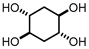
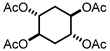
Abstract
Cyclohexanetetrols belong to the family of cyclitols, a class of natural products known for their diverse bioactivity. Their synthesis has been reported using hydrogen peroxide as a green oxidant and water or tert-butanol as a solvent. Due to the high polarity of those compounds, a green approach for their isolation from aqueous solutions can be challenging. Here, we report the stereoselective synthesis of (±)-trans,trans-cyclohexane-1,2,4,5-tetraol combined with a novel isolation method, where is possible the isolation of the product in excellent yield without the need for derivatization, column chromatography or organic solvent extraction.
1. Introduction
Cyclohexanetetrols are of interest because of their close relationship to the naturally occurring betitol, quercitols and inositols [1] that are important bioactive compounds. In particular, trans,trans-cyclohexane-1,2,4,5-tetraol has been isolated from A. modestus Diels ssp macranthus Verdc. stem and root bark extracts and has shown antimicrobial activity against both S. aureus and E. coli [2]. Besides this, trans-diols are important building blocks for the synthesis of pharmaceuticals and agrochemicals, and can also be used as chiral auxiliaries or ligands for asymmetric synthesis [3]. In particular trans,trans-cyclohexane-1,2,4,5-tetraol is important in the industry since it can be used in the total synthesis of biologically active compounds, such as aminocyclitols and analogs, that can be synthetized by less demanding functional group transformations from the intermediates amino-1,2,4,5-cyclohexane-tetrols [4].
Considerable efforts have been devoted to finding more environmentally friendly chemical processes to reduce the emissions of volatile organic compounds (VOCs). Reduction or elimination of the traditional solvents, usually toxic and inflammable, provides an approach to prevent pollution. To assess the sustainability of a chemical process we have to consider the overall process, not just the reaction conditions. In addition, the work up can contribute considerably to the green metrics of the overall process. The synthesis of alcohols from the dihydroxylation of alkenes is a traditional methodology to obtain diols or tetrols, although only a few of the reported methods are metal-free or use non-organic solvents as the reaction medium [5,6]. For example, trans,trans-cyclohexane-1,2,4,5-tetraol synthesis has been reported using selenium(IV) oxide as a catalyst; however, this methodology uses organic solvents in the synthesis and isolation of the product [7,8,9]. Nafion was also reported in the synthesis of cyclohexane-1,2,4,5-tetraol in water, although the obtained stereochemistry was not specified [6].
The possibility to perform this reaction using water as a solvent is a highly attractive approach due to the reduction of organic solvents; nevertheless, it has the additional challenge of isolating the highly polar final product from the aqueous reaction mixture (including the reaction catalyst). For that, several groups have reported the derivatization by acetylation of the hydroxyl groups in order to isolate the final product [7]. This approach is time consuming and decreases the sustainability of the process exponentially. For this reason, we developed a chemical process for the production of trans,trans-cyclohexane-1,2,4,5-tetraol on a large scale where no organic solvents or metal catalysts were used, with the highly effective isolation step being the main breakthrough. It was possible to isolate the highly polar trans,trans-cyclohexane-1,2,4,5-tetraol from the reaction mixture in only one step, using an Amberlite column that retains the reaction catalyst.
2. Materials and Methods
All the solvents were distilled before use. All chemicals were purchased from Aldrich (1,4-cyclohexadiene (CAS 628-41-1); toluene-4-sulfonic acid monohydrate, PTSA (CAS 6192-52-5); hydrogen peroxide 30% wt. (CAS 7722-84-1) and amberlite IRA400 hydroxide form (can be replaced by Ambersep 900 hydroxide from Aldrich)). The closed-vessel reactor (SYNP160002) was purchased from Aldrich. 1H and 13C-NMR spectra were recorded in CDCl3 on a Bruker Fourier 300 spectrometer at 300 and 75 MHz, respectively. Chemical shifts are expressed in parts per million (ppm) relative to tetramethylsilane (TMS). The coupling constants (J) are reported in Hertz (Hz).
2.1. Synthesis of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol
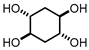
PTSA (1.21 g, 20 mol%, 6.34 mmol) and H2O2 (30% aq. sol., 14.38 g, 2 equiv.) were added into a closed-vessel reactor. After complete dissolution of PTSA, 1,4-Cyclohexadiene (3 mL, 31 mmol) was added and stirred for 21 h at 50 °C using a protection metal grid. This is a biphasic reaction, so a vigorous agitation is necessary to ensure a maximum yield. Performing the reaction with this oxidant at this temperature in a closed vessel requires special attention, due to their instability and the possibility of explosion.
After that, the reaction mixture was cooled to room temperature, sodium bicarbonate (until pH 7) was added to the solution and the solution was reduced with Na2SO3.
2.2. Isolation of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol without the Use of Organic Solvents
Without further treatment, the crude reaction mixture was added to a column filled with Amberlite® IRA400 (Figure 1) and the column was washed with 30 mL of water. After that, the final solution was evaporated and the product dried under vacuum. trans,trans-cyclohexane-1,2,4,5-tetraol was obtained pure by NMR without chromatographic purification. (±)-trans,trans-cyclohexane-1,2,4,5-tetraol was obtained as a white solid with a yield of 98.0%, with a melting point of 203–204 °C (Lit. 208 °C [9,10,11]). 1H NMR (300 MHz, D2O) δ 3.62 (m, 4H), 1.69 (m, 4H). 13C NMR (75 MHz, D2O) δ 69.4, 33.61.
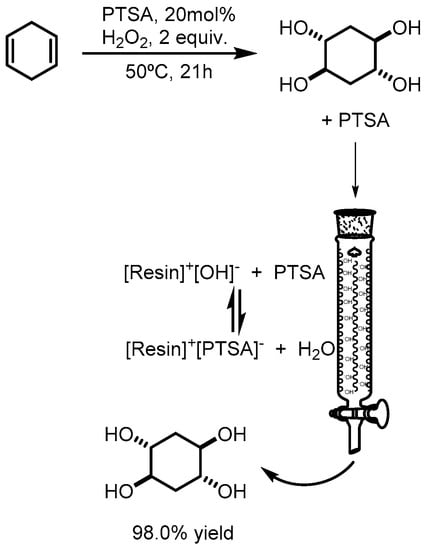
Figure 1.
Isolation of 1,2,4,5-cyclohexatetrol from the crude reaction media.
2.3. Synthesis of (±)-trans,trans-Cyclohexane-1,2,4,5-tetrayltetraacetate
Tetraacetate was synthesized according to a literature procedure, with small changes [12]. Triethylamine (9.03 mL, 8 equiv.) and acetic anhydride (4.56 mL, 6 equiv.) were added to the crude product of cyclohexane-1,2,4,5-tetraol (1.19 g, 8.01 mmol) and stirred for one day. After that, the triethylamine excess was removed by evaporation and 10 mL of aqueous HCl 10% (v/v) was added, which was extracted with 2 × 25 mL of dichloromethane. The organic layer was dried with Na2SO4 and evaporated. The final product was purified by column chromatography (EtOAc/Hexane 80:20), providing (±)-trans,trans-cyclohexane-1,2,4,5-tetrayltetraacetate as a white solid (1.85 g, 73.3%) with a melting point of 148 °C (lit. 148 °C [11], 147–148 °C [12]). 1H NMR (300 MHz, CDCl3) δ 5.03 (m, 4H), 2.02 (m, 16H). 13C NMR (75 MHz, CDCl3) δ 169.77, 69.07, 30.10, 20.94.
3. Discussion
Trans-dihydroxylation of 1,4-cyclohexadiene was studied using a reported methodology, where p-toluenosulfonic acid (PTSA) was used as a catalyst and water as a solvent [13,14]. After reaction optimization, it was possible to obtain (±)-trans,trans-cyclohexane-1,2,4,5-tetraol with high stereoselectivity and 98% yield, without using any metal catalysts or organic solvents. The challenge was to isolate the product from the acidic reaction media and two different approaches were performed: derivatization or using Ambertile as an ion exchange resin. The first approach for the isolation of the trans,trans-cyclohexane-1,2,4,5-tetraol was carried out by acetylation resulting in trans,trans-cyclohexane-1,2,4,5-tetrayltetraacetate (Scheme 1), which was isolated and purified by column chromatography (EtOAc/Hexane 80:20) with a yield of 73.3%.
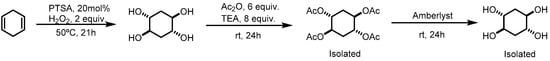
Scheme 1.
First approach for the isolation of (±)-trans,trans-cyclohexane-1,2,4,5-tetraol.
After the isolation of the tetraacetate, hydrolysis was performed using the reported methodology [15] to obtain tetrol in 78% yield. To do so, Amberlyst was used as an acid catalyst in an aqueous solution at 80 °C overnight. Furthermore, this is not an efficient approach since it requires two additional steps to obtain the pure tetrol. Thus, the isolation by derivatization was abandoned.
A more efficient approach for the separation of the tetraol from the catalyst (PTSA) was achieved using an ion exchange resin. By this way the PTSA is retained on the anionic resin and the tetraol is eluted out from the column with excellent yields (98% yield, Figure 1). This is an efficient methodology where it is possible to isolate the product in excellent yield without the need for derivatization, column chromatography or extraction with organic solvents. To show the reproducibility of the process, it was performed several times on a 31 mmol scale (3 mL of cyclehexadiene) with 96–98% yield.
It is interesting to note that this is a highly stereoselective reaction since trans,trans-cyclohexane-1,2,4,5-tetraol is the major product; however, it is possible to observe by NMR that the meso isomer is also formed, although in a residual amount (less than 1%). The trans-trans stereochemistry of the final product, was confirmed by the comparison of the NMR and melting point data with the literature (Table 1) [7].

Table 1.
Observed 1H and 13C NMR and melting point data of compounds (±)-trans,trans-cyclohexane-1,2,4,5-tetraol and (±)-trans,trans-cyclohexane-1,2,4,5-tetraacetate and comparison with the literature data.
4. Conclusions
This study presented a new strategy for the preparation and isolation of (±)-trans,trans-cyclohexane-1,2,4,5-tetraol from its highly polar reaction media (PTSA and water) using Ambertite resin, without the need for derivatization, column chromatography or extraction with organic solvents. This methodology can be useful for carbohydrate chemistry, where the isolation of polar compounds from the reaction mixture is needed. In this work, we obtained (±)-trans,trans-cyclohexane-1,2,4,5-tetraol in high yields and high purity.
Author Contributions
Conceptualization, A.A.R. and C.A.M.A.; methodology, A.A.R. and C.A.M.A.; validation, C.A.M.A.; investigation, A.A.R. and C.A.M.A.; writing—original draft preparation, A.A.R.; writing—review and editing, A.A.R. and C.A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e Tecnologia (FCT) (Ref. PTDC/QUI-QOR/32008/2017, PTDC/CTM-CTM/29869/2017, UIDB/04138/2020, UIDP/04138/2020, UIDB/04567/2020 and UIDP/04567/2020). This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 951996. The NMR spectrometer is part of the National NMR Network (PTNMR) and are partially supported by Infrastructure Project No 022161 (co-financed by FEDER through COMPETE 2020, POCI and PORL and FCT through PIDDAC).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
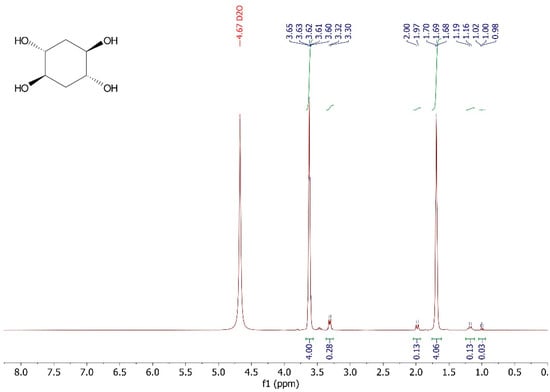
Figure A1.
1H NMR (300 MHz, D2O) spectrum of (±) -trans,trans-cyclohexane-1,2,4,5-tetraol.
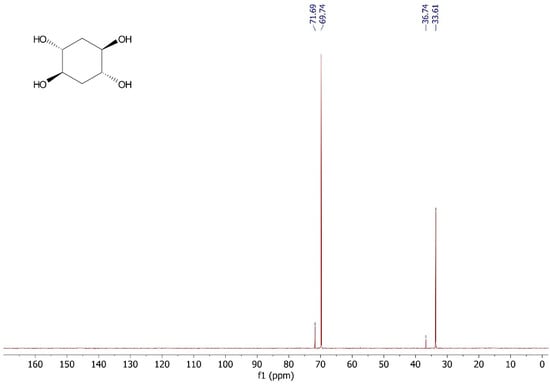
Figure A2.
13C NMR (75 MHz, D2O) spectrum of (±)-trans,trans-cyclohexane-1,2,4,5-tetraol.
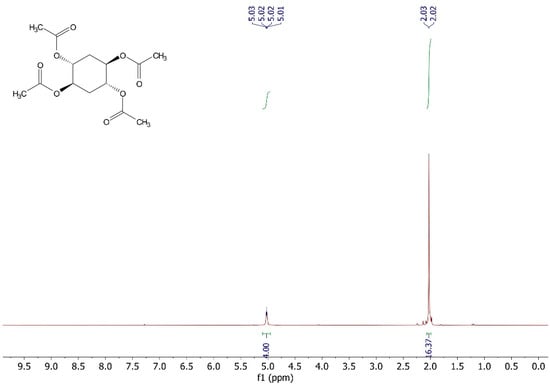
Figure A3.
1H NMR (300 MHz, CDCl3) spectrum of (±)-trans,trans-cyclohexane-1,2,4,5-tetraacetate.
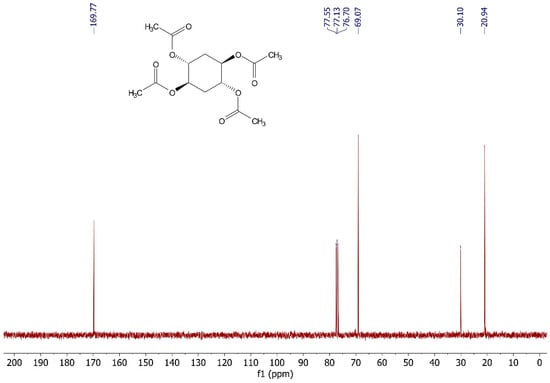
Figure A4.
13C NMR (75 MHz, CDCl3) spectrum of (±)-trans,trans-cyclohexane-1,2,4,5-tetraacetate.
References
- Sun, Y.D.; Zhang, G.H.; Hawkes, C.A.; Shaw, J.E.; McLaurin, J.; Nitz, M. Synthesis of scyllo-inositol derivatives and their effects on amyloid beta peptide aggregation. Bioorg. Med. Chem. 2008, 16, 7177–7184. [Google Scholar] [CrossRef] [PubMed]
- Nyandoro, S.S.; Joseph, C.C.; Nkunya, M.H.; Hosea, K.M. New antimicrobial, mosquito larvicidal and other metabolites from two Artabotrys species. Nat. Prod. Res. 2013, 27, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Ogura, M.; Tsuda, S.; Maemoto, S.; Kutsuki, H.; Ohashi, T. High-yield Production of Optically Active 1,2-Diols from the Corresponding Racemates by Microbial Stereoinversion. Agric. Biol. Chem. Tokyo 1990, 54, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Kurbanoglu, N.I.; Celik, M.; Kilic, H.; Alp, C.; Sahin, E.; Balci, M. Stereospecific synthesis of a DL-gala-aminoquercitol derivative. Tetrahedron 2010, 66, 3485–3489. [Google Scholar] [CrossRef]
- Gogoi, P.; Sharma, S.D.; Konwar, D. SeO2/H2O2/H2O-dioxane: A new catalytic system for trans dihydroxylation of olefins. Lett. Org. Chem. 2007, 4, 249–252. [Google Scholar] [CrossRef]
- Usui, Y.; Sato, K.; Tanaka, M. Catalytic dihydroxylation of olefins with hydrogen peroxide: An organic-solvent- and metal-free system. Angew. Chem. Int. Ed. 2003, 42, 5623–5625. [Google Scholar] [CrossRef] [PubMed]
- Maras, A.; Erden, M.; Secen, H.; Sutbeyaz, P. One-pot synthesis from 1,4-cyclohexadiene of (+/−)-1,4/2,5-cyclohexanetetrol, a naturally occurring cyclitol derivative. Carbohydr. Res. 1998, 308, 435–437. [Google Scholar] [CrossRef]
- Geary, L.M.; Chen, T.-Y.; Montgomery, T.P.; Krische, M.J. Benzannulation via Ruthenium-Catalyzed Diol–Diene [4+2] Cycloaddition: One- and Two-Directional Syntheses of Fluoranthenes and Acenes. J. Am. Chem. Soc. 2014, 136, 5920–5922. [Google Scholar] [CrossRef] [PubMed]
- Kasun, Z.A.; Geary, L.M.; Krische, M.J. Ring expansion of cyclic 1,2-diols to form medium sized rings via ruthenium catalyzed transfer hydrogenative [4+2] cycloaddition. Chem. Commun. 2014, 50, 7545–7547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suemune, H.; Hasegawa, A.; Sakai, K. Highly enantio- and diastereo-selective synthesis of C2-symmetric 3,5-cyclohexadiene-1,2-diol and D2-symmetric cyclohexane-1,2,4,5-tetrol. Tetrahedron Asymmetry 1995, 6, 55–58. [Google Scholar] [CrossRef]
- McCasland, G.E.; Furuta, S.; Johnson, L.F.; Shoolery, J.N. Synthesis of the Five Diastereomeric 1,2,4,5-Cyclohexanetetrols. Nuclear Magnetic Resonance Configurational Proofs1,2. J. Org. Chem. 1963, 28, 894–900. [Google Scholar] [CrossRef]
- Cavdar, H.; Saracoglu, N. Ring opening of epoxides with NaHSO4: Isolation of beta-hydroxy sulfate esters and an effective synthesis for trans-diols. Tetrahedron 2009, 65, 985–989. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Afonso, C.A.M.; Branco, L.C. Oxidation of Cyclohexene to trans-1,2-Cyclohexanediol Promoted by p-Toluenesulfonic Acid without Organic Solvents. J. Chem. Educ. 2011, 88, 1002–1003. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Afonso, C.A.M. Brønsted Acid-Catalyzed Dihydroxylation of Olefins in Aqueous Medium. Adv. Synth. Catal. 2011, 353, 2920–2926. [Google Scholar] [CrossRef]
- Tschamber, T.; Backenstrass, F.; Fritz, H.; Streith, J. Catalytic One-Pot Osmylation of Cyclohexadienes: Stereochemical and conformational studies of the resulting polyols. Helv. Chim. Acta 1992, 75, 1052–1060. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).