodactylus punctatus (Pisces: Aplodactylidae) en la costa centro-norte de Chile. Rev. Chil. de Hist. Nat. 1993, 66, 185–194. [Google Scholar]
- Richmond, A.; Kaufmann, R.K.; Myneni, R.B. Valuing ecosystem services: A shadow price for net primary production. Ecol. Econ. 2007, 64, 454–462. [Google Scholar] [CrossRef]
- Villegas, M.J.; Laudien, J.; Sielfeld, W.; Arntz, W.E. Macrocystis integrifolia and Lessonia trabeculata (Laminariales; Phaeophyceae) kelp habitat structures and associated macrobenthic community off northern Chile. Helgol. Mar. Res. 2008, 62, 33–43. [Google Scholar] [CrossRef]
- Rodriguez, S.R.; Ojeda, F.P. Distribution patterns of Tetrapygus niger (Echinodermata: Echinoidea) off the central Chilean coast. Mar. Ecol. Prog. Ser. 1993, 101, 157–162. [Google Scholar] [CrossRef]
- Ruiz, J.; Ibáñez, C.M.; Cáceres, C.W. Morfometría del tubo digestivo y alimentación del pepino de mar Athyonidium chilensis (Semper, 1868) (Echinodermata: Holothuroidea). Rev. Biol. Mar. Oceanogr. 2007, 42, 269–274. [Google Scholar] [CrossRef]
- Miranda, L.; Thiel, M. Active and passive migration in boring isopods Limnoria spp. (Crustacea, Peracarida) from kelp holdfasts. J. Sea Res. 2008, 60, 176–183. [Google Scholar] [CrossRef]
- Duarte, C.; Jaramillo, E.; Contreras, H. Macroalgas varadas sobre la superficie de una playa arenosa del sur de Chile: Preferencias alimentarias y de hábitat de juveniles y adultos de Orchestoidea tuberculata (Nicolet), (Amphipoda, Talitridae). Rev. Chil. de Hist. Nat. 2008, 81, 69–81. [Google Scholar] [CrossRef]
- Ortega, K.J.; Avaria, C.A.S.; Macaya, E.C. Changes in invertebrate assemblages inhabiting Lessonia spicata (Phaeophyceae) holdfasts after the 2010 earthquake-mediated coastal uplift in Chile. Rev. Biol. Mar. Oceanogr. 2014, 49, 129–134. [Google Scholar] [CrossRef]
- Pérez-Schultheiss, J. Ampliación del rango de distribución de Sunamphitoe lessoniophila (Conlan y Bousfield, 1982) (Amphipoda: Senticaudata: Ampithoidae) en la costa de Chile. Bol. Mus. Nac. Hist. Nat. 2018, 67, 173–179. [Google Scholar]
- Álvarez-Campos, P.; Verdes, A. Syllids inhabiting holdfasts of Lessonia spicata in Central Chile: Diversity, systematics, and description of three new species. Syst. Biodivers. 2017, 15, 520–531. [Google Scholar] [CrossRef]
- Munoz, M.; Santelices, B. Determination of the distribution and abundance of the limpet Scurria scurra on the stipes of the kelp Lessonia nigrescens in Central Chile. Mar. Ecol. Prog. Ser. 1989, 54, 277–285. [Google Scholar]
- Winkler, N.S.; Pérez-Matus, A.; Villena, Á.A.; Thiel, M. Seasonal variation in epifaunal communities associated with giant kelp (Macrocystis pyrifera) at an upwelling-dominated site. Austral Ecol. 2017, 42, 132–144. [Google Scholar] [CrossRef]
- Cerda, O.; Hinojosa, I.A.; Thiel, M. Nest-building behavior by the amphipod Peramphithoe femorata (Krøyer) on the kelp Macrocystis pyrifera (Linnaeus) C. Agardh from northern-central Chile. Biol. Bull. 2010, 218, 248–258. [Google Scholar] [CrossRef]
- Gutow, L.; Long, J.D.; Cerda, O.; Hinojosa, I.A.; Rothäusler, E.; Tala, F.; Thiel, M. Herbivorous amphipods inhabit protective microhabitats within thalli of giant kelp Macrocystis pyrifera. Mar. Biol. 2012, 159, 141–149. [Google Scholar] [CrossRef]
- Gutow, L.; Poore, A.G.; Díaz Poblete, M.A.; Villalobos, V.; Thiel, M. Small burrowing amphipods cause major damage in a large kelp. Proc. Royal Soc. B 2020, 287, 20200330. [Google Scholar] [CrossRef]
- Pérez-Matus, A.; Shima, J.S. Density-and trait-mediated effects of fish predators on amphipod grazers: Potential indirect benefits for the giant kelp Macrocystis pyrifera. Mar. Ecol. Prog. Ser. 2010, 417, 151–158. [Google Scholar] [CrossRef]
- Orejas, C.; Gili, J.M.; Alvà, V.; Arntz, W. Predatory impact of an epiphytic hydrozoan in an upwelling area in the Bay of Coliumo (Dichato, Chile). J. Sea Res. 2000, 44, 209–220. [Google Scholar] [CrossRef]
- Carbajal, P.; Gamarra-Salazar, A.; Moore, P.J.; Pérez-Matus, A. Different kelp species support unique macroinvertebrate assemblages, suggesting the potential community-wide impacts of kelp harvesting along the Humboldt Current System. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 14–27. [Google Scholar] [CrossRef]
- Cancino, J.; Santelices, B. Importancia ecológica de los discos adhesivos de Lessonia nigrescens Bory (Phaeophyta). Rev. Chil. de Hist. Nat. 1984, 57, 23–33. [Google Scholar]
- Bularz, B.; Fernández, M.; Subida, M.D.; Wieters, E.A.; Pérez-Matus, A. Effects of harvesting on subtidal kelp forests (Lessonia trabeculata) in central Chile. Ecosphere 2022, 13, e3958. [Google Scholar] [CrossRef]
- Rabosky, D.L.; Chang, J.; Cowman, P.F.; Sallan, L.; Friedman, M.; Kaschner, K.; Garilao, C.; Near, T.J.; Coll, M.; Alfaro, M.E. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 2018, 559, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Thyrring, J.; Peck, L.S. Global gradients in intertidal species richness and functional groups. eLife 2021, 10, e64541. [Google Scholar] [CrossRef] [PubMed]
- Angel, A.; Ojeda, F.P. Structure and trophic organization of subtidal fish assemblages on the northern Chilean coast: The effect of habitat complexity. Mar. Ecol. Prog. Ser. 2021, 217, 81–91. [Google Scholar] [CrossRef]
- Medina-Vogel, G.; Rodriguez, C.D.; Alvarez, R.P.; Bartheld, J.L.V. Feeding ecology of the marine otter (Lutra felina) in a rocky seashore of the south of Chile. Mar. Mamm. Sci. 2004, 20, 134–144. [Google Scholar] [CrossRef]
- Pérez-Matus, A.; Ferry-Graham, L.A.; Cea, A.; Vásquez, J.A. Community structure of temperate reef fishes in kelp-dominated subtidal habitats of northern Chile. Mar. Freshw. Res. 2017, 58, 1069–1085. [Google Scholar] [CrossRef]
- Gelcich, S.; Fernández, M.; Godoy, N.; Canepa, A.; Prado, L.; Castilla, J.C. Territorial user rights for fisheries as ancillary instruments for marine coastal conservation in Chile. Conservar. Biol. 2012, 26, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Matus, A.; Pledger, S.; Díaz, F.J.; Ferry, L.A.; Vásquez, J.A. Plasticidad en la selección de alimento y estructura trófica de los peces asociados a bosques de macroalgas pardas del norte de Chile. Rev. Chil. de Hist. Nat. 2012, 85, 29–48. [Google Scholar] [CrossRef]
- Pérez-Matus, A.; Sánchez, F.; González-But, J.C.; Lamb, R.W. Understory algae associations and predation risk influence broad-scale kelp habitat use in a temperate reef fish. Mar. Ecol. Prog. Ser. 2016, 559, 147–158. [Google Scholar] [CrossRef]
- Ruz, C.S.; Muth, A.F.; Tala, F.; Pérez-Matus, A. The herbivorous fish, Aplodactylus punctatus, as a potential facilitator of dispersal of kelp, Lessonia trabeculata, in Chile. J. Exp. Mar. Biol. Ecol. 2018, 500, 112–119. [Google Scholar] [CrossRef]
- Lozano-Muñoz, I.; Giorgio, C.; Jurij, W.; German, B. Herbivore Fish as Sustainable Alternative for Nutrition Security: Food Habits and Nutritional Composition of the Acha Fish (Medialuna Ancietae) in Northern Chile. Sci. Rep. 2021; in submitted. [Google Scholar]
- Ruz, C.S.; Garmendia, V.; Muñoz-Cordovez, R.; Wieters, E.; Pérez-Matus, A. Observaciones del desarrollo temprano de la doncellita, Myxodes viridis (Clinidae), y la primera descripción de su hábitat de desove en bosques de macroalgas pardas submareales (Lessonia trabeculata). Rev. Biol. Mar. Oceanogr. 2021, 56, 66–73. [Google Scholar] [CrossRef]
- Benavides, A.G.; Cancino, J.M.; Ojeda, F.P. Ontogenetic Changes in Gut Dimensions and Macroalgal Digestibility in the Marine Herbivorous Fish, Aplodactylus punctatus. Funct. Ecol. 1994, 8, 46–51. [Google Scholar] [CrossRef]
- Vásquez-Castillo, S.; Hinojosa, I.A.; Colin, N.; Poblete, A.A.; Górski, K. The presence of kelp Lessonia trabeculata drives isotopic niche segregation of redspotted catshark Schroederichthys chilensis. Estuar. Coast. Shelf Sci. 2021, 258, 107435. [Google Scholar] [CrossRef]
- Fariña, J.M.; Ojeda, F.P. Abundance, activity, and trophic patterns of the redspotted catshark, Schroederichthys chilensis, on the Pacific temperate coast of Chile. Copeia 1993, 1993, 545–549. [Google Scholar] [CrossRef]
- Flores, D.; Adams, G.D. Observaciones sobre el comportamiento de Schroederichthys chilensis (Carcharhiniformes, Scyliorhinidae). Rev. Peru. Biol. 2014, 21, 275–276. [Google Scholar] [CrossRef][Green Version]
- Hockey, P.A.R. Kelp gulls Larus dominicanus as predators in kelp Macrocystis pyrifera beds. Oecologia 1988, 76, 155–157. [Google Scholar] [CrossRef]
- Castilla, J.C.; Bahamondes, I. Observaciones conductuales y ecológicas sobre Lutra felina (Molina) 1782 (Carnivora: Mustelidae) en las zonas central y centro-norte de Chile. Arch. Biol. Med. Exp. 1979, 12, 119–132. [Google Scholar]
- Reynolds, L.K.; McGlathery, K.J.; Waycott, M. Genetic diversity enhances restoration success by augmenting ecosystem services. PLoS ONE 2012, 7, e38397. [Google Scholar] [CrossRef]
- Berkeley, S.A.; Hixon, M.A.; Larson, R.J.; Love, M.S. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries 2004, 29, 23–32. [Google Scholar] [CrossRef]
- Jaramillo, E.; De la Huz, R.; Duarte, C.; Contreras, H. Algal wrack deposits and macroinfaunal arthropods on sandy beaches of the Chilean coast. Rev. Chil. Hist. Nat. 2006, 79, 337–351. [Google Scholar] [CrossRef]
- Thiel, M.; Vásquez, J.A. Are kelp holdfasts islands on the ocean floor?—Indication for temporarily closed aggregations of peracarid crustaceans. In Island, Ocean and Deep-Sea Biology. Developments in Hydrobiology; Jones, M.B., Azevedo, J.M.N., Neto, A.I., Costa, A.C., Martins, A.M.F., Eds.; Springer: Dordrecht, The Netherlands, 2000; Volume 152, pp. 45–54. [Google Scholar]
- Hinojosa, I.; Boltaña, S.; Lancellotti, D.; Macaya, E.; Ugalde, P.; Valdivia, N.; Vásquez, N.; Newman, W.A.; Thiel, M. Distribución geográfica y descripción de cuatro especies de cirripedios pelágicos a lo largo de la costa chilena del Pacífico sur este-una aproximación zoogeográfica. Rev. Chil. Hist. Nat. 2006, 79, 13–27. [Google Scholar]
- Duarte, C.; Jaramillo, E.; Contreras, H.; Acuña, K.; Navarro, J.M. Importancia del subsidio de macroalgas sobre la abundancia y biología poblacional del anfípodo Orchestoidea tuberculata (Nicolet) en playas arenosas del centro sur de Chile. Rev. Biol. Mar. Oceanogr. 2009, 44, 691–702. [Google Scholar] [CrossRef]
- Duarte, C.; Acuña, K.; Navarro, J.M.; Gómez, I.; Jaramillo, E.; Quijón, P. Variable feeding behavior in Orchestoidea tuberculata (Nicolet 1849): Exploring the relative importance of macroalgal traits. J. Sea Res. 2014, 87, 1–7. [Google Scholar] [CrossRef]
- Hinojosa, I.A.; González, E.R.; Macaya, E.; Thiel, M. Macroalgas flotantes en el mar interior de Chiloé, Chile y su fauna asociada con énfasis en peracarida y estados temprano de desarrollo de decapoda (crustacea). Tecnol. y Cienc. del Agua 2010, 33, 71–86. [Google Scholar]
- González, S.A.; Yáñez-Navea, K.; Muñoz, M. Effect of coastal urbanization on sandy beach coleoptera Phaleria maculata (Kulzer, 1959) in northern Chile. Mar. Pollut. Bull. 2014, 83, 265–274. [Google Scholar] [CrossRef]
- Tala, F.; Velásquez, M.; Mansilla, A.; Macaya, E.C.; Thiel, M. Latitudinal and seasonal effects on short-term acclimation of floating kelp species from the South-East Pacific. J. Exp. Mar. Biol. Ecol. 2016, 483, 31–41. [Google Scholar] [CrossRef]
- Carrasco, S.A.; Vandecasteele, L.; Rivadeneira, M.M.; Fernández, M.; Pérez-Matus, A. Spatial and short-term variability of larval, post-larval and macrobenthic assemblages associated with subtidal kelp forest ecosystems in Central Chile. Mar. Biol. Res. 2017, 13, 1041–1058. [Google Scholar] [CrossRef]
- Aller-Rojas, O.; Moreno, B.; Aponte, H.; Zavala, J. Carbon storage estimation of Lessonia trabeculata kelp beds in Southern Peru: An analysis from the San Juan de Marcona region. Carbon Mang. 2020, 11, 525–532. [Google Scholar] [CrossRef]
- Manriquez, P.H.; Cancino, J.M. Bryozoan-macroalgal interactions: Do epibionts benefit? Mar. Ecol. Prog. Ser. 1996, 138, 189–197. [Google Scholar] [CrossRef][Green Version]
- Venegas, M.; Matsuhiro, B.; Edding, M.E. Alginate composition of Lessonia trabeculata (Phaeophyta: Laminariales) growing in exposed and sheltered habitats. Bot. Mar. 1993, 36, 47–52. [Google Scholar] [CrossRef]
- Zuniga-Jara, S.; Marín-Riffo, M.C.; Bulboa-Contador, C. Bioeconomic analysis of giant kelp Macrocystis pyrifera cultivation (Laminariales; Phaeophyceae) in northern Chile. J. Appl. Phycol. 2016, 28, 405–416. [Google Scholar] [CrossRef]
- Dantagnan, P.; Hernández, A.; Borquez, A.; Mansilla, A. Inclusion of macroalgae meal (Macrocystis pyrifera) as feed ingredient for rainbow trout (Oncorhynchus mykiss): Effect on flesh fatty acid composition. Aquac. Res. 2009, 41, 87–94. [Google Scholar] [CrossRef]
- Valiente, O.; Mogollón, E. Contenido de Ácido Algínico, Manitol y Laminarano en Algas Pardas de Importancia Económica. Boletín Investig. Inst. Tecnológico Prod. Perú 2013, 11, 91–98. [Google Scholar]
- Camus, C.; Ballerino, P.; Delgado, R.; Olivera-Nappa, Á.; Leyton, C.; Buschmann, A.H. Scaling up bioethanol production from the farmed brown macroalga Macrocystis pyrifera in Chile. Biofuels Bioprod. Biorefin. 2016, 10, 673–685. [Google Scholar] [CrossRef]
- Westermeier, R.; Murúa, P.; Patiño, D.J.; Muñoz, L.; Ruiz, A.; Atero, C.; Muller, D. Utilization of holdfast fragments for vegetative propagation of Macrocystis integrifolia in Atacama, Northern Chile. J. Appl. Phycol. 2013, 25, 639–642. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Hernández-González, M.C.; Varela, D.A. Seaweed future cultivation in Chile: Perspectives and challenges. Int. J. Environ. Pollut. 2008, 33, 432–456. [Google Scholar] [CrossRef]
- PRODUCE. Anuario Estadístico Pesquero y Acuícola 2015; Ministerio de la Produccion: Lima, Peru, 2015. Available online: https://www.produce.gob.pe/documentos/estadisticas/anuarios/anuario-estadistico-pesca-2015.pdf (accessed on 23 July 2022).
- SERNAPESCA. In Anuario Estadístico de Pesca y Acuicultura 2020; Servicio Nacional de Pesca y Acuicultura de Chile: Valparaíso, Chile, 2020. Available online: http://www.sernapesca.cl/informes/estadisticas (accessed on 23 July 2022).
- Dhargalkar, V.K.; Verlecar, X.N. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Piaget, N.; Vega, J.M. The Lessonia nigrescens fishery in northern Chile: “How you harvest is more important than how much you harvest”. J. Appl. Phycol. 2012, 24, 417–426. [Google Scholar] [CrossRef]
- Gelcich, S.; Godoy, N.; Prado, L.; Castilla, J.C. Add-on conservation benefits of marine territorial user rights fishery policies in central Chile. Ecol. Appl. 2008, 18, 273–281. [Google Scholar] [CrossRef]
- Camus, C.; Buschmann, A.H. Macrocystis pyrifera aquafarming: Production optimization of rope-seeded juvenile sporophytes. Aquaculture 2017, 468, 107–114. [Google Scholar] [CrossRef]
- Madariaga, D.J.; Ortiz, M.; Thiel, M. Demography and feeding behavior of the kelp crab Taliepus marginatus in subtidal habitats dominated by the kelps Macrocystis pyrifera or Lessonia trabeculata. Invertebr. Biol. 2013, 2, 133–144. [Google Scholar] [CrossRef]
- Godoy, N.; Gelcich, S.; Vásquez, J.A.; Castilla, J.C. Spearfishing to depletion: Evidence from temperate reef fishes in Chile. Ecol. Appl. 2010, 20, 1504–1511. [Google Scholar] [CrossRef]
- Vásquez, J.A.; Donoso, G.A. Loxechinus albus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elservier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 285–296. [Google Scholar]
- Gonzalez, S.J.; Cáceres, C.W.; Ojeda, F.P. Feeding and nutritional ecology of the edible sea urchin Loxechinus albus in the northern Chilean coast. Rev. Chil. Hist. Nat. 2008, 81, 575–584. [Google Scholar] [CrossRef]
- Erlandson, J.M.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Estes, J.A.; Steneck, R.S. The kelp highway hypothesis: Marine ecology, the coastal migration theory, and the Peopling of the Americas. J. Island Coast. Archaeol. 2007, 2, 161–174. [Google Scholar] [CrossRef]
- Vásquez, J.A. Ecology of Loxechinus albus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 37, pp. 227–241. [Google Scholar]
- Dillehay, T.D.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, food, medicine, and the peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef]
- Yacovleff, E.; Muelle, J.C. Un fardo funerario de Paracas. Rev. Mus. Nac. 1934, 3, 63–153. [Google Scholar]
- Gaitán-Espitia, J.D.; Hancock, J.R.; Padilla-Gamiño, J.L.; Rivest, E.B.; Blanchette, C.A.; Reed, D.C.; Hofmann, G.E. Interactive effects of elevated temperature and pCO2 on early-life-history stages of the giant kelp Macrocystis pyrifera. J. Exp. Mar. Biol. Ecol. 2014, 457, 51–58. [Google Scholar] [CrossRef]
- González, C.P.; Edding, M.; Tala, F.; Torres, R.; Manríquez, P.H. Exposure time modulates the effects of climate change-related stressors on fertile sporophytes and early-life stage performance of a habitat-forming kelp species. Environ. Pollut. 2021, 286, 117224. [Google Scholar] [CrossRef]
- González, C.P.; Edding, M.; Torres, R.; Manríquez, P.H. Increased temperature but not pCO2 levels affect early developmental and reproductive traits of the economically important habitat-forming kelp Lessonia trabeculata. Mar. Pollut. Bull. 2018, 135, 694–703. [Google Scholar] [CrossRef]
- Hollarsmith, J.A.; Buschmann, A.H.; Camus, C.; Grosholz, E.D. Varying reproductive success under ocean warming and acidification across giant kelp (Macrocystis pyrifera) populations. J. Exp. Mar. Biol. Ecol. 2020, 522, 151247. [Google Scholar] [CrossRef]
- Vega, J.A.; Vásquez, J.A.; Buschmann, A.H. Population biology of the subtidal kelps Macrocystis integrifolia and Lessonia trabeculata (Laminariales, Phaeophyceae) in an upwelling ecosystem of northern Chile: Interannual variability and El Niño 1997–1998. Rev. Chil. de Hist. Nat. 2005, 78, 33–50. [Google Scholar]
- Vásquez, J.A.; Vega, J.M.; Buschmann, A.H. Long term variability in the structure of kelp communities in northern Chile and the 1997–98 ENSO. In Eighteenth International Seaweed Symposium: Developments in Applied Phycology; Anderson, R., Brodie, J., Onsøyen, E., Critchley, A.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 1, pp. 279–293. [Google Scholar]
- Thiel, M.; Macaya, E.C.; Acuña, E.; Arntz, W.E.; Bastias, H.; Brokordt, K.; Camus, P.A.; Castilla, J.C.; Castro, L.R.; Cortés, M.; et al. The Humboldt Current System of Northern and Central Chile: Oceanographic processes, ecological interactions and socioeconomic feedback. Oceanogr. Mar. Biol. Annu. Rev. 2007, 45, 195–344. [Google Scholar]
- Martínez, E.A.; Cárdenas, L.; Pinto, R. Recovery and genetic diversity of the intertidal kelp Lessonia nigrescens (Phaeophyceae) 20 years after El Niño 1982/831. J. Phycol. 2003, 39, 504–508. [Google Scholar] [CrossRef]
- De Oliveira, L.R.; Meyer, D.; Hoffman, J.; Majluf, P.; Morgante, J.S. Evidence of a genetic bottleneck in an El Niño affected population of South American fur seals, Arctocephalus australis. J. Mar. Biolog. Assoc. UK 2009, 89, 1717–1725. [Google Scholar] [CrossRef]
- Gaymer, C.F.; Palma, A.T.; Vega, J.A.; Monaco, C.J.; Henríquez, L.A. Effects of La Nina on recruitment and abundance of juveniles and adults of benthic community-structuring species in northern Chile. Mar. Freshw. Res. 2010, 61, 1185–1196. [Google Scholar] [CrossRef]
- Perreault, M.C.; Borgeaud, I.A.; Gaymer, C.F. Impact of grazing by the sea urchin Tetrapygus niger on the kelp Lessonia trabeculata in Northern Chile. J. Exp. Mar. Biol. Ecol. 2014, 453, 22–27. [Google Scholar] [CrossRef]
- Uribe, R.A.; Perea, Á.; Ortiz, M. Determining ecosystem properties and short-term dynamical simulations in Eisenia cokeri kelp forest (north-center of Peru): Implications for conservation and monitoring. Estuar. Coast. Shelf Sci. 2022, 269, 107813. [Google Scholar] [CrossRef]
- Paton, D.; Jang, L.J. Earthquake readiness and recovery: An Asia-Pacific perspective. In Earthquakes and Their Impact on Society; D’Amico, S., Ed.; Springer Natural Hazards: Cham, Switzerland, 2016; pp. 647–663. [Google Scholar]
- Tellier, F.; Meynard, A.P.; Correa, J.A.; Faugeron, S.; Valero, M. Phylogeographic analyses of the 30° S south-east Pacific biogeographic transition zone establish the occurrence of a sharp genetic discontinuity in the kelp Lessonia nigrescens: Vicariance or parapatry? Mol. Phylogenet. Evol. 2009, 53, 679–693. [Google Scholar] [CrossRef]
- Cheng, X.; Van Damme, S.; Li, L.; Uyttenhove, P. Evaluation of cultural ecosystem services: A review of methods. Ecosyst. Serv. 2019, 37, 100925. [Google Scholar] [CrossRef]
- Florez, J.Z.; Camus, C.; Hengst, M.B.; Marchant, F.; Buschmann, A.H. Structure of the epiphytic bacterial communities of Macrocystis pyrifera in localities with contrasting nitrogen concentrations and temperature. Algal Res. 2019, 44, 101706. [Google Scholar] [CrossRef]
- Minich, J.J.; Morris, M.M.; Brown, M.; Doane, M.; Edwards, M.S.; Michael, T.P.; Dinsdale, E.A. Elevated temperature drives kelp microbiome dysbiosis, while elevated carbon dioxide induces water microbiome disruption. PLoS ONE 2018, 13, e0192772. [Google Scholar] [CrossRef]
- Qiu, Z.; Coleman, M.A.; Provost, E.; Campbell, A.H.; Kelaher, B.P.; Dalton, S.J.; Thomas, T.; Steinberg, P.D.; Marzinelli, E.M. Future climate change is predicted to affect the microbiome and condition of habitat-forming kelp. Proc. Royal Soc. B 2019, 286, 20181887. [Google Scholar] [CrossRef]



| Criteria | Explanation |
|---|---|
| Ecosystem Service (ES) concept | The paper applies the ES concept in a meaningful way, or the main findings are related to, at least, one of the ES. |
| Type of article | The paper is an academic peer-reviewed research article. |
| Species | The content is discussed in relation to Lessonia spp. or Macrocystis pyrifera. |
| Locality | The content is discussed in relation to the Humboldt Current System (HCS). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuba, D.; Guardia-Luzon, K.; Cevallos, B.; Ramos-Larico, S.; Neira, E.; Pons, A.; Avila-Peltroche, J. Ecosystem Services Provided by Kelp Forests of the Humboldt Current System: A Comprehensive Review. Coasts 2022, 2, 259-277. https://doi.org/10.3390/coasts2040013
Cuba D, Guardia-Luzon K, Cevallos B, Ramos-Larico S, Neira E, Pons A, Avila-Peltroche J. Ecosystem Services Provided by Kelp Forests of the Humboldt Current System: A Comprehensive Review. Coasts. 2022; 2(4):259-277. https://doi.org/10.3390/coasts2040013
Chicago/Turabian StyleCuba, Diego, Katerin Guardia-Luzon, Bruno Cevallos, Sabrina Ramos-Larico, Eva Neira, Alejandro Pons, and Jose Avila-Peltroche. 2022. "Ecosystem Services Provided by Kelp Forests of the Humboldt Current System: A Comprehensive Review" Coasts 2, no. 4: 259-277. https://doi.org/10.3390/coasts2040013
APA StyleCuba, D., Guardia-Luzon, K., Cevallos, B., Ramos-Larico, S., Neira, E., Pons, A., & Avila-Peltroche, J. (2022). Ecosystem Services Provided by Kelp Forests of the Humboldt Current System: A Comprehensive Review. Coasts, 2(4), 259-277. https://doi.org/10.3390/coasts2040013








