Carbon–Heteroatom Bond Formation via Coupling Reactions Performed on a Magnetic Nanoparticle Bed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Preparation of Fe3O4 Superparamagnetic Nanoparticle MNPs
2.3. Preparation of KF/Fe3O4
2.4. General Procedure for the Synthesis of C–N Materials
2.5. General Procedure for the Synthesis of Diaryl Ethers
3. Results and Discussion
3.1. Characterization of the Catalyst
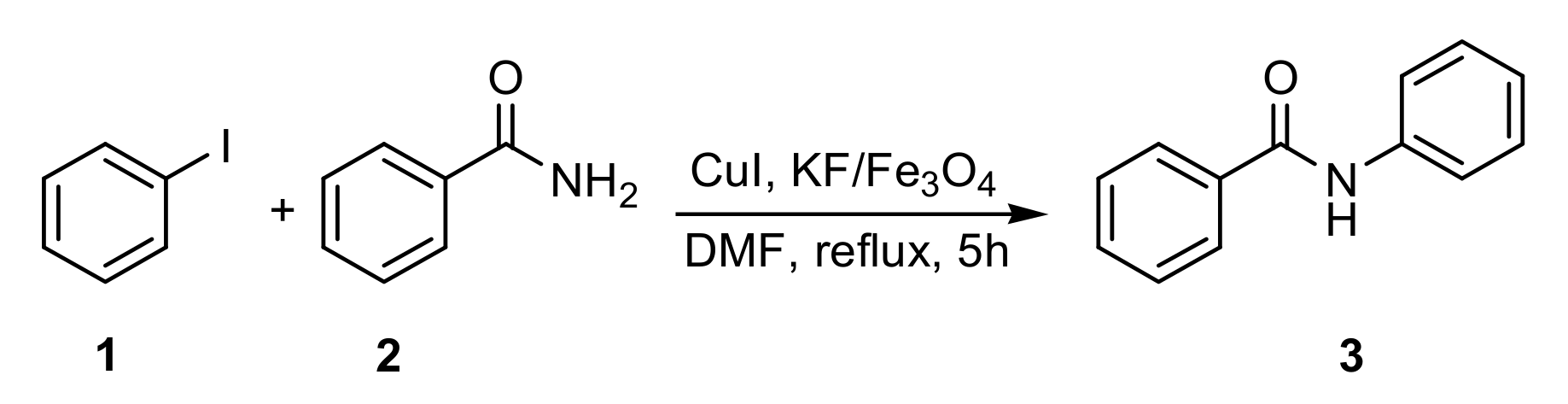
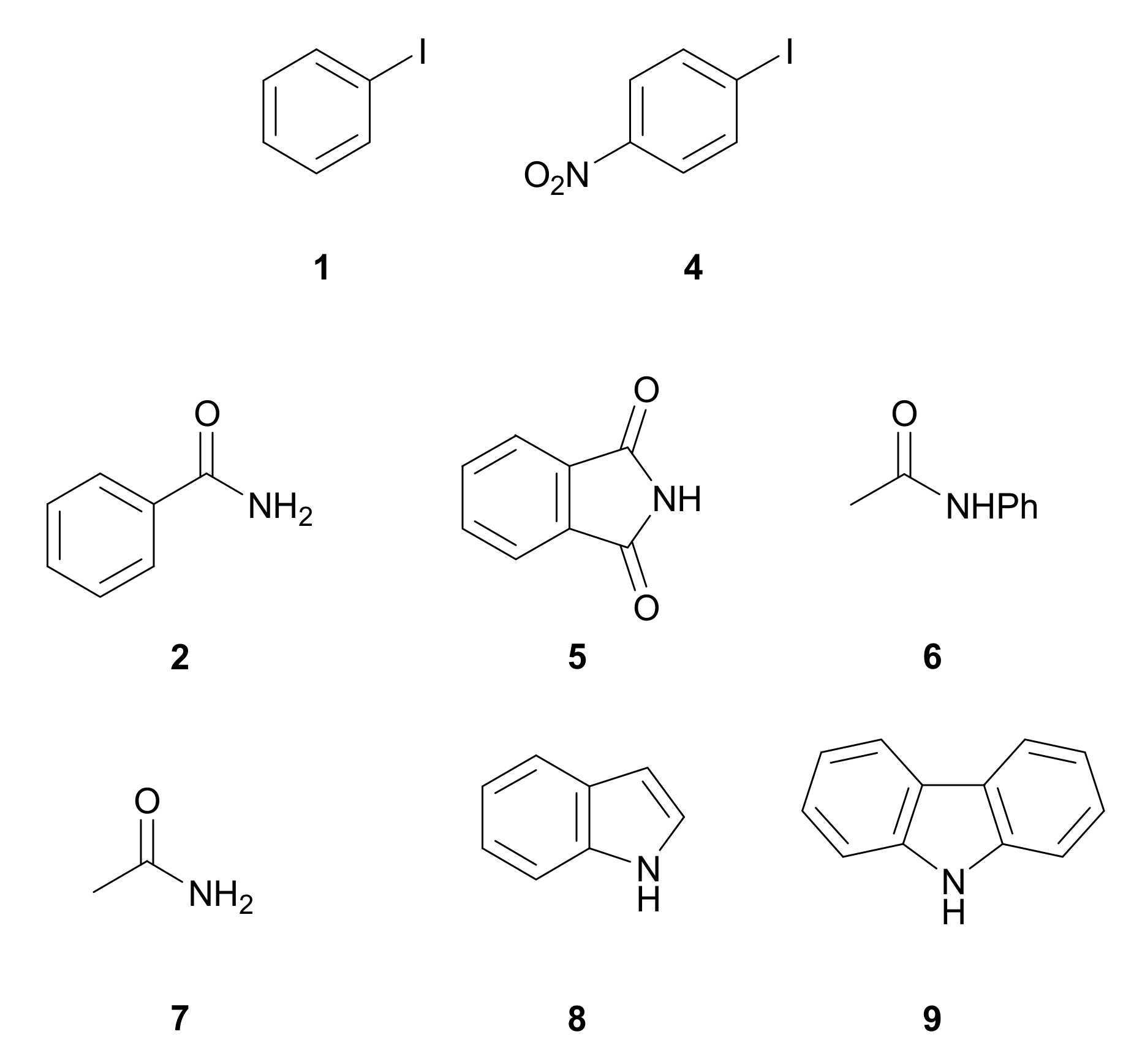
3.2. Formation of C–N Bond
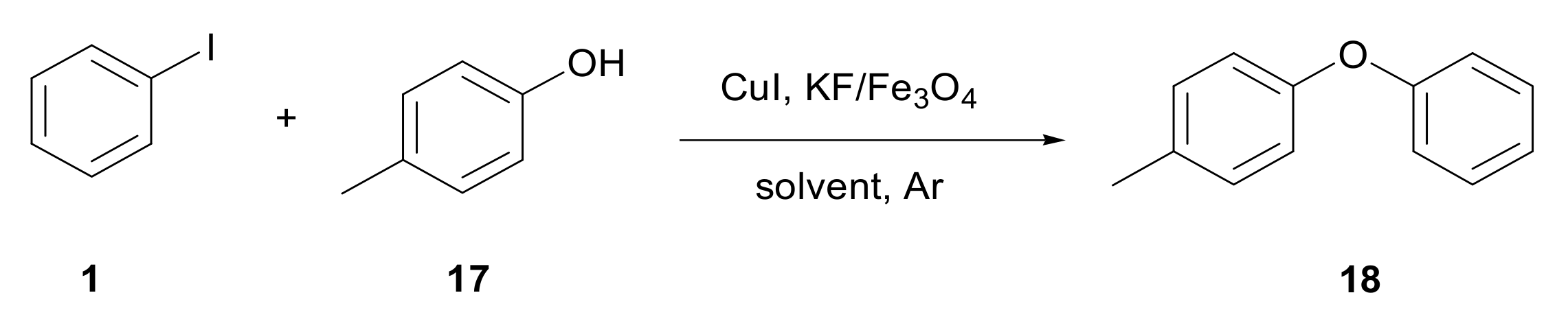
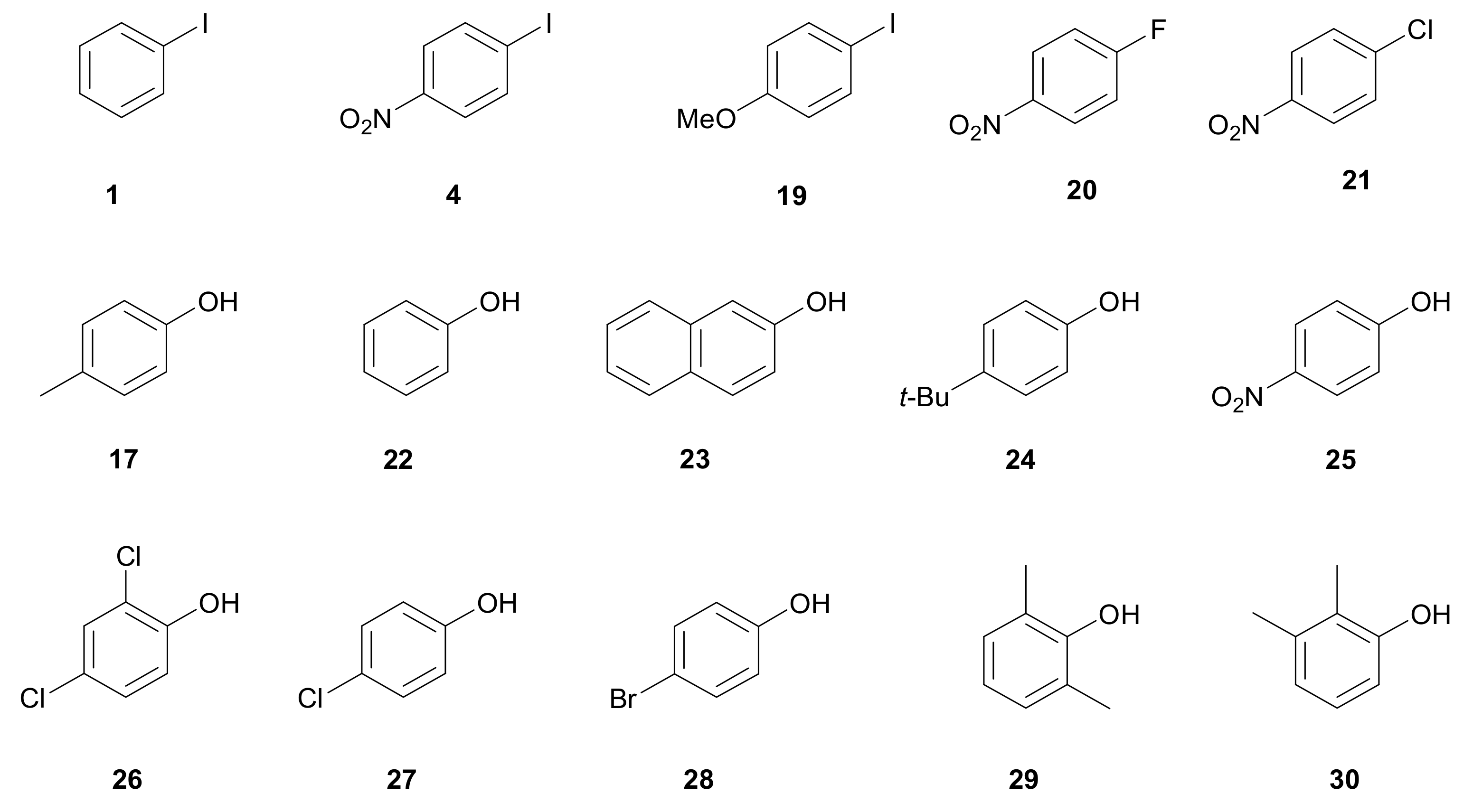
3.3. Formation of C–O Bond
3.4. Role of the Copper and Recyclability of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, D.A.; Wood, M.R.; Trotter, B.W.; Richardson, T.I.; Barrow, J.C.; Katz, J.L. Total Syntheses of Vancomycin and Eremomycin Aglycons. Angew. Chem. Int. Ed. 1998, 37, 2700–2704. [Google Scholar] [CrossRef]
- Boger, D.L.; Miyazaki, S.; Kim, S.H.; Wu, J.H.; Castle, S.L.; Loiseleur, O.; Jin, Q. Total synthesis of the vancomycin aglycon. J. Am. Chem. Soc. 1999, 121, 10004–10011. [Google Scholar] [CrossRef]
- Procter, D.J. The synthesis of thiols, selenols, sulfides, selenides, sulfoxides, selenoxides, sulfones and selenones. J. Chem. Soc. Perkin Trans. 2001, 1, 335–354. [Google Scholar] [CrossRef]
- Ley, S.V.; Thomas, A.W. Modern Synthetic Methods for Copper-Mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S Bond Formation. Angew. Chem.-Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef]
- Scott Sawyer, J. Recent advances in diaryl ether synthesis. Tetrahedron 2000, 56, 5045–5065. [Google Scholar] [CrossRef]
- Bariwal, J.; Van Der Eycken, E. C-N bond forming cross-coupling reactions: An overview. Chem. Soc. Rev. 2013, 42, 9283–9303. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Hosseini, A.; Pilevar, A. Potassium Fluoride Supported on Natural Nanoporous Zeolite: A New Solid Base for the Synthesis of Diaryl Ethers. Eur. J. Org. Chem. 2011, 2011, 1587–1592. [Google Scholar] [CrossRef]
- Agawane, S.M.; Nagarkar, J.M. Nano ceria catalyzed Ullmann type coupling reactions. Tetrahedron Lett. 2011, 52, 5220–5223. [Google Scholar] [CrossRef]
- Ono, Y.; Baba, T. Strong solid bases for organic reactions. In Catalysis; Royal Society of Chemistry: Cambridge, UK, 2000; Volume 15, pp. 1–39. ISBN 9781847553270. [Google Scholar]
- Albouy, D.; Laspéras, M.; Etemad-Moghadam, G.; Koenig, M. Role of base catalysts upon the Pudovik reaction: Unexpected synthesis of 1,2-dihydropyridine phosphonate derivatives. Tetrahedron Lett. 1999, 40, 2311–2314. [Google Scholar] [CrossRef]
- Smith, G.; Notheisz, F. Heterogeneous Catalysis in Organic Chemistry; Academic Press: San Diego, CA, USA, 1999; ISBN 9780126516456. [Google Scholar]
- Arzamendi, G.; Campo, I.; Arguiñarena, E.; Sánchez, M.; Montes, M.; Gandía, L.M. Synthesis of biodiesel with heterogeneous NaOH/alumina catalysts: Comparison with homogeneous NaOH. Chem. Eng. J. 2007, 134, 123–130. [Google Scholar] [CrossRef]
- Tedeschi, L.; Enders, D. Asymmetric synthesis of β-phosphono malonates via Fe2O3-mediated phospha-Michael addition to Knoevenagel acceptors. Org. Lett. 2001, 3, 3515–3517. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Z.; Liu, Y.; He, Q. Preparation and characterization of a novel solid base catalyst hydroxyapatite loaded with strontium. Catal. Commun. 2008, 9, 516–521. [Google Scholar] [CrossRef]
- Motokura, K.; Fujita, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. An acidic layered clay is combined with a basic layered clay for one-pot sequential reactions. J. Am. Chem. Soc. 2005, 127, 9674–9675. [Google Scholar] [CrossRef] [PubMed]
- Okachi, T.; Fujimoto, K.; Onaka, M. Practical carbonyl-ene reactions of α-methylstyrenes with paraformaldehyde promoted by a combined system of boron trifluoride and molecular sieves 4A. Org. Lett. 2002, 4, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Perozo-Rondón, E.; Calvino-Casilda, V.; Martín-Aranda, R.M.; Casal, B.; Durán-Valle, C.J.; Rojas-Cervantes, M.L. Catalysis by basic carbons: Preparation of dihydropyridines. Appl. Surf. Sci. 2006, 252, 6080–6083. [Google Scholar] [CrossRef]
- Choi, M.K.W.; Yu, W.Y.; So, M.H.; Zhou, C.Y.; Deng, Q.H.; Che, C.M. A non-cross-linked soluble polystyrene-supported ruthenium catalyst for carbenoid transfer reactions. Chem.-An Asian J. 2008, 3, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- So, M.H.; Liu, Y.; Ho, C.M.; Che, C.M. Graphite-supported gold nanoparticles as efficient catalyst for aerobic oxidation of benzylic amines to imines and N-substituted 1,2,3,4-tetrahydroisoquinolines to amides: Synthetic applications and mechanistic study. Chem.-Asian J. 2009, 4, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.N. Chemical Catalysis by Colloids and Clusters. Chem. Rev. 1993, 93, 2693–2730. [Google Scholar] [CrossRef]
- Trnka, T.M.; Grubbs, R.H. The development of L2X2RU=CHR olefin metathesis catalysts: An organometallic success story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef]
- Malinsky, M.D.; Kelly, K.L.; Schatz, G.C.; Van Duyne, R.P. Chain length dependence and sensing capabilities of the localized surface plasmon resonance of silver nanoparticles chemically modified with alkanethiol self-assembled monolayers. J. Am. Chem. Soc. 2001, 123, 1471–1482. [Google Scholar] [CrossRef]
- McConnell, W.P.; Novak, J.P.; Brousseau, L.C.; Fuierer, R.R.; Tenent, R.C.; Feldheim, D.L. Electronic and optical properties of chemically modified metal nanoparticles and molecularly bridged nanoparticle arrays. J. Phys. Chem. B 2000, 104, 8925–8930. [Google Scholar] [CrossRef]
- Shi, F.; Tse, M.K.; Pohl, M.M.; Radnik, J.; Brückner, A.; Zhang, S.; Beller, M. Nano-iron oxide-catalyzed selective oxidations of alcohols and olefins with hydrogen peroxide. J. Mol. Catal. A Chem. 2008, 292, 28–35. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; So, M.H.; Che, C.M.; Wang, R.; Chen, R. One-pot solvothermal synthesis of Pd/Fe3O4 nanocomposite and its magnetically recyclable and efficient catalysis for Suzuki reactions. J. Mol. Catal. A Chem. 2012, 359, 81–87. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.K.C.; McEvoy, S. Novel Photocatalyst: Titania-Coated Magnetite. Activity and Photodissolution. J. Phys. Chem. B 2000, 104, 4387–4396. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Shi, A.-H.; Yang, Y.-S.; Li, C.-L. Impregnated copper on magnetite as catalyst for the O-arylation of phenols with aryl halides. Chin. Chem. Lett. 2014, 25, 141–145. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO-Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Nejati, K.; Ahmadi, S.; Nikpassand, M.; Kheirollahi Nezhad, P.D.; Vessally, E. Diaryl ethers synthesis: Nano-catalysts in carbon-oxygen cross-coupling reactions. RSC Adv. 2018, 8, 19125–19143. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.; Qi, H.; Zhao, W.; Chen, J.; Li, L.; Wu, Q. Phase transfer catalysis: Synthesis of monodispersed FePt nanoparticles and its electrocatalytic activity. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 312, 73–78. [Google Scholar] [CrossRef]
- Ames, L.L., Jr. The Cation Sieve Properties of Clinoptilolite. Am. Mineral. 1960, 45, 689–700. [Google Scholar]
- Hosseinzadeh, R.; Tajbakhsh, M.; Mohadjerani, M.; Alikarami, M. Copper-catalysed N-arylation of arylsulfonamides with aryl bromides and aryl iodides using KF/Al2O3. J. Chem. Sci. 2010, 122, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Elazab, H.A.; Sadek, M.A.; El-Idreesy, T.T. Microwave-assisted synthesis of palladium nanoparticles supported on copper oxide in aqueous medium as an efficient catalyst for Suzuki cross-coupling reaction. Adsorpt. Sci. Technol. 2018, 36, 1352–1365. [Google Scholar] [CrossRef]
- Panahi, F.; Daneshgar, F.; Haghighi, F.; Khalafi-Nezhad, A. Immobilized Pd nanoparticles on silica-starch substrate (PNP-SSS): Efficient heterogeneous catalyst in Buchwald–Hartwig C–N cross coupling reaction. J. Organomet. Chem. 2017, 851, 210–217. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Khorsandi, Z.; Fatemeh Mohammadi Metkazini, S. Palladium nanoparticles supported on cysteine-functionalized MNPs as robust recyclable catalysts for fast O- and N-arylation reactions in green media. J. Organomet. Chem. 2019, 899, 120793. [Google Scholar] [CrossRef]
- Veisi, H.; Safarimehr, P.; Hemmati, S. Buchwald–Hartwig C–N cross coupling reactions catalyzed by palladium nanoparticles immobilized on thio modified-multi walled carbon nanotubes as heterogeneous and recyclable nanocatalyst. Mater. Sci. Eng. C 2019, 96, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Tajbakhsh, M.; Alikarami, M. Copper-catalyzed N-arylation of diazoles with aryl bromides using KF/Al2O3: An improved protocol. Tetrahedron Lett. 2006, 47, 5203–5205. [Google Scholar] [CrossRef]
- Kim, K.D.; Kim, S.S.; Choa, Y.-H.; Kim, H.T. Formation and Surface Modification of Fe3O4 Nanoparticles by Co-precipitation and Sol-gel Method. J. Ind. Eng. Chem. 2007, 13, 1137–1141. [Google Scholar]
- Xie, X.; Cai, G.; Ma, D. CuI/L-proline-catalyzed coupling reactions of aryl halides with activated methylene compounds. Org. Lett. 2005, 7, 4693–4695. [Google Scholar] [CrossRef]
- Marcoux, J.F.; Doye, S.; Buchwald, S.L. A general copper-catalyzed synthesis of diaryl ethers. J. Am. Chem. Soc. 1997, 119, 10539–10540. [Google Scholar] [CrossRef]
- Cai, Q.; Zou, B.; Ma, D. Mild Ullmann-type biaryl ether formation reaction by combination of ortho-substituent and ligand effects. Angew. Chem.-Int. Ed. 2006, 45, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Song, Z.J.; Tschaen, D.; Dormer, P.G.; Volante, R.P.; Reider, P.J. Ullmann Diaryl Ether Synthesis: Rate Acceleration by 2,2,6,6-Tetramethylheptane-3,5-dione. Org. Lett. 2002, 4, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Sun, H.; Li, L.; Zhang, H. Ullmann reaction in tetraethyl orthosilicate: A novel synthesis of triarylamines and diaryl ethers. Chem. Commun. 2007, 3186–3188. [Google Scholar] [CrossRef]
- Miao, T.; Wang, L. Immobilization of copper in organic-inorganic hybrid materials: A highly efficient and reusable catalyst for the Ullmann diaryl etherification. Tetrahedron Lett. 2007, 48, 95–99. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q. N,N-Dimethyl Glycine-Promoted Ullmann Coupling Reaction of Phenols and Aryl Halides. Org. Lett. 2003, 5, 3799–3802. [Google Scholar] [CrossRef]
- Lv, X.; Bao, W. A β-keto ester as a novel, efficient, and versatile ligand for copper(I)-catalyzed C-N, C-O, and C-S coupling reactions. J. Org. Chem. 2007, 72, 3863–3867. [Google Scholar] [CrossRef] [PubMed]
- Bistri, O.; Correa, A.; Bolm, C. Iron-catalyzed C-O cross-couplings of phenols with aryl iodides. Angew. Chem.-Int. Ed. 2008, 47, 586–588. [Google Scholar] [CrossRef]
- Fagan, P.J.; Hauptman, E.; Shapiro, R.; Casalnuovo, A. Using intelligent/random library screening to design focused libraries for the optimization of homogeneous catalysts: Ullmann ether formation. J. Am. Chem. Soc. 2000, 122, 5043–5051. [Google Scholar] [CrossRef]
- Gujadhur, R.K.; Bates, C.G.; Venkataraman, D. Formation of aryl-nitrogen, aryl-oxygen, and aryl-carbon bonds using well-defined copper(I)-based catalysts. Org. Lett. 2001, 3, 4315–4317. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.B.; Jaseer, E.A.; Sekar, G. General, mild, and intermolecular Ullmann-type synthesis of diaryl and alkyl aryl ethers catalyzed by diol-copper(I) complex. J. Org. Chem. 2009, 74, 3675–3679. [Google Scholar] [CrossRef]
- Naidu, A.B.; Raghunath, O.R.; Prasad, D.J.C.; Sekar, G. An efficient BINAM-copper(II) catalyzed Ullmann-type synthesis of diaryl ethers. Tetrahedron Lett. 2008, 49, 1057–1061. [Google Scholar] [CrossRef]



| Entry | KF (g) | Fe3O4 (g) | KF/Fe3O4 (g:g) | Yield % [b] |
|---|---|---|---|---|
| 1 | 0.5 | 1 | 0.5 | 20 |
| 2 | 1 | 0.5 | 2 | 60 |
| 3 | 2 | 0.5 | 4 | 85 |
| 4 | 2.5 | 0.5 | 5 | 90 |
| 5 | 3 | 0.5 | 6 | 90 |
| Entry | Solvent | T (°C) | CuI (mol%) | L-Proline (mol%) | Yield (%) [b] |
|---|---|---|---|---|---|
| 1 | o-Xylene | Reflux | 15 | - | Trace |
| 2 | Toluene | Reflux | 15 | - | Trace |
| 3 | THF | Reflux | 15 | - | Trace |
| 4 | Dioxane | Reflux | 15 | - | 20 |
| 5 | DMSO | Reflux | 15 | - | 80 |
| 6 | DMF | Reflux | 15 | - | 90 |
| 7 | DMF | Reflux | 10 | 10 | 90 |
| 8 | DMF | Reflux | 5 | 10 | 60 |
| 9 | DMF | 120 | 15 | - | 90 |
| 10 | DMF | 120 | 10 | - | 90 |
| 11 | DMF | 80 | 15 | - | 30 |
| 12 | DMF | r.t. | 15 | - | Trace |
| Entry | Aryl Iodide | Nitrogenated Compound | Product | Time (h) | Yield (%) [b] |
|---|---|---|---|---|---|
| 1 | 1 | 2 | 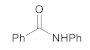 3 | 5 | 90 |
| 2 | 1 | 5 | 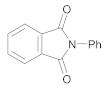 10 | 7 | 90 |
| 3 | 1 | 6 | 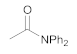 11 | 7 | 80 |
| 4 | 1 | 7 | 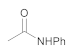 12 | 6 | 85 |
| 5 | 1 | 8 |  13 | 7 | 90 |
| 6 | 4 | 8 | 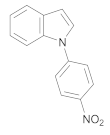 14 | 6 | 90 |
| 7 | 1 | 9 | 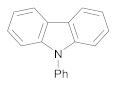 15 | 3 | 90 |
| 8 | 4 | 9 | 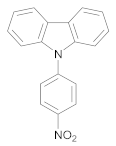 16 | 2 | 90 |
| Entry | Solvent | T (°C) | CuI (mol%) | L-Proline (mol%) | Yield (%) [b] |
|---|---|---|---|---|---|
| 1 | o-Xylene | Reflux | 15 | - | Trace |
| 2 | Toluene | Reflux | 15 | - | Trace |
| 3 | THF | Reflux | 15 | - | Trace |
| 4 | Dioxane | Reflux | 15 | - | 30 |
| 5 | DMSO | Reflux | 15 | - | 80 |
| 6 | DMF | Reflux | 15 | - | 95 |
| 7 | DMF | 120 | 15 | - | 95 |
| 8 | DMF | 80 | 15 | - | 30 |
| 9 | DMF | r.t. | 15 | - | Trace |
| 10 | DMF | 120 | 10 | - | 95 |
| 11 | DMF | 120 | 5 | 5 | 70 |
| 12 | DMF | 120 | 10 | 10 | 95 |
| Entry | Aryl Iodide | Phenol | Product | Time (h) | Yield (%) [b] |
|---|---|---|---|---|---|
| 1 | 1 | 22 | 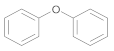 31 | 5 | 95 |
| 2 | 1 | 17 |  18 | 5 | 95 |
| 3 | 1 | 23 |  32 | 5 | 88 |
| 4 | 1 | 24 | 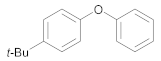 33 | 5.5 | 95 |
| 5 | 4 | 25 |  34 | 4 | 95 |
| 6 | 4 | 17 | 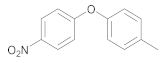 35 | 4 | 92 |
| 7 | 18 | 17 | 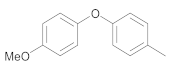 36 | 3.5 | 90 |
| 8 | 4 | 24 | 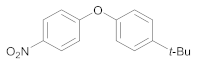 37 | 4 | 95 |
| 9 | 1 | 25 | 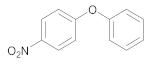 34 | 12 | 95 |
| Entry | Phenol | Product | Time (min) | Yield (%) [b] |
|---|---|---|---|---|
| 1 | 22 | 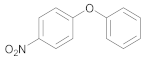 34 | 10 | 95 |
| 2 | 17 | 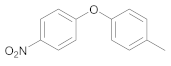 35 | 10 | 95 |
| 3 | 23 | 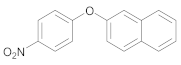 38 | 25 | 88 |
| 4 | 24 | 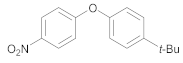 37 | 50 | 95 |
| 5 | 26 | 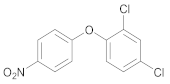 39 | 30 | 95 |
| 6 | 27 | 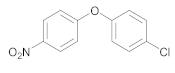 40 | 20 | 92 |
| 7 | 25 |  41 | 60 | 90 |
| 8 | 28 | 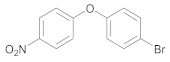 42 | 25 | 95 |
| 9 | 29 | 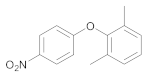 43 | 15 | 95 |
| 10 | 30 |  44 | 45 | 95 |
| Entry | Phenol | Product | Time (h) | Yield (%) [b] |
|---|---|---|---|---|
| 1 | 22 | 34 | 1.5 | 90 |
| 2 | 17 | 35 | 0.9 | 95 |
| 3 | 23 | 38 | 4.5 | 85 |
| 4 | 24 | 37 | 5 | 90 |
| 5 | 26 | 39 | 3 | 95 |
| 6 | 27 | 40 | 4.5 | 90 |
| 7 | 25 | 41 | 5 | 85 |
| 8 | 28 | 42 | 4.5 | 92 |
| 9 | 29 | 43 | 1.5 | 94 |
| 10 | 30 | 44 | 3 | 90 |
| Reaction Cycle | Yield (%) 3 [c] | Yield (%) 35 [c] |
|---|---|---|
| 1 | 90 | 95 |
| 2 | 90 | 95 |
| 3 | 88 | 90 |
| 4 | 84 | 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajbakhsh, M.; Ramezani, A.; Qandalee, M.; Falahati, M.; Durán-Valle, C.J.; Izquierdo, S.; López-Coca, I.M. Carbon–Heteroatom Bond Formation via Coupling Reactions Performed on a Magnetic Nanoparticle Bed. AppliedChem 2021, 1, 75-89. https://doi.org/10.3390/appliedchem1020007
Tajbakhsh M, Ramezani A, Qandalee M, Falahati M, Durán-Valle CJ, Izquierdo S, López-Coca IM. Carbon–Heteroatom Bond Formation via Coupling Reactions Performed on a Magnetic Nanoparticle Bed. AppliedChem. 2021; 1(2):75-89. https://doi.org/10.3390/appliedchem1020007
Chicago/Turabian StyleTajbakhsh, Mahmood, Ali Ramezani, Mohammad Qandalee, Mobina Falahati, Carlos J. Durán-Valle, Silvia Izquierdo, and Ignacio M. López-Coca. 2021. "Carbon–Heteroatom Bond Formation via Coupling Reactions Performed on a Magnetic Nanoparticle Bed" AppliedChem 1, no. 2: 75-89. https://doi.org/10.3390/appliedchem1020007
APA StyleTajbakhsh, M., Ramezani, A., Qandalee, M., Falahati, M., Durán-Valle, C. J., Izquierdo, S., & López-Coca, I. M. (2021). Carbon–Heteroatom Bond Formation via Coupling Reactions Performed on a Magnetic Nanoparticle Bed. AppliedChem, 1(2), 75-89. https://doi.org/10.3390/appliedchem1020007









