1. Introduction
Currently, the mining industry has developed processes to extract valuable minerals and elements from primary sources. In the case of copper, it is mainly obtained from copper oxides. However, as these primary sources become depleted over time, there is a growing need to explore alternative methods for copper extraction. One promising alternative is the extraction of copper from sulfide minerals, such as chalcopyrite [
1]. Chalcopyrite is the most common and abundant copper sulfide mineral in the Earth’s crust, accounting for approximately 70% of global copper reserves [
2]. However, due to its complex structure, chalcopyrite is considered a refractory mineral for copper recovery using conventional hydrometallurgical methods.
Chile, one of the leading mining countries in Latin America and a major producer of metallic copper, expects an increase in the production of copper concentrates from ores with high sulfur content. It is estimated that concentrates will account for 89.9% of total copper production by 2027, up from 69.2% in 2015 [
3]. Sulfide minerals are typically processed using pyrometallurgical techniques due to their high refractoriness, while hydrometallurgy is employed for the treatment of lower-grade ores [
4]. Hydrometallurgy is considered less environmentally damaging than pyrometallurgy, as pyrometallurgical processes generate significant amounts of pollutant gases that must be treated before being released into the atmosphere.
In the study of chalcopyrite leaching in acidic media, the use of ferric ions as oxidizing agents has been explored. These ions are derived from compounds such as ferric sulfate (Fe
2(SO
4)
3), dissolved in acidic solutions. According to research by Liu et al. [
5], a mechanism is proposed in which ferric ions act as the oxidizing agents, with the primary dissolution reaction of chalcopyrite in ferric/ferrous sulfate systems being the oxidation of chalcopyrite by ferric ions. The general chemical equations representing this dissolution are presented below [
5].
Chalcopyrite, with the general formula CuFeS
2, has a tetragonal crystal structure in which sulfide ions are coordinated by copper or iron atoms. This structure is similar to that of sphalerite, although there is a difference in the c-lattice parameter [
6,
7,
8]. However, chalcopyrite exhibits slow dissolution kinetics due to its composition and crystal structure. A key factor in this slow dissolution is the phenomenon of passivation, which is illustrated by the formation of a low-porosity layer of elemental sulfur on the chalcopyrite surface, as proposed by Muñoz et al. [
9]. This passivation reduces the contact between the chalcopyrite and reactive ions, thus hindering further dissolution. The chalcopyrite oxidation model by Fe
3+ involves the conversion of sulfur atoms in chalcopyrite from a −2 state to 0, as shown in Equation (2), resulting in the formation of an elemental sulfur layer as a byproduct. This sulfur layer acts as a passivating barrier, reducing the contact between chalcopyrite and reactive ions, thereby slowing down the dissolution process. The dissolution rate is controlled by the formation of this sulfur layer and the diffusion of ferric ions through it [
10,
11].
The leaching of chalcopyrite involves a series of redox reactions, with copper dissolution being significantly influenced by the redox potential. Studies [
4,
12,
13,
14] suggest that higher recovery rates are achieved under strong oxidizing conditions, particularly at redox potentials above 0.45 V. The copper leaching rate increases within a specific potential range, with some studies indicating that the dissolution rate is higher in media with potentials between 0.42 and 0.6 V [
15]. However, more recent research has concluded that the leaching rate improves at potential values above 0.5 V [
2,
16]. The Pourbaix diagram for chalcopyrite dissolution in acidic media, highlighting the formation of various intermediate sulfides, such as bornite (Cu
5FeS
4), covellite (CuS), and chalcocite (Cu
2S), which become progressively richer in copper, can be found in the study by Córdoba et al. [
10]. Optimal conditions for copper dissolution to Cu
2+ are suggested to be pH < 4 and an oxidizing redox potential (Eh) > +0.4 V. To achieve these conditions, oxidizing agents are employed, with ferric ions, typically in the form of sulfate or chloride, being commonly used.
Another crucial factor is temperature, which has a significant impact on this type of leaching. The relation between temperature and copper dissolution from chalcopyrite has been observed, with increased dissolution occurring at higher temperatures. In some studies, leaching tests conducted in a chlorinated acidic medium, where a previously sulfurized copper concentrate was leached, achieved a copper recovery of over 95% at a temperature of 100 °C [
17]. It is important to note that chalcopyrite leaching is a complex process and may require specific conditions to achieve efficient copper dissolution. Factors such as temperature, lixiviant concentration, and contact time can significantly affect the effectiveness of the process.
Recent studies have highlighted the challenges associated with leaching chalcopyrite-rich concentrates, which exhibit refractory characteristics that make them difficult to process using conventional hydrometallurgical methods. The recoveries observed in existing processes are generally low. For instance, Yang [
14] emphasized the crucial role of mixed potential in leaching kinetics, suggesting that optimizing this factor could improve recovery rates. Similarly, Tian [
2] discussed the effects of redox potential on the leaching process, indicating that adjustments in this parameter can lead to significant improvements. Additionally, Liu [
18] investigated the speciation of copper, iron, and sulfur during bioleaching, revealing that microbial interactions can influence dissolution rates, which may further impact recovery efficiency. Furthermore, it is essential to consider the environmental aspects of chalcopyrite leaching, as the use of sulfuric acid or other lixiviants can generate acidic waste that must be properly managed to avoid negative environmental impacts.
In this context, during the acid leaching of chalcopyrite, a copper and iron solution is obtained, which is then subjected to a cementation process to recover the copper. In this final step, a residual solution is produced with a high content of dissolved iron, predominantly in the form of Fe2+, along with smaller amounts of Fe3+.
The residual solution can be treated by catalytic oxidation to regenerate the oxidative potential of the solution. Fe
2+ ions are oxidized to Fe
3+ ions using activated carbon. The surface of the activated carbon has particular characteristics depending on the activation treatment it has undergone, and it has been shown that the functional groups present on its surface are responsible for its catalytic activity [
19]. The interactions that occur in the oxidation of Fe
2+ to Fe
3+ suggest a two-step mechanism. In the first step, hydrogen peroxide is formed when the carbon comes into contact with the oxygen in the aqueous solution. In the second step, the ability of the carbon to reoxidize is considered [
20].
The objective of this study was to determine the optimal working conditions to achieve higher copper recovery through acid leaching using ferric ion as the oxidizing agent. Additionally, the residual solution obtained during the first leaching cycle was regenerated to be recirculated in a second cycle, with the aim of evaluating the copper recovery obtained with this recirculated leaching solution.
2. Materials and Methods
2.1. Materials and Reagents
The leaching medium was prepared using reagent-grade sulfuric acid (PanReac, Castellar del Vallès, Spain, 98%). The oxidizing agents employed were ferric sulfate (Fe2(SO4)3·nH2O, Baker, 75%) and ferric chloride (FeCl3·6H2O, LOBAChemie, Mumbai, India, 97%).
The samples analyzed consisted of a copper and iron concentrate obtained from flotation processes in the southern region of Ecuador. A total mass of 50 kg of concentrate was used. Initially, a representative sample of the copper sulfide mineral was collected. This initial sample was quartered and homogenized to produce a final representative sample weighing approximately 2 kg, which was used for the subsequent assays and analyses described below.
2.2. Analysis and Characterization of the Concentrate
Mineralogical characterization was performed using X-ray diffraction (XRD) on a D8 ADVANCE diffractometer (Bruker, Karlsruhe, Germany). The chemical composition was then determined using X-ray fluorescence (XRF) with the S8 Tiger equipment (Bruker, Karlsruhe, Germany).
The contents of gold and silver were determined using fire assay and atomic absorption techniques with the Perkin Elmer AAnalyst 300 (Perkin Elmer, Shelton, CT, USA). Due to the high copper and iron content in the concentrate, a pretreatment step was required. Specifically, 20 g of copper concentrate were weighed and roasted in a muffle furnace at 500 °C for 3 h, followed by an additional hour at 600 °C. After pretreatment, the sample was leached in the pulp with a 10% solids sulfuric acid solution (concentration 60 g/L) with magnetic stirring for 2 h. The leached sample was then washed with distilled water (35 °C), filtered, dried, and subjected to fire assay for gold and silver analysis.
The physical characterization of the concentrate included the evaluation of three parameters: particle size, moisture content, and volatile material content. A particle size analysis was performed using sieve analysis to determine the distribution of particle sizes within the sample. For moisture content, a 30 g sample of the concentrate was dried at 90 °C for 48 h, and the moisture was measured by mass difference. The volatile material content was determined by calcining the sample at 600 °C for 6 h.
2.3. Pretreatment of the Concentrate
To eliminate residual chemicals from the flotation process, including surfactants, frothers, and other reagents, the concentrate was washed with water at 35 °C. After washing, the sample was dried at 90 °C for 48 h to remove any remaining moisture before being used in the leaching experiments.
2.4. Leaching Experiments with Different Oxidants—Preliminar Test
In the initial stage, leaching experiments were conducted under the ambient pressure (0.72 atm) and temperature conditions typical of Quito, Ecuador (20 °C). The leaching medium was prepared using sulfuric acid at a concentration of 0.5 M. An oxidizing agents (Fe
2(SO
4)
3 or FeCl
3) was added to increase the initial electrochemical potential of the leaching solution to Eh > 550 mV, with a concentration of 0.1 M. Leaching tests were performed with Fe
2(SO
4)
3 or FeCl
3 as oxidizing agents to facilitate copper dissolution, as recommended in the literature [
4,
13,
14].
The operating conditions included a pulp volume of 500 mL with 15% solids, mechanical agitation at 450 rpm, pH < 1.5, and Eh > 450 mV during the process, with continuous monitoring using a HANNA HI98121 m, equipped with an Ag/AgCl reference electrode. The experiments lasted for a total of 80 h. Solution samples were taken at 24, 48, 72, and 80 h to evaluate the copper dissolution kinetics.
Each test generated the following fractions: pregnant solution, wash solution, and tailings. To determine the copper content in the solutions, atomic absorption analysis was performed using a Perkin Elmer AA 300 (Perkin Elmer, Shelton, CT, USA). For the tailings (sludges), acid digestion (using nitric, hydrochloric, and hydrofluoric acids) was conducted prior to copper content analysis via atomic absorption with the Perkin Elmer AA 300. If the copper concentration in the tailings was above 1%, copper analysis was repeated using X-ray fluorescence (XRF) with the Bruker S8 Tiger (Bruker, Karlsruhe, Germany). This approach allowed for the determination of copper concentration in each fraction and the monitoring of copper dissolution in solution during the leaching process.
2.5. Influence of Temperature and Solid Content on Copper Recovery During Leaching with Ferric Sulfate
In the second stage, experiments were conducted to evaluate the impact of temperature, solid content (1%), a solid content of 1% was selected based on previous studies reporting improved leaching kinetics at low pulp densities [
1,
21]. Preliminary tests at 15% solids resulted in lower copper recovery, confirming that higher pulp density limits mass transfer and reduces leaching efficiency; and electrochemical potential (>450 mV) on copper recovery using sulfuric acid leaching with ferric sulfate as the oxidizing agent. Ferric sulfate (Fe
2(SO
4)
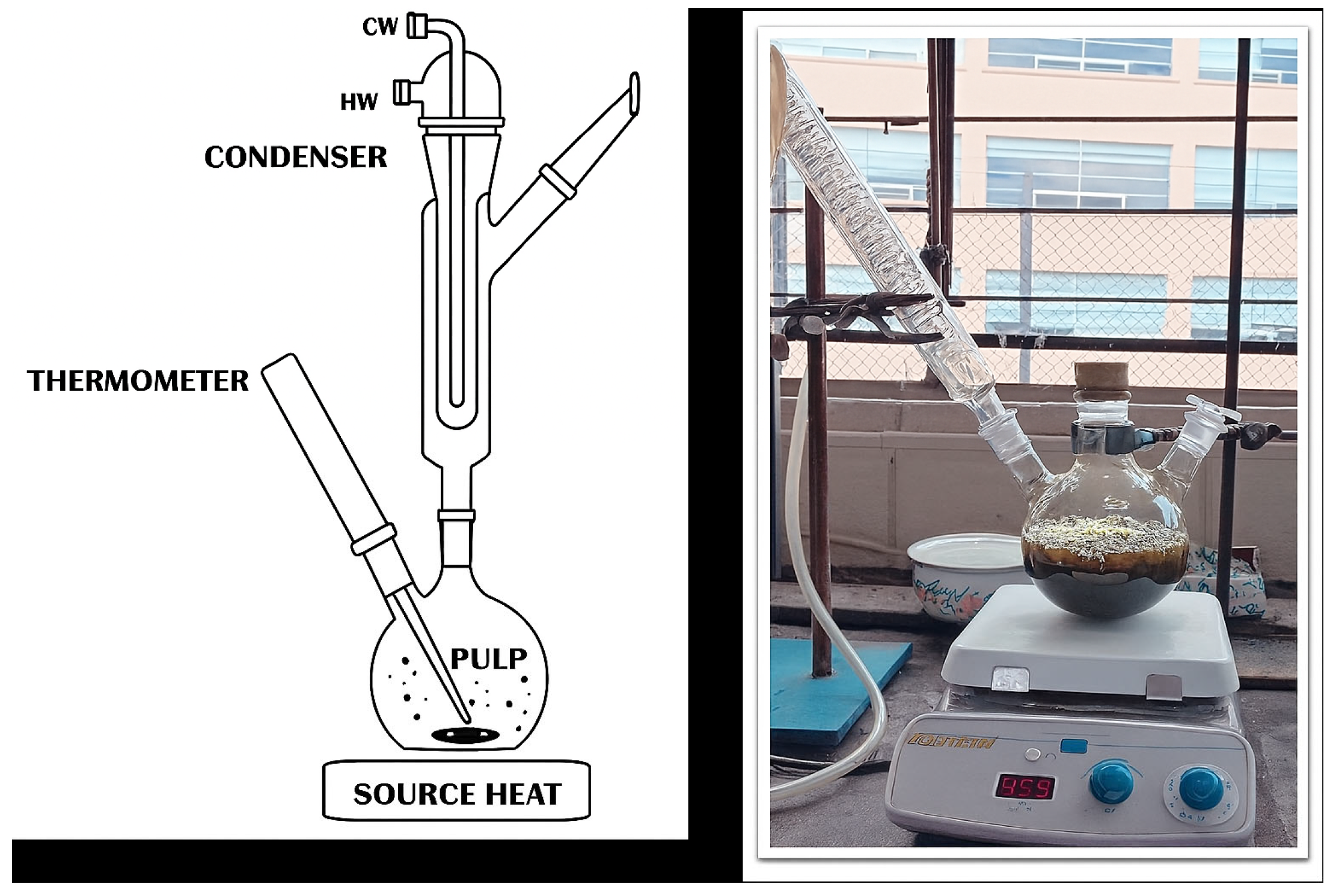
3) was used as the oxidizing agent at a concentration of [0.1 M] in the pulp. The leaching temperature was increased to 92 °C, with a heating system employed to achieve this temperature, and a cooling system was adapted to the reactor to prevent solution loss due to evaporation (
Figure 1). The solid content was set at 1%, and the leaching was conducted with magnetic stirring at 450 rpm, using a pulp volume of 500 mL. Aliquots were taken at 24, 48, 72, and 80 h to determine copper dissolution. The resulting fractions were collected and analyzed for copper content. Copper content in the solution was determined using atomic absorption spectroscopy with a Perkin Elmer AA 300 (Perkin Elmer, Shelton, CT, USA). For the obtained tailings, an acid digestion (nitric, hydrochloric, and hydrofluoric acids) was performed before copper content analysis using atomic absorption with the Perkin Elmer AA 300 (Perkin Elmer, Shelton, CT, USA). If the copper concentration was above 1%, copper analysis was repeated using X-ray fluorescence (XRF) with a Bruker S8 Tiger (Bruker, Karlsruhe, Germany). After obtaining the copper content values for each fraction, a metallurgical balance was performed to determine the recovery.
2.5.1. Copper Cementation
Once the pregnant leach solution (PLS) was obtained from the leaching experiment, copper recovery was carried out through a cementation process using solid iron. During this operation, the PLS was continuously agitated at 500 RPM to ensure thorough mixing and effective contact between the solution and the solid iron. Solid iron filings were gradually introduced into the agitated solution. As the cementation reaction occurred, copper in the leach solution reacted with the iron filings, leading to the precipitation of metallic copper (
Figure 2). The precipitated copper was then separated from the solution, collected, and further processed as needed.
2.5.2. Regeneration of the Oxidizing Potential of the Iron Residual Solution
Before the catalytic oxidation stage, the residual solution obtained after copper cementation was conditioned by dilution with 0.1 M H2SO4 in a 1:1 ratio. This adjustment was performed to match the iron concentration of the fresh leach solution and ensure comparable conditions. The pH of the solution was measured immediately after the cementation stage and again after dilution with 0.1 M H2SO4; in both cases, the pH remained below 1.5. Therefore, no additional acid was required to restore the pH conditions of the fresh leaching solution.
Catalytic oxidation was conducted under the following conditions: pH < 1.5; 3 g of activated carbon per 100 mL of residual solution; mechanical agitation at 500 rpm; and ambient temperature. The activated carbon used was a steam-activated microporous coconut shell carbon, with a specific surface area of 1200 m
2/g, more than 95 % of which is in micropores, and an ash content of 2 %. Boehm titration indicated that the main surface functional groups were carboxyl, lactone, and phenol groups with a total acidic group content of 0.16 mmol/g [
22].
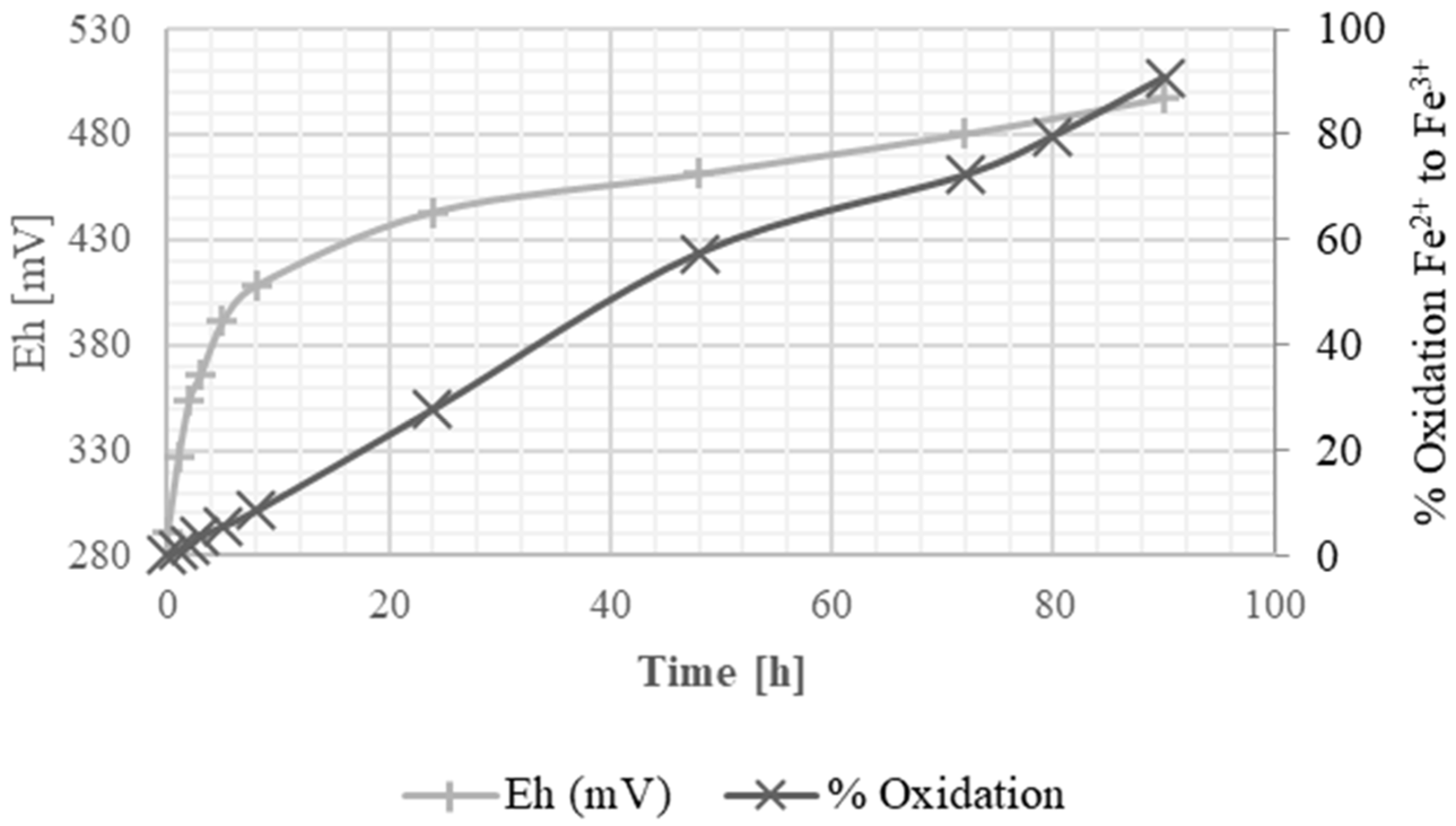
The progression of Fe2+ oxidation was monitored by titration with 0.1 N potassium permanganate (KMnO4) at 1, 2, 3, 5, 8, 24, 48, 72, 80, and 90 h. For each measurement, a 1 mL aliquot of the solution was titrated until a pale pink endpoint was reached. Once the maximum oxidation was achieved, the regenerated solution was recirculated in a second leaching cycle to assess copper recovery.
The residual solution, both before and after catalytic oxidation, was analyzed for iron and copper contents using Atomic Absorption Spectroscopy (Perkin Elmer AA 300, Shelton, CT, USA). Additionally, pH and oxidation potential (Eh) were measured with a HANNA HI98121 m equipped with an Ag/AgCl reference electrode. The reported potentials were not corrected to the standard hydrogen electrode (SHE). Measurements were performed at ambient temperature (~20 °C) in the conditioned residual solution.
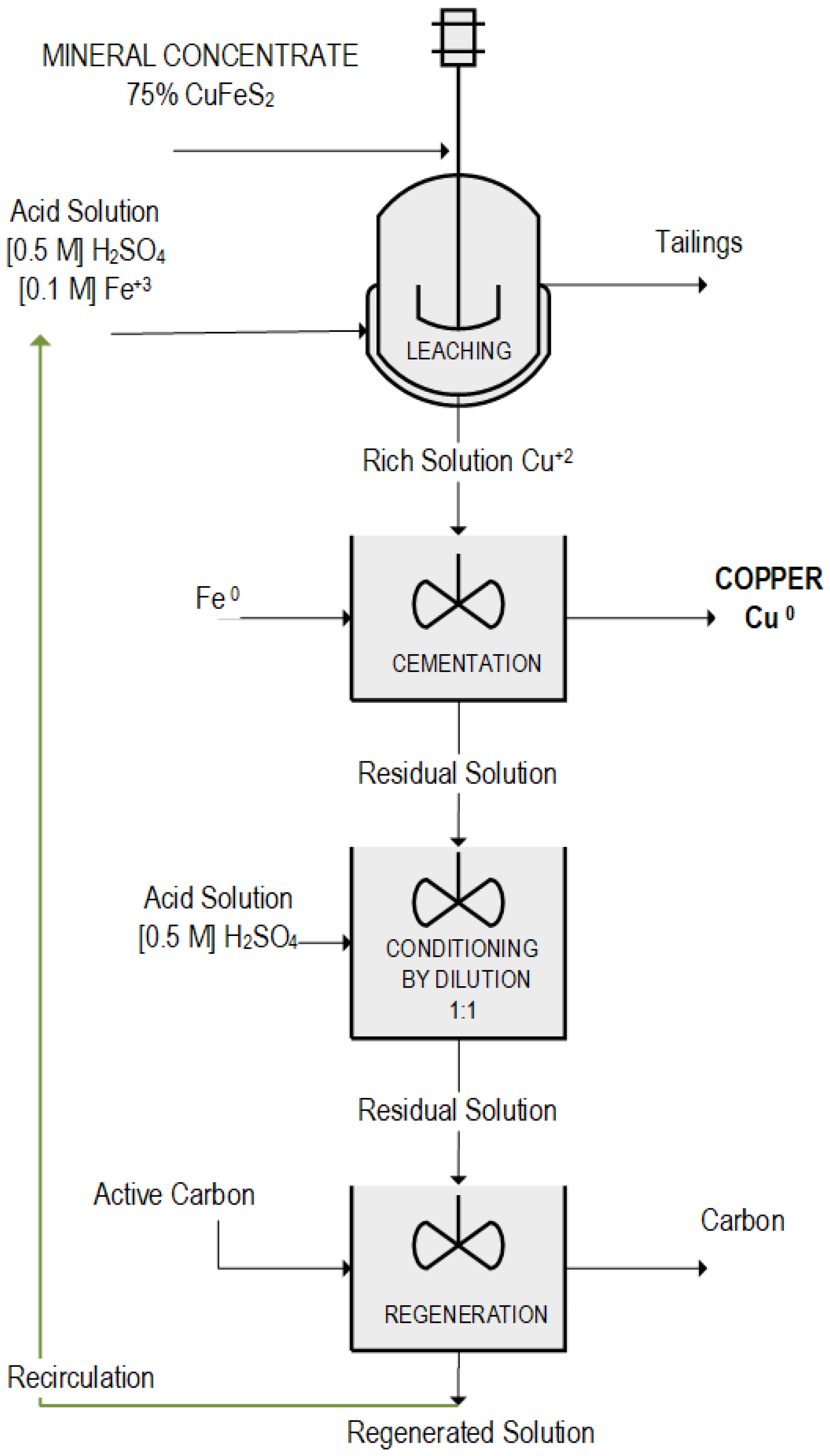
Figure 3 shows a schematic of the overall process carried out, illustrating the stages of ferric leaching with sulfuric acid, copper cementation, and regeneration of the oxidizing potential of the residual solution.
All experiments under optimized conditions were performed in triplicate, and the reported values correspond to the mean of the most consistent replicates.
5. Conclusions
The recovery of copper from chalcopyrite-rich concentrates is significantly influenced by the mineralogical characteristics of the concentrate. The results obtained in this study are specific to the chalcopyrite-rich concentrate analyzed, reflecting its unique mineral composition and properties. The concentrate composition was 75% chalcopyrite and 10% pyrite. Additionally, there are concentrations of 15% copper (Cu), 16% iron (Fe), and 14% sulfur (S). Furthermore, the precious metal content is notable, with 4 g/ton of gold (Au) and 74 g/ton of silver (Ag).
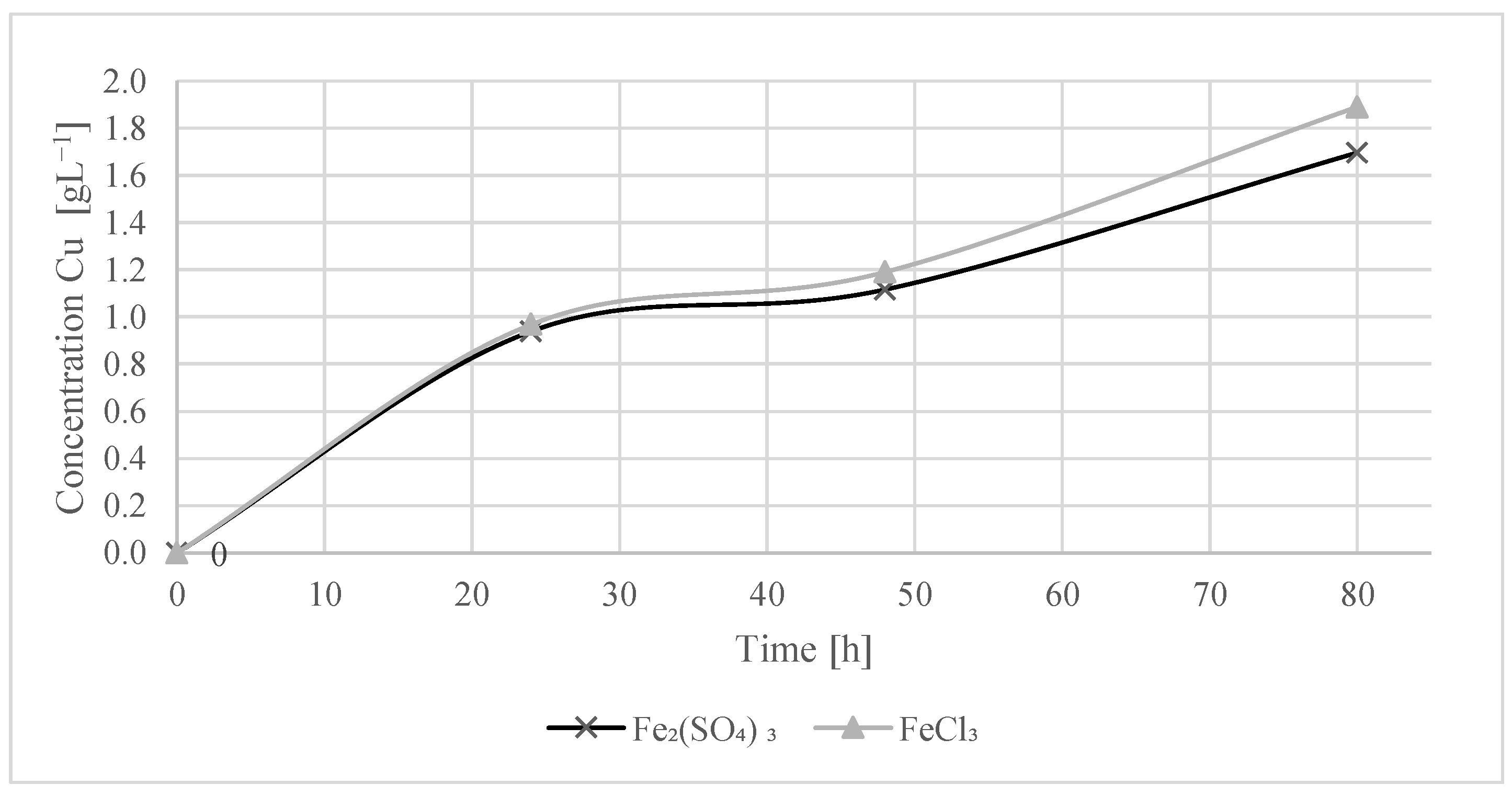
Ferric sulfate (Fe2(SO4)3) and ferric chloride (FeCl3) as oxidizing agents, under ambient conditions (17 °C) and with a 15% solids pulp, show that the copper dissolution is relatively low. Both oxidizing agents exhibited similar effectiveness, achieving copper recoveries of 7.5% and 8.2%, respectively, after a period of 80 h. In subsequent trials, ferric sulfate was selected as the oxidizing agent to avoid adding more species to the system.
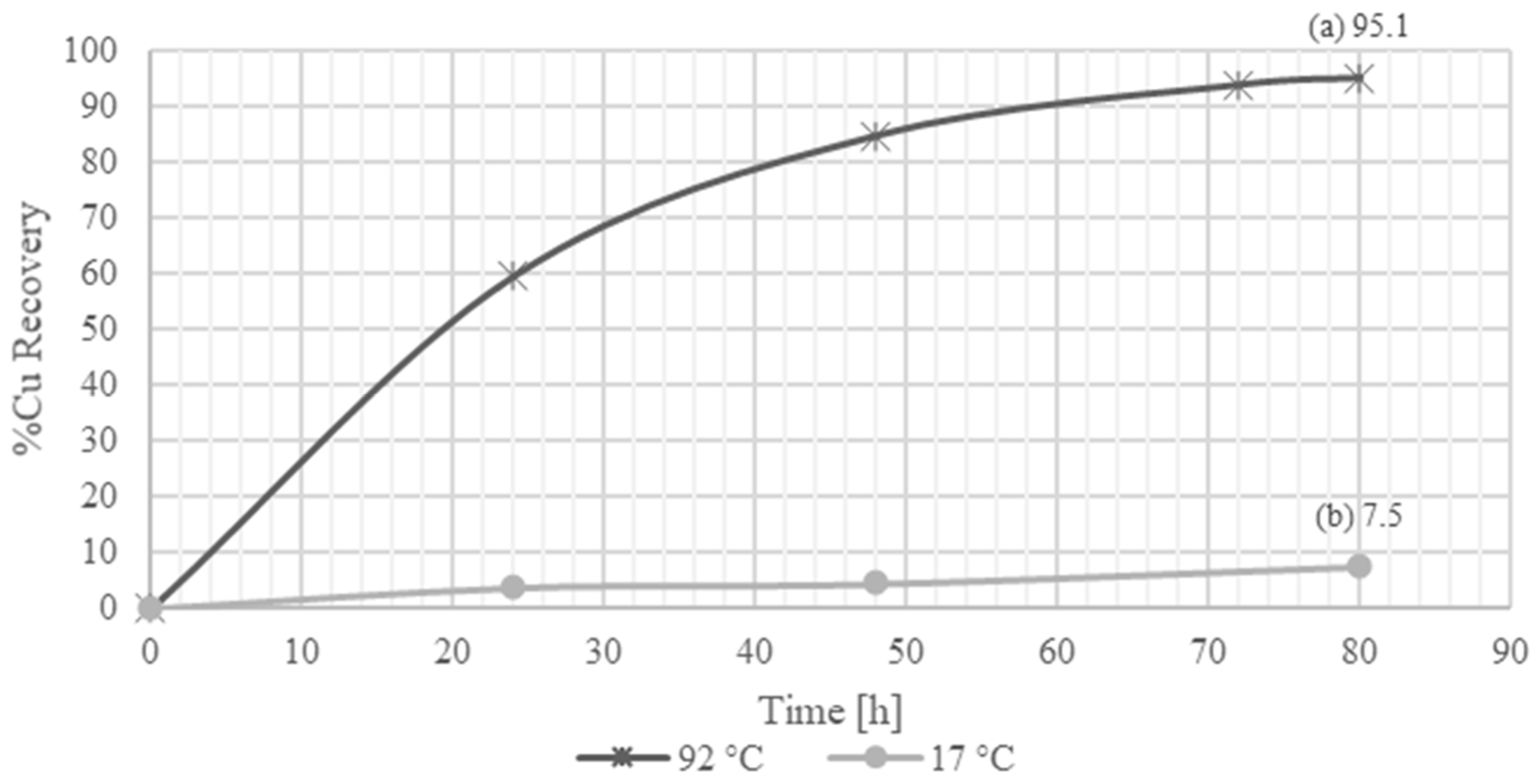
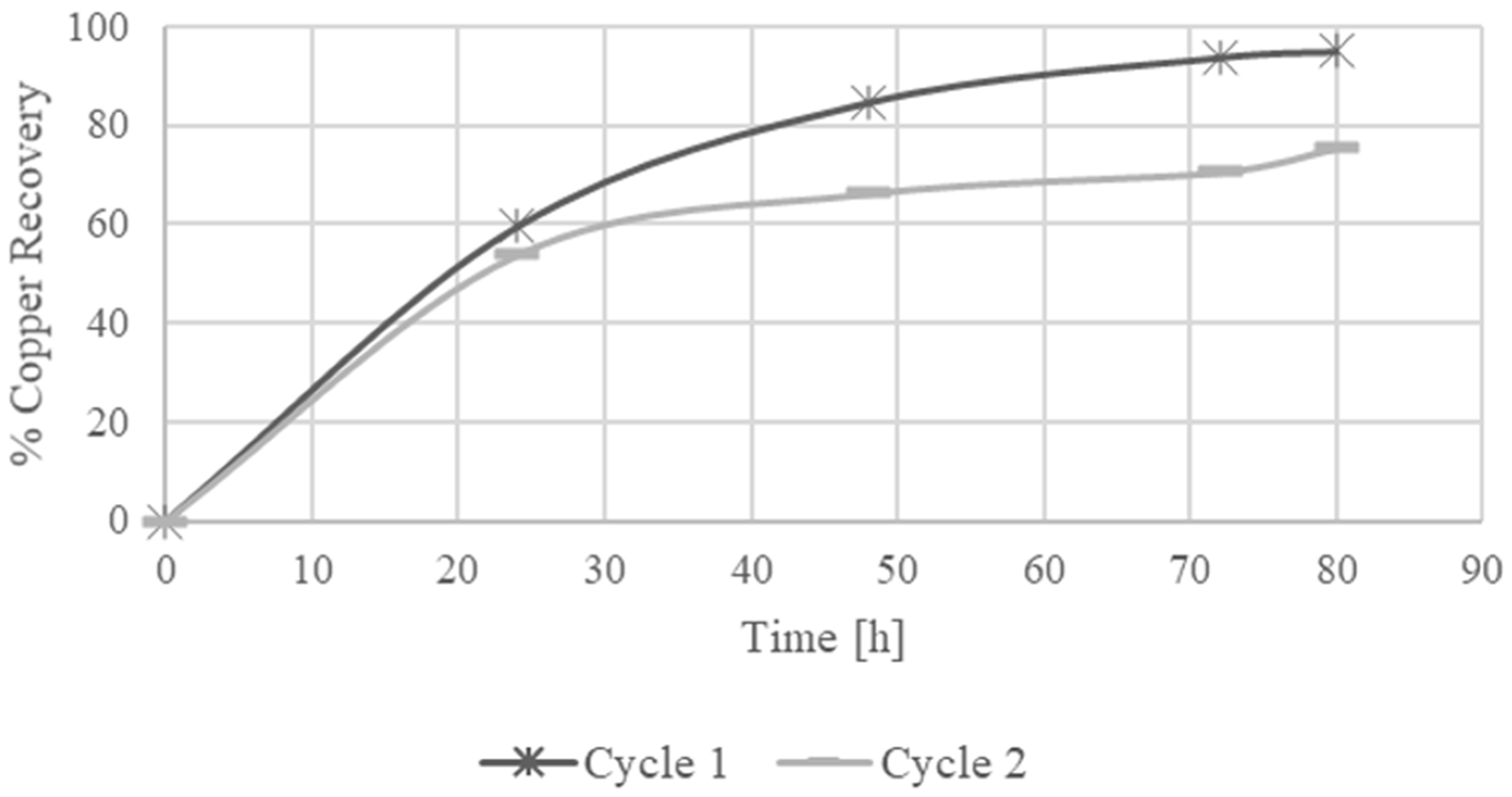
The recovery rates for copper leaching with sulfuric acid and ferric sulfate as the oxidizing agent varied significantly with the leaching conditions. At 17 °C with 15% solids, the copper recovery was 7.5%. In contrast, at 92 °C with 1% solids, the recovery rate improved substantially to 95.1% after 80 h. This demonstrates a strong positive correlation between temperature and copper dissolution, indicating that higher temperatures enhance copper recovery.
The cementation process effectively recovered copper, reducing the final copper concentration in the solution to less than 0.1 g/L and achieving a solid copper recovery rate of 98.4%.
To enhance the oxidizing potential of the residual solution, catalytic oxidation with activated carbon was performed, effectively converting ferrous ions (Fe2+) into ferric ions (Fe3+). The initial potential of the residual solution was 291 mV, and through the catalytic oxidation process, it was regenerated to 497 mV, restoring 90.7% of its oxidizing potential. The catalytic oxidation method demonstrates potential for industrial regeneration of ferric solutions by restoring the oxidizing potential of the leaching solution. Compared with traditional pyrometallurgical processes, it offers advantages including lower energy consumption, reduced environmental impact, and the potential for reuse of leaching solutions, thereby enhancing sustainability.
The fresh leaching solution initially exhibited a potential of >550 mV due to the action of ferric ions, achieving a copper recovery of 95.1% during the first leaching cycle. The regenerated solution enabled a second leaching cycle, yielding a copper recovery of 75.6%.
As this work represents an initial laboratory-scale approach, future developments based on these findings could explore strategies to optimize the handling of the excess leaching solution generated during the conditioning step by dilution. Such considerations would contribute to improving process efficiency and scalability under industrial conditions.