Evaluation of Individual Rearing of a Genetically Improved Giant River Prawn Macrobrachium rosenbergii Broodstock as an Alternate Approach to Group Rearing During the Post-Selection Rearing Phase
Abstract
1. Introduction
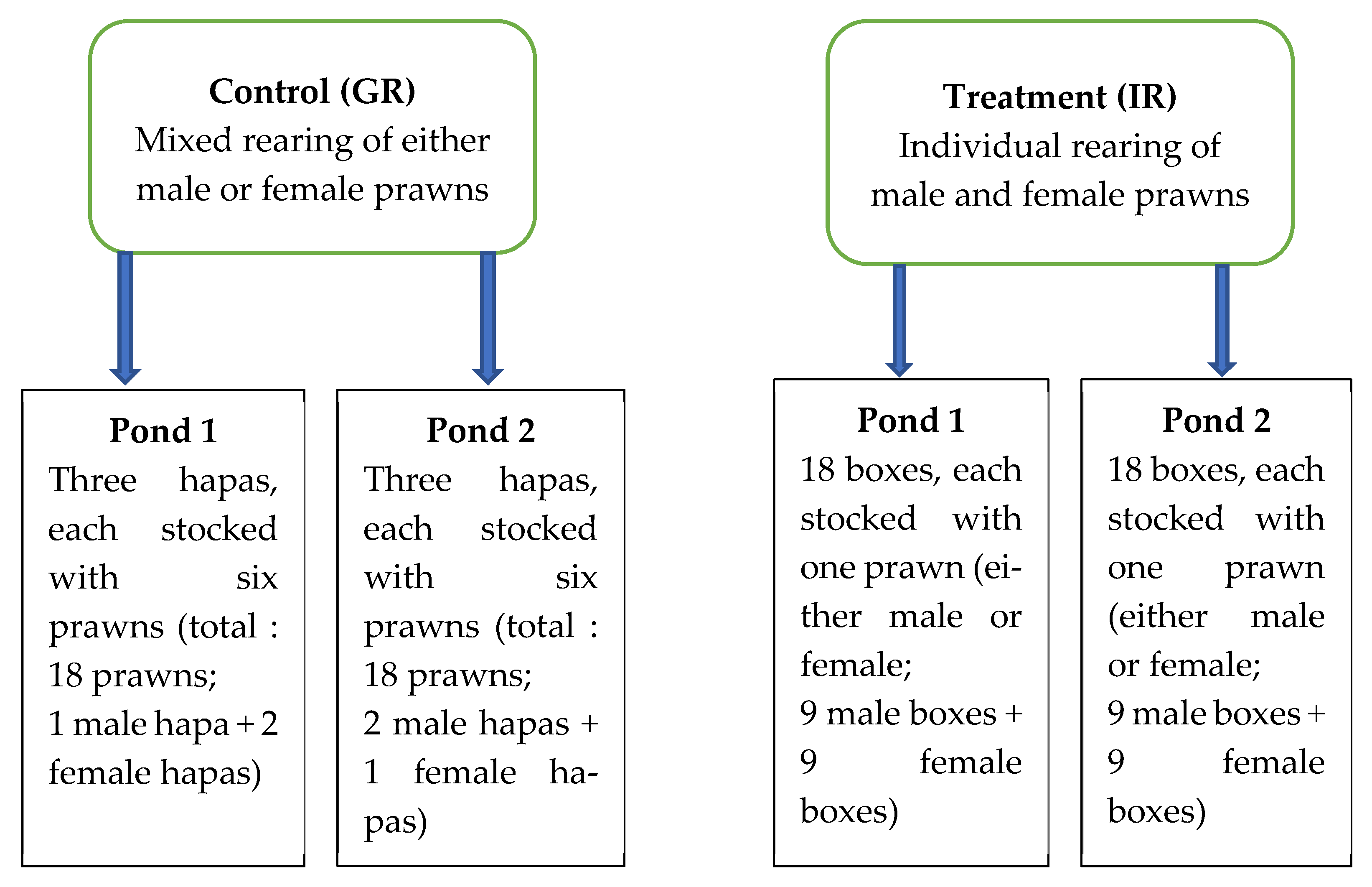
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- New, M.B. Farming freshwater prawn. A manual for the culture of the giant river prawn, Macrobrachium rosenbergii. In FAO Fisheries Technical Paper 428; FAO: Rome, Italy, 2002. [Google Scholar]
- Pillai, B.R.; Panda, D. Global status of Giant prawn, Macrobrachium rosenbergii farming with special reference to India and measures for enhancing production. J. Aquac. 2024, 33, 1–14. [Google Scholar] [CrossRef]
- Pillai, B.R.; Ponzoni, W.R.; Mahapatra, K.D.; Panda, D. Genetic improvement of giant freshwater prawn Macrobrachium rosenbergii: A review of global status. Rev. Aquac. 2022, 14, 1285–1299. [Google Scholar] [CrossRef]
- FAO. FishStat: Global aquaculture production 1950–2023. In FishStatJ; FAO: Rome, Italy, 2025; Available online: www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 30 May 2025).
- Gjedrem, T. Genetic improvement of coldwater fish species. Aquac. Res. 2000, 31, 25–33. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Rodriguez, B.M., Jr. Considerations about effective dissemination of improved fish strains. In WorldFish Working Paper; WorldFish: Penang, Malaysia, 2012. [Google Scholar]
- Eknath, A.E.; Bentsen, H.B.; Ponzoni, R.W.; Rye, M.; Nguyen, H.N.; Thodesen, J.; Gjerde, B. Genetic improvement of farmed tilapias: Composition and genetic parameters of a synthetic base population of Oreochromis niloticus for selective breeding. Aquaculture 2007, 273, 1–14. [Google Scholar] [CrossRef]
- Mahapatra, K.D.; Jana, R.K.; Saha, J.N.; Gjerde, B.; Sarangi, N. Lessons from the breeding program of Rohu. In Development of Aquatic Animal Genetic Improvement and Dissemination Programs: Current Status and Action Plans; Ponzoni, R.W., Acosta, B.O., Ponniah, A.G., Eds.; WorldFsih Center: Penang, Malaysia, 2006; pp. 34–40. [Google Scholar]
- Argue, B.J.; Arce, S.M.; Lotz, J.M.; Moss, S.M. Selective breeding of Pacific white shrimp, Litopenaeus vannamei for growth and resistance to Taura Syndrome Virus. Aquaculture 2002, 204, 447–460. [Google Scholar] [CrossRef]
- Pillai, B.R.; Mahapatra, K.D.; Ponzoni, R.W.; Sahoo, L.; Lalrinsanga, P.L.; Nguyen, N.H.; Mohanty, S.; Sahu, S.; Kumar, V.; Sahu, S.; et al. Genetic evaluation of a complete diallel cross involving three populations of freshwater prawn (Macrobrachium rosenbergii) from different geographical regions of India. Aquaculture 2011, 319, 347–354. [Google Scholar] [CrossRef]
- Pillai, B.R.; Lalrinsanga, P.L.; Ponzoni, R.W.; Khaw, H.L.; Das Mohapatra, K.; Mohanty, S.; Patra, G.; Naik, N.; Pradhan, H.; Jayasankar, P. Phenotypic and genetic parameters for body traits in the giant freshwater prawn (Macrobrachium rosenbergii) in India. Aquac. Res. 2017, 48, 5741–5750. [Google Scholar] [CrossRef]
- Pillai, B.R.; Lalrinsanga, P.L.; Ponzoni, R.W.; Khaw, H.L.; Mahapatra, K.D.; Sahu, S.; Mohanty, S.; Patra, G.; Naik, N.; Pradhan, H. Selective breeding of giant freshwater prawn (Macrobrachium rosenbergii) in India: Response to selection for harvest body weight and on-farm performance evaluation. Aquac. Res. 2020, 51, 4874–4880. [Google Scholar] [CrossRef]
- Smith, T.I.J.; Sandifer, P.A. Population structure of Malaysian prawn Macrobrachium rosenbergii (de Man) reared in earthen ponds in South California, 1974–1976. Proc. World Maric. Soc. 1978, 9, 21–38. [Google Scholar]
- Sandifer, P.A.; Smith, T.I.J.; Stokes, A.D.; Jenkins, W.E. Semi-intensive grow-out of prawns (Macrobrachium rosenbergii): Preliminary results and prospects. In Giant Prawn Farming, Developments in Aquaculture and Fisheries; New, M.B., Ed.; Elsevier Scientific Publishing: Amsterdam, The Netherlands, 1982; Volume 10, pp. 161–172. [Google Scholar]
- D’Abramo, L.R.; Robinette, H.R.; Heinen, J.M.; Ra’anan, Z.; Cohen, D. Polyculture of the freshwater prawn (Macrobrachium rosenbergii) with a mixed-size population of channel Catfish (Ictalurus punctatus). Aquaculture 1986, 59, 71–80. [Google Scholar] [CrossRef]
- Karplus, I.; Hulata, G.; Wohlfarth, G.W.; Halvey, A. The effect of density of Macrobrachium rosenbergii raised in earthen ponds on their population structure and weight distribution. Aquaculture 1986, 52, 307–320. [Google Scholar] [CrossRef]
- Siddiqui, A.Q.; Hafedh, Y.S.A.; Harbi, A.H.A.; Ali, S.A. Effects of stocking density and monosex culture of freshwater prawn Macrobrachium rosenbergii on growth and production in concrete tanks in Saudi Arabia. J. Aqua. Soc. 1997, 28, 106–112. [Google Scholar] [CrossRef]
- Jose, S.; Joseph, A.; Shyama, S.; Mohan, M.V.; Nair, C.M. Production trends of the giant freshwater prawn, Macrobrachium rosenbergii (De Man) in two culture strategies. In Proceedings of the International Symposium on Freshwater Prawns; Nair, C.M., Nambudiri, D.D., Jose, S., Sankaran, T.M., Jayachandran, K.V., Salin, K.R., Eds.; Allied Publishers: New Delhi, India, 2007; pp. 404–409. [Google Scholar]
- Peebles, J.B. Molting and mortality in Macrobrachium rosenbergii. Proc. World Maric. Soc. 1978, 9, 39–46. [Google Scholar] [CrossRef]
- Hopkins, K.D. Reporting fish growth: A review of the basics. J. World Aquac. Soc. 1992, 23, 173–179. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; 541p. [Google Scholar]
- New, M.B.; Valenti, W.C. Freshwater Prawn Culture: The Farming of Macrobrachium rosenbergii; Blackwell Science: Oxford, UK, 2000; 443p. [Google Scholar]
- Hung, D.; Nguyen, N.H.; Ponzoni, R.W.; Hurwood, D.A.; Mather, P.B. Quantitative genetic parameter estimates for body and carcass traits in a cultured stock of giant freshwater prawn (Macrobrachium rosenbergii) selected for harvest weight in Vietnam. Aquaculture 2013, 404–405, 122–129. [Google Scholar] [CrossRef]
- Luan, S.; Yang, G.; Wang, J.; Luo, K.; Zhang, Y.; Gao, Q.; Hu, H.; Kong, J. Genetic parameters and response to selection for harvest body weight of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 2012, 262–263, 88–96. [Google Scholar] [CrossRef]
- Ra’anan, Z.; Cohen, D. The ontogeny of social structure in the freshwater prawn Macrobrachium rosenbergii. In Crustaceans Issues 2: Crustacean Growth; Wenner, A., Schram, F.R., Eds.; A. A. Balkema: Rotterdam, The Netherlands, 1985; pp. 277–311. [Google Scholar]
- Ranjeet, K.; Kurup, B. Heterogeneous individual growth of Macrobrachium rosenbergii male morphotypes. Naga 2002, 25, 13–18. [Google Scholar]
- Karplus, I. Social control of growth in Macrobrachium rosenbergii (De Man): A review and prospects for future research. Aquac. Res. 2005, 36, 238–254. [Google Scholar] [CrossRef]
- Rahman, S.M.S.; Islam, M.A.; Wahab, M.A.; Banu, M.R.; Kunda, M.; Azim, M.E. Effects of stocking density of all male Macrobrachium rosenbergii (De Man, 1879) in polyculture ponds on production and economics. Asian Fish. Sci. 2012, 25, 133–143. [Google Scholar] [CrossRef]
- Nagarathinam, N.; Kumar, J.S.S.; Sundararaj, V. Influence of stocking density on growth, production and survival of Macrobrachium rosenbergii (de man) in a monoculture grow-out pond. Indian J. Fish. 2000, 47, 103–108. [Google Scholar]
- Paul, P.; Rahman, A.; Hossain, M.M.; Islam, S.; Mondal, S.; Haq, S. Effect of stocking density on the growth and production of freshwater prawn (Macrobrachium rosenbergii). Int. J. Fish. Aquac. Sci. 2016, 6, 77–86. [Google Scholar]
- Sui, J.; Luan, S.; Yang, G.; Xia, Z.; Tang, Q.; Luo, K.; Meng, X.; Kong, J. Effects of the individual rearing stage on the growth traits of candidate giant freshwater prawns (Macrobrachium rosenbergii). Aquac. Int. 2022, 30, 1659–1673. [Google Scholar] [CrossRef]
- Nair, C.M.; Salin, K.R.; Raju, M.S.; Sebastian, M. Economic analysis of monosex culture of giant freshwater prawn (Macrobrachium rosenbergii de man): A case study. Aquac. Res. 2006, 37, 949–954. [Google Scholar] [CrossRef]
- New, M.B.; Valenti, W.C.; Tidwell, J.H.; D’Abramo, L.R.; Kutty, M.N. (Eds.) Freshwater Prawns: Biology and Farming; John Wiley & Sons: Bognor Regis, UK, 2009. [Google Scholar]
- Ra’anan, Z.; Sagi, A.; Wax, Y.; Karplus, I.; Hulata, G.; Kuris, A. Growth, size rank, and maturation of the freshwater prawn, Macrobrachium rosenbergii: Analysis of marked prawns in an experimental population. Biol. Bull. 1991, 181, 379–386. [Google Scholar] [CrossRef]
- Karplus, I.; Hulata, G. Social control of growth in Macrobrachium rosenbergii. Isr. J. Aquacult. 1992, 44, 148. [Google Scholar] [CrossRef]
- Karplus, I.; Sagi, A. The biology and management of size variation. In Freshwater Prawns: Biology and Farming; New, M.B., Valenti, W.C., Tidwell, J.H., D’Abramo, L.R., Kutty, M.N., Eds.; John Wiley & Sons: Bognor Regis, UK, 2009; pp. 316–345. [Google Scholar]
- Karplus, I.; Malecha, S.; Sagi, A. The biology and management of size variation. In Freshwater Prawn Culture; New, M.B., Valenti, W.C., Eds.; Blackwell Science: New York, NY, USA, 2000; pp. 259–289. [Google Scholar]
- D’Abramo, L.R.; Heinen, J.M.; Robinett, H.R.; Collins, J.S. Production of the freshwater prawn Macrobrachium rosenbergii stocked as juveniles at different. densities in temperature zone ponds. J. Aqua. Soc. 1989, 20, 81–89. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Webster, C.D.; Tiu, L.G.; D’Abramo, L.R. Population characteristics of Macrobrachium rosenbergii fed diets containing different protein sources under cool water conditions in earthen ponds. Aquaculture 1994, 126, 271–281. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Coyle, S.D.; Schulmeister, G. Effects of added substrate on the production and population characteristics of freshwater prawns Macrobrachiurn rosenbergii in ponds. J. World Aquac. Soc. 1998, 29, 17–22. [Google Scholar] [CrossRef]
- Pillai, B.R.; Mahapatra, K.D.; Ponzoni, R.W.; Sahoo, L.; Lalrinsanga, P.L.; Mekkawy, W.; Khaw, H.L.; Nguyen, N.H.; Mohanty, S.; Sahu, S.; et al. Survival male morphotypes and female proportion, female reproductive status and tag loss in crosses among three populations of freshwater prawn Macrobrachium rosenbergii (de Man) in India. Aquac. Res. 2015, 46, 2644–2655. [Google Scholar] [CrossRef]
- Aflalo, E.D.; Raju, D.V.S.N.; Bommi, N.A.; Verghese, J.T.; Samraj, T.Y.C.; Hulata, G.; Ovadia, O.; Sahi, A. Towards a sustainable production of genetically improved all-male prawn (Macrobrachium rosenbergii): Evaluation of production traits and obtaining neo-females in three Indian strains. Aquaculture 2012, 338, 197–207. [Google Scholar] [CrossRef]
| Parameters | Group Rearing (GR-Control) | Individual Rearing (IR-Treatment) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Initial weight (g) (Mean ± SD) | 24.39 ± 4.78 | 25.00 ± 2.95 | 21.50 ± 3.13 | 21.39 ± 3.24 |
| Final weight (g) (Mean ± SD) | 56.11 ± 2.571 | 46.93 ± 13.41 | 43.50 ± 7.79 | 32.67 ± 5.96 |
| Duration of experiment (d) | 70 | 70 | 70 | 70 |
| Weight gain (g) | 33.33 ± 2.83 c | 21.71 ± 6.90 b | 22 ± 7.43 b | 11.28 ± 4.44 a |
| Weight gain % | 134.42 ± 27.37 c | 88.55 ± 25.02 b | 104.51 ± 35.72 b | 53.39 ± 20.92 a |
| ADG (g/day) | 0.48 ± 0.04 c | 0.31 ± 0.06 b | 0.31 ± 0.11 b | 0.16 ± 0.06 a |
| SGR (%) | 1.17 ± 0.16 c | 0.89 ± 0.19 b | 1.00 ± 0.27 b | 0.60 ± 0.19 a |
| Survival (%) | 50 ± 1.73 a | 77.8 ± 0.58 b | 100.00 c | 100.00 c |
| Maturity Stages | Group Rearing (GR-Control) | Individual Rearing (IR-Treatment) |
|---|---|---|
| Immature | 21.43 | 16.67 |
| Maturing | 28.57 | 27.78 |
| Fully mature | 50.00 | 55.55 |
| Morphotypes | Group Rearing (GR-Control) | Individual Rearing (IR-Treatment) |
|---|---|---|
| Blue claw | 30 | 44.44 |
| Orange claw | 40 | 22.22 |
| Small male | 20 | 16.67 |
| No claw | 10 | 16.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, T.; Panda, D.; Naik, N.; Udgata, S.K.; Choudhury, D.; Sahu, S.; Pillai, B.R. Evaluation of Individual Rearing of a Genetically Improved Giant River Prawn Macrobrachium rosenbergii Broodstock as an Alternate Approach to Group Rearing During the Post-Selection Rearing Phase. Aquac. J. 2025, 5, 16. https://doi.org/10.3390/aquacj5030016
Nayak T, Panda D, Naik N, Udgata SK, Choudhury D, Sahu S, Pillai BR. Evaluation of Individual Rearing of a Genetically Improved Giant River Prawn Macrobrachium rosenbergii Broodstock as an Alternate Approach to Group Rearing During the Post-Selection Rearing Phase. Aquaculture Journal. 2025; 5(3):16. https://doi.org/10.3390/aquacj5030016
Chicago/Turabian StyleNayak, Tanisha, Debabrata Panda, Namita Naik, Santosh Kumar Udgata, Dharitri Choudhury, Sovan Sahu, and Bindu R. Pillai. 2025. "Evaluation of Individual Rearing of a Genetically Improved Giant River Prawn Macrobrachium rosenbergii Broodstock as an Alternate Approach to Group Rearing During the Post-Selection Rearing Phase" Aquaculture Journal 5, no. 3: 16. https://doi.org/10.3390/aquacj5030016
APA StyleNayak, T., Panda, D., Naik, N., Udgata, S. K., Choudhury, D., Sahu, S., & Pillai, B. R. (2025). Evaluation of Individual Rearing of a Genetically Improved Giant River Prawn Macrobrachium rosenbergii Broodstock as an Alternate Approach to Group Rearing During the Post-Selection Rearing Phase. Aquaculture Journal, 5(3), 16. https://doi.org/10.3390/aquacj5030016






