Determination of Antioxidant Activity and Proximate Composition of a Variety of Red Pigmented Zea mays L. from Puebla, Mexico
Abstract
1. Introduction
2. Materials and Methods
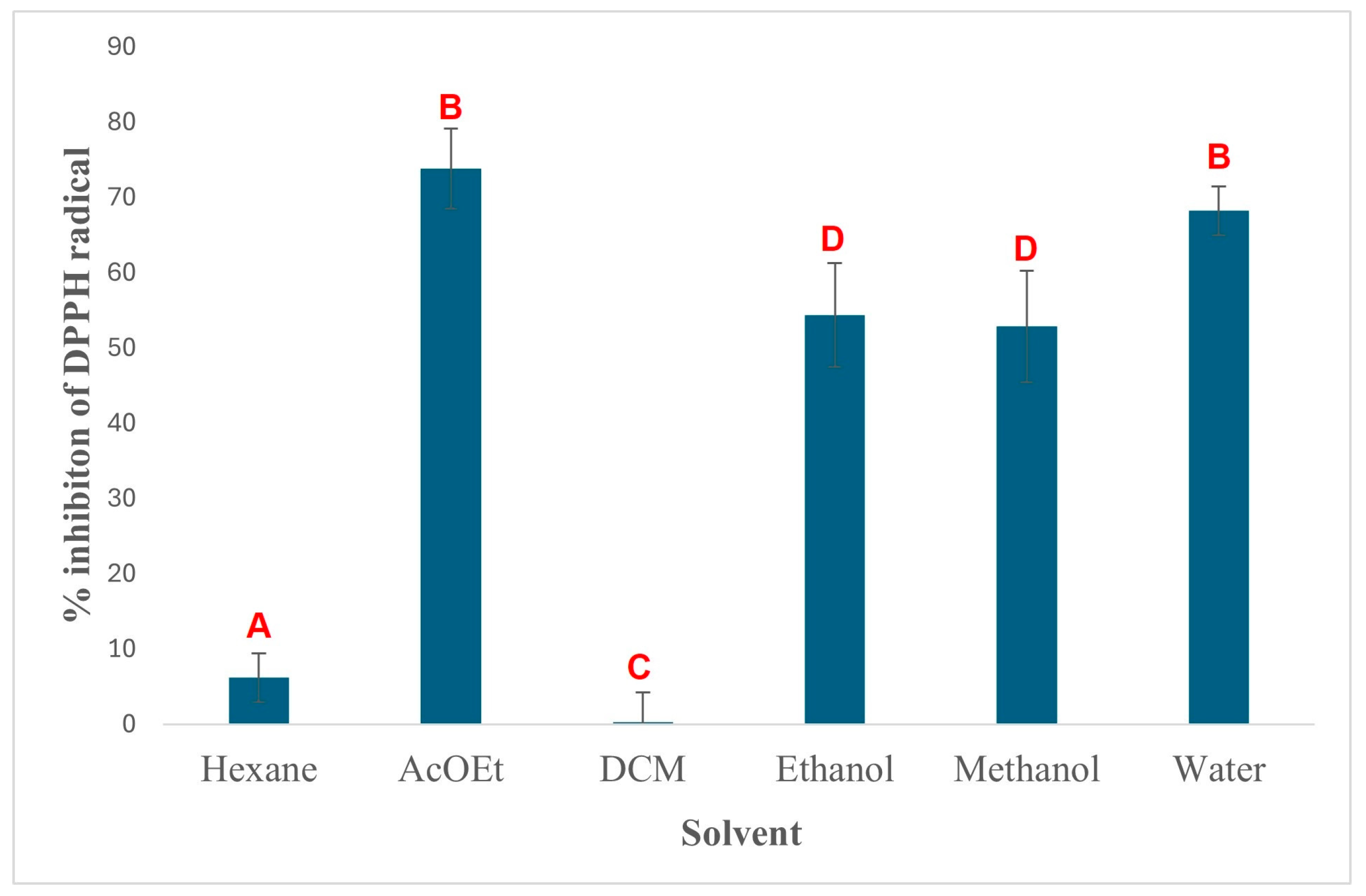
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcOEt | Ethyl acetate |
| DCM | Dichloromethane |
| TPC | Total phenolic content |
| TFC | Total flavonoids content |
| TMAC | Total monomeric anthocyanin content |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| cyd-3-glu | Cyanidin-3-glucoside |
| QE | Quercetin equivalents |
| GAE | Gallic acid equivalents |
References
- CONABIO Razas de maíz de México. Available online: https://www.biodiversidad.gob.mx/diversidad/alimentos/maices/razas-de-maiz (accessed on 30 March 2025).
- FOLLETO DE DIVULGACIÓN. Potencial Nutracéutico de Los Maíces Pigmentados. Available online: https://www.researchgate.net/publication/272294102_FOLLETO_DE_DIVULGACION_Potencial_nutraceutico_de_los_Maices_pigmentados (accessed on 30 March 2025).
- Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Maíces Nativos, Producto Gourmet y Precio Especial. Available online: http://www.gob.mx/inifap/articulos/maices-nativos-producto-gourmet-y-precio-especial (accessed on 30 March 2025).
- Tang, J.; Li, X.; Zhang, Y.; Yang, Y.; Sun, R.; Li, Y.; Gao, J.; Han, Y. Differential Flavonoids and Carotenoids Profiles in Grains of Six Poaceae Crops. Foods 2022, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic Compounds, Carotenoids, Anthocyanins, and Antioxidant Capacity of Colored Maize (Zea mays L.) Kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Frountzas, M.; Karanikki, E.; Toutouza, O.; Sotirakis, D.; Schizas, D.; Theofilis, P.; Tousoulis, D.; Toutouzas, K.G. Exploring the Impact of Cyanidin-3-Glucoside on Inflammatory Bowel Diseases: Investigating New Mechanisms for Emerging Interventions. Int. J. Mol. Sci. 2023, 24, 9399. [Google Scholar] [CrossRef] [PubMed]
- Sendri, N.; Bhandari, P. Anthocyanins: A Comprehensive Review on Biosynthesis, Structural Diversity, and Industrial Ap-plications. Phytochem. Rev. 2024, 23, 1913–1974. [Google Scholar] [CrossRef]
- Reyes-Pavón, D.; Soto-Sigala, K.S.; Cano-Sampedro, E.; Méndez-Trujillo, V.; Navarro-Ibarra, M.J.; Pérez-Pasten-Borja, R.; Olvera-Sandoval, C.; Torres-Maravilla, E. Pigmented Native Maize: Unlocking the Potential of Anthocyanins and Bioactive Compounds from Traditional to Functional Beverages. Beverages 2024, 10, 69. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium Vitis-Idaea L. and Their Antioxidant and An-ticancer Activities Assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as Promising Molecules and Dietary Bioactive Components against Diabetes – A Review of Recent Advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Vidana Gamage, G.C.; Cintrat, J.-C.; Choo, W.S. Acylated and Non-Acylated Anthocyanins as Antibacterial and Antibiofilm Agents. Discov. Food 2023, 3, 21. [Google Scholar] [CrossRef]
- Moreira, V.; Stanquevis, R.; Amaral, E.P.; Lajolo, F.M.; Hassimotto, N.M.A. Anthocyanins from Purple Maize (Zea mays L.) Downregulate Lipopolysaccharide-Induced Peritonitis in Mice by Modulating the MyD88 Signaling Pathway. PharmaNutrition 2021, 16, 100265. [Google Scholar] [CrossRef]
- Ijinu, T.P.; De Lellis, L.F.; Shanmugarama, S.; Pérez-Gregorio, R.; Sasikumar, P.; Ullah, H.; Buccato, D.G.; Di Minno, A.; Baldi, A.; Daglia, M. Anthocyanins as Immunomodulatory Dietary Supplements: A Nutraceutical Perspective and Micro-/Nano-Strategies for Enhanced Bioavailability. Nutrients 2023, 15, 4152. [Google Scholar] [CrossRef]
- Sánchez-Nuño, Y.A.; Zermeño-Ruiz, M.; Vázquez-Paulino, O.D.; Nuño, K.; Villarruel-López, A. Bioactive Compounds from Pigmented Corn (Zea mays L.) and Their Effect on Health. Biomolecules 2024, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Agricultura y Desarrollo Rural. Maíz, Semilla de Vida. Available online: http://www.gob.mx/agricultura/es/articulos/maiz-semilla-de-vida (accessed on 30 March 2025).
- El maíz Representa Cultura e Identidad y Conserva Biodiversidad. Available online: https://conap.gob.gt/el-maiz-representa-cultura-e-identidad-y-conserva-biodiversidad/ (accessed on 20 May 2025).
- Gogoi, P.; Sharma, P.; Mahajan, A.; Goudar, G.; Chandragiri, A.K.; Sreedhar, M.; Singh, M.; Longvah, T. Exploring the Nutritional Potential, Anti-Nutritional Components and Carbohydrate Fractions of Indian Pigmented Maize. Food Chem. Ad-Vances 2023, 2, 100176. [Google Scholar] [CrossRef]
- Rodríguez-Salinas, P.A.; Zavala-García, F.; Urías-Orona, V.; Muy-Rangel, D.; Heredia, J.B.; Niño-Medina, G. Chromatic, Nutritional and Nutraceutical Properties of Pigmented Native Maize (Zea mays L.) Genotypes from the Northeast of Mexico. Arab J. Sci. Eng. 2020, 45, 95–112. [Google Scholar] [CrossRef]
- Ramírez-García, O.; Salinas-Moreno, Y.; Santillán-Fernández, A.; Sumaya-Martínez, M.T. Screening Antioxidant Capacity of Mexican Maize (Zea mays L.) Landraces with Colored Grain Using ABTS, DPPH and FRAP Methods. Cereal Res. Commun. 2022, 50, 1075–1083. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Martínez-Ortiz, M.Á.; Padilla-Camberos, E.; Ramírez-Díaz, J.L.; Ledesma-Miramontes, A.; Alemán de la Torre, I.; Santillán-Fernández, A. Effect of Solvent and Grain Color on the Biological Activities of Maize Grain. Foods 2025, 14, 1163. [Google Scholar] [CrossRef]
- Coyotl-Martinez, E.; Hernández-Rivera, J.A.; Parra-Suarez, J.L.A.; Reyes-Carmona, S.R.; Carrasco-Carballo, A. Phytochem-ical Profile, Antioxidant and Antimicrobial Activity of Two Species of Oak: Quercus Sartorii and Quercus Rysophylla. Appl. Biosci. 2025, 4, 13. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R.; Khan, Z.; Banerjee, K. Anthocyanin Profiling Using UV-Vis Spectroscopy and Liquid Chromatography Mass Spectrometry. J. AOAC Int. 2020, 103, 23–39. [Google Scholar] [CrossRef]
- Ghafar, F.; Tengku Nazrin, T.N.N.; Mohd Salleh, M.R.; Nor Hadi, N.; Ahmad, N.; Hamzah, A.A.; Mohd Yusof, Z.A.; Azman, I.N. Total Phenolic Content And Total Flavonoid Content In Moringa Oleifera Seed. Sci. Herit. J. 2017, 1, 23–25. [Google Scholar] [CrossRef]
- Ayala-Cid, J.P.; Hernández-Rivera, J.A.; Pérez-Xochipa, I.; Carrasco-Carballo, A. Antioxidant Activity of Vaccinium Oxy-coccus in Commercial, Natural and Extract Juices. GSC Biol. Pharm. Sci. 2025, 30, 203–209. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, M.; Park, E. Antioxidant Activity of Orange Flesh and Peel Extracted with Various Solvents. Prev. Nutr Food Sci. 2014, 19, 291–298. [Google Scholar] [CrossRef]
- Comparative Study on the Nutritional Value of Local Maize Variety (Hakorin Hajiya) and Improved Varieties of Maize (Sammaz14 and Golden Strawberry). Available online: https://www.researchgate.net/publication/371256125_Comparative_study_on_the_nutritional_value_of_local_maize_variety_Hakorin_Hajiya_and_improved_varieties_of_maize_sammaz14_and_golden_strawberry (accessed on 30 March 2025).
- Uzoekwe, N.M.; Ukhun, M.E.; Ejidike, P.P. Proximate Analysis, Vitamins, Moisture Content and Mineral Elements Deter-mination in Leaves of Solanum Erianthum and Glyphaea Brevis. J. Chem. Soc. Niger. 2021, 46, 149–159. [Google Scholar] [CrossRef]
- Terefe, Z.K.; Omwamba, M.N.; Nduko, J.M. Effect of Solid State Fermentation on Proximate Composition, Antinutritional Factors and in Vitro Protein Digestibility of Maize Flour. Food Sci. Nutr. 2021, 9, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, S.S.; Oyeyode, J.O.; Shafik, W.; Sunusi, Z.A.; Adeyemi, A.A. Proximate Analysis of Poultry-Mix Formed Feed Using Maize Bran as a Base. Int. J. Anal. Chem. 2021, 2021, 8894567. [Google Scholar] [CrossRef] [PubMed]
- Kotue, T.C.; Jayamurthy, P.; Nisha, P.; Pieme, A.C.; Kansci, G.; Fokou, E.; Ashok, P. Proximate Analysis and Minerals of Black Bean Seeds (Phaseolus vulgaris L.) Used to Manage Sickle Cell Disease in West Region of Cameroon. Asian Food Sci. J. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Pirian, K.; Jeliani, Z.Z.; Arman, M.; Sohrabipour, J.; Yousefzadi, M. Proximate Analysis of Selected Macroalgal Species from the Persian Gulf as a Nutritional Resource. Trop. Life Sci. Res. 2020, 31, 1–17. [Google Scholar] [CrossRef]
- Shakir, M.I. Comparative Study for the Determination of Nutritional Composition in Commercial and Noncommercial Maize Flours. Pak. J. Bot 2017, 49, 519–523. [Google Scholar]
- Rajesh, Y.; Khan, N.M.; Raziq Shaikh, A.; Mane, V.S.; Daware, G.; Dabhade, G. Investigation of Geranium Oil Extraction Performance by Using Soxhlet Extraction. Mater. Today Proc. 2023, 72, 2610–2617. [Google Scholar] [CrossRef]
- Phan, K.; Den Broeck, E.V.; Raes, K.; De Clerck, K.; Speybroeck, V.V.; De Meester, S. A Comparative Theoretical Study on the Solvent Dependency of Anthocyanin Extraction Profiles. J. Mol. Liq. 2022, 351, 118606. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Zhang, Q.; Gonzalez de Mejia, E.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of Phenolic Composition of Selected Purple Maize (Zea mays L.) Genotypes with Their Anti-Inflammatory, An-ti-Adipogenic and Anti-Diabetic Potential. Food Chem. 2019, 289, 739–750. [Google Scholar] [CrossRef]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of Total Polyphenols, Profile Anthocyanins, Color Analysis, Ca-rotenoids and Tocols in Pigmented Maize. LWT 2021, 144, 111257. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-Based pH-Sensitive Smart Packaging Films for Monitoring Food Freshness. J. Agric. Food Res. 2022, 9, 100340. [Google Scholar] [CrossRef]
- da Silva Oliveira, J.P.; de Oliveira, R.T.; Guedes, A.L.; da Costa Oliveira, M.; Macedo, A.F. Metabolomic Studies of Antho-cyanins in Fruits by Means of a Liquid Chromatography Coupled to Mass Spectrometry Workflow. Curr. Plant Biol. 2022, 32, 100260. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Pérez, A.A.; Mercado-Luna, A.; Murillo-Cárdenas, C.A.; González-Santos, R.; Chávez-Servín, J.L.; Vargas-Madriz, A.F.; Luna-Sánchez, E. Polyphenolic Compounds and Antioxidant Capacity in Native Maize of the Sierra Gorda of Querétaro. Agronomy 2024, 14, 142. [Google Scholar] [CrossRef]
- Zhang, Q.; Luna-Vital, D.; Gonzalez de Mejia, E. Anthocyanins from Colored Maize Ameliorated the Inflammatory Paracrine Interplay between Macrophages and Adipocytes through Regulation of NF-κB and JNK-Dependent MAPK Pathways. J. Funct. Foods 2019, 54, 175–186. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Antho-cyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Márquez, K.; Ramírez, V.; González-Córdova, A.F.; Ramírez-Rodríguez, Y.; García-Ortega, L.; Trujillo, J. Research Opportunities: Traditional Fermented Beverages in Mexico. Cultural, Microbiological, Chemical, and Functional Aspects. Food Res. Int. 2021, 147, 110482. [Google Scholar] [CrossRef]
- Chauhan, D.; Kumar, K.; Ahmed, N.; Singh, T.P.; Thakur, P. Effect of Processing Treatments on the Nutritional, An-ti-Nutritional, and Bioactive Composition of Blue Maize (Zea mays L.). Curr. Res. Nutr. Food Sci. J. 2022, 10, 171–182. [Google Scholar] [CrossRef]
- Adeniyi, O.O.; Ariwoola, O.S. Comparative Proximate Composition of Maize (Zea mays L.) Varieties Grown in South-Western Nigeria. Int. Ann. Sci. 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Rivera-Castro, V.M.; Muy-Rangel, M.D.; Gutiérrez-Dorado, R.; Escobar-Álvarez, J.L.; Hernández-Castro, E.; Valenzue-la-Lagarda, J.L. Nutritional, Physicochemical and Anatomical Evaluation of Creole Corn Varieties from the Region of the Costa Chica of Guerrero. Food Sci. Technol. 2020, 40, 938–944. [Google Scholar] [CrossRef]
- Likhayo, P.; Bruce, A.Y.; Tefera, T.; Mueke, J. Maize Grain Stored in Hermetic Bags: Effect of Moisture and Pest Infestation on Grain Quality. J. Food Qual. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Michel, M.R.; Aguilar-Zárate, P.; Espinoza-Velázquez, J.; Aguilar, C.N.; Rodríguez-Herrera, R.; Michel, M.R.; Aguilar-Zárate, P.; Espinoza-Velázquez, J.; Aguilar, C.N.; Rodríguez-Herrera, R. Environmental Effects on Chemical Composition and Physical Properties of Polyembryonic Maize Grain. TIP. Rev. Espec. En Cienc. Químico-Biológicas 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Li, D.; Hao, A.; Shao, W.; Zhang, W.; Jiao, F.; Zhang, H.; Dong, X.; Zhan, Y.; Liu, X.; Mu, C.; et al. Maize Kernel Nutritional Quality—An Old Challenge for Modern Breeders. Planta 2025, 261, 43. [Google Scholar] [CrossRef]
- Kabir, S.H.; Das, A.K.; Rahman, M.S.; Singh, M.S.; Morshed, M.; Marma, A.S.H. Effect of Genotype on Proximate Composition and Biological Yield of Maize (Zea mays L.). Arch. Agri. Environ. Sci. 2019, 4, 185–189. [Google Scholar] [CrossRef]
| Solvent | Extract (g Extract/kg ± std. dev.) 1 |
|---|---|
| Hexane | 31.40 ± 5.38 A |
| AcOEt | 8.54 ± 4.27 B |
| DCM | 6.55 ± 3.64 B |
| Ethanol | 19.04 ± 4.55 C |
| Methanol | 14.51 ± 3.84 C |
| Water | 6.06 ± 0.66 B |
| Solvent | Folin–Ciocalteu (mg GAE/kg ± std. dev.) 1 |
|---|---|
| Hexane | 46.618 ± 9.636 A |
| AcOEt | 54.474 ± 10.344 A |
| DCM | 69.054 ± 10.498 B |
| Ethanol | 1292.080 ± 126.571 C |
| Methanol | 1368.420 ± 104.094 C |
| Water | 846.154 ± 98.140 D |
| Solvent | Flavonoids (mg QE/kg ± std. dev.) 1 |
|---|---|
| Hexane | 349.349 ± 32.341 A |
| AcOEt | 106.872 ± 21.436 B |
| DCM | 84.706 ± 12.987 B |
| Ethanol | 613.813 ± 68.854 C |
| Methanol | 573.706 ± 32.787 C |
| Water | 833.984 ± 65.218 D |
| Solvent | Anthocyanins (mg cyd-3-glu Equivalent/kg ± std. dev.) 1 |
|---|---|
| Hexane | No monomeric anthocyanins detected |
| AcOEt | No monomeric anthocyanins detected |
| DCM | No monomeric anthocyanins detected |
| Ethanol | 772.240 ± 83.136 A |
| Methanol | 822.884 ± 43.885 A |
| Water | 47.796 ± 8.84 B |
| Parameter | Red-Pigmented Corn Studied (%) |
|---|---|
| Moisture | 6.49 ± 0.10 |
| Ash | 3.07 ± 0.35 |
| Lipids | 7.11 ± 0.59 |
| Crude Fiber | 1.15 ± 0.30 |
| Protein | 4.21 ± 0.21 |
| Carbohydrates | 77.97 ± 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Quiroz, J.; Hernández-Rivera, J.A.; Pérez-Xochipa, I.; Antonio-López, P.; Carrasco-Carballo, A. Determination of Antioxidant Activity and Proximate Composition of a Variety of Red Pigmented Zea mays L. from Puebla, Mexico. AppliedChem 2025, 5, 18. https://doi.org/10.3390/appliedchem5030018
Pineda-Quiroz J, Hernández-Rivera JA, Pérez-Xochipa I, Antonio-López P, Carrasco-Carballo A. Determination of Antioxidant Activity and Proximate Composition of a Variety of Red Pigmented Zea mays L. from Puebla, Mexico. AppliedChem. 2025; 5(3):18. https://doi.org/10.3390/appliedchem5030018
Chicago/Turabian StylePineda-Quiroz, Jesabel, Juan Alex Hernández-Rivera, Ivonne Pérez-Xochipa, Pedro Antonio-López, and Alan Carrasco-Carballo. 2025. "Determination of Antioxidant Activity and Proximate Composition of a Variety of Red Pigmented Zea mays L. from Puebla, Mexico" AppliedChem 5, no. 3: 18. https://doi.org/10.3390/appliedchem5030018
APA StylePineda-Quiroz, J., Hernández-Rivera, J. A., Pérez-Xochipa, I., Antonio-López, P., & Carrasco-Carballo, A. (2025). Determination of Antioxidant Activity and Proximate Composition of a Variety of Red Pigmented Zea mays L. from Puebla, Mexico. AppliedChem, 5(3), 18. https://doi.org/10.3390/appliedchem5030018







