Removal of Radio and Stable Isotopes of Cobalt and Cesium from Contaminated Aqueous Solutions by Isatin-Derived Ligand
Abstract
1. Introduction
2. Experimental Approach
2.1. Materials
2.1.1. Adsorptive Materials
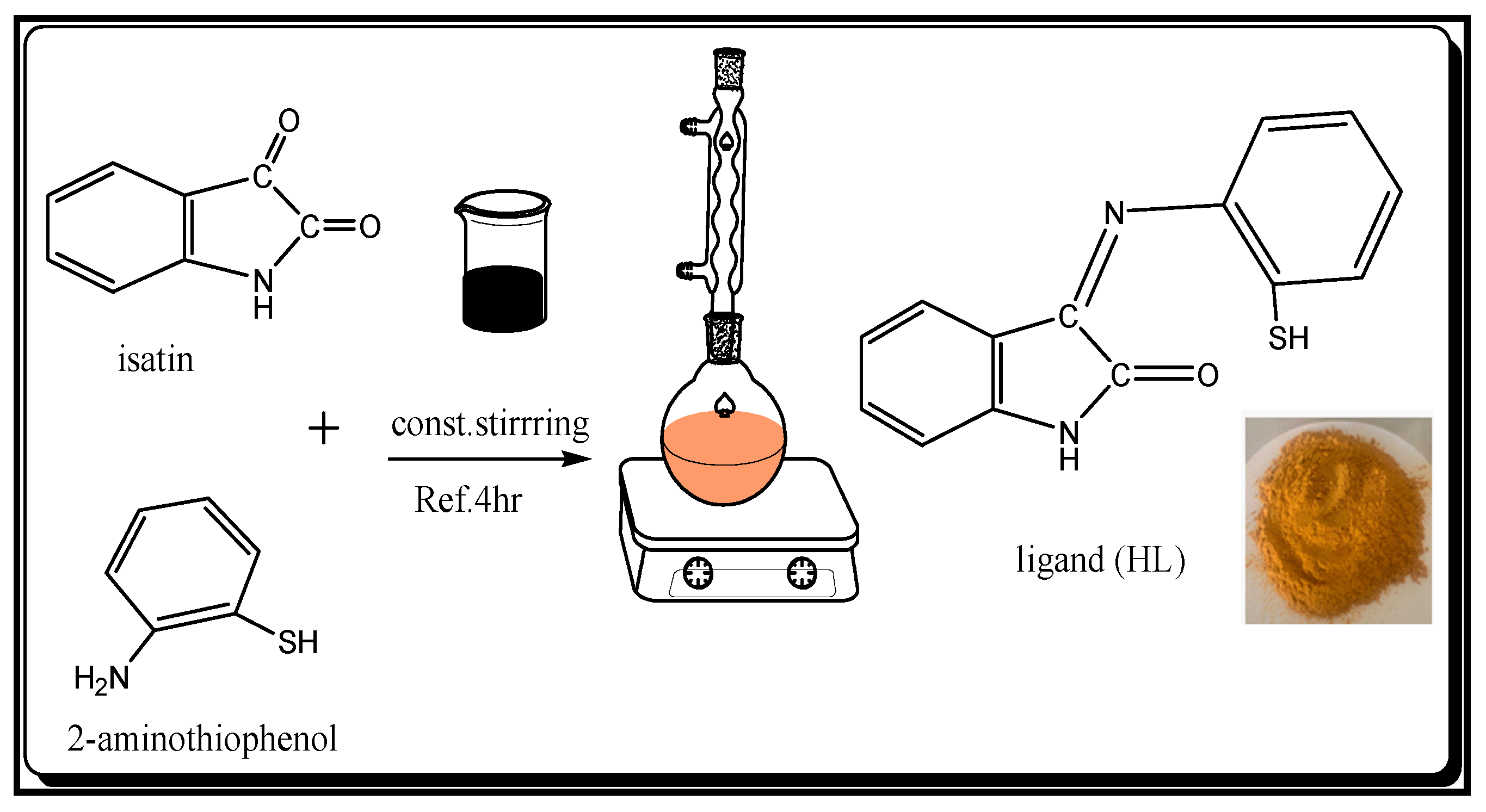
2.1.2. Preparation of Adsorbent Ligand (HL)
2.1.3. Adsorbate Elements
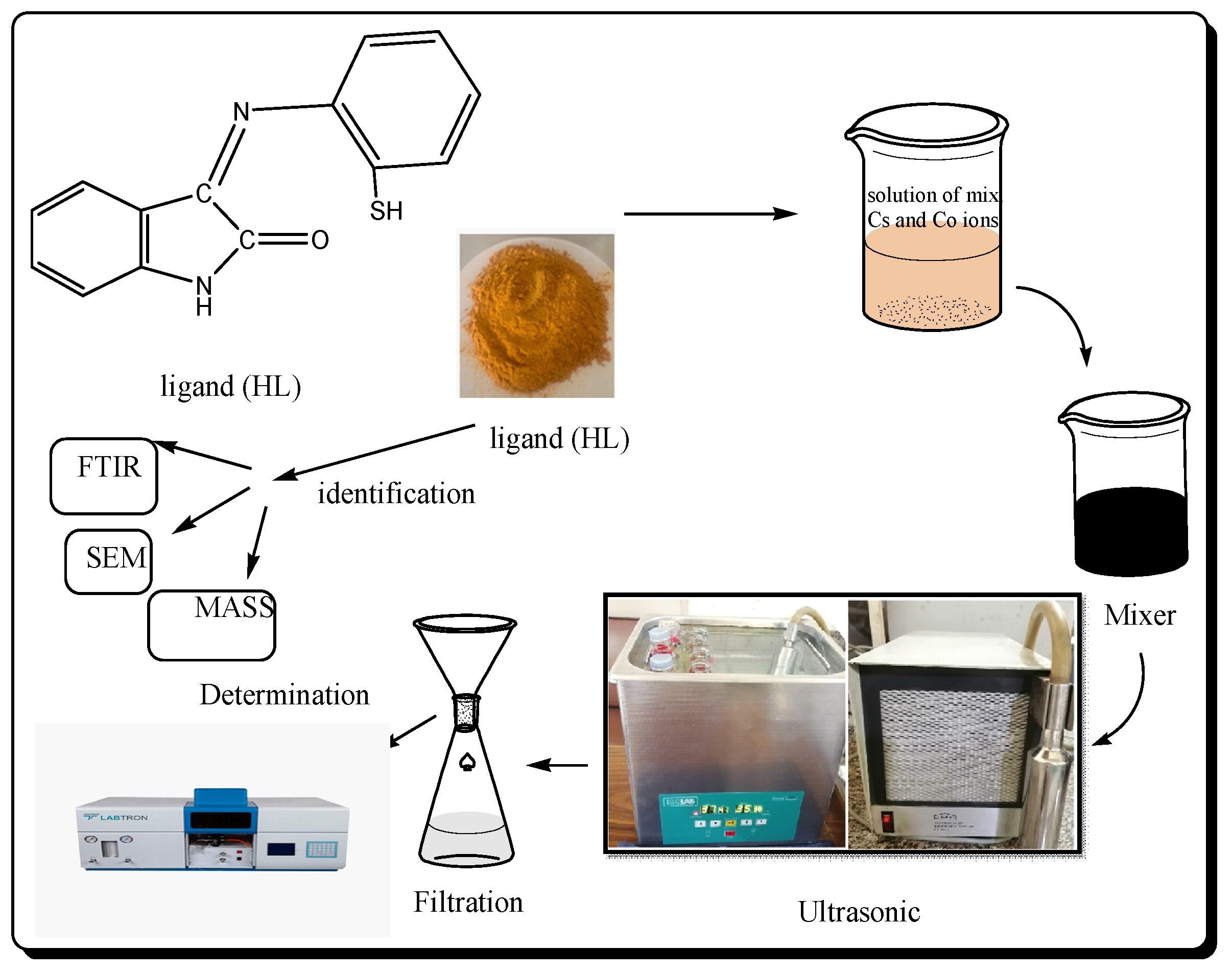
2.2. Experimentation
2.2.1. Analyses of Adsorption
Contact Time
pH Effect
Adsorbent Dose
2.2.2. Optical Emissions Spectroscopy with Inductively Coupled Plasma (ICP-OES)
2.2.3. Adsorption Isotherms and Data Analysis
2.2.4. Adsorption of Radioisotopes (60Co or 134Cs)
2.2.5. Statistical Analysis
3. Results and Discussions
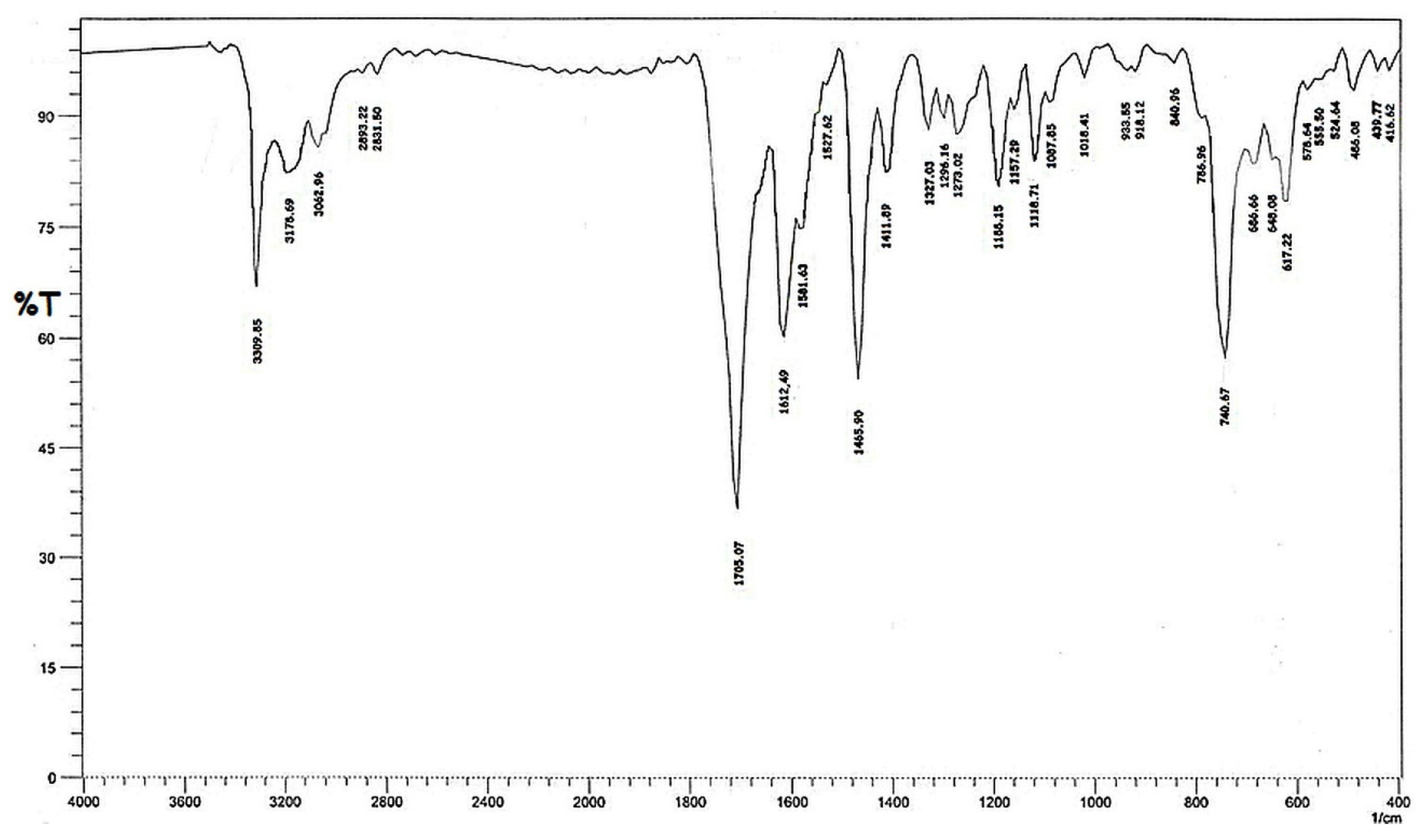
3.1. Characterization of the Synthesized Materials
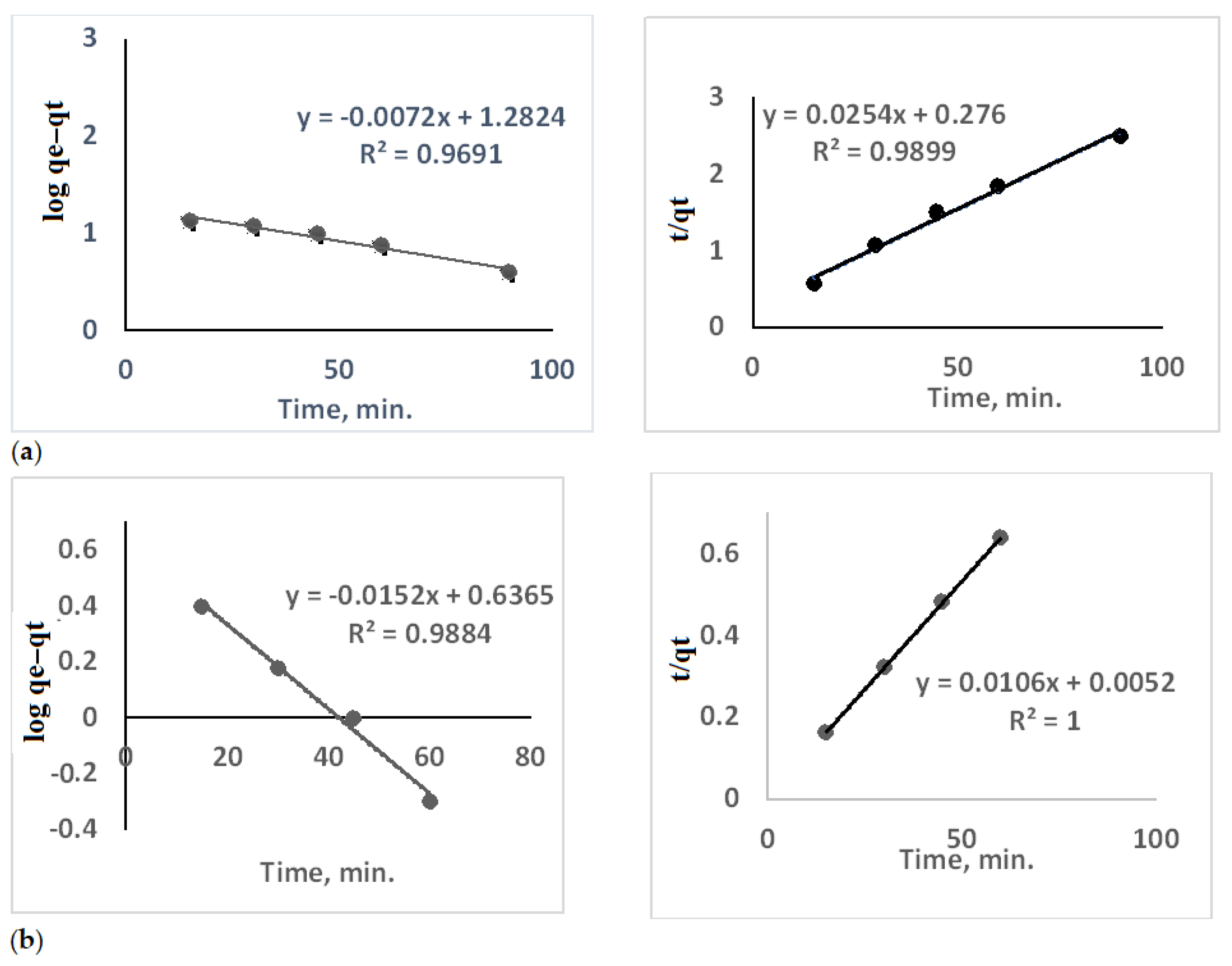
3.2. Contact Time
- (a)
- Cs+ adsorption; the pseudo-first-order kinetic model, showing a linear relationship with a correlation coefficient (R2) of 0.9691, indicating good fitting to the model but suggesting that the adsorption rate is relatively slow. The pseudo-second-order kinetic model, which exhibits an excellent fit with R2 = 0.9899, suggests that Cs+ adsorption follows a chemisorption mechanism dominated by valence forces or electron sharing.
- (b)
- Co2+ adsorption; the pseudo-first-order model with an R2 value of 0.9884, indicating reasonable fitting but showing a negative slope, which might reflect a desorption tendency or low interaction strength. The pseudo-second-order model, showing a perfect linear fit (R2 = 1), suggests that Co2+ adsorption is highly consistent with a chemisorption mechanism, indicating strong binding between the ligand and Co2+ ions. These results confirm that the adsorption of both Cs+ and Co2+ is better described by the pseudo-second-order kinetic model, implying that chemical interactions are the primary driving force for metal ion uptake.
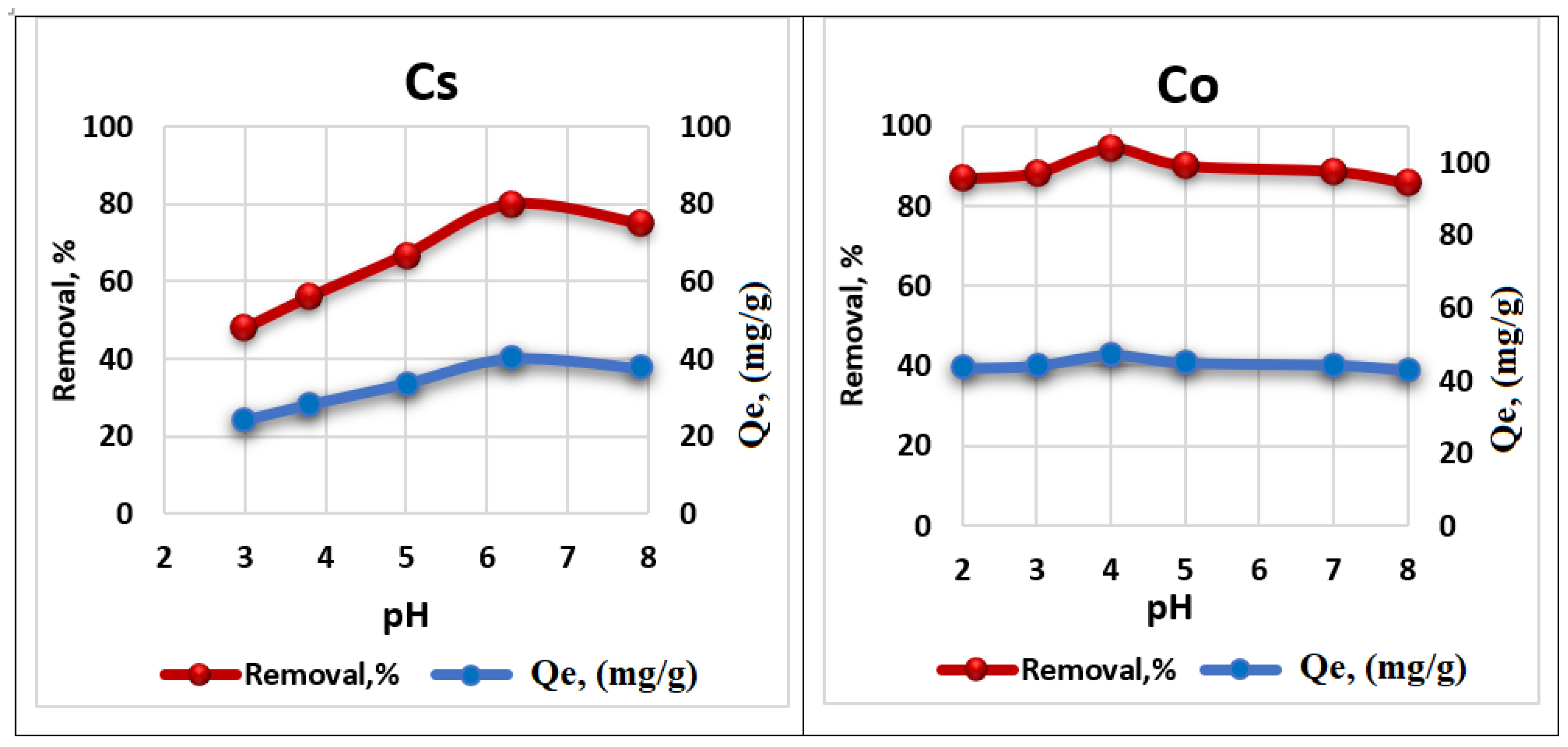
3.3. pH Effect
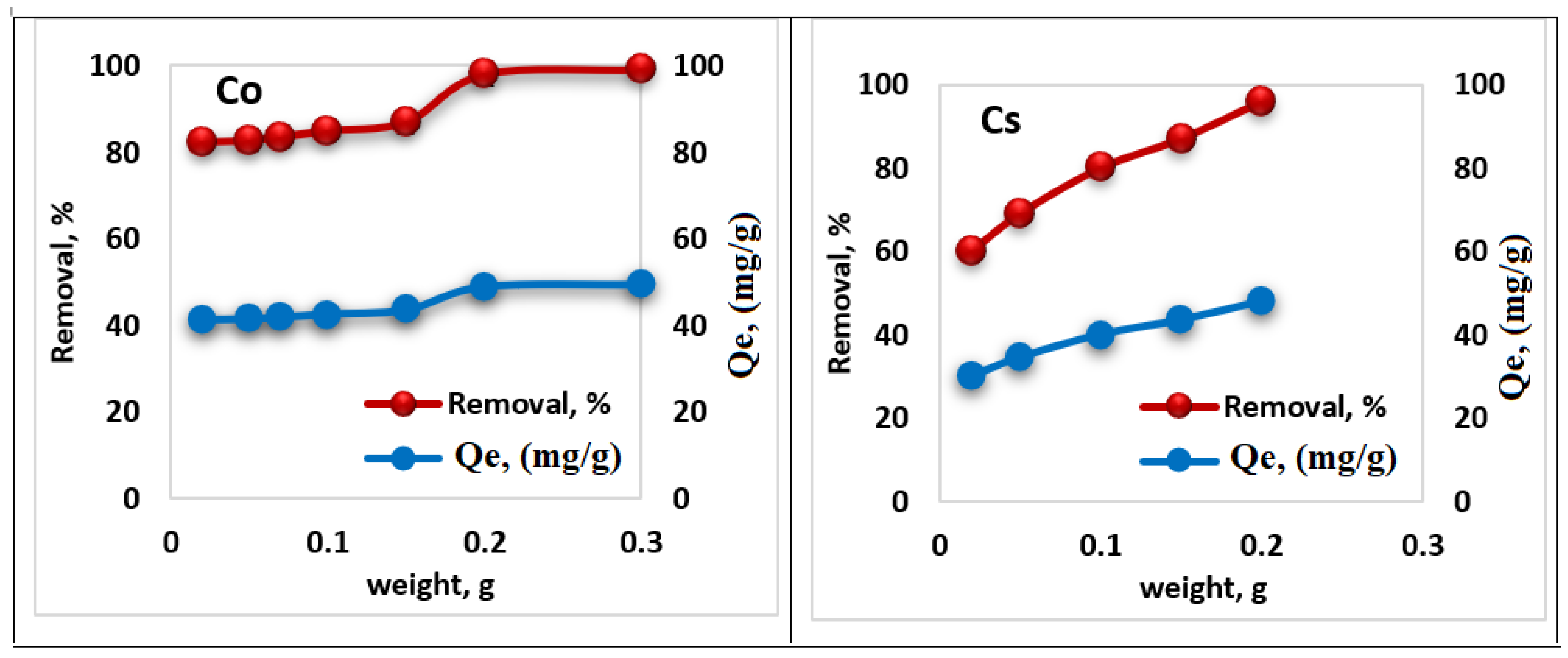
3.4. Dosage Weight of Dried Ligand (HL)
3.5. Comparison of the Study Findings with Other Similar Published Work
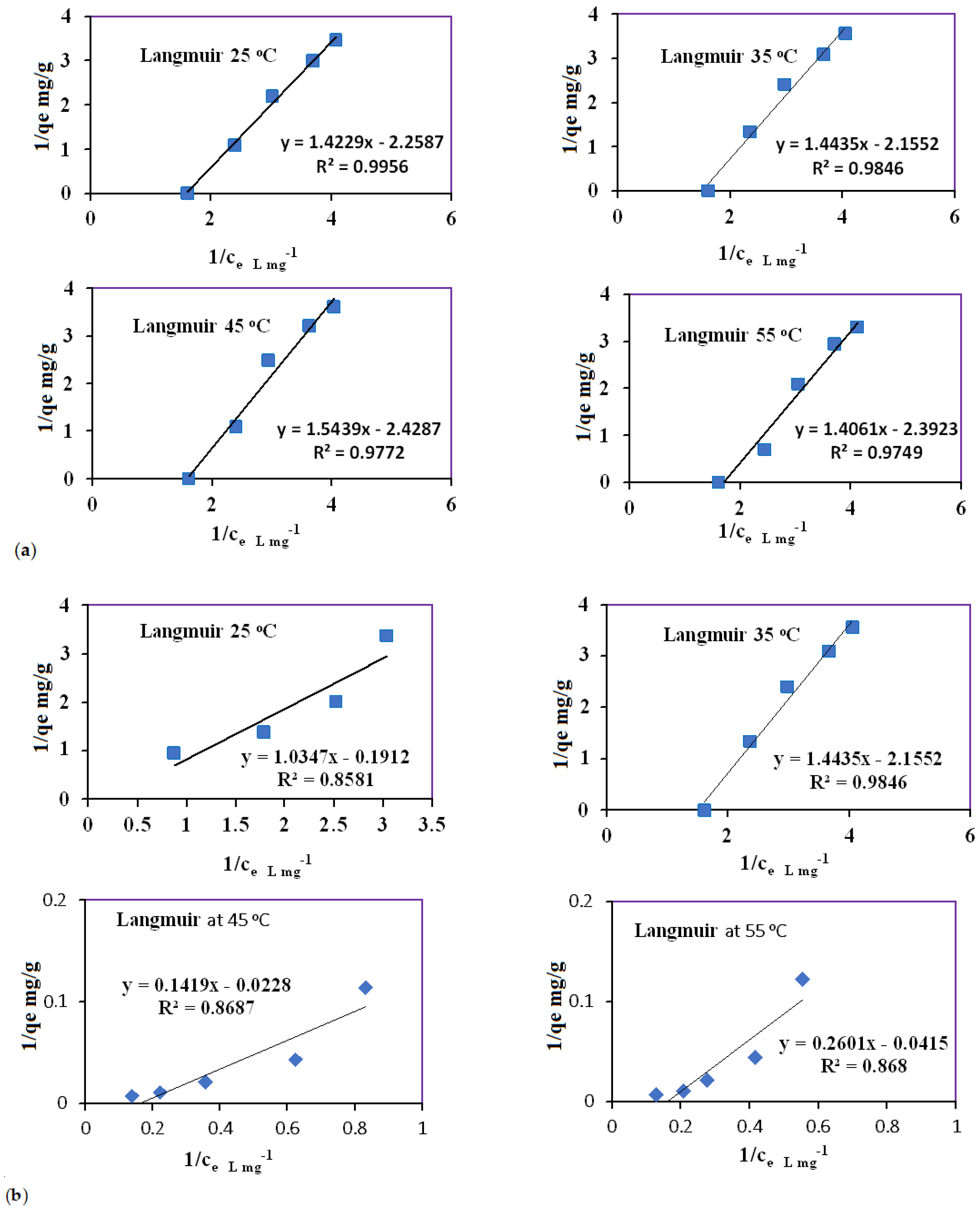
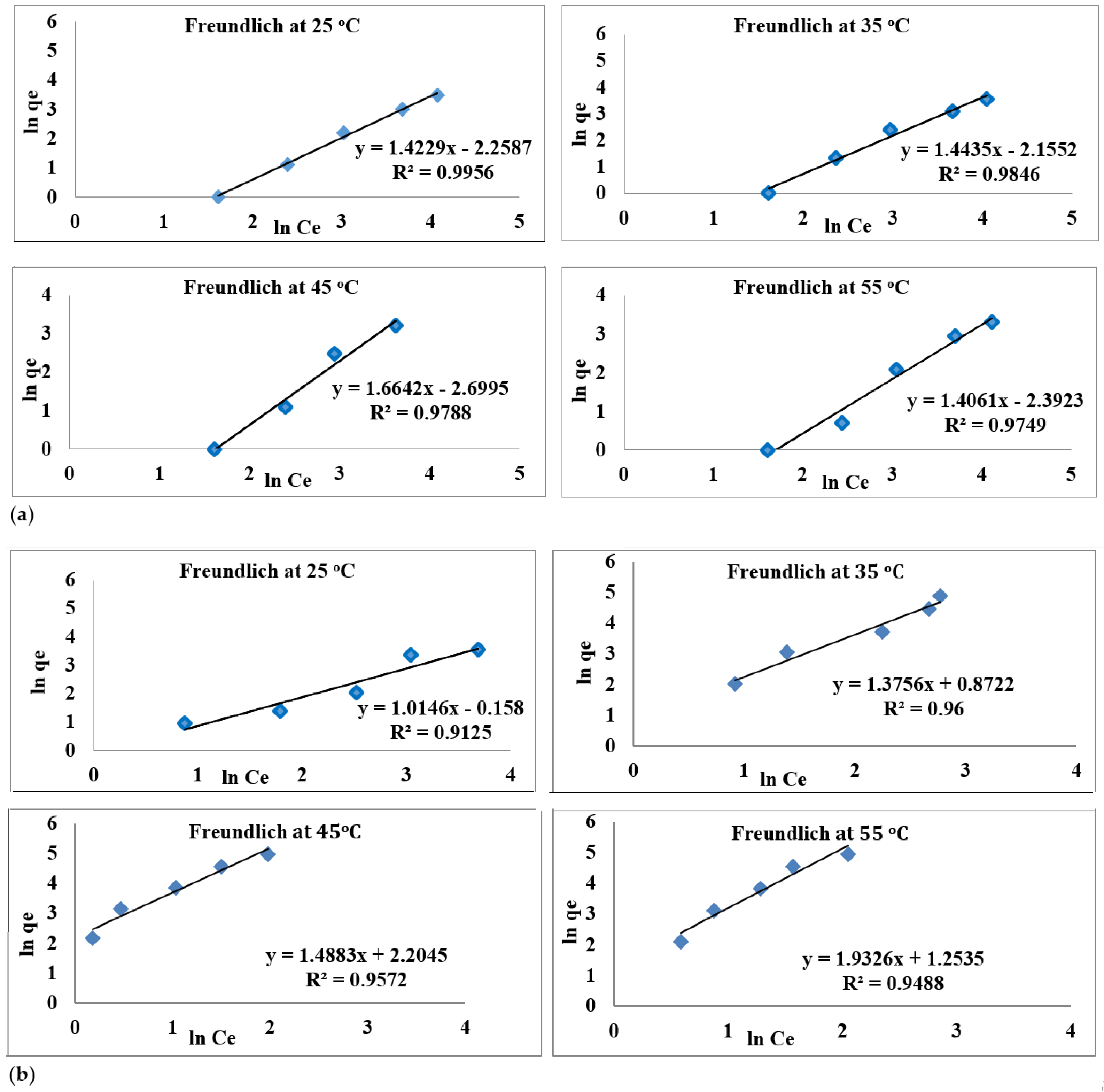
3.6. Adsorption Isotherm at Different Temperatures
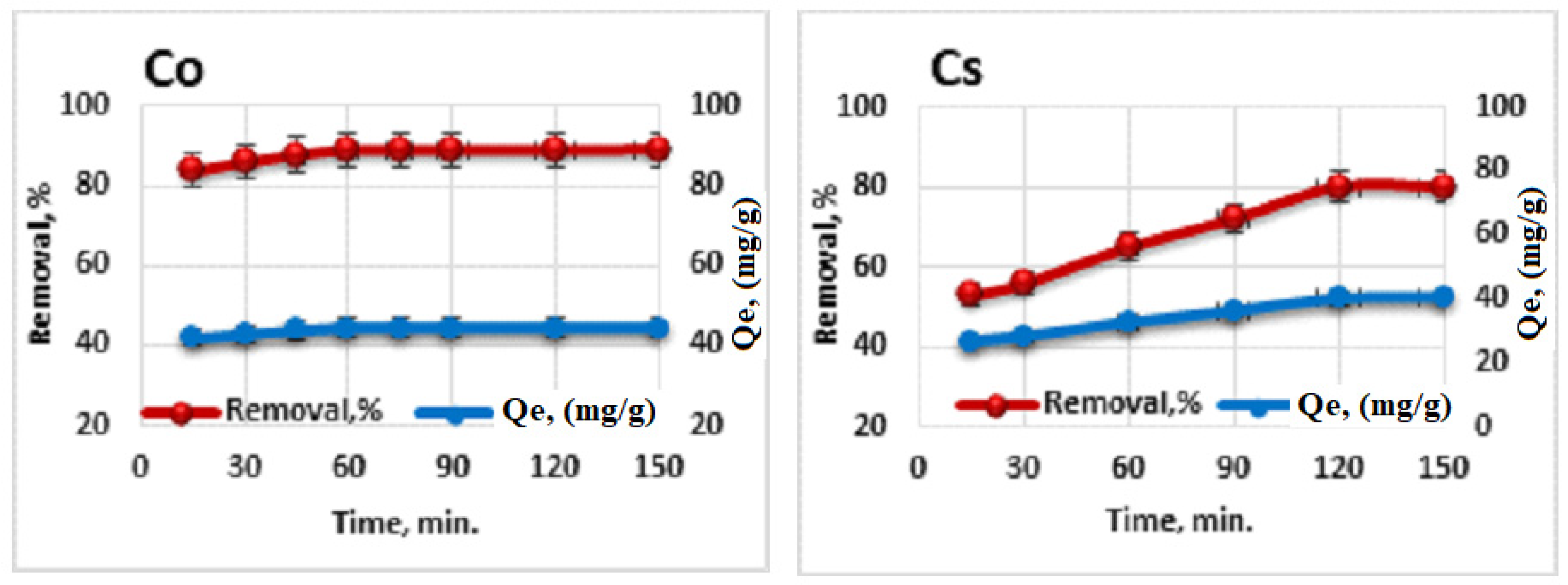
3.7. Adsorption of Radioisotopes (60Co and 134Cs) on Ligand (HL)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monier, M.; Youssef, I.; El-Mekabaty, A. Preparation of Functionalized Ion-Imprinted Phenolic Polymer for Efficient Removal of Copper Ions. Polym. Int. 2020, 69, 31–40. [Google Scholar] [CrossRef]
- Ren, Z.; Zhu, X.; Du, J.; Kong, D.; Wang, N.; Wang, Z.; Wang, Q.; Liu, W.; Li, Q.; Zhou, Z. Facile and Green Preparation of Novel Adsorption Materials by Combining Sol-Gel with Ion Imprinting Technology for Selective Removal of Cu(II) Ions from Aqueous Solution. Appl. Surf. Sci. 2018, 435, 574–584. [Google Scholar] [CrossRef]
- Atubi, A.O. Effects of Warri Refinery Effluents on Water Quality from the Iffie River, Delta State, Nigeria. Am. Rev. Polit. Econ. 2011, 9, 45–56. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Q.; Li, X.; Huang, X. A Critical Review on Chemical Analysis of Heavy Metal Complexes in Water/Wastewater and the Mechanism of Treatment Methods. Chem. Eng. J. 2022, 429, 131688. [Google Scholar] [CrossRef]
- Saleh, H.M.; Moussa, H.R.; Mahmoud, H.H.; El-Saied, F.A.; Dawoud, M.; Abdel Wahed, R.S. Potential of the Submerged Plant Myriophyllum Spicatum for Treatment of Aquatic Environments Contaminated with Stable or Radioactive Cobalt and Cesium. Prog. Nucl. Energy 2020, 118, 103147. [Google Scholar] [CrossRef]
- Soni, S.; Bajpai, P.K.; Mittal, J.; Arora, C. Utilisation of Cobalt Doped Iron Based MOF for Enhanced Removal and Recovery of Methylene Blue Dye from Waste Water. J. Mol. Liq. 2020, 314, 113642. [Google Scholar] [CrossRef]
- Maazinejad, B.; Mohammadnia, O.; Ali, G.A.M.; Makhlouf, A.S.H.; Nadagouda, M.N.; Sillanpää, M.; Asiri, A.M.; Agarwal, S.; Gupta, V.K.; Sadegh, H. Taguchi L9 (34) Orthogonal Array Study Based on Methylene Blue Removal by Single-Walled Carbon Nanotubes-Amine: Adsorption Optimization Using the Experimental Design Method, Kinetics, Equilibrium and Thermodynamics. J. Mol. Liq. 2020, 298, 112001. [Google Scholar] [CrossRef]
- Beyki, M.H.; Shemirani, F.; Shirkhodaie, M. Aqueous Co(II) Adsorption Using 8-Hydroxyquinoline Anchored γ-Fe2O3@ Chitosan with Co(II) as Imprinted Ions. Int. J. Biol. Macromol. 2016, 87, 375–384. [Google Scholar] [CrossRef]
- Dil, N.N.; Sadeghi, M. Free Radical Synthesis of Nanosilver/Gelatin-Poly (Acrylic Acid) Nanocomposite Hydrogels Employed for Antibacterial Activity and Removal of Cu(II) Metal Ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar] [CrossRef]
- Cai, Y.; Li, C.; Wu, D.; Wang, W.; Tan, F.; Wang, X.; Wong, P.K.; Qiao, X. Highly Active MgO Nanoparticles for Simultaneous Bacterial Inactivation and Heavy Metal Removal from Aqueous Solution. Chem. Eng. J. 2017, 312, 158–166. [Google Scholar]
- Chowdhury, M.N.K.; Khan, M.W.; Mina, M.F.; Beg, M.D.H.; Khan, M.R.; Alam, A. Synthesis and Characterization of Radiation Grafted Films for Removal of Arsenic and Some Heavy Metals from Contaminated Water. Radiat. Phys. Chem. 2012, 81, 1606–1611. [Google Scholar]
- Al Abdullah, J.; Al Lafi, A.G.; Al Masri, W.; Amin, Y.; Alnama, T. Adsorption of Cesium, Cobalt, and Lead onto a Synthetic Nano Manganese Oxide: Behavior and Mechanism. Water. Air. Soil Pollut. 2016, 227, 241. [Google Scholar] [CrossRef]
- Eskander, S.B.; Saleh, H.M. Cement mortar-degraded spinney waste composite as a matrix for immobilizing some low and intermediate level radioactive wastes: Consistency under frost attack. J. Nucl. Mater. 2012, 420, 491–496. [Google Scholar]
- Saleh, H.M.; Eskander, S.B. Characterizations of mortar-degraded spinney waste composite nominated as solidifying agent for radwastes due to immersion processes. J. Nucl. Mater. 2012, 430, 106–113. [Google Scholar]
- Saleh, H.M.; Eskander, S.B. Long-term effect on the solidified degraded cellulose-based waste slurry in cement matrix. Acta Montan. Slovaca 2009, 14, 291–297. [Google Scholar]
- Saleh, H.M. Some Applications of Clays in Radioactive Waste Management. In Clays and Clay Minerals: Geological Origin, Mechanical Properties and Industrial Applications; Wesley, L.R., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 403–415. [Google Scholar]
- Abdelhamid, A.A.; Badr, M.H.; Mohamed, R.A.; Saleh, H.M. Using Agricultural Mixed Waste as a Sustainable Technique for Removing Stable Isotopes and Radioisotopes from the Aquatic Environment. Sustainability 2023, 15, 1600. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Jiang, B.; Li, J.; Li, T.; Shao, X.; Cai, W.; Wang, H.; Zhang, Y. Molecular Dynamics Simulation of Ion Adsorption and Ligand Exchange on an Orthoclase Surface. ACS Omega 2021, 6, 14952–14962. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Detailed Macro-/Micro-Scale Exploration of the Excellent Active Corrosion Inhibition of a Novel Environmentally Friendly Green Inhibitor for Carbon Steel in Acidic Environments. J. Taiwan Inst. Chem. Eng. 2019, 100, 239–261. [Google Scholar]
- Khalaf, R.N.; Hassan, A.I.; El-Shafiey, Z.A.; Faheim, A.A.; Ibrahim, S.S.; Saleh, H.M. Evaluation of an Isatin-Derived Ligand and Its Metal Complexes as Potential Anticancer Agents in Breast Adenocarcinoma Cells. Chem. Pap. 2025, 79, 1539–1560. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Roby, O.; Hammoudan, I.; Saddik, R.; Garcia, Y.; Almarhoon, Z.M.; Mabkhot, Y.N. Kinetics, Thermodynamics, Equilibrium, Surface Modelling, and Atomic Absorption Analysis of Selective Cu(Ii) Removal from Aqueous Solutions and Rivers Water Using Silica-2-(Pyridin-2-Ylmethoxy)Ethan-1-Ol Hybrid Material. RSC Adv. 2021, 12, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Misak, N.Z. Langmuir isotherm and its application in ion-exchange reactions. React. Polym. 1993, 21, 53–64. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Evrendilek, G.A.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. ICP-OES Technical Guide: Principles and Instrumentation; Thermo Fisher White Paper. 2019. Available online: https://www.thermofisher.com (accessed on 25 March 2025).
- Liu, Y.; Diwu, C.; Zhao, Y.; Liu, X.; Yuan, H.; Wang, J. Determination of Trace and Rare-Earth Elements in Chinese Soil and Clay Reference Materials by ICP-MS. Chin. J. Geochem. 2014, 33, 95–102. [Google Scholar]
- Nasri, S.; Guergueb, M.; Brahmi, J.; Al-Ghamdi, Y.O.; Molton, F.; Loiseau, F.; Turowska-Tyrk, I.; Nasri, H. Synthesis of New Cobalt(III) Meso-Porphyrin Complex, Photochemical, X-Ray Diffraction, and Electrical Properties for Photovoltaic Cells. Molecules 2022, 27, 8866. [Google Scholar] [CrossRef]
- Saad, F.A.; Al-Fahemi, J.H.; El-Ghamry, H.; Khedr, A.M.; Elghalban, M.G.; El-Metwaly, N.M. Elaborated Spectral, Modeling, QSAR, Docking, Thermal, Antimicrobial and Anticancer Activity Studies for New Nanosized Metal Ion Complexes Derived from Sulfamerazine Azodye. J. Therm. Anal. Calorim. 2018, 131, 1249–1267. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of infrared and Raman spectroscopies to characterize metal-organic frameworks and investigate their interaction with guest molecules. Chem. Rev. 2020, 121, 1286–1424. [Google Scholar]
- Fakoya, T.O.; Oyekunle, J.A.O.; Adekunle, A.S.; Oyinloye, A.J.; Ejidike, I.P. Removal of Hexachlorocyclohexane Isomers from Wastewater Using Activated Carbon from Musa Paradisiaca Peel: Adsorption Isotherms, Kinetic, and Thermodynamic Studies. Chem. Thermodyn. Therm. Anal. 2024, 15, 100140. [Google Scholar] [CrossRef]
- Svobodová, E.; Tišler, Z.; Peroutková, K.; Strejcová, K.; Abrham, J.; Šimek, J.; Gholami, Z.; Vakili, M. Adsorption of Cu(II) and Ni(II) from Aqueous Solutions Using Synthesized Alkali-Activated Foamed Zeolite Adsorbent: Isotherm, Kinetic, and Regeneration Study. Molecules 2024, 29, 2357. [Google Scholar] [CrossRef]
- Ghanavati, B.; Bozorgian, A.; Ghanavati, J. Removal of Copper (II) Ions from the Effluent by Carbon Nanotubes Modified with Tetrahydrofuran. Chem. Rev. Lett. 2022, 5, 68–75. [Google Scholar] [CrossRef]
- Xu, Z.; Xing, Y.; Ren, A.; Ma, D.; Li, Y.; Hu, S. Study on Adsorption Properties of Water Hyacinth-Derived Biochar for Uranium (VI). J. Radioanal. Nucl. Chem. 2020, 324, 1317–1327. [Google Scholar] [CrossRef]
- Hanaf, R.A.K. Effect of Salinity and PH on the Absorption of Cadmium in Lemna Minor. Int. J. Aquat. Biol. 2021, 9, 383–387. [Google Scholar] [CrossRef]
- Adeolu, A.T.; Okareh, O.T.; Dada, A.O. Adsorption of Chromium Ion from Industrial Effluent Using Activated Carbon Derived from Plantain (Musa paradisiaca) Wastes. Am J Env. Prot. 2016, 4, 7–20. [Google Scholar]
- Sartape, A.S.; Mandhare, A.M.; Jadhav, V.V.; Raut, P.D.; Anuse, M.A.; Kolekar, S.S. Removal of Malachite Green Dye from Aqueous Solution with Adsorption Technique Using Limonia Acidissima (Wood Apple) Shell as Low Cost Adsorbent. Arab. J. Chem. 2017, 10, S3229–S3238. [Google Scholar] [CrossRef]
- Khodaie, M.; Ghasemi, N.; Moradi, B.; Rahimi, M. Removal of Methylene Blue from Wastewater by Adsorption onto Znclactivated Corn Husk Carbon Equilibrium Studies. J. Chem. 2013, 383985. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Altunkaynak, Y.; Güzel, F. Removal of Copper, Nickel, Cobalt and Manganese from Aqueous Solution by Kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Rawat, J.P.; Iraqi, S.M.U.; Singh, R.P. Sorption Equilibria of Cobalt(II) on Two Types of Indian Soils-The Natural Ion Exchangers. Colloids Surf. A Physicochem. Eng. Asp. 1996, 117, 183–188. [Google Scholar] [CrossRef]
- Iyer, A.; Mody, K.; Jha, B. Sorption of Heavy Metals by a Marine Bacterium. Mar. Pollut. Bull. 2005, 50, 340–343. [Google Scholar] [CrossRef]
- van Hullebusch, E.D.; Peerbolte, A.; Zandvoort, M.H.; Lens, P.N.L. Sorption of Cobalt and Nickel on Anaerobic Granular Sludges: Isotherms and Sequential Extraction. Chemosphere 2005, 58, 493–505. [Google Scholar] [CrossRef]
- Parab, H.; Joshi, S.; Shenoy, N.; Lali, A.; Sarma, U.S.; Sudersanan, M. Determination of Kinetic and Equilibrium Parameters of the Batch Adsorption of Co(II), Cr(III) and Ni(II) onto Coir Pith. Process. Biochem. 2006, 41, 609–615. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. Sorption of Cobalt(II) and Nickel(II) by Seaweeds: Batch and Column Studies. Sep. Purif. Technol. 2005, 44, 53–59. [Google Scholar] [CrossRef]
- Saleh, H.M.; Moussa, H.R.; El-Saied, F.A.; Dawoud, M.; Nouh, E.S.A.; Wahed, R.S.A. Adsorption of Cesium and Cobalt onto Dried Myriophyllum spicatum L. from Radio-Contaminated Water: Experimental and Theoretical Study. Prog. Nucl. Energy 2020, 125, 103393. [Google Scholar] [CrossRef]
- Miah, M.Y.; Volchek, K.; Kuang, W.; Tezel, F.H. Kinetic and Equilibrium Studies of Cesium Adsorption on Ceiling Tiles from Aqueous Solutions. J. Hazard. Mater. 2010, 183, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-H.; Li, M.-H.; Yeh, W.-C.; Wei, Y.-Y.; Teng, S.-P. Removal of Cesium Ions from Aqueous Solution by Adsorption onto Local Taiwan Laterite. J. Hazard. Mater. 2008, 160, 638–642. [Google Scholar] [CrossRef]
- Melkior, T.; Yahiaoui, S.; Motellier, S.; Thoby, D.; Tevissen, E. Cesium Sorption and Diffusion in Bure Mudrock Samples. Appl. Clay Sci. 2005, 29, 172–186. [Google Scholar] [CrossRef]
- Ding, D.; Lei, Z.; Yang, Y.; Zhang, Z. Efficiency of Transition Metal Modified Akadama Clay on Cesium Removal from Aqueous Solutions. Chem. Eng. J. 2014, 236, 17–28. [Google Scholar] [CrossRef]
- Rani, R.D.; Sasidhar, P. Sorption of cesium on clay colloids: Kinetic and thermodynamic studies. Aquat. Geochem. 2012, 18, 281–296. [Google Scholar]
- Parab, H.; Sudersanan, M. Engineering a Lignocellulosic Biosorbent–Coir Pith for Removal of Cesium from Aqueous Solutions: Equilibrium and Kinetic Studies. Water Res. 2010, 44, 854–860. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Qin, L.; Liang, W.; Chen, K.; Li, K.; Yan, R. Adsorption properties of cesium by natural Na-bentonite and Ca-bentonite. J. Radioanal. Nucl. Chem. 2024, 333, 5347–5361. [Google Scholar]
- Wang, C.; Myshkin, V.F.; Khan, V.A.; Poberezhnikov, A.D.; Baraban, A.P. Effect of Temperature on the Diffusion and Sorption of Cations in Clay Vermiculite. ACS Omega 2022, 7, 11596–11605. [Google Scholar] [CrossRef]
- Chen, X. Modeling of Experimental Adsorption Isotherm Data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Obayomi, K.S.; Auta, M.; Kovo, A.S. Isotherm, kinetic and thermodynamic studies for adsorption of lead (II) onto modified Aloji clay. Desalination Water Treat. 2020, 181, 376–384. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Hu, H. Adsorption characteristics of lead on cotton-stalk-derived activated carbon fibre by steam activation. Desalination Water Treat. 2011, 30.1-3, 1–9. [Google Scholar]
- Win, M.M.M. Synthesis of Nanomaterials for Their Applications in Nuclear Research and Radioisotope Production. Master’s Thesis, Simon Fraser University, Burnaby, BC, Canada, 2022. [Google Scholar]
- Dakroury, G.A.R.; Abo-Zahra, S.F.; Hassan, H.S.; Ali, H.E.A. Improvement of the Sorption Behavior of Aluminum Silicate Composite toward 134Cs and 60Co Radionuclides by Non-Living Biomass of Chlorella vulgaris. Environ. Sci. Pollut. Res. 2020, 27, 21109–21125. [Google Scholar]



| Material | Adsorption Capacity (qe) of Co, (mg/g) | Literature |
| Kaolinite | 0.92 | [38] |
| Soil | 1.50 | [39] |
| Marine bacterium | 4.38 | [40] |
| Nedalco sludge | 11.71 | [40] |
| Eerbeek sludge | 12.34 | [41] |
| Coir pith | 12.82 | [42] |
| Brown seaweed | 20.63 | [43] |
| Myriophyllum spicatum | 43.40 | [44] |
| Mixed waste | 37.45 | [18] |
| Complexes | 62.11 | Current study |
| Material | Adsorption Capacity (qe) of Cs, (mg/g) | Literature |
| Ceiling tiles | 0.21 | [45] |
| Coal and chitosan | 3.00 | [46] |
| Bure mudrock | 13.30 | [47] |
| Modified akadama clay | 16.10 | [48] |
| Kaolinite clay | 17.10 | [49] |
| Coir pith | 32.00 | [50] |
| Bentonites | 92.3 | [51] |
| Myriophyllum spicatum | 58.00 | [44] |
| Mixed waste | 48.30 | [18] |
| Complexes | 15.10 | Current study |
| Temp. | Cs+ | Co2+ | ||||||
|---|---|---|---|---|---|---|---|---|
| qe | qmax | kL | R2 | qe | qmax | kL | R2 | |
| 25 °C | 59 | 9.36 | 0.019 | 0.987 | 40.066 | 30.1120 | 0.037 | 0.908 |
| 35 °C | 57.5 | 7.48 | 0.017 | 0.963 | 134 | 62.118 | 0.046 | 0.937 |
| 45 °C | 56.5 | 7.62 | 0.023 | 0.982 | 142.8 | 43.859 | 0.160 | 0.868 |
| 55 °C | 61 | 15.10 | 0.012 | 0.979 | 142.224 | 24.096 | 0.159 | 0.868 |
| Temp. | Cs+ | Co2+ | ||||
|---|---|---|---|---|---|---|
| n | Kf | R2 | n | Kf | R2 | |
| 25 °C | 0.702 | 0.005 | 0.995 | 0.985 | 0.695 | 0.9125 |
| 35 °C | 0.692 | 0.007 | 0.984 | 0.726 | 7.450 | 0.96 |
| 45 °C | 0.64 | 0.003 | 0.977 | 0.671 | 60.140 | 0.95 |
| 55 °C | 0.711 | 0.004 | 0.974 | 0.517 | 17.926 | 0.948 |
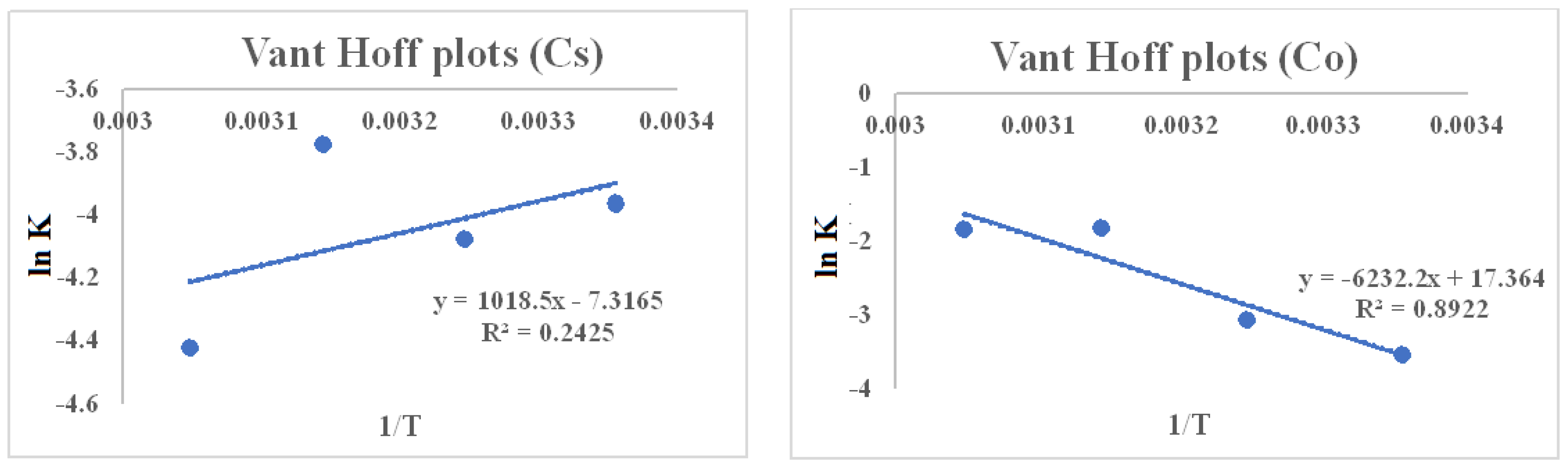
| Element | ∆S° | ∆H° | T (K) | T∆S° | ∆G° |
|---|---|---|---|---|---|
| Co2+ | 298 | 43.020 | −8.771 | ||
| 0.144 | 51.814 | 308 | 44.46 | 7.884 | |
| Endothermic | 318 | 45.90 | 4.845 | ||
| 328 | 47.351 | 5.014 | |||
| Cs+ | −60.829381 | −8.467809 | 298 | −18.1272 | 9.819 |
| Exothermic | 308 | −18.7354 | 10.433 | ||
| 318 | −19.3437 | 9.9732 | |||
| 328 | −19.952 | 12.0610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, R.N.; Ibrahim, S.S.; El-Shafiey, Z.A.; Faheim, A.A.; Saleh, H.M. Removal of Radio and Stable Isotopes of Cobalt and Cesium from Contaminated Aqueous Solutions by Isatin-Derived Ligand. AppliedChem 2025, 5, 8. https://doi.org/10.3390/appliedchem5020008
Khalaf RN, Ibrahim SS, El-Shafiey ZA, Faheim AA, Saleh HM. Removal of Radio and Stable Isotopes of Cobalt and Cesium from Contaminated Aqueous Solutions by Isatin-Derived Ligand. AppliedChem. 2025; 5(2):8. https://doi.org/10.3390/appliedchem5020008
Chicago/Turabian StyleKhalaf, Riyam N., Sattar S. Ibrahim, Zeinab A. El-Shafiey, Abeer A. Faheim, and Hosam M. Saleh. 2025. "Removal of Radio and Stable Isotopes of Cobalt and Cesium from Contaminated Aqueous Solutions by Isatin-Derived Ligand" AppliedChem 5, no. 2: 8. https://doi.org/10.3390/appliedchem5020008
APA StyleKhalaf, R. N., Ibrahim, S. S., El-Shafiey, Z. A., Faheim, A. A., & Saleh, H. M. (2025). Removal of Radio and Stable Isotopes of Cobalt and Cesium from Contaminated Aqueous Solutions by Isatin-Derived Ligand. AppliedChem, 5(2), 8. https://doi.org/10.3390/appliedchem5020008







