Impact of Global Warming on the Management of Mussel Fouling: Can the Use of Different Air Exposure Facilities Mitigate the Effects of Temperature? A Preliminary Experimental Trial in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea)
Abstract
1. Introduction
2. Materials and Methods
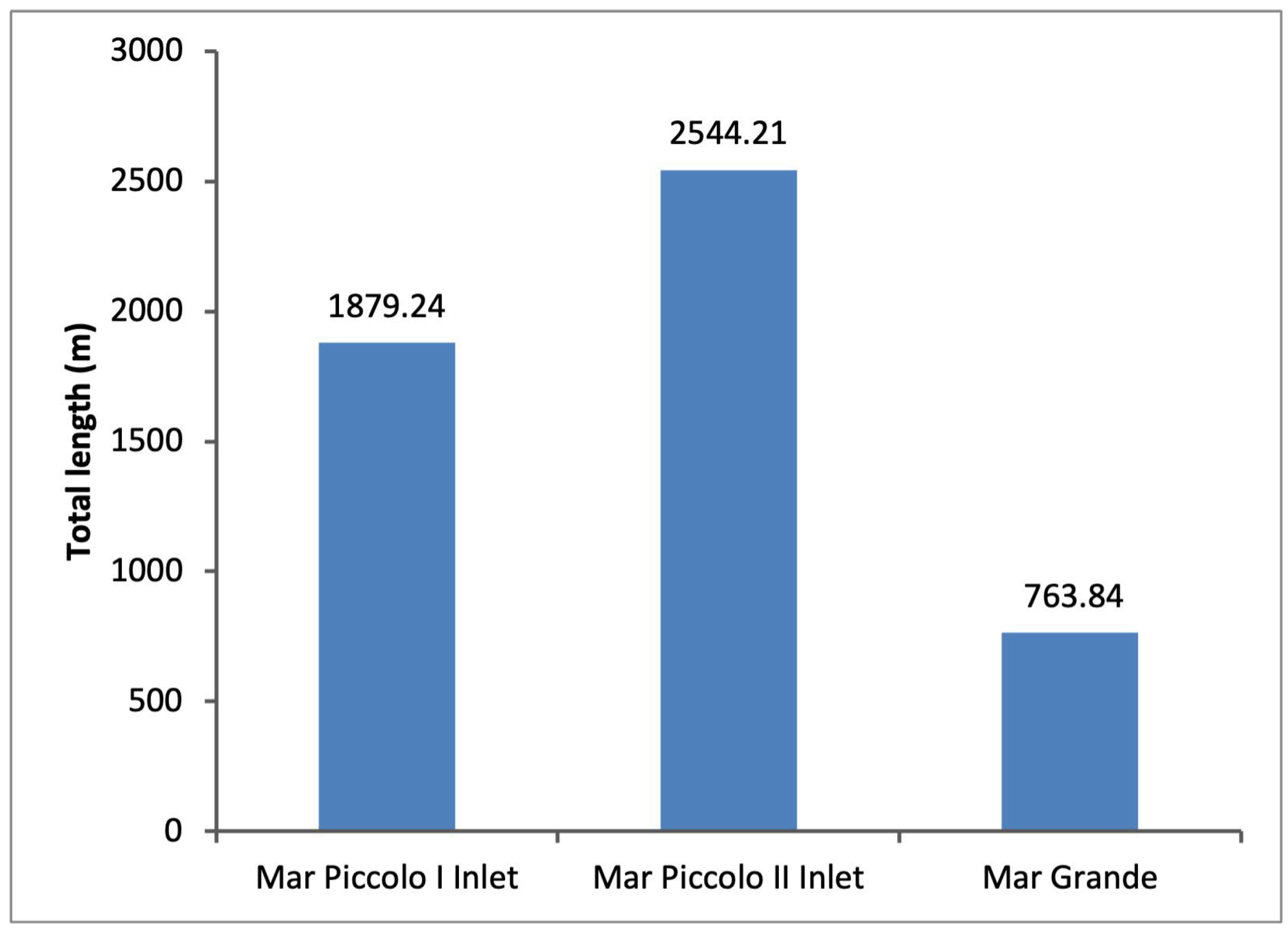
2.1. Study Area
2.2. Production Cycle in Mar Piccolo
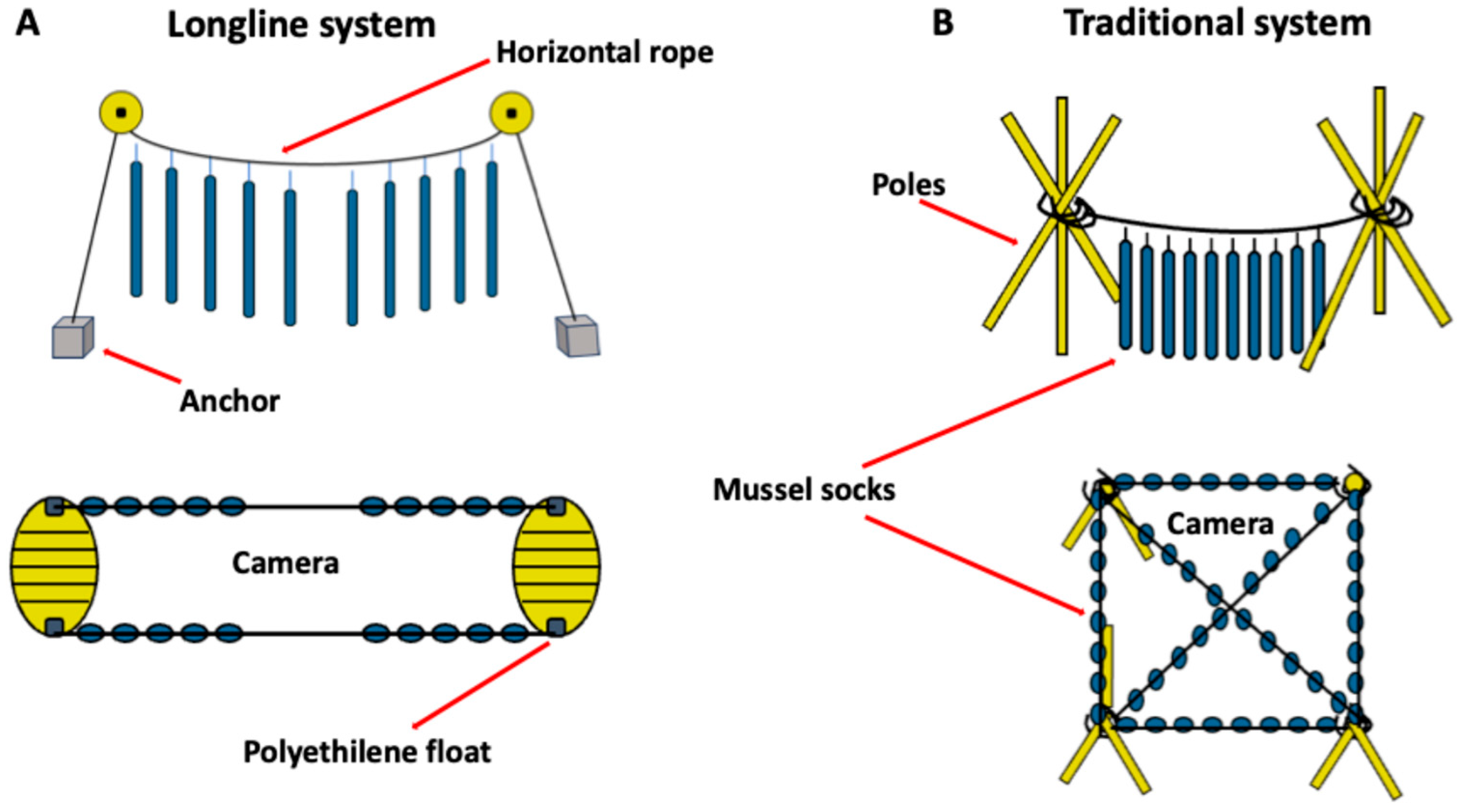
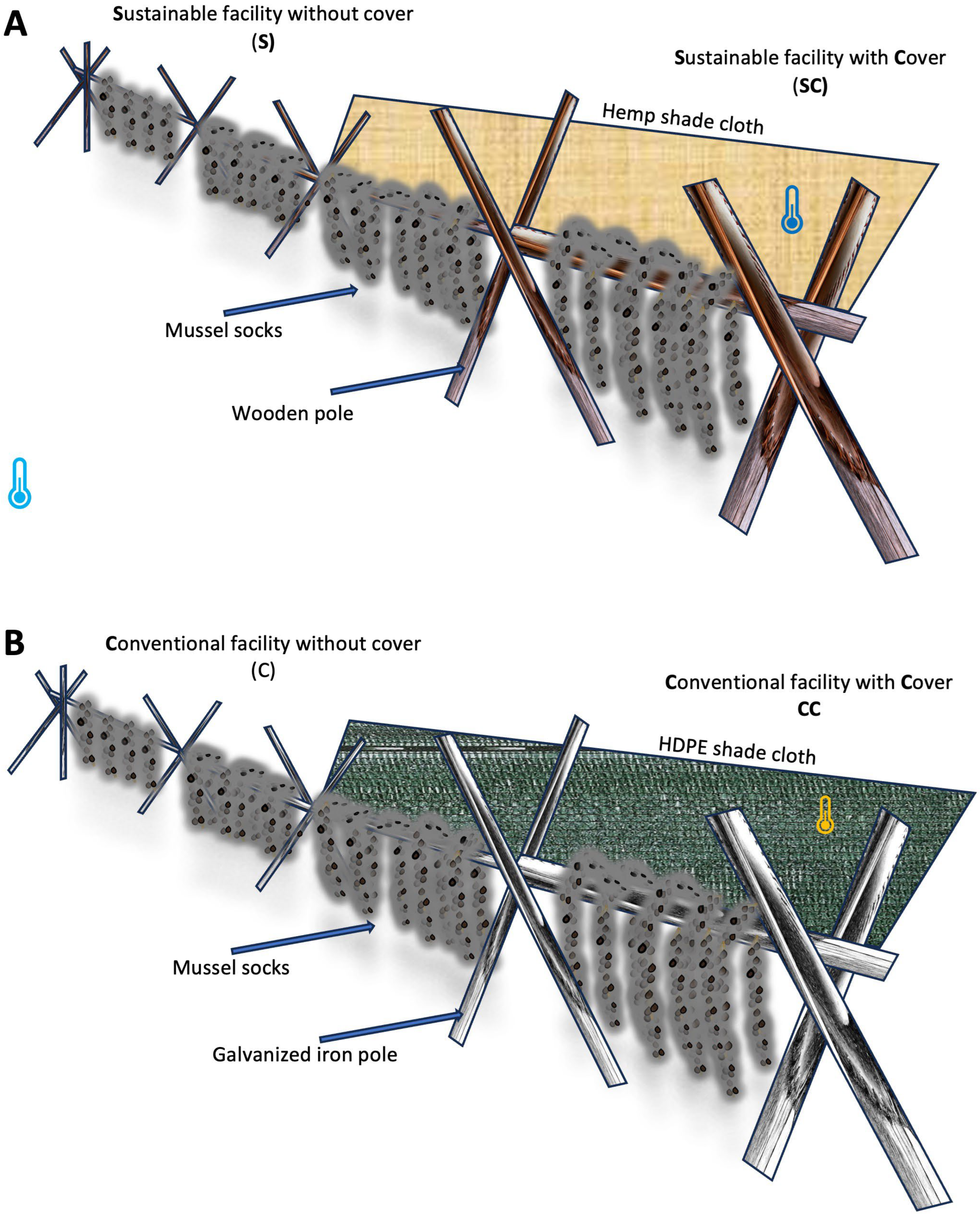
2.3. Experimental Facilities
2.4. Experimental Trial
2.5. Statistical Analysis
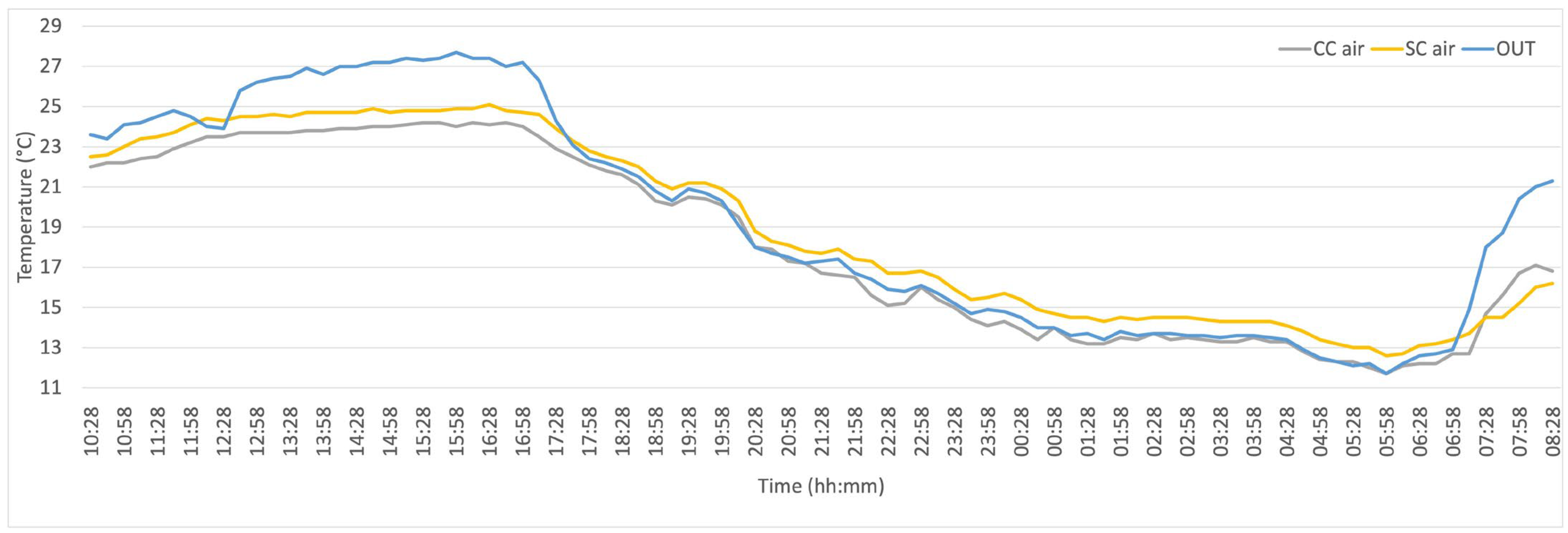
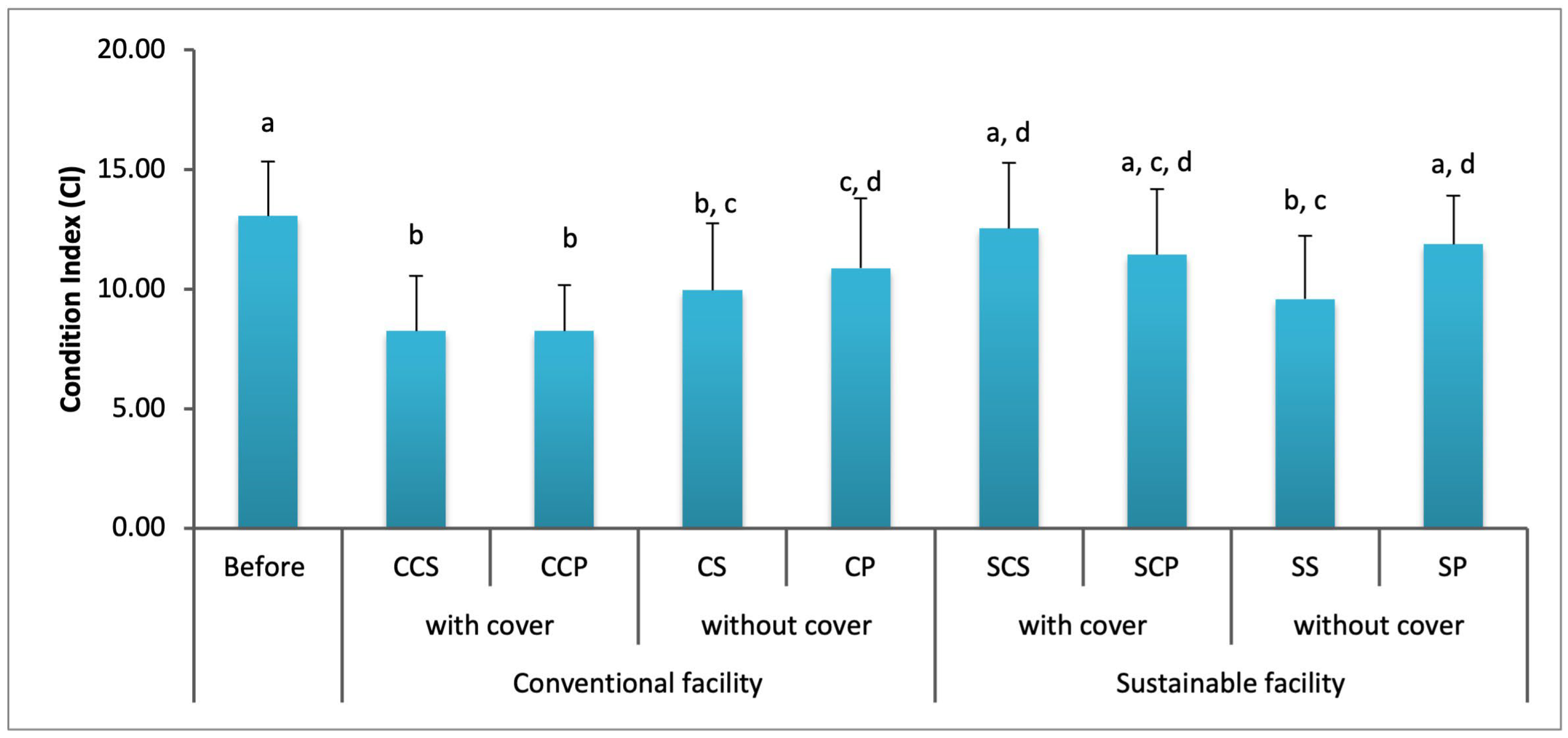
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.L.; Asche, F.; Garlock, T.; Chu, J. Aquaculture: Its role in the future of food: International food security. Front. Econ. Glob. 2017, 17, 59–173. [Google Scholar] [CrossRef]
- Veiga, P.; Moreira, J.; Sampaio, L.; Troncoso, J.S.; Rubal, M. Effects of habitat homogenisation on assemblages associated with mussel clumps. PLoS ONE 2022, 17, e0269308. [Google Scholar] [CrossRef]
- Yoo, C.; Wilms, T.; Stoehr, S.; Latuta, L.; Timmermann, K.; Molteson, M.; Svendsen, J. Mussel reefs promote taxonomic biodiversity and host a unique assemblage of mobile marine fauna in a coastal bay of poor ecological status. J. Sea Res. 2024, 202, 102544. [Google Scholar] [CrossRef]
- Timmermann, K.; Maar, M.; Bolding, K.; Larsen, J.; Nielsen, P.; Petersen, J.K. Mussel production as a nutrient mitigation tool for improving marine water quality. Aquacult. Environ. Interact. 2019, 11, 191–204. [Google Scholar] [CrossRef]
- EUMOFA—European Market Observatory for Fisheries and Aquaculture Products. Study on the Challenges of Aquaculture Products in Food Outlets. 2024. Available online: https://eumofa.eu/documents/20124/115068/Aquaculture+outlets+study+_final.pdf/5aa4920a-525b-6ad8-bbe9-cf32d8f25be6?t=1718282885395 (accessed on 16 June 2025).
- Avdelas, L.; Avdic-Mravlje, E.; Borges Marques, A.C.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Fernández Polanco, J.M.; et al. The decline of mussel aquaculture in the European Union: Causes, economic impacts and opportunities. Rev. Aquacult. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Ben Cheikh, Y.; Massol, F.; Giusti-Petrucciani, N.; Travers, M.A. Impact of epizootics on mussel farms: Insights into microbiota composition of Mytilus species. Microbiol. Res. 2024, 280, 127593. [Google Scholar] [CrossRef]
- EUMOFA—European Market Observatory for Fisheries and Aquaculture Products. Mussel in the EU. Price Structure in the Supply Chain. Focus on Spain, France, Italy and Ireland. 2022. Available online: https://eumofa.eu/documents/20124/35713/PTAT_Mussels_FV_EN.pdf/553ae567-b9a2-b6fc-0197-74e80eb6120a?version=1.0&t=1674035465415&download=true (accessed on 16 June 2025).
- Anestis, A.; Lazou, A.; Pörtner, H.O.; Michaelidis, B. Behavioural, metabolic and molecular stress indicators in the marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. 2007, 293, R911–R921. [Google Scholar] [CrossRef]
- Boni, R.; Gallo, A.; Montanino, M.; Macina, A.; Tosti, E. Dynamic changes in the sperm quality of Mytilus galloprovincialis under continuous thermal stress. Mol. Reprod. Dev. 2016, 83, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Hajer, A.; Sforzini, S.; Oliveri, C.; Boussetta, H.; Viarengo, A. Transcriptional expression levels and biochemical markers of oxidative stress in Mytilus galloprovincialis exposed to nickel and heat stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 160, 23–29. [Google Scholar] [CrossRef]
- Fearman, J.; Moltschaniwskyj, N. Warmer temperatures reduce rates of gametogenesis in temperate mussels, Mytilus galloprovincialis. Aquaculture 2010, 305, 20–25. [Google Scholar] [CrossRef]
- Nardi, A.; Mezzelani, M.; Costa, S.; d’Errico, G.; Benedetti, M.; Gorbi, S.; Freitas, R.; Regoli, F. Marine heatwaves hamper neuro-immune and oxidative tolerance toward carbamazepine in Mytilus galloprovincialis. Environ. Pollut. 2022, 300, 118970. [Google Scholar] [CrossRef]
- Jansen, J.M.; Hummel, H.; Bonga, S.W. The respiratory capacity of marine mussels (Mytilus galloprovincialis) in relation to the high temperature threshold. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 399–402. [Google Scholar] [CrossRef]
- Mesas, A.; Tarifeño, E. Upper lethal temperatures for the mussel Mytilus galloprovincialis (Lamarck, 1819), in central coast of Chile. Lat. Am. J. Aquat. Res. 2017, 43, 473–483. [Google Scholar] [CrossRef]
- Schneider, K.; Thiel, L.; Helmuth, B. Interactive effects of food availability and aerial body temperature on the survival of two intertidal Mytilus species. J. Therm. Biol. 2010, 35, 161–166. [Google Scholar] [CrossRef]
- Kamermans, P.; Saurel, C. Interacting climate change effects on mussels (Mytilus edulis and M. galloprovincialis) and oysters (Crassostrea gigas and Ostrea edulis): Experiments for bivalve individual growth models. Aquat. Living Resour. 2022, 35, 1. [Google Scholar] [CrossRef]
- De Marco, A.; Baldassarro, V.A.; Quadalti, C.; Burato, V.; Calzà, L.; Giardino, L.; Montroni, D.; Falini, G.; Graziani, G.; Greggio, N.; et al. Thermal tipping points in mediterranean mussel adhesion: Molecular mechanisms and mechanical consequences of heatwave on byssus production in Mytilus galloprovincialis. Sci. Rep. 2025, 15, 24752. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.; Attig, H.; Dagnino, A.; Boussetta, H.; Banni, M. Increased temperatures affect oxidative stress markers and detoxification response to benzo[a]pyrene exposure in mussel Mytilus galloprovincialis. Arch. Environ. Contam. Toxicol. 2012, 63, 534–543. [Google Scholar] [CrossRef]
- Coppola, F.; Henriques, B.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Freitas, R. Influence of temperature rise on the recovery capacity of Mytilus galloprovincialis exposed to mercury pollution. Ecol. Indic. 2018, 93, 1060–1069. [Google Scholar] [CrossRef]
- Lattos, A.; Papadopoulos, D.K.; Feidantsis, K.; Karagiannis, D.; Giantsis, I.A.; Michaelidis, B. Are Marine Heatwaves Responsible for Mortalities of Farmed Mytilus galloprovincialis? A Pathophysiological Analysis of Marteilia Infected Mussels from Thermaikos Gulf, Greece. Animals 2022, 12, 2805. [Google Scholar] [CrossRef]
- Balbi, T.; Bozzo, M.; Auguste, M.; Montagna, M.; Miglioli, A.; Drouet, K.; Vezzulli, L.; Canesi, L. Impact of ocean warming on early development of the Mediterranean mussel Mytilus galloprovincialis: Effects on larval susceptibility to potential vibrio pathogens. Fish Shellfish Immunol. 2024, 154, 109937. [Google Scholar] [CrossRef]
- Arduini, D.; Portacci, G.; Giangrande, A.; Acquaviva, M.I.; Borghese, J.; Calabrese, C.; Giandomenico, S.; Quarta, E.; Stabili, L. Growth Performance of Mytilus galloprovincialis Lamarck, 1819 under an Innovative Integrated Multi-Trophic Aquaculture System (IMTA) in the Mar Grande of Taranto (Mediterranean Sea, Italy). Water 2023, 15, 1922. [Google Scholar] [CrossRef]
- Bracchetti, L.; Capriotti, M.; Fazzini, M.; Cocci, P.; Palermo, F.A. Mass Mortality Event of Mediterranean Mussels (Mytilus galloprovincialis) in the Middle Adriatic: Potential Implications of the Climate Crisis for Marine Ecosystems. Diversity 2024, 16, 130. [Google Scholar] [CrossRef]
- Estaque, T.; Richaume, J.; Bianchimani, O.; Schull, Q.; Mérigot, B.; Bensoussan, N.; Bonhomme, P.; Vouriot, P.; Sartoretto, S.; Monfort, T.; et al. Marine heatwaves on the rise: One of the strongest ever observed mass mortality event in temperate gorgonians. Glob. Change Biol. 2023, 29, 6159–6162. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Change Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Masanja, F.; Yang, K.; Xu, Y.; He, G.; Liu, X.; Xu, X.; Xiaoyan, J.; Xin, L.; Mkuye, R.; Deng, Y.; et al. Impacts of marine heat extremes on bivalves. Front. Mar. Sci. 2023, 10, 1159261. [Google Scholar] [CrossRef]
- Kralj, M.; De Vittor, C.; Comici, C.; Relitti, F.; Auriemma, R.; Alabiso, G.; Del Negro, P. Recent evolution of the physical–chemical characteristics of a Site of National Interest—The Mar Piccolo of Taranto (Ionian Sea)—And changes over the last 20 years. Environ. Sci. Pollut. Res. 2016, 23, 12675–12690. [Google Scholar] [CrossRef]
- Caroppo, C.; Giordano, L.; Palmieri, N.; Bellio, G.; Bisci, A.P.; Portacci, G.; Sclafani, P.; Hopkins, T.S. Progress Toward Sustainable Mussel Aquaculture in Mar Piccolo, Italy. Ecol. Soc. 2012, 17, 10. [Google Scholar] [CrossRef]
- Massarelli, C.; Galeone, C.; Savino, I.; Campanale, C.; Uricchio, V.F. Towards Sustainable Management of Mussel Farming through High-Resolution Images and Open Source Software—The Taranto Case Study. Remote Sens. 2021, 13, 2985. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale n. 291 del 14 Dicembre 1998. Legge n. 426/1998. Nuovi Interventi in Campo Ambientale. Available online: https://www.mase.gov.it/portale/documents/d/guest/legge_09121998_426-pdf (accessed on 18 June 2025).
- Cotecchia, F.; Vitone, C.; Sollecito, F.; Mali, M.; Miccoli, D.; Petti, R.; Milella, D.; Ruggieri, G.; Bottiglieri, O.; Santaloia, F.; et al. A geo-chemo-mechanical study of a highly polluted marine system (Taranto, Italy) for the enhancement of the conceptual site model. Sci. Rep. 2021, 11, 4017. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 1259/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Dioxins, Dioxin-Like PCBs and Non Dioxin-like PCBs in Foodstuffs Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2011/1259/oj/eng (accessed on 18 June 2025).
- Giordano, L.; Portacci, G.; Caroppo, C. Multidisciplinary tools for sustainable management of an ecosystem service: The case study of mussel farming in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea). Ocean Coast. Manag. 2019, 176, 11–23. [Google Scholar] [CrossRef]
- CMCC. Marine Heat Wave in the Mediterranean: Observations and Predictions. 2022. Available online: https://www.cmcc.it/article/marine-heat-wave-in-the-mediterranean-observations-and-predictions (accessed on 18 June 2025).
- CMCC. Over 30 °C: Marine Heatwaves Currently Hitting the Mediterranean. 2023. Available online: https://www.cmcc.it/article/over-30c-marine-heatwaves-currently-hitting-the-mediterranean (accessed on 18 June 2025).
- Levina, E.; Tirpak, T.; Adaptation to Climate Change: Key Terms. OECD and International Energy Agency COM/ENV/EPOC/IEA/SLT(2006)1. Available online: https://www.oecd.org/content/dam/oecd/en/publications/reports/2006/01/adaptation-to-climate-change_83dd1f1c/326a53e2-en.pdf (accessed on 20 June 2025).
- Santacroce, M.P.; Conversano, M.C.; Vlora, A.; Colao, V.; Centoducati, G. The impact of hangingcleaning husbandry practices on Mediterranean mussels, Mytilus galloprovincialis Lmk, cultivated in the Mar Piccolo (Taranto, Ionian Sea, Italy). Ital. J. Anim. Sci. 2008, 7, 449–464. [Google Scholar] [CrossRef]
- Andrady, A. Weathering and fragmentation of plastic debris in the ocean environment. Mar. Pollut. Bull. 2022, 180, 113761. [Google Scholar] [CrossRef]
- Zuffianò, L.E.; Basso, A.; Casarano, D.; Dragone, V.; Limoni, P.P.; Romanazzi, A.; Polemio, M. Coastal hydrogeological system of Mar Piccolo (Taranto, Italy). Environ. Sci. Pollut. Res. 2016, 23, 12502–12514. [Google Scholar] [CrossRef]
- Interreg Greece-Italy. Fish & Chips—Fisheries and Cultural Heritage, Identity and Participated Societies. Available online: https://2014-2020.greece-italy.eu/rlb-funded-projects/fishchips/ (accessed on 20 June 2025).
- Caroppo, C.; Portacci, G.; Giordano, L. Produzione di serie storiche con il telerilevamento satellitare: Uno strumento innovativo per la gestione sostenibile della molluschicoltura? Biol. Mar. Mediterr. 2018, 25, 64–67. [Google Scholar]
- Noor, A.R.; Shakil, A.; Hoque, N.F.; Rahman, M.M.; Akter, S.; Talukder, A.; Ahmad-Al-Nahid, S.; Wahab, M.A.; Nahiduzzaman, M.; Rahman, M.J.; et al. Effect of eco-physiological factors on biometric traits of green mussel Perna viridis cultured in the south-east coast of the Bay of Bengal, Bangladesh. Aquac. Rep. 2021, 19, 100562. [Google Scholar] [CrossRef]
- Boscolo, R.; Cornello, M.; Giovanardi, O. A condition index applied to mussel cultured mussel in the Northen Adriatic: A preliminary study. Biol. Mar. Mediterr. 2002, 11, 243–254. [Google Scholar]
- StatsCalculators Team. Three-Way ANOVA Calculator. StatsCalculators. 2025. Available online: https://www.statscalculators.com/calculators/hypothesis-testing/three-way-anova-calculator (accessed on 8 July 2025).
- Orban, E.; Di Lena, G.; Nevigato, T.; Casini, I.; Marzetti, A.; Caproni, R. Seasonal changes in meat content, condition index and chemical composition of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem. 2002, 77, 57–65. [Google Scholar] [CrossRef]
- Pantea, E.-D.; Oros, A.; Rosioru, D.M.; Rosoiu, N. Condition Index of Mussel Mytilus Galloprovincialis (Lamarck, 1819) as a Physiological Indicator of Heavy Metals Contamination. Ann. Acad. Rom. Sci. Ser. Biol. Sci. 2020, 9, 20–36. [Google Scholar] [CrossRef]
- Andrade, M.; Soares, A.; Figueira, E.; Freitas, R. Biochemical changes in mussels submitted to different time periods of air exposure. Environ. Sci. Pollut. Res. 2018, 25, 8903–8913. [Google Scholar] [CrossRef]
- Andrade, M.; De Marchi, L.; Soares, A.M.V.M.; Rocha, R.J.M.; Figueira, E.; Freitas, R. Are the effects induced by increased temperature enhanced in Mytilus galloprovincialis submitted to air exposure? Sci. Total Environ. 2019, 647, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.D.; Toone, T.A.; Hillman, J.R.; Handley, S.J.; Jeffs, A. Aerial exposure and critical temperatures limit the survival of restored intertidal mussels. Restor. Ecol. 2024, 32, e14105. [Google Scholar] [CrossRef]
- Seuront, L.; Nicastro, K.R.; Zardi, G.I.; Goberville, E. Decreased thermal tolerance under recurrent heat stress conditions explains summer mass mortality of the blue mussel Mytilus edulis. Sci. Rep. 2019, 9, 17498. [Google Scholar] [CrossRef] [PubMed]
- Helmuth, B.S.T. Intertidal mussel microclimates: Predicting the body temperature of a sessile invertebrate. Ecol. Monog. 1998, 68, 51–74. [Google Scholar] [CrossRef]
- Dowd, W.W.; Somero, G.N. Behavior and survival of Mytilus congeners following episodes of elevated body temperature in air and seawater. J. Exp. Biol. 2013, 216 Pt 3, 502–514. [Google Scholar] [CrossRef]
- Georgoulis, I.; Bock, C.; Lannig, G.; Pörtner, H.O.; Feidantsis, K.; Giantsis, I.A.; Sokolova, I.M.; Michaelidis, B. Metabolic remodeling caused by heat hardening in the Mediterranean mussel Mytilus galloprovincialis. J. Exp. Biol. 2022, 225, jeb244795. [Google Scholar] [CrossRef]
- Bordignon, F.; Bertolini, C.; Bernardini, I.; Rovere, G.; Iori, S.; Breggion, C.; Pastres, R.; Boffo, L.; Xiccato, G.; Matozzo, V.; et al. Spatio-temporal variations of growth, chemical composition, and gene expression in Mediterranean mussels (Mytilusgalloprovincialis): A two-year study in the Venice lagoon under anthropogenic and climate changing scenarios. Aquaculture 2023, 578, 740111. [Google Scholar] [CrossRef]



| Experimental Units | Treatments | Abbreviation |
|---|---|---|
| Conventional facility without cover (C) | on pole (P) | CP |
| suspended (S) | CS | |
| Conventional facility with cover (CC) | on pole (P) | CCP |
| suspended (S) | CCS | |
| Sustainable facility without cover (S) | on pole (P) | SP |
| suspended (S) | SS | |
| Sustainable facility with cover (SC) | on pole (P) | SCP |
| suspended (S) | SCS |
| Mean ± S.D. | Minimum | Maximum | ||
|---|---|---|---|---|
| Air temperature (°C) | OUT | 19.3 ± 5.4 | 11.7 | 27.7 |
| CC | 18.1 ± 4.5 | 11.7 | 24.2 | |
| SC | 18.9 ± 4.5 | 12.6 | 25.1 |
| SS | df | Mean Squares | F | p-Value | |
|---|---|---|---|---|---|
| Between groups: | 0.0956281 | 8 | 0.0119535 | 18.61 | <0.001 |
| Within groups: | 0.224137 | 349 | 0.0006422 | ||
| Total: | 0.319765 | 367 | 0.00001 |
| Source | SS | df | F | p-Value |
|---|---|---|---|---|
| Facility | 0.033 | 1 | 51.3841 | <0.001 |
| Cover | 0.0016 | 1 | 2.4699 | 0.1171 |
| Mussel sock section | 0.0022 | 1 | 3.4283 | 0.065 |
| Facility × Cover | 0.0232 | 1 | 36.1235 | <0.001 |
| Facility × Mussel sock section | 0 | 1 | 0.0509 | 0.8216 |
| Cover × Mussel sock section | 0.0093 | 1 | 14.4833 | <0.001 |
| Facility × Cover × Mussel sock section | 0.0031 | 1 | 4.8041 | <0.05 |
| Residual | 0.1999 | 311 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portacci, G.; Parlapiano, I.; Narracci, M.; Di Leo, A. Impact of Global Warming on the Management of Mussel Fouling: Can the Use of Different Air Exposure Facilities Mitigate the Effects of Temperature? A Preliminary Experimental Trial in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea). Aquac. J. 2025, 5, 24. https://doi.org/10.3390/aquacj5040024
Portacci G, Parlapiano I, Narracci M, Di Leo A. Impact of Global Warming on the Management of Mussel Fouling: Can the Use of Different Air Exposure Facilities Mitigate the Effects of Temperature? A Preliminary Experimental Trial in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea). Aquaculture Journal. 2025; 5(4):24. https://doi.org/10.3390/aquacj5040024
Chicago/Turabian StylePortacci, Giuseppe, Isabella Parlapiano, Marcella Narracci, and Antonella Di Leo. 2025. "Impact of Global Warming on the Management of Mussel Fouling: Can the Use of Different Air Exposure Facilities Mitigate the Effects of Temperature? A Preliminary Experimental Trial in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea)" Aquaculture Journal 5, no. 4: 24. https://doi.org/10.3390/aquacj5040024
APA StylePortacci, G., Parlapiano, I., Narracci, M., & Di Leo, A. (2025). Impact of Global Warming on the Management of Mussel Fouling: Can the Use of Different Air Exposure Facilities Mitigate the Effects of Temperature? A Preliminary Experimental Trial in the Mar Piccolo of Taranto (Mediterranean, Ionian Sea). Aquaculture Journal, 5(4), 24. https://doi.org/10.3390/aquacj5040024




