Processing Water-Based Lithium Iron Phosphate (LiFePO4) Cathodes with CMC Binder: The Impact of Dispersing Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Slurry Production
2.3. Slurry Characterization
2.4. Coating Process
2.5. Electrode Calendering
2.6. Coating Characterization
2.7. Electrode Punching and Drying
2.8. Cell Assembly
2.9. Electrochemical Characterization
3. Results
3.1. Slurry Properties
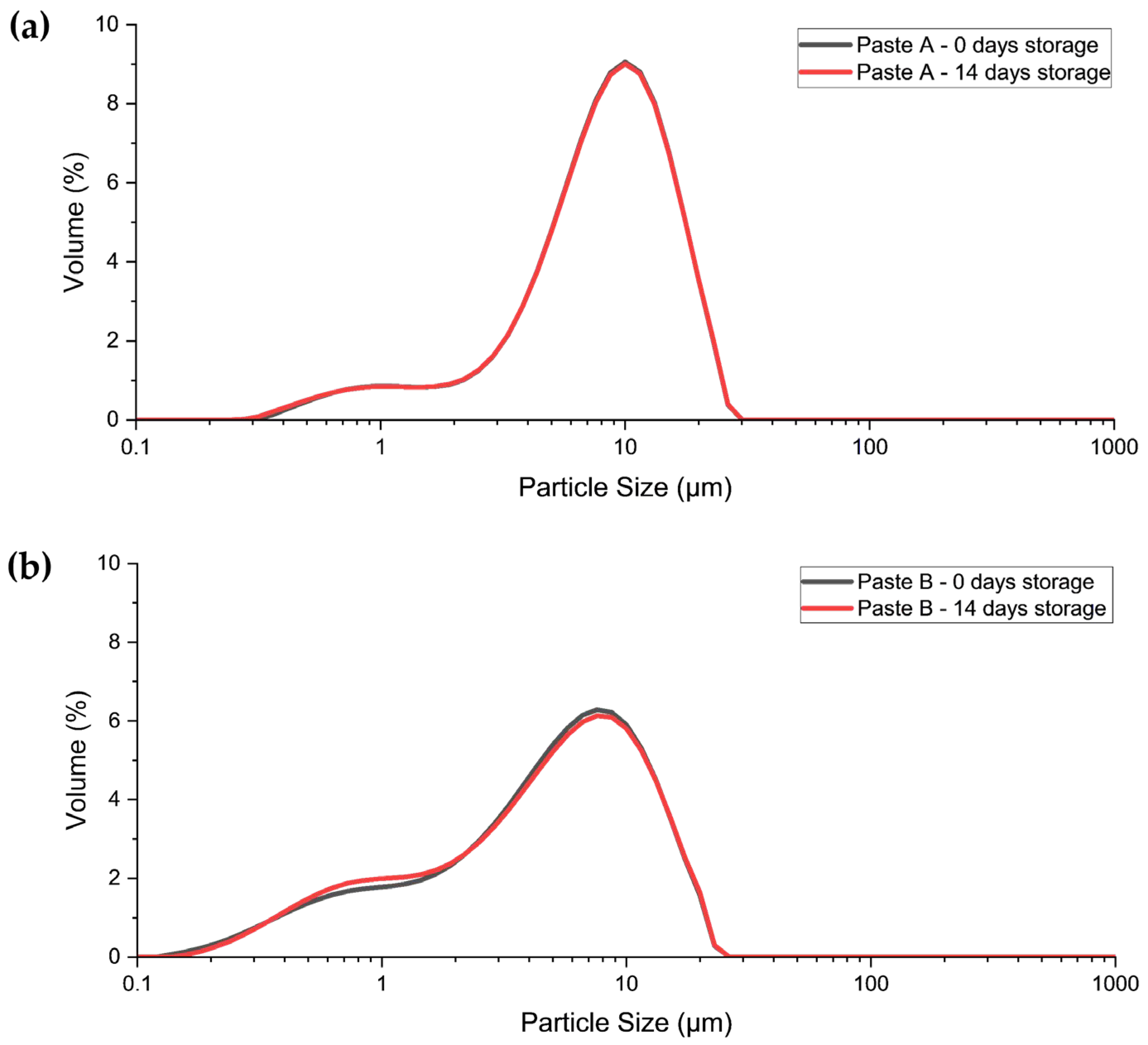
3.1.1. Particle Size Distribution
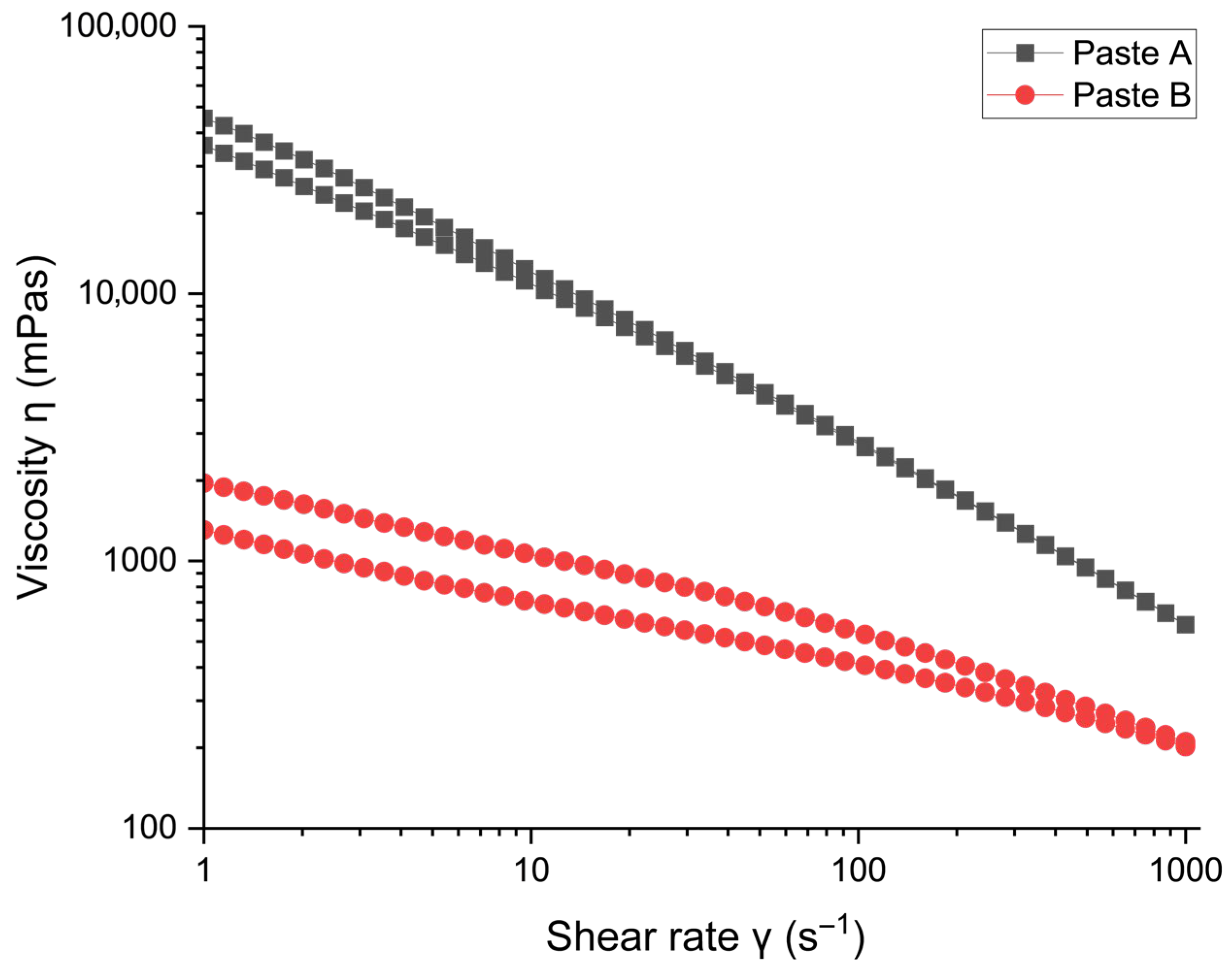
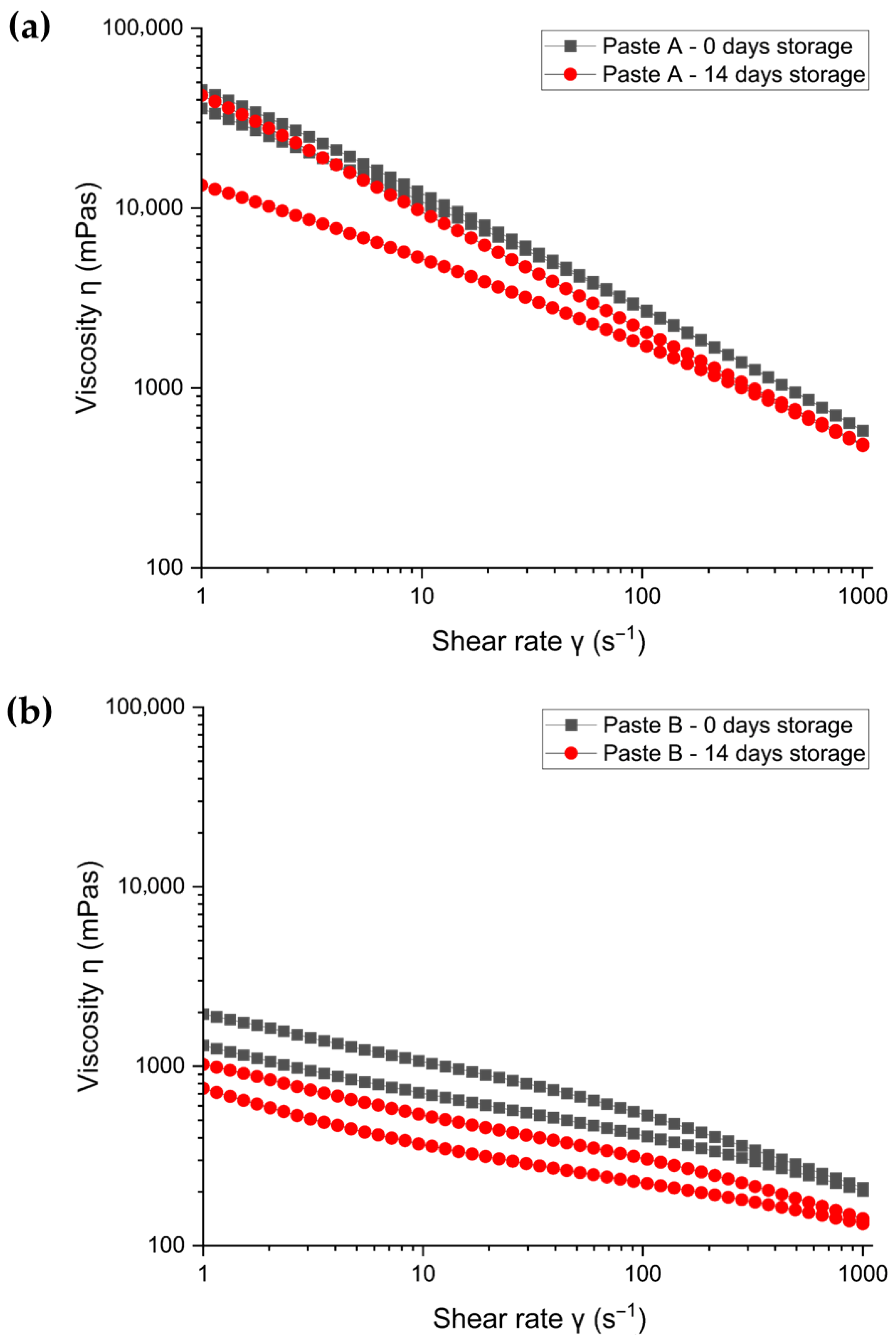
3.1.2. Rheology
3.2. Electrode Coating Characteristics
3.2.1. Electrode Layer Thickness
3.2.2. SEM Analysis
3.3. Electrochemical Performance
C-Rate Tests
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMC | Carboxymethyl cellulose |
| LCO | Lithium cobalt oxide |
| LFP | Lithium iron phosphate |
| LIBs | Lithium-ion batteries |
| NMC | Nickel manganese cobalt oxide |
| NMP | N-methyl-2-pyrrolidone |
| PVDF | Polyvinylidene fluoride |
| SBR | Styrene butadiene rubber |
| SD | Standard deviation |
| SEI | Solid electrolyte interface |
| SEM | Scanning electron microscopy |
References
- Ahmed, D.; Maraz, K.M. Revolutionizing energy storage: Overcoming challenges and unleashing the potential of next generation Lithium-ion battery technology. Mater. Eng. Res. 2023, 5, 265–278. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.-Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.-P. High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries. J. Power Sources 2020, 458, 228033. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and optimization of lithium-ion battery as an efficient energy storage device for electric vehicles: A comprehensive review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Jeon, J.-W.; Biswas, M.C.; Patton, C.L.; Wujcik, E.K. Water-processable, sprayable LiFePO4/graphene hybrid cathodes for high-power lithium ion batteries. J. Ind. Eng. Chem. 2020, 84, 72–81. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of batteries and battery management for electric vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-Energy Batteries: Beyond Lithium-Ion and Their Long Road to Commercialisation. Nano-Micro Lett. 2022, 14, 94. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Suttison, S.; Pengpat, K.; Intatha, U.; Fan, J.; Zhang, W.; Eitssayeam, S. Preparation of LFP-based cathode materials for lithium-ion battery applications. Mater. Today Proc. 2022, 65, 2347–2350. [Google Scholar] [CrossRef]
- Wulandari, T.; Fawcett, D.; Majumder, S.B.; Poinern, G.E.J. Lithium-based batteries, history, current status, challenges, and future perspectives. Battery Energy 2023, 2, 20230030. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Bae, J. Recent Advances in Lithium Iron Phosphate Battery Technology: A Comprehensive Review. Batteries 2024, 10, 424. [Google Scholar] [CrossRef]
- Boozula, A.R.; Bagheri, K.; Lampuse, R.G.; Shah, S.; Thakkar, J.J. Review of thermal runaway risks in Na-ion and Li-ion batteries: Safety improvement suggestions for Na-ion batteries. J. Eng. Appl. Sci. 2025, 72, 106. [Google Scholar] [CrossRef]
- Weng, J.; Peng, L. Improving the electrochemical performance of LiFePO4 cathode with novel water-soluble binders. Mater. Chem. Phys. 2022, 290, 126530. [Google Scholar] [CrossRef]
- Zhao, T.; Mahandra, H.; Marthi, R.; Ji, X.; Zhao, W.; Chae, S.; Traversy, M.; Li, W.; Yu, F.; Li, L.; et al. An overview on the life cycle of lithium iron phosphate: Synthesis, modification, application, and recycling. Chem. Eng. J. 2024, 485, 149923. [Google Scholar] [CrossRef]
- El Moutchou, S.; Aziam, H.; Mansori, M.; Saadoune, I. Thermal stability of Lithium-ion batteries: Case study of NMC811 and LFP cathode materials. Mater. Today Proc. 2022, 51, A1–A7. [Google Scholar] [CrossRef]
- Bitsch, B.; Dittmann, J.; Schmitt, M.; Scharfer, P.; Schabel, W.; Willenbacher, N. A novel slurry concept for the fabrication of lithium-ion battery electrodes with beneficial properties. J. Power Sources 2014, 265, 81–90. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Jalowy, L.; Nemec, D.; Ilhan, O. Comparison of Dispersing Processes of Bio-Based and Synthetic Materials: A Review. ChemEngineering 2025, 9, 36. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Pu, W.; Li, J.; Wan, C. Effect of slurry preparation and dispersion on electrochemical performances of LiFePO4 composite electrode. Ionics 2011, 17, 473–477. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, S.; Jeon, Y.; Jung, J.; Han, D.-Y.; Park, S. Improving silicon anode durability through uniform dispersion and binding enhancement with polyacrylamide-grafted carbon nanotubes. Energy Mater. 2025, 5, 500071. [Google Scholar] [CrossRef]
- Komoda, Y.; Ishibashi, K.; Kuratani, K.; Suzuki, K.; Ohmura, N.; Kobayashi, H. Effects of drying rate and slurry microstructure on the formation process of LiB cathode and electrochemical properties. J. Power Sources 2023, 568, 232983. [Google Scholar] [CrossRef]
- Baboo, J.P.; Yatoo, M.A.; Dent, M.; Hojaji Najafabadi, E.; Lekakou, C.; Slade, R.; Hinder, S.J.; Watts, J.F. Exploring Different Binders for a LiFePO4 Battery, Battery Testing, Modeling and Simulations. Energies 2022, 15, 2332. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—The transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Cuesta, N.; Ramos, A.; Cameán, I.; Antuña, C.; García, A.B. Hydrocolloids as binders for graphite anodes of lithium-ion batteries. Electrochim. Acta 2015, 155, 140–147. [Google Scholar] [CrossRef]
- Kuenzel, M.; Choi, H.; Wu, F.; Kazzazi, A.; Axmann, P.; Wohlfahrt-Mehrens, M.; Bresser, D.; Passerini, S. Co-Crosslinked Water-Soluble Biopolymers as a Binder for High-Voltage LiNi0.5Mn1.5O4|Graphite Lithium-Ion Full Cells. ChemSusChem 2020, 13, 2650–2660. [Google Scholar] [CrossRef]
- Liu, M.; Ye, C.; Peng, L.; Weng, J. Influence of Binder on Impedance of Lithium Batteries: A Mini-review. J. Electr. Eng. Technol. 2022, 17, 1281–1291. [Google Scholar] [CrossRef]
- Li, C.-C.; Lin, Y.-S. Interactions between organic additives and active powders in water-based lithium iron phosphate electrode slurries. J. Power Sources 2012, 220, 413–421. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Jhang, J.-H.; Hsieh, H.-W.; Li, C.-C. Dispersion, agglomeration, and gelation of LiFePO4 in water-based slurry. J. Power Sources 2016, 310, 47–53. [Google Scholar] [CrossRef]
- Chang, H.J.; Rodríguez-Pérez, I.A.; Fayette, M.; Canfield, N.L.; Pan, H.; Choi, D.; Li, X.; Reed, D. Effects of water-based binders on electrochemical performance of manganese dioxide cathode in mild aqueous zinc batteries. Carbon Energy 2021, 3, 473–481. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, X.; Shen, Z.; Ma, H.; Wang, J.; Wang, S.; Liu, L.; Liu, B.; Liu, L.; Zhao, Y. Green water-based binders for LiFePO4/C cathodes in Li-ion batteries: A comparative study. New J. Chem. 2021, 45, 9846–9855. [Google Scholar] [CrossRef]
- Gordon, R.; Kassar, M.; Willenbacher, N. Effect of Polymeric Binders on Dispersion of Active Particles in Aqueous LiFePO4-Based Cathode Slurries as well as on Mechanical and Electrical Properties of Corresponding Dry Layers. ACS Omega 2020, 5, 11455–11465. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.H.; Ahn, K.H. Role of carboxymethyl cellulose binder and its effect on the preparation process of anode slurries for Li-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131130. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, J.; Kim, H.; Kim, J.; Jin, H.-J. Polymeric Binder Design for Sustainable Lithium-Ion Battery Chemistry. Polymers 2024, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- NETZSCH-Feinmahltechnik GmbH. MasterMix® Dissolver. Available online: https://grinding.netzsch.com/en/products-and-solutions/mixing-de-aerating/mastermix-dissolver (accessed on 4 August 2025).
- Sugino Machine Limited. Star Burst Wet Pulverizing and Dispersing. Available online: https://www.suginocorp.com/star-burst/ (accessed on 4 August 2025).
- Bauer, W.; Çetinel, F.A.; Müller, M.; Kaufmann, U. Effects of pH control by acid addition at the aqueous processing of cathodes for lithium ion batteries. Electrochim. Acta 2019, 317, 112–119. [Google Scholar] [CrossRef]
- Reynolds, C.D.; Hare, S.D.; Slater, P.R.; Simmons, M.J.H.; Kendrick, E. Rheology and Structure of Lithium-Ion Battery Electrode Slurries. Energy Technol. 2022, 10, 2200545. [Google Scholar] [CrossRef]
- Ibing, L.; Gallasch, T.; Schneider, P.; Niehoff, P.; Hintennach, A.; Winter, M.; Schappacher, F.M. Towards water based ultra-thick Li ion battery electrodes—A binder approach. J. Power Sources 2019, 423, 183–191. [Google Scholar] [CrossRef]
- Ouyang, L.; Wu, Z.; Wang, J.; Qi, X.; Li, Q.; Wang, J.; Lu, S. The effect of solid content on the rheological properties and microstructures of a Li-ion battery cathode slurry. RSC Adv. 2020, 10, 19360–19370. [Google Scholar] [CrossRef]
- Reynolds, C.D.; Walker, H.; Mahgoub, A.; Adebayo, E.; Kendrick, E. Battery electrode slurry rheology and its impact on manufacturing. Energy Adv. 2025, 4, 84–93. [Google Scholar] [CrossRef]





| Paste A | Solids (g) | wsolid (%) 1 | Total Weight (g) | wtotal (%) 2 | |
|---|---|---|---|---|---|
| LFP | 237.5 | 91.0 | Solids | 261.1 | 37.1 |
| Carbon black | 15.6 | 6.0 | |||
| CMC | 8.0 | 3.0 | |||
| Deionized water | 0 | 0 | Solvents | 442.5 | 62.9 |
| Total | 261.1 | 100.0 | 703.6 | 100.0 | |
| Paste B | Solids (g) | wsolid (%) 1 | Total Weight (g) | wtotal (%) 2 | |
|---|---|---|---|---|---|
| LFP | 237.5 | 91.0 | Solids | 261.1 | 41.1 |
| Carbon black | 15.6 | 6.0 | |||
| CMC | 8.0 | 3.0 | |||
| Deionized water | 0 | 0 | Solvents | 374.0 | 58.9 |
| Total | 261.1 | 100.0 | 635.1 | 100.0 | |
| Paste A | ||||||
|---|---|---|---|---|---|---|
| Total Electrode Thickness | Electrode Layer Thickness | |||||
| Measuring Position | Uncalendared (µm) | Calendered (µm) | Compaction (%) | Uncalendared (µm) | Calendered (µm) | Compaction (%) |
| Start | 112 | 86 | 23.2 | 92 | 66 | 28.3 |
| Middle left | 108 | 80 | 25.9 | 88 | 60 | 31.8 |
| Middle right | 109 | 81 | 25.7 | 89 | 61 | 31.5 |
| End | 105 | 80 | 23.8 | 85 | 60 | 29.4 |
| Mean ± SD 1 | 108.5 ± 2.9 | 81.8 ± 2.9 | 24.7 ± 1.4 | 88.5 ± 2.9 | 61.8 ± 2.9 | 30.2 ± 1.7 |
| Paste B | ||||||
|---|---|---|---|---|---|---|
| Total Electrode Thickness | Electrode Layer Thickness | |||||
| Measuring Position | Uncalendared (µm) | Calendered (µm) | Compaction (%) | Uncalendared (µm) | Calendered (µm) | Compaction (%) |
| Start | 105 | 80 | 23.8 | 85 | 60 | 29.4 |
| Middle left | 112 | 86 | 23.2 | 92 | 66 | 28.3 |
| Middle right | 113 | 82 | 27.4 | 93 | 62 | 33.3 |
| End | 101 | 76 | 24.8 | 81 | 56 | 30.9 |
| Mean ± SD 1 | 107.8 ± 5.7 | 81.0 ± 4.2 | 24.8 ± 1.9 | 87.8 ± 5.7 | 61.0 ± 4.2 | 30.5 ± 2.2 |
| Cell | Mass Loading (mg cm−2) | Areal Capacity (mAh cm−2) | |
|---|---|---|---|
| Paste A | Cell 1 | 9.05 | 1.36 |
| Cell 2 | 9.14 | 1.37 | |
| Cell 3 | 8.50 | 1.28 | |
| Mean ± SD 1 | 8.90 ± 0.35 | 1.34 ± 0.05 | |
| Paste B | Cell 1 | 9.28 | 1.39 |
| Cell 2 | 9.28 | 1.39 | |
| Cell 3 | 8.68 | 1.30 | |
| Mean ± SD 1 | 9.08 ± 0.35 | 1.36 ± 0.05 |
| Discharge Capacity (mAh g−1) at | |||||||
|---|---|---|---|---|---|---|---|
| 0.1 C | 0.33 C | 0.5 C | 1.0 C | 3.0 C | 0.1 C | ||
| Paste A | Cell 1 | 114.9 ± 2.6 | 99.3 ± 6.5 | 92.7 ± 7.2 | 73.0 ± 12.6 | 1.7 ± 0.2 | 112.0 ± 8.0 |
| Cell 2 | 116.6 ± 0.7 | 99.7 ± 6.0 | 92.4 ± 6.8 | 72.6 ± 12.5 | 1.7 ± 0.2 | 111.4 ± 7.7 | |
| Cell 3 | 111.6 ± 5.1 | 99.2 ± 6.0 | 92.5 ± 5.8 | 73.6 ± 11.7 | 1.7 ± 0.2 | 110.7 ± 8.0 | |
| Mean ± SD 1 | 114.4 ± 2.8 | 99.4 ± 6.2 | 92.5 ± 6.6 | 73.1 ± 12.3 | 1.7 ± 0.2 | 111.4 ± 7.9 | |
| Paste B | Cell 1 | 112.5 ± 4.2 | 102.9 ± 4.6 | 95.9 ± 3.3 | 84.5 ± 3.5 | 1.5 ± 0.3 | 120.2 ± 4.8 |
| Cell 2 | 114.1 ± 4.1 | 102.5 ± 3.4 | 96.6 ± 4.0 | 83.6 ± 2.6 | 1.5 ± 0.2 | 117.0 ± 5.9 | |
| Cell 3 | 114.6 ± 4.2 | 102.0 ± 3.3 | 97.0 ± 4.1 | 83.2 ± 2.8 | 1.5 ± 0.1 | 115.8 ± 7.4 | |
| Mean ± SD 1 | 113.7 ± 4.2 | 102.5 ± 3.8 | 96.5 ± 3.8 | 83.8 ± 3.0 | 1.5 ± 0.2 | 117.7 ± 6.1 | |
| Initial Coulombic Efficiency (%) at 0.1 C | ||||
|---|---|---|---|---|
| 1st Cycle | 2nd Cycle | 3rd Cycle | ||
| Paste A | Cell 1 | 96.81 | 102.30 | 95.10 |
| Cell 2 | 96.02 | 101.52 | 100.57 | |
| Cell 3 | 102.10 | 99.70 | 94.46 | |
| Mean ± SD 1 | 98.31 ± 3.31 | 101.17 ± 1.33 | 96.71 ± 3.36 | |
| Paste B | Cell 1 | 97.76 | 100.84 | 100.06 |
| Cell 2 | 99.12 | 101.09 | 100.48 | |
| Cell 3 | 100.34 | 101.35 | 100.49 | |
| Mean ± SD 1 | 99.07 ± 1.29 | 101.09 ± 0.26 | 100.34 ± 0.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalowy, L.; Lehmann, H.; Rassek, P.; Fromm, O.; Entenmann, M.; Nemec, D. Processing Water-Based Lithium Iron Phosphate (LiFePO4) Cathodes with CMC Binder: The Impact of Dispersing Methods. AppliedChem 2025, 5, 33. https://doi.org/10.3390/appliedchem5040033
Jalowy L, Lehmann H, Rassek P, Fromm O, Entenmann M, Nemec D. Processing Water-Based Lithium Iron Phosphate (LiFePO4) Cathodes with CMC Binder: The Impact of Dispersing Methods. AppliedChem. 2025; 5(4):33. https://doi.org/10.3390/appliedchem5040033
Chicago/Turabian StyleJalowy, Leah, Henry Lehmann, Patrick Rassek, Olga Fromm, Marc Entenmann, and Dominik Nemec. 2025. "Processing Water-Based Lithium Iron Phosphate (LiFePO4) Cathodes with CMC Binder: The Impact of Dispersing Methods" AppliedChem 5, no. 4: 33. https://doi.org/10.3390/appliedchem5040033
APA StyleJalowy, L., Lehmann, H., Rassek, P., Fromm, O., Entenmann, M., & Nemec, D. (2025). Processing Water-Based Lithium Iron Phosphate (LiFePO4) Cathodes with CMC Binder: The Impact of Dispersing Methods. AppliedChem, 5(4), 33. https://doi.org/10.3390/appliedchem5040033







