Hydrothermal Carbonization of Sugarcane Tip (Saccharum officinarum L.) for Pb (II) Removal: Synthesis, Characterization, and Adsorption Equilibrium
Abstract
1. Introduction
2. Materials and Methods
2.1. Harvesting and Processing Sugarcane Tip
2.2. FTIR-ATR and TGA Analyses
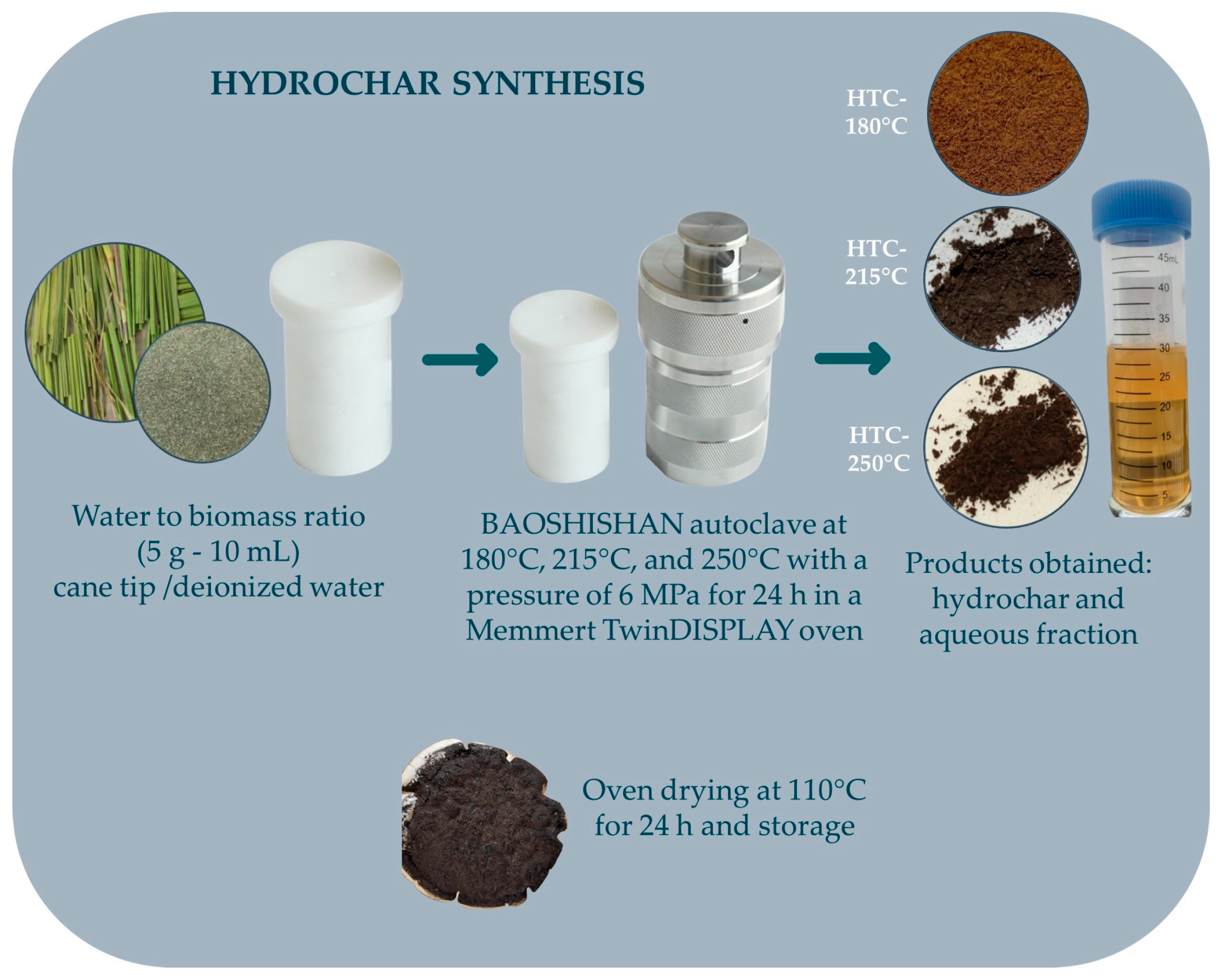
2.3. Hydrochar Synthesis
2.4. Proximate Analysis of ST Hydrochar
2.5. Adsorption Equilibrium Experiment
2.6. Active Site Analysis of ST Biomass and Hydrochar
2.7. Texture Analysis of ST Hydrochar
3. Results and Discussion
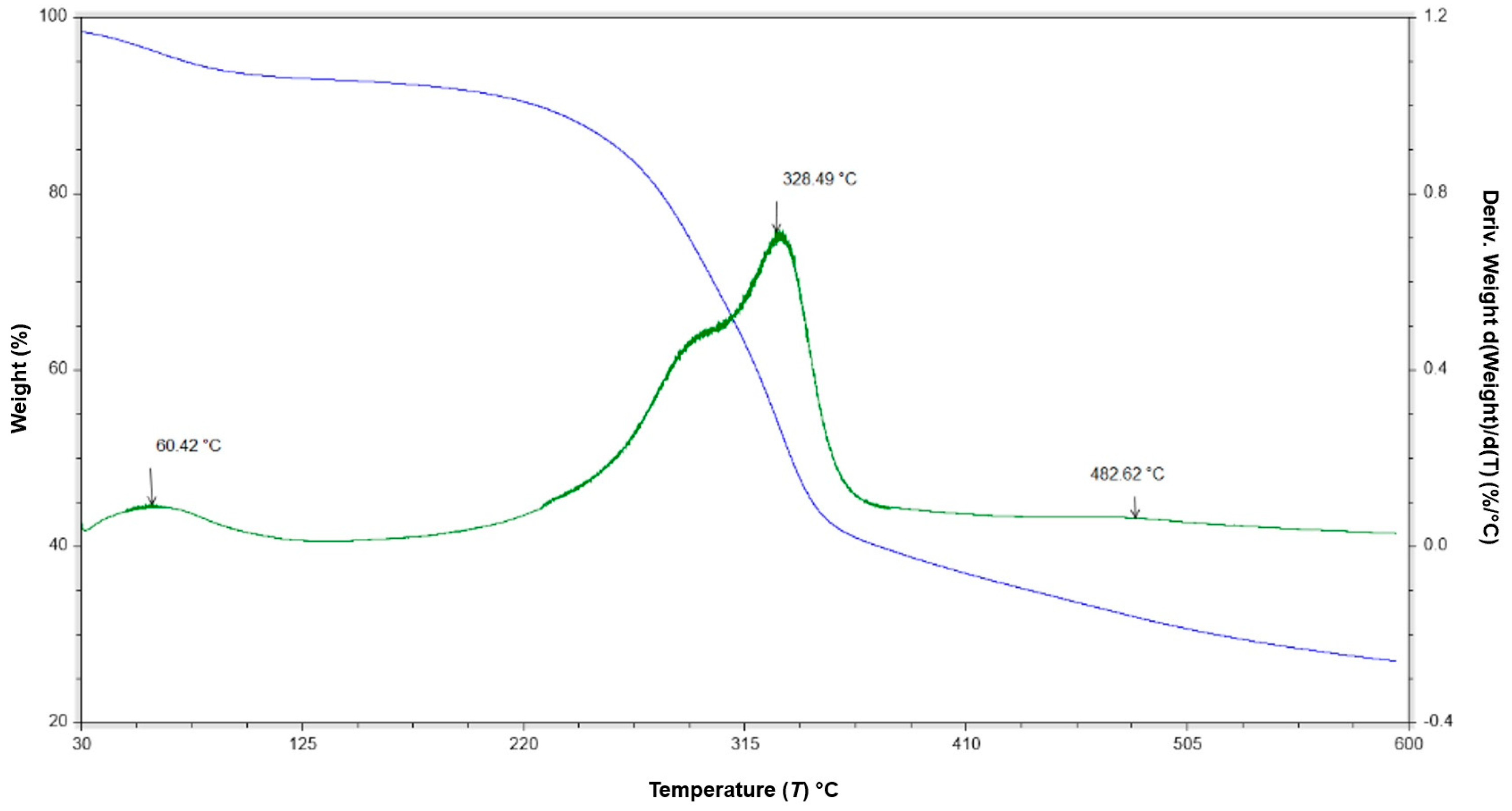
3.1. FTIR-ATR and TGA Analyses
3.2. ST Hydrochar Synthesis Yields
3.3. Characterization of ST Hydrochar
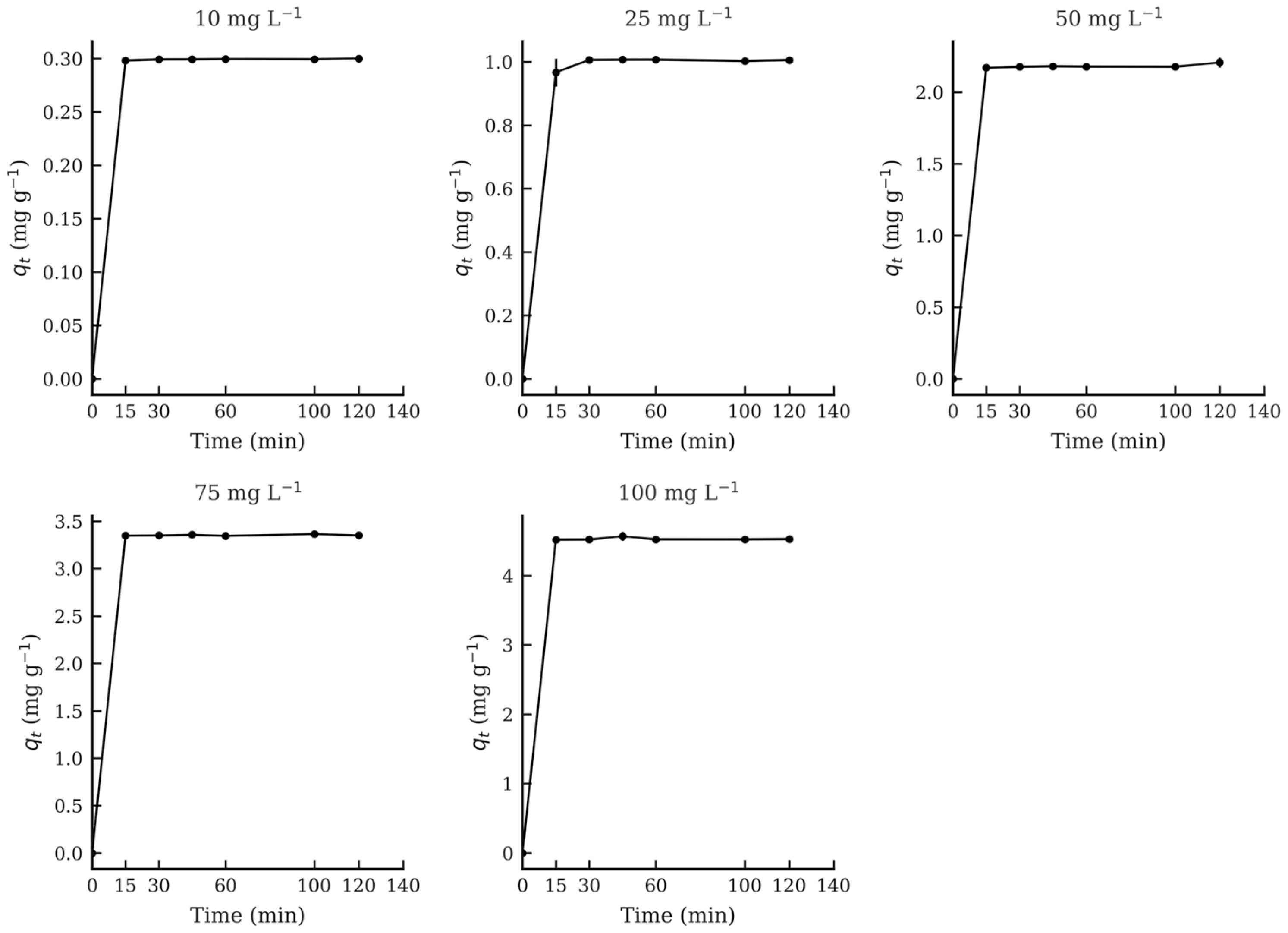
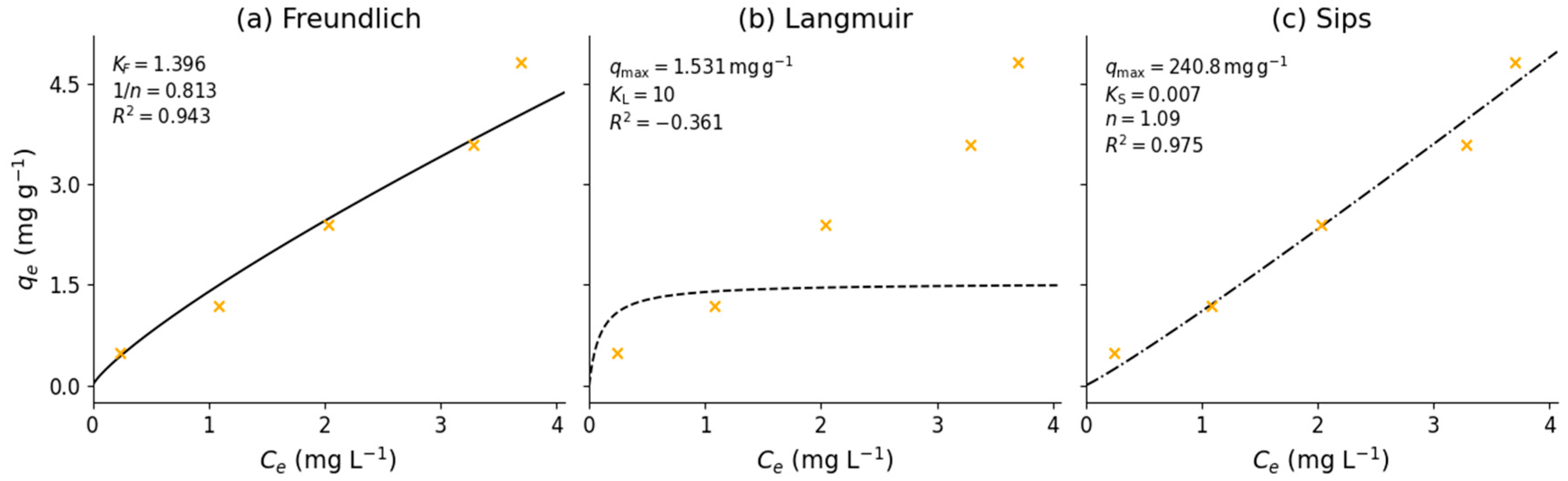
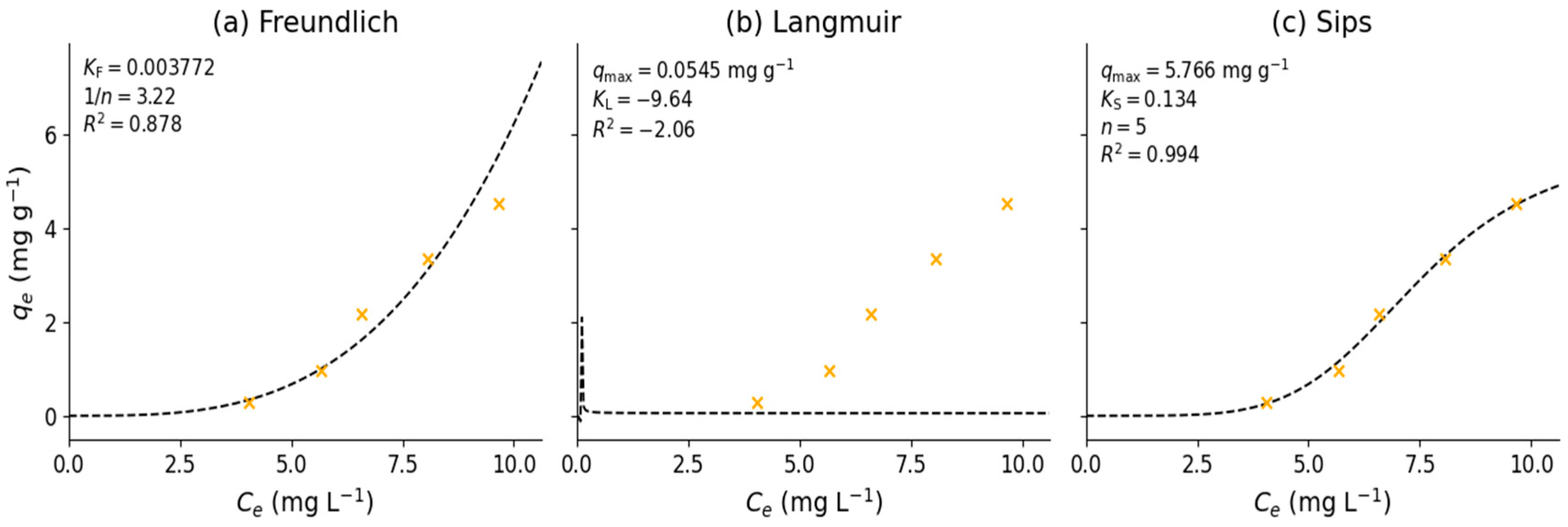
3.4. Adsorption Equilibrium Experiment
3.5. Active Sites in ST and Hydrochar Biomass
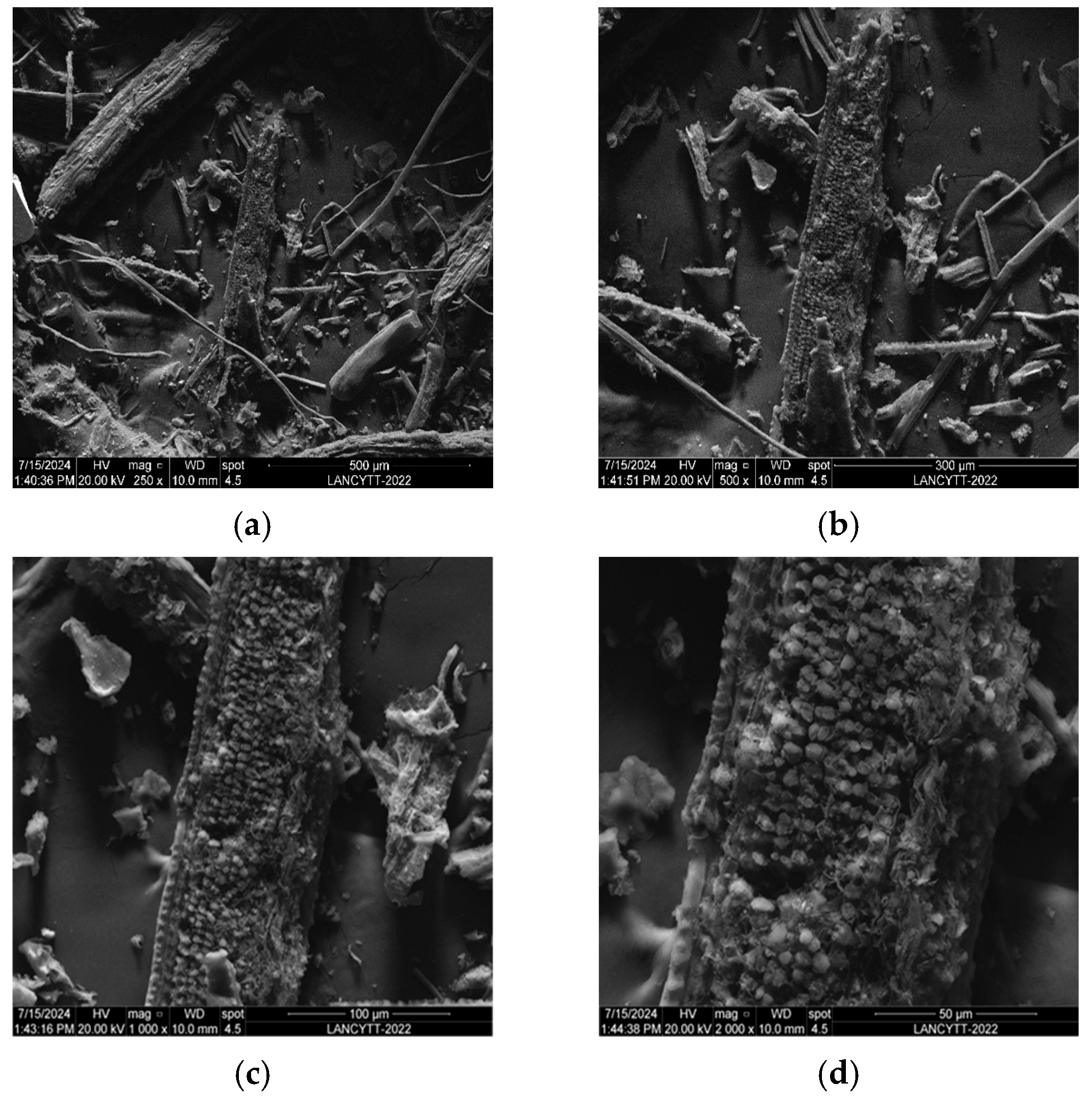
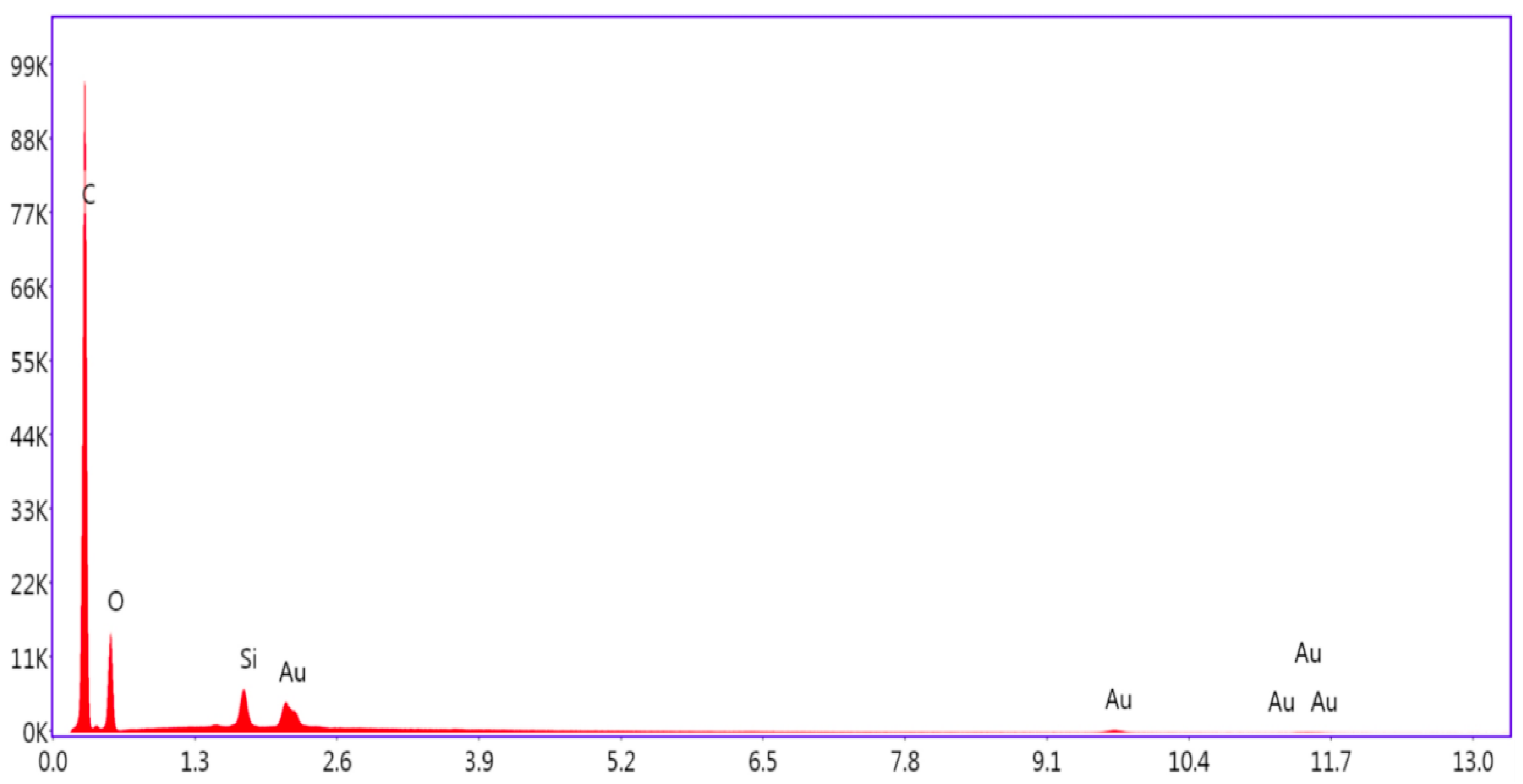
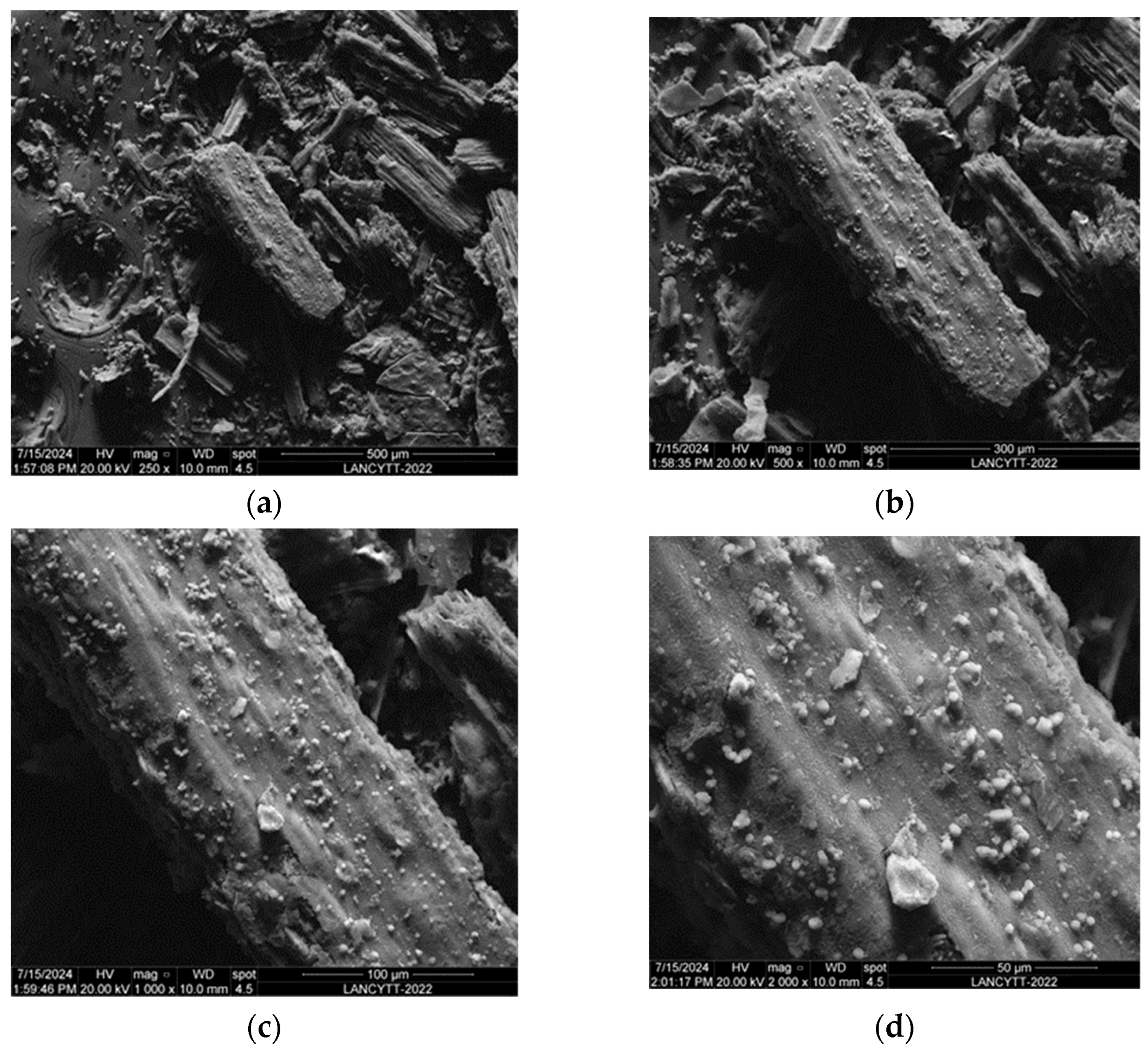
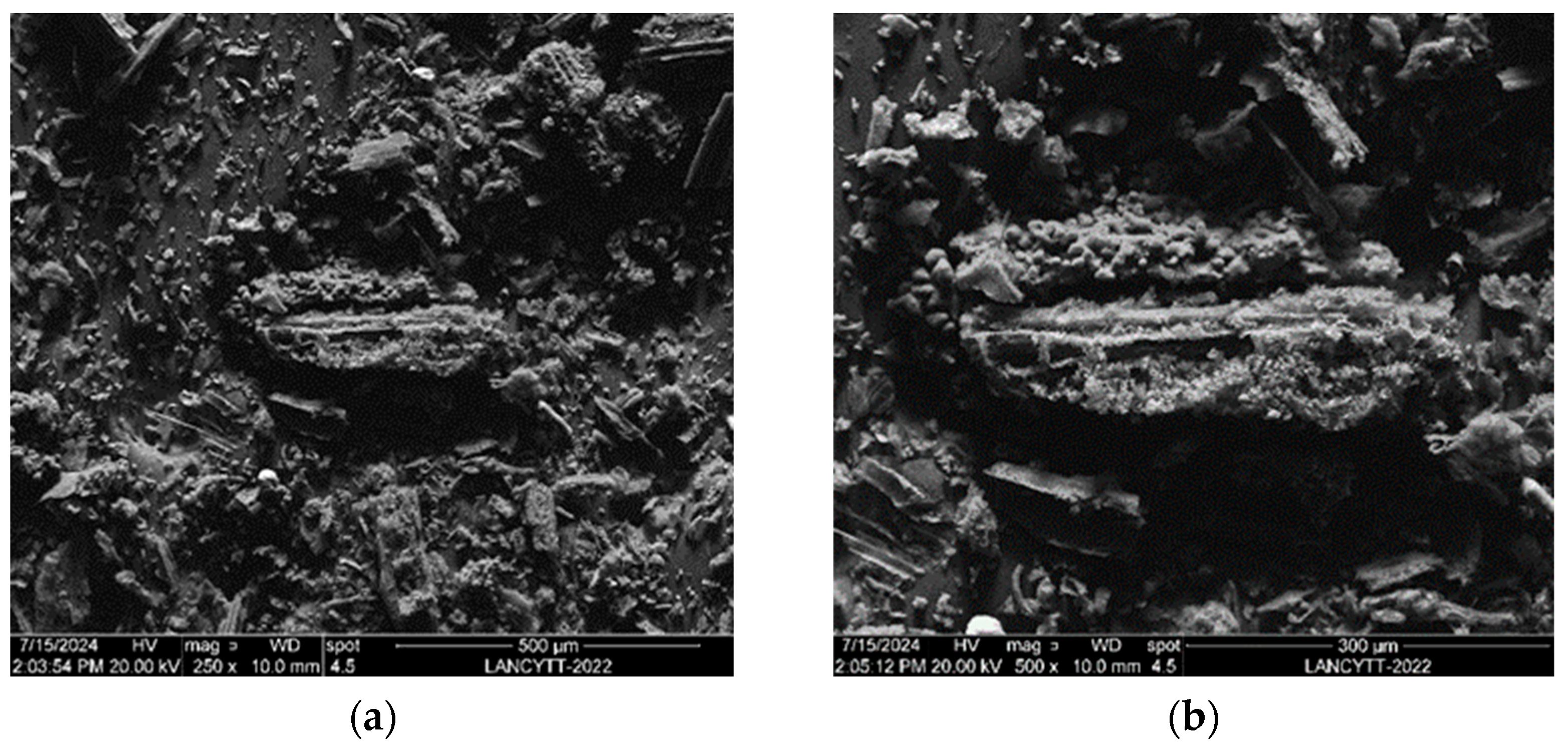
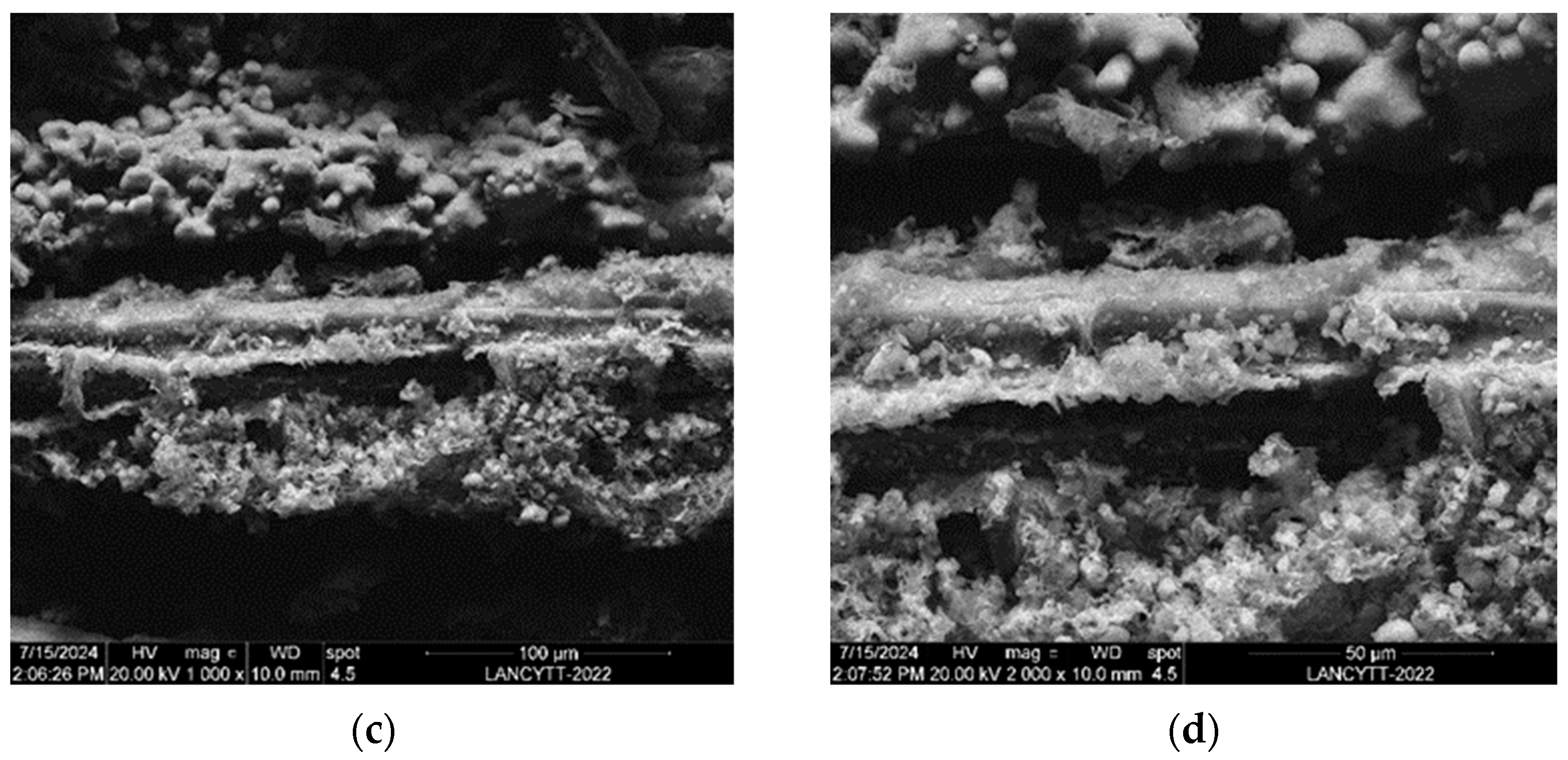
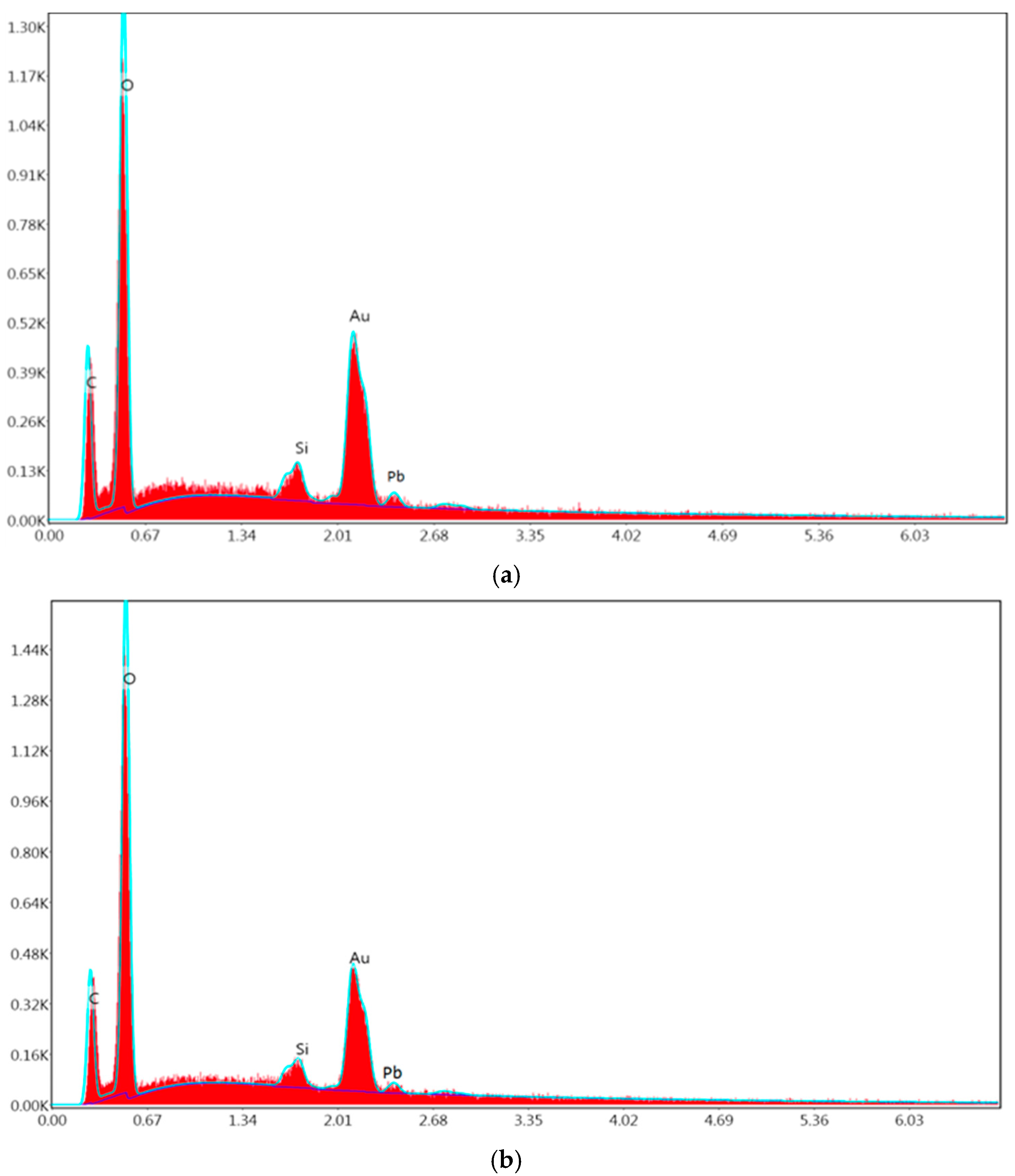
3.6. SEM and EDAX Analysis
3.7. Proposed Adsorption Mechanism Based on Experimental Evidence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Brito, G.M.; Cipriano, D.F.; Schettino, M.Â.; Cunha, A.G.; Coelho, E.R.C.; Freitas, J.C.C. One-Step Methodology for Preparing Physically Activated Biocarbons from Agricultural Biomass Waste. Environ. Chem. Eng. 2019, 7, 103113. [Google Scholar] [CrossRef]
- Hoang, A.T.; Kumar, S.; Lichtfouse, E.; Cheng, C.K.; Varma, R.S.; Senthilkumar, N.; Nguyen, P.Q.P.; Nguyen, X.P. Remediation of Heavy Metal Polluted Waters Using Activated Carbon from Lignocellulosic Biomass: An Update of Recent Trends. Chemosphere 2022, 302, 134825. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, A.A.; Li, J. Removal of Pb (II) from Aqueous Solution by Using Biochars Derived from Sugar Cane Bagasse and Orange Peel. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.S. Agricultural Waste of Sugarcane Bagasse as Efficient Adsorbent for Lead and Nickel Removal from Untreated Wastewater: Biosorption, Equilibrium Isotherms, Kinetics and Desorption Studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef]
- Secretaría de Agricultura y Desarrollo Rural. Planeación Agrícola Nacional 2017–2030. Gobierno de México. 2017. Available online: https://www.gob.mx/agricultura/acciones-y-programas/planeacion-agricola-nacional-2017-2030-126813 (accessed on 15 March 2024).
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Sistema de Información Agroalimentaria de Consulta (SIACON). Gobierno de México. 2023. Available online: https://www.gob.mx/agricultura/dgsiap/prensa/sistema-de-informacion-agroalimentaria-de-consulta-siacon?idiom=es (accessed on 20 March 2024).
- Aguilar, R.N. Value Chain of the Diversification of the Sugar Cane Agroindustry in México. Agroproductividad 2017, 10, 21–27. [Google Scholar]
- Sampaio, I.L.M.; Cardoso, T.F.; Souza, N.R.D.; Watanabe, M.D.B.; Carvalho, D.J.; Bonomi, A.; Junqueira, T.L. Electricity Production from Sugarcane Straw Recovered Through Bale System: Assessment of Retrofit Projects. BioEnergy Res. 2019, 12, 865–877. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Hydrothermal Processing of Algal Biomass for the Production of Biofuels and Chemicals. Biofuels 2012, 3, 603–623. [Google Scholar] [CrossRef]
- Wang, T.; Yunbo, Z.; Zhu, Y.; Caiting, L.; Guangming, Z. A Review of the Hydrothermal Carbonization of Biomass Waste for Hydrochar Formation: Process Conditions, Fundamentals, and Physicochemical Properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Malool, M.E.; Keshavarz, M.; Shayegan, J. Co-hydrothermal Carbonization of Digested Sewage Sludge and Sugarcane Bagasse: Integrated Approach for Waste Management, Optimized Production, Characterization and Pb(II) Adsorption. Alexandria Eng. J. 2023, 74, 79–105. [Google Scholar] [CrossRef]
- Izquierdo, S.; Pacheco, N.; Durán-Valle, C.J.; López-Coca, I.M. From Waste to Resource: Utilizing Sweet Chestnut Waste to Produce Hydrothermal Carbon for Water Decontamination. C 2023, 9, 57. [Google Scholar] [CrossRef]
- Koprivica, M.; Simić, M.; Petrović, J.; Ercegović, M.; Dimitrijević, J. Evaluation of Adsorption Efficiency on Pb(II) Ions Removal Using Alkali-Modified Hydrochar from Paulownia Leaves. Processes 2023, 11, 1327. [Google Scholar] [CrossRef]
- Khushk, S.; Zhang, L.; Li, A.; Irfan, M.; Zhang, X. Microwave-Assisted Hydrothermal Carbonization of Furfural Residue for Adsorption of Cr(VI): Adsorption and Kinetic Study. Pol. J. Environ. Stud. 2020, 29, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Prasannamedha, G.; Senthil Kumar, P.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced Adsorptive Removal of Sulfamethoxazole from Water Using Biochar Derived from Hydrothermal Carbonization of Sugarcane Bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Malool, M.E.; Keshavarz Moraveji, M.; Shayegan, J. Optimized Production, Pb(II) Adsorption and Characterization of Alkali Modified Hydrochar from Sugarcane Bagasse. Sci. Rep. 2021, 11, 22328. [Google Scholar] [CrossRef]
- Dos Santos, W.N.L.; Cavalcante, D.D.; da Silva, E.G.P.; das Virgens, C.F.; de Souza Dias, F. Biosorption of Pb(II) and Cd(II) ions by Agave sisalana (sisal fiber). Microchem. J. 2011, 97, 269–273. [Google Scholar] [CrossRef]
- Medellín, N.A.; Hernández, M.G.; Salazar, J.J.; Labrada, G.J.; Aragón, A. Bioadsorción de Plomo (II) Presente en Solución Acuosa sobre Residuos de Fibras Naturales Procedentes de la Industria Ixtlera (Agave lechuguilla Torr. y Yucca carnerosana). Rev. Int. Contam. Ambient. 2017, 33, 269–280. [Google Scholar] [CrossRef]
- Xiang, A.; Ebdon, J.R.; Horrocks, A.R.; Kandola, B.K. On the Utility of Thermogravimetric Analysis for Exploring the Kinetics of Thermal Degradation of Lignins. Bioresour. Technol. Rep. 2022, 20, 101214. [Google Scholar] [CrossRef]
- Karn, A.; Thakur, S.; Shrestha, B. Extraction and Characterization of Cellulose from Agricultural Residues: Wheat Straw and Sugarcane Bagasse. J. Nepal Chem. Soc. 2022, 43, 89–94. [Google Scholar] [CrossRef]
- Sofana, I.; Wijayanti, W.; Hamidi, N. Study of Thermal Behavior in Mixed Scrap Tires (ST) and Polypropylene (PP) Plastic Using Thermogravimetry Analysis (TGA) and Differential Scanning Calorimetry (DSC). Int. J. Mech. Eng. Technol. Appl. 2022, 7, 122–129. [Google Scholar] [CrossRef]
- González, J.; Sánchez, M.E.; Martínez, E.J.; Covalski, C.; Alonso-Simón, A.; González, R.; Cara, J. Hydrothermal Carbonization of Olive Tree Pruning as a Sustainable Way for Improving Biomass Energy Potential: Effect of Reaction Parameters on Fuel Properties. Processes 2020, 8, 1201. [Google Scholar] [CrossRef]
- Garrido, R.A.; Lagos, C.; Luna, C.; Sánchez, J.; Díaz, G. Study of the Potential Uses of Hydrochar from Grape Pomace and Walnut Shells Generated from Hydrothermal Carbonization as an Alternative for the Revalorization of AgriWaste in Chile. Sustainability 2021, 13, 12600. [Google Scholar] [CrossRef]
- ISO 18134-3:2015; Solid Biofuels—Determination of Moisture Content. Oven Dry Method. ISO (International Organization for Standardization): Geneva, Switzerland, 2015.
- ISO 18122:2022; Solid Biofuels—Determination of Ash Content. ISO (International Organization for Standardization): Geneva, Switzerland, 2022.
- ISO 18123:2023; Solid Biofuels—Determination of Volatile Matter. ISO (International Organization for Standardization): Geneva, Switzerland, 2023.
- Mahmood-ul-Hassan, M.; Suthar, V.; Rafique, E.; Ahmad, R.; Yasin, M. Kinetics of cadmium, chromium, and lead sorption onto chemically modified sugarcane bagasse and wheat straw. Environ. Monit. Assess. 2015, 187, 470. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Hummadi, K.K.; Luo, S.; He, S. Adsorption of Methylene Blue Dye from the Aqueous Solution via Bio-adsorption in the Inverse Fluidized-Bed Adsorption Column Using the Torrefied Rice Husk. Chemosphere 2022, 287 Pt 1, 131907. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of Biochar for the Removal of Pollutants from Aqueous Solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Cruz, S.A.; Medellín, N.A.; Torres, A. Bone Char from an Invasive Aquatic Specie as a Green Adsorbent for Fluoride Removal in Drinking Water. Water Air Soil Pollut. 2021, 232, 346. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Biohythane Production from Sugarcane Bagasse and Water Hyacinth: A Way towards Promising Green Energy Production. J. Clean. Prod. 2019, 207, 689–701. [Google Scholar] [CrossRef]
- Mohammed, I.Y.; Kabir, G.; Abakr, Y.A.; Apasiku, M.A.A.; Kazi, F.K.; Abubakar, L.G. Bioenergy Potential of Millet Chaff via Thermogravimetric Analysis and Combustion Process Simulation Using Aspen Plus. Clean. Chem. Eng. 2022, 3, 100046. [Google Scholar] [CrossRef]
- Rueda-Ordóñez, Y.J.; Tannous, K. Drying and Thermal Decomposition Kinetics of Sugarcane Straw by Nonisothermal Thermogravimetric Analysis. Bioresour. Technol. 2018, 264, 131–139. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Wei, C.; Hou, H.; Song, G.; Cao, L.; Zhang, J. Hydrothermal Carbonization of Heavy Metal-Contaminated Biomass: Migration, Transformation, and Ecological Stability Changes of Metals. Int. J. Mol. Sci. 2025, 26, 2551. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Hoekman, S.K.; Balasubramanian, R. Chemical, Structural and Combustion Characteristics of Carbonaceous Products Obtained by Hydrothermal Carbonization of Palm Empty Fruit Bunches. Bioresour. Technol. 2013, 135, 683–689. [Google Scholar] [CrossRef]
- Devnath, B.; Khanal, S.; Shah, A.; Reza, T. Influence of Hydrothermal Carbonization (HTC) Temperature on Hydrochar and Process Liquid for Poultry, Swine, and Dairy Manure. Environments 2024, 11, 150. [Google Scholar] [CrossRef]
- Mendoza-Martinez, C.L.; Sermyagina, E.; Saari, J.; de Jesus, M.S.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Hydrothermal Carbonization of Lignocellulosic Agro-Forest Based Biomass Residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Karim, A.A.; Martínez-Cartas, M.L.; Cuevas-Aranda, M. Production of Hydrochar Fuel by Microwave-Hydrothermal Carbonisation of Olive Pomace Slurry from Olive Oil Industry for Combustion Application. J. Anal. Appl. Pyrolysis 2024, 183, 106801. [Google Scholar] [CrossRef]
- Krysanova, K.O.; Krylova, A.Y.; Zaichenko, V.M.; Lavrenov, V.; Khaskhachikh, V. Influence of the parameters of the hydrothermal carbonization of the biomass on the biocoal obtained from peat. E3S Web Conf. 2019, 114, 07003. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Licursi, D.; Raspolli Galletti, A.M.; Bertini, B.; Ardemani, L.; Scotti, N.; Di Fidio, N.; Fulignati, S.; Antonetti, C. Design Approach for the Sustainable Synthesis of Sulfonated Biomass-Derived Hydrochars and Pyrochars for the Production of 5-(Hydroxymethyl)furfural. Sustain. Chem. Pharm. 2023, 35, 101216. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, W.; Liu, Y.; Tan, S.; Cai, H.; Geng, J.; Liu, X. Effective Removal of Pb(II) from Multiple Cationic Heavy Metals—An Inexpensive Lignin-Modified Attapulgite. Sustainability 2024, 16, 5831. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Salazar, J.J.; Leyva, R. Novel biosorbent with high adsorption capacity prepared by chemical modification of white pine (Pinus durangensis) sawdust. Adsorption of Pb (II) from aqueous solutions. J. Environ. Manag. 2016, 169, 303–312. [Google Scholar] [CrossRef]
- Martín–Lorenzo, A.; Hoyos, M.; Álvarez–Gómez, A. The Influence of Hydrothermal Carbonization Parameters on the Textural and Physicochemical Properties of Highly Porous Activated Carbons Derived from Garlic Peel Biowaste. J. Anal. Appl. Pyrolysis 2025, 192, 107280. [Google Scholar] [CrossRef]
- Moreno-Chocontá, L.N.; Lozano-Pérez, A.S.; Guerrero-Fajardo, C.A. Evaluation of the Effect of Particle Size and Biomass-to-Water Ratio on the Hydrothermal Carbonization of Sugarcane Bagasse. ChemEngineering 2024, 8, 43. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michel, J.R.; Ritchie, N.W.; Scott, J.H.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2018; 550p, ISBN 978-1-4939-6674-5. [Google Scholar]





| ST HTC | Temperature (°C) | Time (h) | Hydrochar Yield (%R HYR) | Aqueous Fraction Yield (% R AF) |
|---|---|---|---|---|
| 1 | 180 | 24 | 64.21 | 33.45 |
| 2 | 180 | 24 | 59.11 | 39.82 |
| 3 | 180 | 24 | 60.73 | 36.63 |
| 4 | 180 | 24 | 65.16 | 33.45 |
| 5 | 215 | 24 | 44.97 | 47.78 |
| 6 | 215 | 24 | 41.32 | 55.75 |
| 7 | 215 | 24 | 43.36 | 52.56 |
| 8 | 215 | 24 | 43.20 | 54.15 |
| 9 | 250 | 24 | 41.88 | 58.93 |
| 10 | 250 | 24 | 36.40 | 62.12 |
| 11 | 250 | 24 | 38.23 | 60.52 |
| 12 | 250 | 24 | 39.98 | 60.36 |
| ST HTC | Moisture (%) | Ash (%) | Volatile Matter (%VM) | Fixed Carbon (%CF) |
|---|---|---|---|---|
| 180 °C | 0.57 | 2.86 | 83.09 | 13.48 |
| 215 °C | 0.62 | 5.48 | 71.34 | 22.56 |
| 250 °C | 0.13 | 6.66 | 60.19 | 33.02 |
| ST HTC (°C) | C0 (mg L−1) | Ce (mg L−1) | qt (mg g−1) | ST HTC (°C) | C0 (mg L−1) | Ce (mg L−1) | qt (mg g−1) | ST HTC (°C) | C0 (mg L−1) | Ce (mg L−1) | qt (mg g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 180 | 10 | 0.24 | 0.49 | 215 | 10 | 3.83 | 0.31 | 250 | 10 | 4.04 | 0.30 |
| 180 | 25 | 1.09 | 1.20 | 215 | 25 | 4.80 | 1.01 | 250 | 25 | 5.66 | 0.97 |
| 180 | 50 | 2.04 | 2.40 | 215 | 50 | 6.52 | 2.17 | 250 | 50 | 6.59 | 2.17 |
| 180 | 75 | 3.29 | 3.59 | 215 | 75 | 8.09 | 3.35 | 250 | 75 | 8.06 | 3.35 |
| 180 | 100 | 3.70 | 4.90 | 215 | 100 | 8.42 | 4.58 | 250 | 100 | 9.66 | 4.52 |
| Sample | Lactonic Sites (meq g−1) | Carboxylic Sites (meq g−1) | Phenolic Sites (meq g−1) | Total Acid Sites (meq g−1) | Total Basic Sites (meq g−1) | Point Zero Charge (PZC) |
|---|---|---|---|---|---|---|
| ST | 0.2136 | 0.0996 | 1.0817 | 1.3950 | 0.1433 | 6.9 |
| ST HTC 180 °C | 1.8717 | 0.8866 | 1.0958 | 3.8543 | 0.1757 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Pintor, D.C.; Carranza-Álvarez, C.; Lorenzo-Márquez, H.; Wong-Arguelles, C.; Mojica-Mesinas, C. Hydrothermal Carbonization of Sugarcane Tip (Saccharum officinarum L.) for Pb (II) Removal: Synthesis, Characterization, and Adsorption Equilibrium. AppliedChem 2025, 5, 24. https://doi.org/10.3390/appliedchem5040024
Acosta-Pintor DC, Carranza-Álvarez C, Lorenzo-Márquez H, Wong-Arguelles C, Mojica-Mesinas C. Hydrothermal Carbonization of Sugarcane Tip (Saccharum officinarum L.) for Pb (II) Removal: Synthesis, Characterization, and Adsorption Equilibrium. AppliedChem. 2025; 5(4):24. https://doi.org/10.3390/appliedchem5040024
Chicago/Turabian StyleAcosta-Pintor, Dulce Carolina, Candy Carranza-Álvarez, Habacuc Lorenzo-Márquez, Cynthia Wong-Arguelles, and Cuitláhuac Mojica-Mesinas. 2025. "Hydrothermal Carbonization of Sugarcane Tip (Saccharum officinarum L.) for Pb (II) Removal: Synthesis, Characterization, and Adsorption Equilibrium" AppliedChem 5, no. 4: 24. https://doi.org/10.3390/appliedchem5040024
APA StyleAcosta-Pintor, D. C., Carranza-Álvarez, C., Lorenzo-Márquez, H., Wong-Arguelles, C., & Mojica-Mesinas, C. (2025). Hydrothermal Carbonization of Sugarcane Tip (Saccharum officinarum L.) for Pb (II) Removal: Synthesis, Characterization, and Adsorption Equilibrium. AppliedChem, 5(4), 24. https://doi.org/10.3390/appliedchem5040024








