Effect of Crystallization on Electrochemical and Tribological Properties of High-Velocity Oxygen Fuel (HVOF)-Sprayed Fe-Based Amorphous Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Preparation and Heat-Treatment Process
2.3. Microstructure and Phase Analysis
2.4. Corrosion Tests
2.5. Tribological Tests
3. Results and Discussion
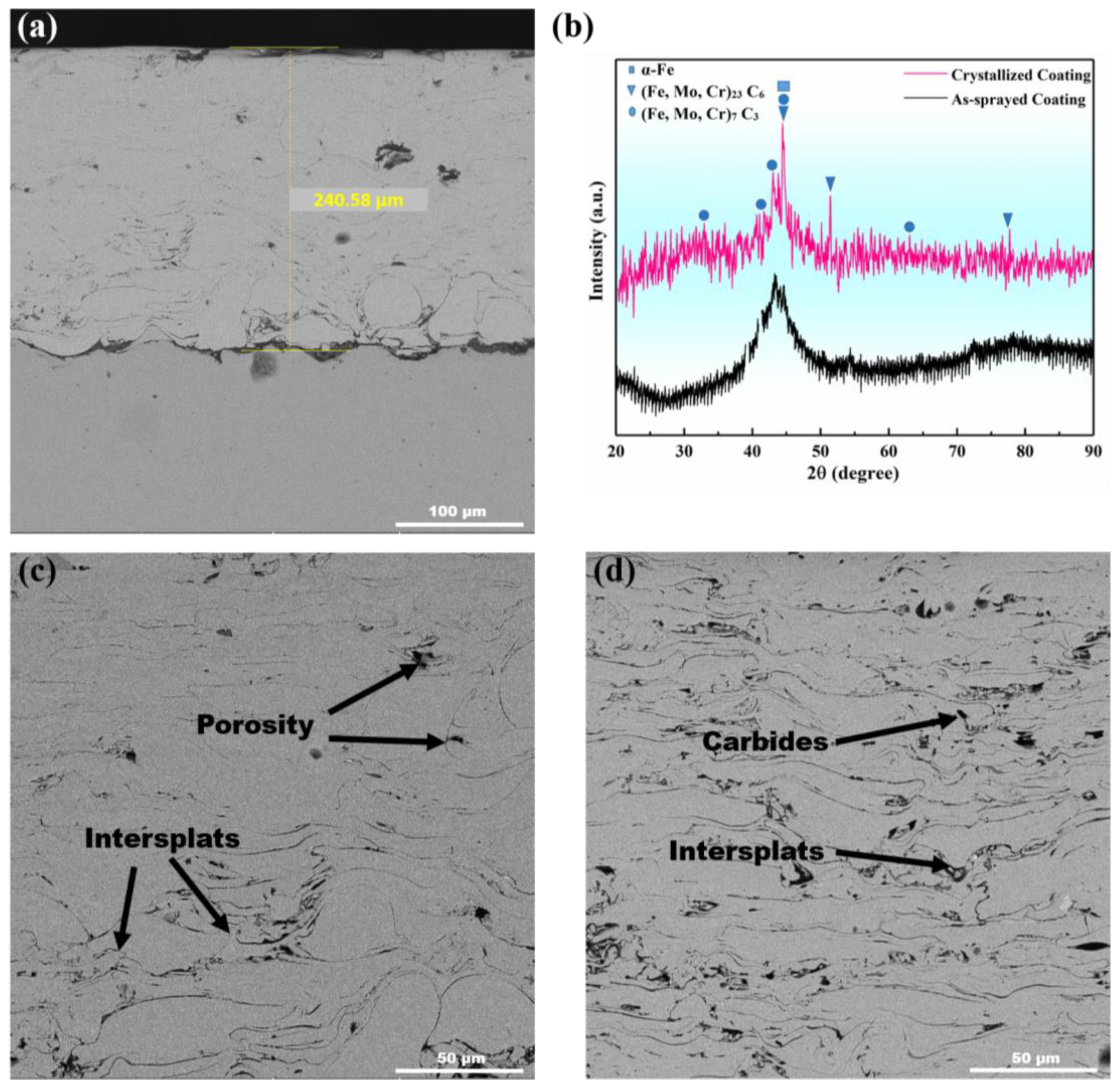
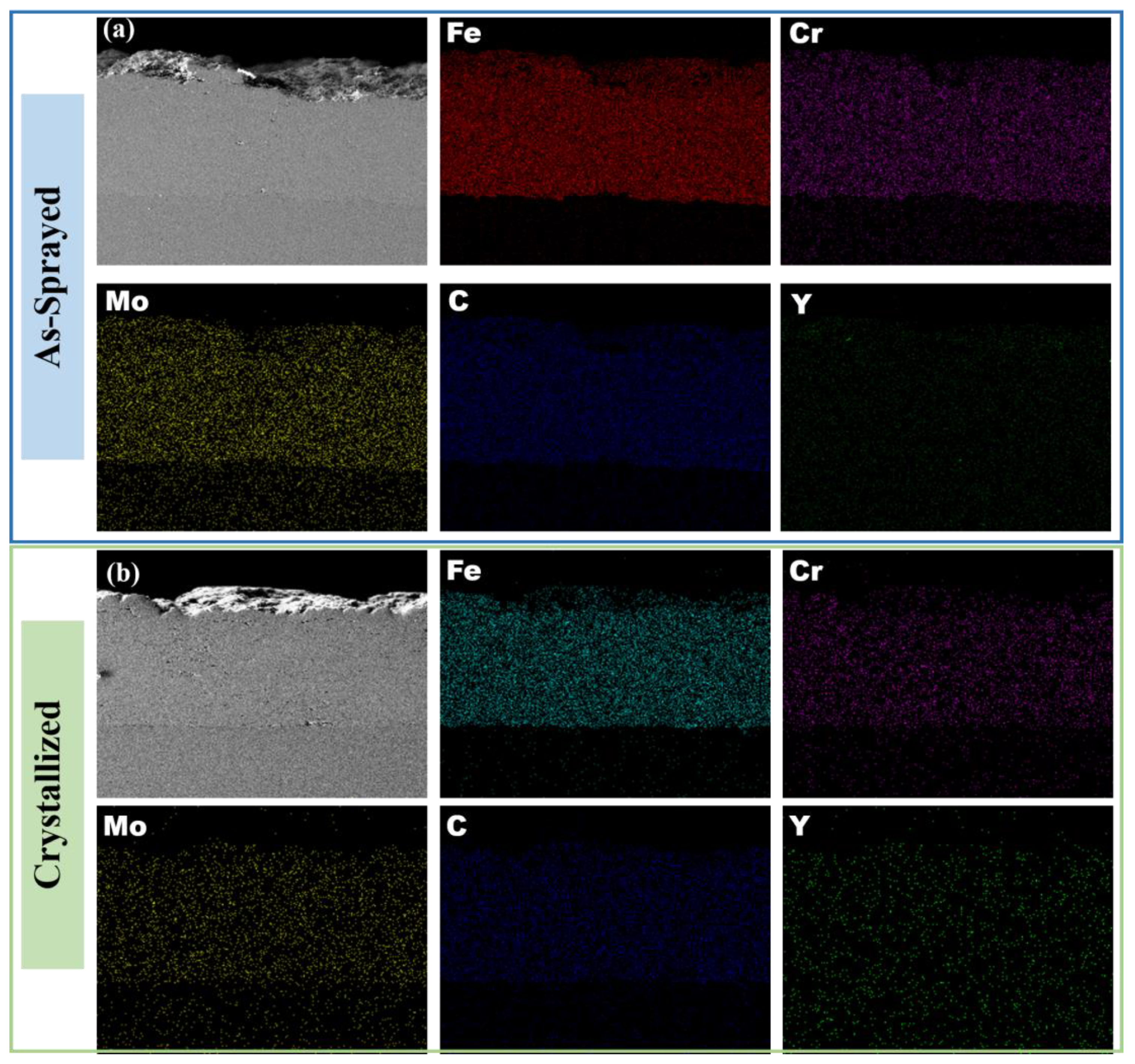
3.1. Microstructural Properties
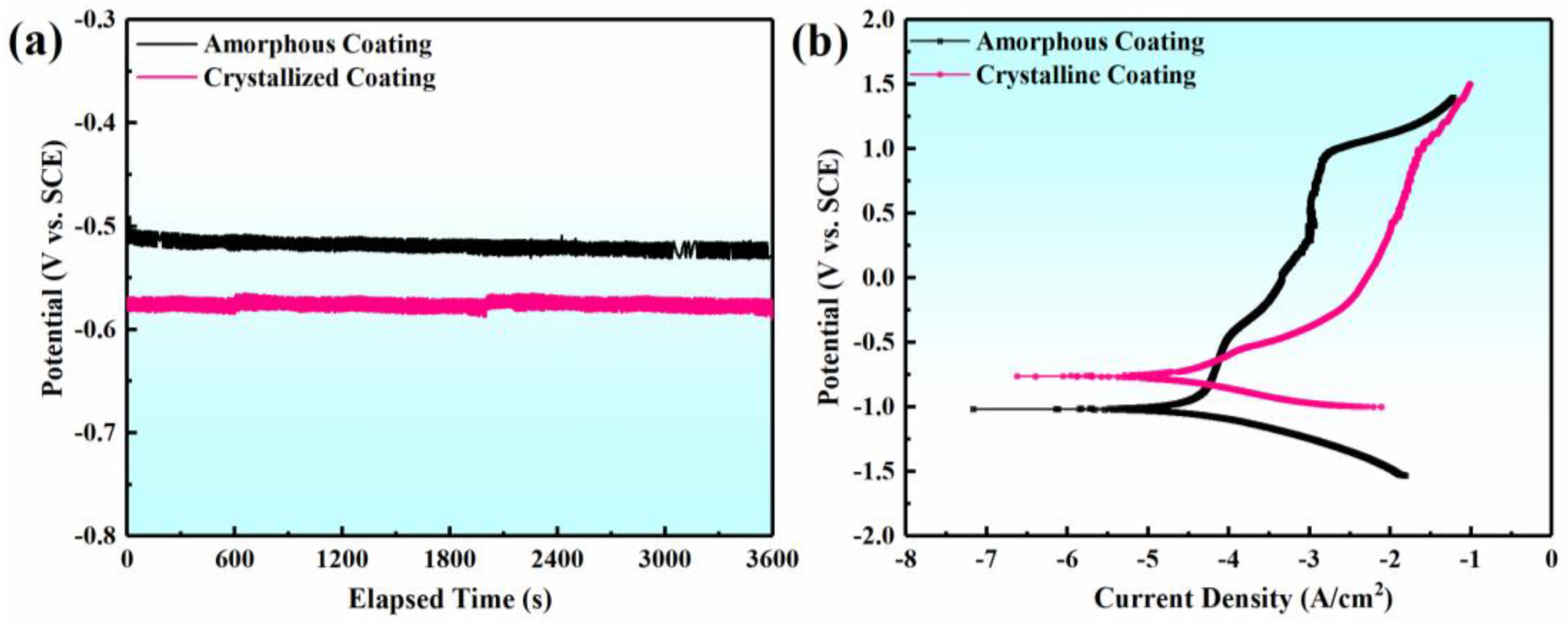
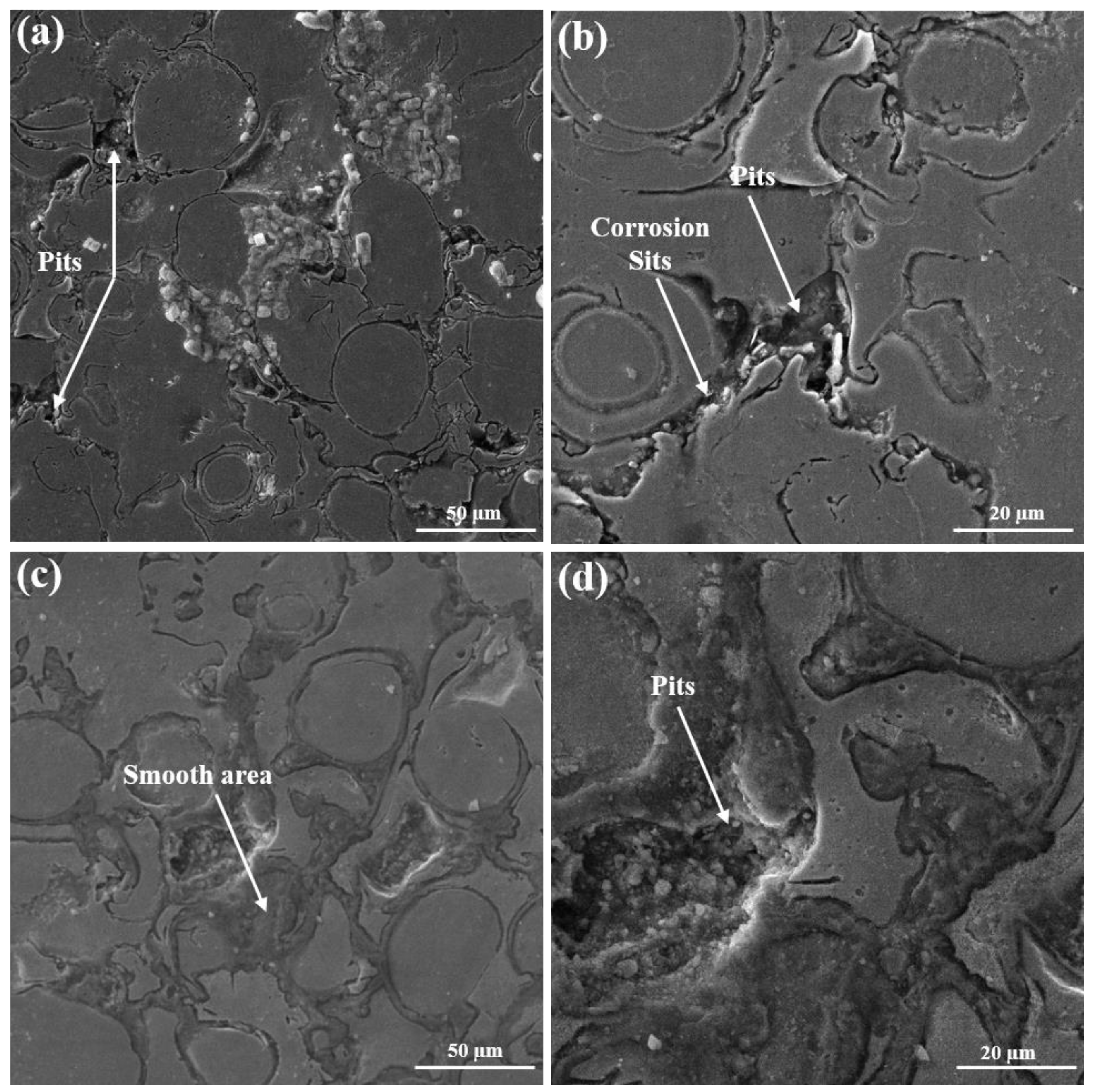
3.2. Potentiodynamic Polarization Analysis
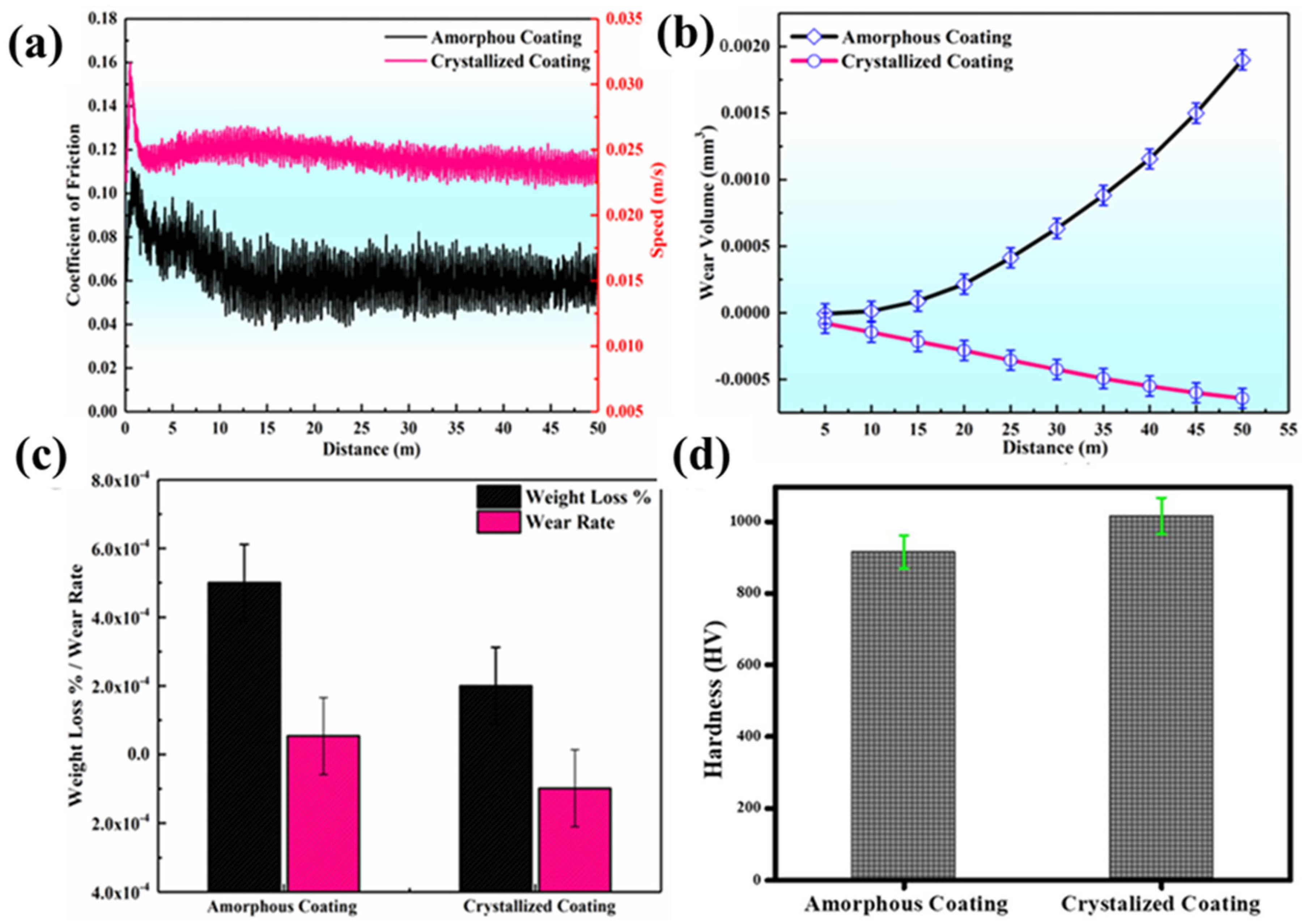
3.3. Tribological Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchtík, M.; Hasoňová, M.; Horník, P.; Březina, M.; Doskočil, L.; Másilko, J.; Mrňa, L.; Filipenský, J.; Kuběna, I.; Fintová, S.; et al. Influence of laser remelting on the microstructure and corrosion behavior of HVOF-sprayed Fe-based coatings on magnesium alloy. Mater. Charact. 2022, 194, 112343. [Google Scholar] [CrossRef]
- Nayak, S.K.; Faridi, M.A.; Gopi, M.; Kumar, A.; Laha, T. Fe-based metallic glass composite coatings by HVOF spraying: Influence of Mo on phase evolution, wear and corrosion resistance. Mater. Charact. 2022, 191, 112149. [Google Scholar] [CrossRef]
- Meghwal, A.; Pinches, S.; King, H.J.; Schulz, C.; Stanford, N.; Hall, C.; Berndt, C.C.; Ang, A.S.M. Fe-based amorphous coating for high-temperature wear, marine and low pH environments. Materialia 2022, 25, 101549. [Google Scholar] [CrossRef]
- Varis, T.; Lagerbom, J.; Suhonen, T.; Raami, L.; Terho, S.; Laurila, J.; Peura, P.; Vuoristo, P. Effect of heat treatments on the wear resistance of HVAF and HVOF sprayed tool steel coatings. Surf. Coat. Technol. 2023, 462, 129508. [Google Scholar] [CrossRef]
- Inoue, A.; Kong, F.; Zhu, X.; Chen, J.; Men, H.; Botta, W.J. Development and industrialization of Zr- and Fe-based bulk metallic glasses and light metal-based metastable alloys. J. Alloys Compd. 2024, 979, 173546. [Google Scholar] [CrossRef]
- Al-Abboodi, H.; Fan, H.; Mhmood, I.A.; Al-Bahrani, M. The dry sliding wear rate of a Fe-based amorphous coating prepared on mild steel by HVOF thermal spraying. J. Mater. Res. Technol. 2022, 18, 1682–1691. [Google Scholar] [CrossRef]
- Ham, G.-S.; Cho, Y.-H.; Park, S.-Y.; Kim, C.P.; Ko, W.-S.; Lee, K.-A. Fabrication, microstructure, and wear properties of novel Fe–Cr–B–Nb–Mo metamorphic alloy coatings manufactured by the HVOF thermal spray process. Intermetallics 2023, 162, 108038. [Google Scholar] [CrossRef]
- Zhai, H.; Li, X.; Zhang, Y.; Li, W.; He, D.; Cheng, B.; Zhang, X.; Viktor, Z.; Seniuts, U. Non-isothermal crystallization behavioral analysis of detonation sprayed Fe-based amorphous coating. J. Mater. Res. Technol. 2023, 23, 6115–6126. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C. Fe-based amorphous coatings: Structures and properties. Thin Solid Film. 2014, 561, 70–86. [Google Scholar] [CrossRef]
- Zhai, F.; Pineda, E.; Duarte, M.J.; Crespo, D. Role of Nb in glass formation of Fe–Cr–Mo–C–B–Nb BMGs. J. Alloys Compd. 2014, 604, 157–163. [Google Scholar] [CrossRef]
- Xiao, M.; Jiang, F.; Guo, C.; Song, H.; Dong, T. Investigation on microstructure and mechanical properties of Fe-based amorphous coatings prepared via laser cladding assisted with ultrasonic vibration. Opt. Laser Technol. 2023, 162, 109294. [Google Scholar] [CrossRef]
- Meghwal, A.; Schulz, C.; Hall, C.; Vogli, E.; Berndt, C.C.; Ang, A.S.M. Microstructural, mechanical and high-temperature tribological performance of Fe-based fully amorphous and amorphous/crystalline coatings. Surf. Coat. Technol. 2023, 475, 130114. [Google Scholar] [CrossRef]
- Bhushan, B.; Banerjee, A.; Patel, S.N.; Banik, D.; Godbole, K.; Vishwanath, K.; Mandal, S.; Mondal, K. Electrochemical response and passivation affinity of Fe-based amorphous HVOF coatings prepared from pig iron on mild steel. Surf. Coat. Technol. 2023, 452, 129082. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, H.; Tong, W.; Li, W.; Li, X.; Fan, X.; Xiong, D. Corrosion and tribo-corrosion behaviors of detonation sprayed Fe-based amorphous coating. Surf. Coat. Technol. 2024, 482, 130717. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Wan, Y.; Jeyaprakash, N.; Wang, Y.; Ma, K.; Yang, J. Influence of scanning speed on microstructure and corrosion resistance of Fe-based amorphous coatings by high-speed laser cladding. Surf. Coat. Technol. 2024, 479, 130449. [Google Scholar] [CrossRef]
- Zhu, P.-Y.; Feng, D.-Q.; Yasir, M.; Song, W.-L.; Hafeez, M.A.; Zhang, C.; Liu, L. Enhanced antifouling capability of PDMS/Cu2O-anchored Fe-based amorphous coatings. Surf. Coat. Technol. 2023, 475, 130192. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Z.; Zhang, K.; Wu, L.; Zhang, X.; Shi, X. Study on corrosion resistance of passive sealant to Fe-based amorphous coating at atomic-scale. Constr. Build. Mater. 2023, 408, 133661. [Google Scholar] [CrossRef]
- Iqbal, A.; Iqbal, A.; Moskal, G.; Yasir, M.; Al-Mansour, A.I.; Khan, M.A.; Alam, S.; Shahbaz, M.; Zia, A.; Ejaz, A. Long-Term Potentiodynamic Testing and Tribometric Properties of Amorphous Alloy Coatings under Saline Environment. Molecules 2022, 27, 1421. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Peng, Y.; Yu, Y.; Liu, L. Effects of crystallization on the corrosion resistance of Fe-based amorphous coatings. Corros. Sci. 2012, 59, 10–19. [Google Scholar] [CrossRef]
- Song, H.; Guo, C.; Li, J.; Jiang, F.; Diao, M.; Xiao, M.; Li, L.; Sun, Q. Study the influence of laser energy density on the amorphous content and properties of Fe-based amorphous coatings. Surf. Coat. Technol. 2024, 478, 130420. [Google Scholar] [CrossRef]
- Subbiah, R.; Arun, A.; Lakshmi, A.A.; Naga Sai Harika, A.; Ram, N.; Sateesh, N. Experimental Study of Wear Behaviour on Al-2014 Alloy Coated with Thermal Spray HVOF (High Velocity Oxy-Fuel) and Plasma Spray Process—A Review. Mater. Today Proc. 2019, 18, 5151–5157. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Z.; Zhang, Y.; Wu, H.; Liao, H.; Deng, S. Prediction and analysis of high velocity oxy fuel (HVOF) sprayed coating using artificial neural network. Surf. Coat. Technol. 2019, 378, 124988. [Google Scholar] [CrossRef]
- Ma, H.R.; Li, J.W.; Jiao, J.; Chang, C.T.; Wang, G.; Shen, J.; Wang, X.M.; Li, R.W. Wear resistance of Fe-based amorphous coatings prepared by AC-HVAF and HVOF. Mater. Sci. Technol. 2017, 33, 65–71. [Google Scholar] [CrossRef]
- Sun, Y.J.; Yang, R.; Xie, L.; Li, Y.B.; Wang, S.L.; Li, H.X.; Wang, W.R.; Zhang, J.S. Interfacial bonding and corrosion behaviors of HVOF-sprayed Fe-based amorphous coating on 8090 Al-Li alloy. Surf. Coat. Technol. 2022, 436, 128316. [Google Scholar] [CrossRef]
- Sun, Y.J.; Yang, R.; Xie, L.; Wang, W.R.; Li, Y.B.; Wang, S.L.; Li, H.X.; Zhang, J.M.; Zhang, J.S. Interfacial bonding mechanism and properties of HVOF-sprayed Fe-based amorphous coatings on LA141 magnesium alloy substrate. Surf. Coat. Technol. 2021, 426, 127801. [Google Scholar] [CrossRef]
- Sweitzer, J.E.; Shiflet, G.J.; Scully, J.R. Localized corrosion of Al90Fe5Gd5 and Al87Ni8.7Y4.3 alloys in the amorphous, nanocrystalline and crystalline states: Resistance to micrometer-scale pit formation. Electrochim. Acta 2003, 48, 1223–1234. [Google Scholar] [CrossRef]
- Lucente, A.M.; Scully, J.R. Pitting of Al-Based Amorphous-Nanocrystalline Alloys with Solute-Lean Nanocrystals. Electrochem. Solid-State Lett. 2007, 10, C39. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.Y.; Scenini, F.; Jiao, J.; Qu, S.J.; Luo, Q.; Shen, J. The effect of residual stress on the electrochemical corrosion behavior of Fe-based amorphous coatings in chloride-containing solutions. Surf. Coat. Technol. 2016, 302, 27–38. [Google Scholar] [CrossRef]
- Yoo, Y.H.; Lee, S.H.; Kim, J.G.; Kim, J.S.; Lee, C. Effect of heat treatment on the corrosion resistance of Ni-based and Cu-based amorphous alloy coatings. J. Alloys Compd. 2008, 461, 304–311. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.C.; Zhang, H.F.; Liu, Y.B.; Xu, S.H. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Ha, H.M.; Miller, J.R.; Payer, J.H. Devitrification of Fe-Based Amorphous Metal SAM 1651 and the Effect of Heat-Treatment on Corrosion Behavior. J. Electrochem. Soc. 2009, 156, C246. [Google Scholar] [CrossRef]
- Conshohocken, W.J.W. Standard test method for wear testing with a pin-on-disk apparatus 1. Wear 2007, 5, 1–5. [Google Scholar]
- Inam, A.; Hafeez, M.A.; Atif, M.; Ishtiaq, M.; Hassan, M.H.; Hussain, T.; Mughal, M.S.; Raza, M.A.; Abbas, M.A.Q.; Ullah, I. Microstructural, Mechanical, and Electrochemical Properties of Quenched and Partitioned 3 wt% Mn Steel. Arab. J. Sci. Eng. 2021, 46, 417–423. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Zhang, Z.-W.; Li, Y.-C.; Yasir, M.; Wang, H.-T.; Liu, L. Toughening Fe-based Amorphous Coatings by Reinforcement of Amorphous Carbon. Sci. Rep. 2017, 7, 4084. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R. Impedance spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Zhang, C.; Wang, W.; Zhang, Z.-W.; Liu, L. Tribocorrosion Behavior of Fe-Based Amorphous Composite Coating Reinforced by Al2O3 in 3.5% NaCl Solution. J. Therm. Spray Technol. 2016, 25, 1554–1560. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Xu, P.; Yasir, M.; Liu, L. Enhancement of oxidation and wear resistance of Fe-based amorphous coatings by surface modification of feedstock powders. Mater. Des. 2015, 73, 35–41. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, C.; Wang, W.; Yasir, M.; Liu, L. Microstructure and Mechanical Properties of Fe-based Amorphous Composite Coatings Reinforced by Stainless Steel Powders. J. Mater. Sci. Technol. 2015, 31, 43–47. [Google Scholar] [CrossRef]
- Henao, J.; Concustell, A.; Cano, I.G.; Cinca, N.; Dosta, S.; Guilemany, J.M. Influence of Cold Gas Spray process conditions on the microstructure of Fe-based amorphous coatings. J. Alloys Compd. 2015, 622, 995–999. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, J.; Bae, G.; Kim, B.; Lee, C. Formation of coating and tribological behavior of kinetic sprayed Fe-based bulk metallic glass. J. Alloys Compd. 2011, 509, 347–353. [Google Scholar] [CrossRef]
- Abbas, A.; Huang, S.J.; Ballóková, B.; Sülleiová, K. Tribological effects of carbon nanotubes on magnesium alloy AZ31 and analyzing aging effects on CNTs/AZ31 composites fabricated by stir casting process. Tribol. Int. 2020, 142, 105982. [Google Scholar] [CrossRef]
- Lu, T.-t.; Hua, D.-p.; An, B.-L.; Hafeez, M.A.; Pan, J.; Chen, L.-X.; Lu, J.-Y.; Zhou, Q.; Zhang, C.; Liu, L. Improved anti-adhesive wear performance of rail/armature pair via interfacial energy modulation for electromagnetic launching applications. Scr. Mater. 2023, 236, 115677. [Google Scholar] [CrossRef]


| Coatings | OCP (mV) | Icorr (μAcm−2) | Ecorr (mV) | Corrosion Rat (mpy) |
|---|---|---|---|---|
| Amorphous | −505.6 | 04.95 | −1006.0 | 4.28 |
| Crystallized | −586.4 | 11.57 | −768.79 | 9.99 |
| Coating | OCP (mV) | Rs (Ωcm2) | CPE-c (Ω−1snm−2) | Rp (Ωcm2) | W | CPE-ct (Ω−1sn cm−2) |
|---|---|---|---|---|---|---|
| Amorphous | −505.6 | 4.3 | 18.0 | 120 | 3.0 | − |
| Crystallized | −586.4 | 1.7 | 2.5 × 104 | 65.1 | − | 1.1 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.Q.; Hafeez, M.A.; Zhang, C.; Atiq-ur-Rehman, M.; Yasir, M. Effect of Crystallization on Electrochemical and Tribological Properties of High-Velocity Oxygen Fuel (HVOF)-Sprayed Fe-Based Amorphous Coatings. AppliedChem 2024, 4, 270-281. https://doi.org/10.3390/appliedchem4030017
Abbas AQ, Hafeez MA, Zhang C, Atiq-ur-Rehman M, Yasir M. Effect of Crystallization on Electrochemical and Tribological Properties of High-Velocity Oxygen Fuel (HVOF)-Sprayed Fe-Based Amorphous Coatings. AppliedChem. 2024; 4(3):270-281. https://doi.org/10.3390/appliedchem4030017
Chicago/Turabian StyleAbbas, Abdul Qadir, Muhammad Arslan Hafeez, Cheng Zhang, Muhammad Atiq-ur-Rehman, and Muhammad Yasir. 2024. "Effect of Crystallization on Electrochemical and Tribological Properties of High-Velocity Oxygen Fuel (HVOF)-Sprayed Fe-Based Amorphous Coatings" AppliedChem 4, no. 3: 270-281. https://doi.org/10.3390/appliedchem4030017
APA StyleAbbas, A. Q., Hafeez, M. A., Zhang, C., Atiq-ur-Rehman, M., & Yasir, M. (2024). Effect of Crystallization on Electrochemical and Tribological Properties of High-Velocity Oxygen Fuel (HVOF)-Sprayed Fe-Based Amorphous Coatings. AppliedChem, 4(3), 270-281. https://doi.org/10.3390/appliedchem4030017








