Evaluation of the Effect of Three Florfenicol Doses Against Salmonid Rickettsial Septicemia (SRS) in Atlantic Salmon (Salmo salar Linnaeus) Challenged by Intraperitoneal Injection
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Culture Conditions
2.2. Minimum Inhibitory Concentration
2.3. Production of the Medicated Feed
2.4. Fish and Fish Husbandry
2.5. Pre-Study for Inoculum Dose Determination
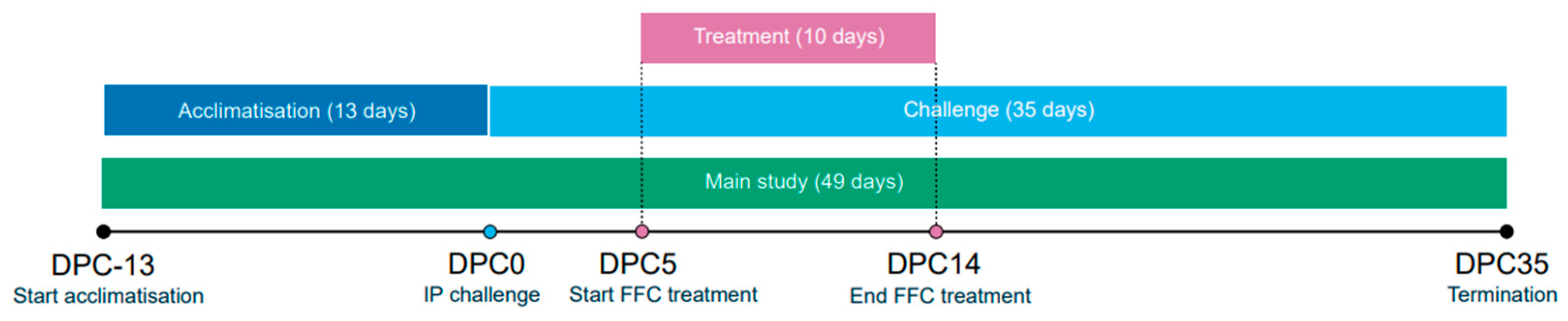
2.6. Treatment Study
2.7. Sampling
2.8. Statistical Analysis
3. Results
3.1. Production of the Medicated Feed
3.2. Minimum Inhibitory Concentration (MIC)
3.3. Pre-Study for Inoculum Determination
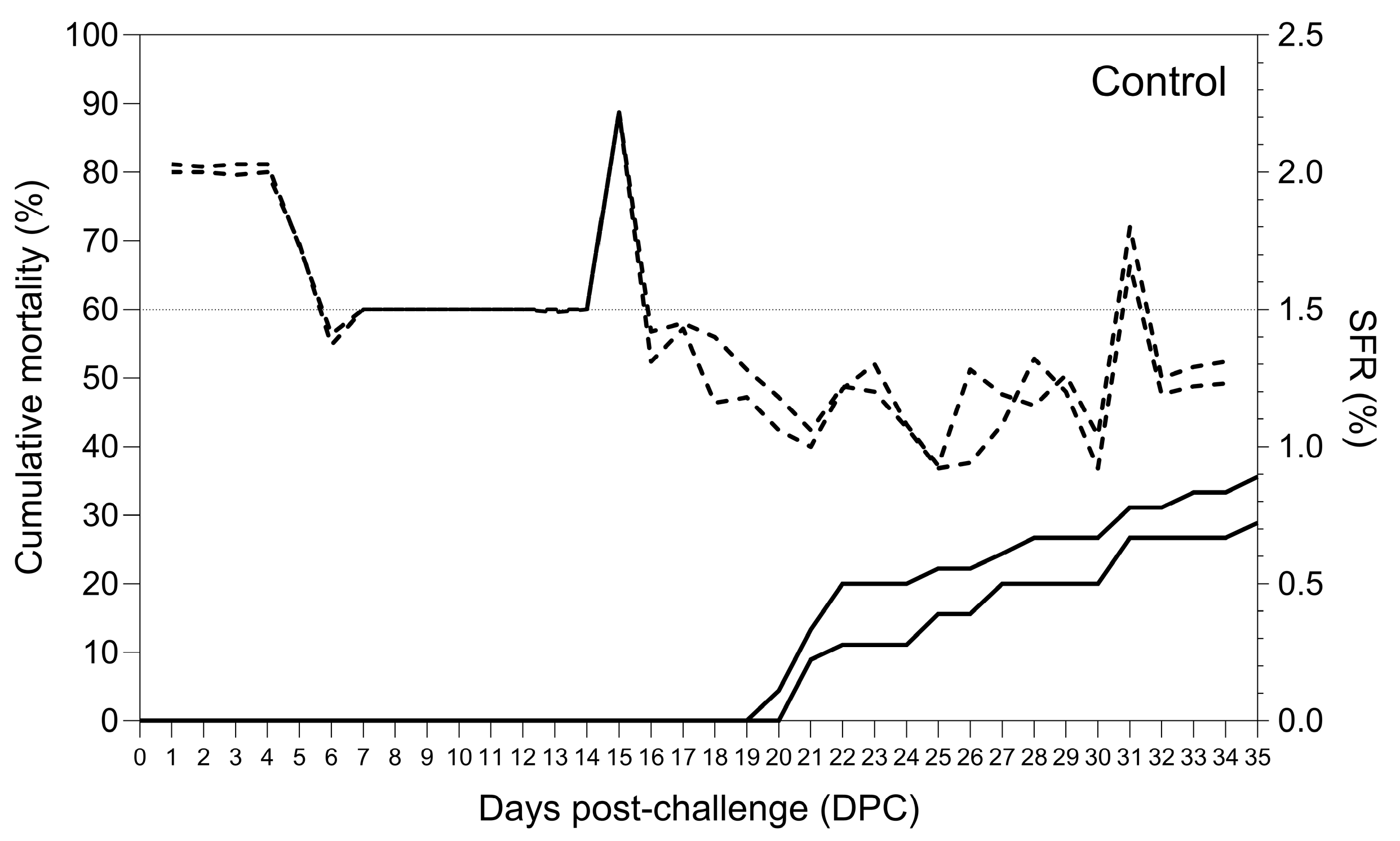
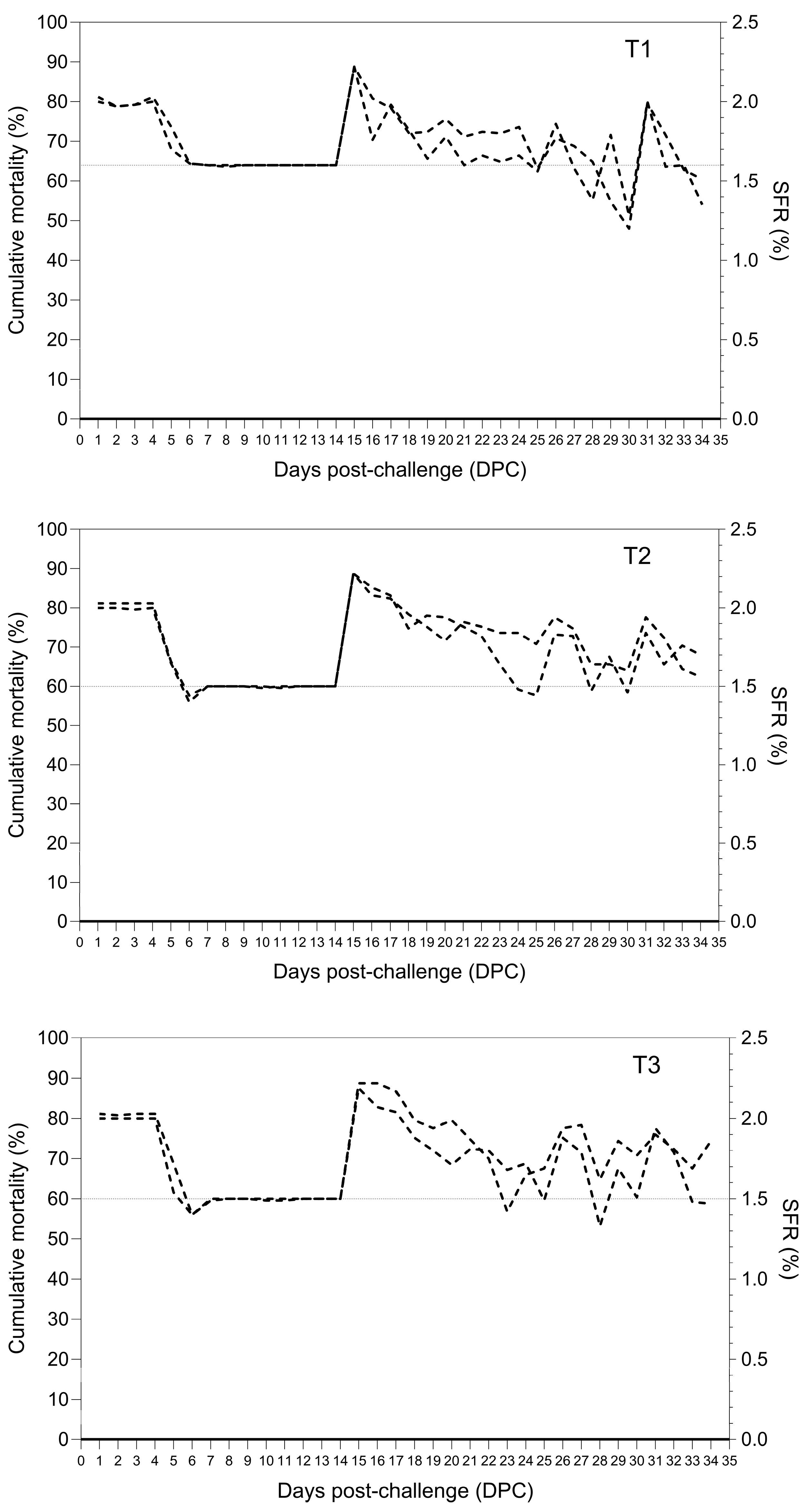
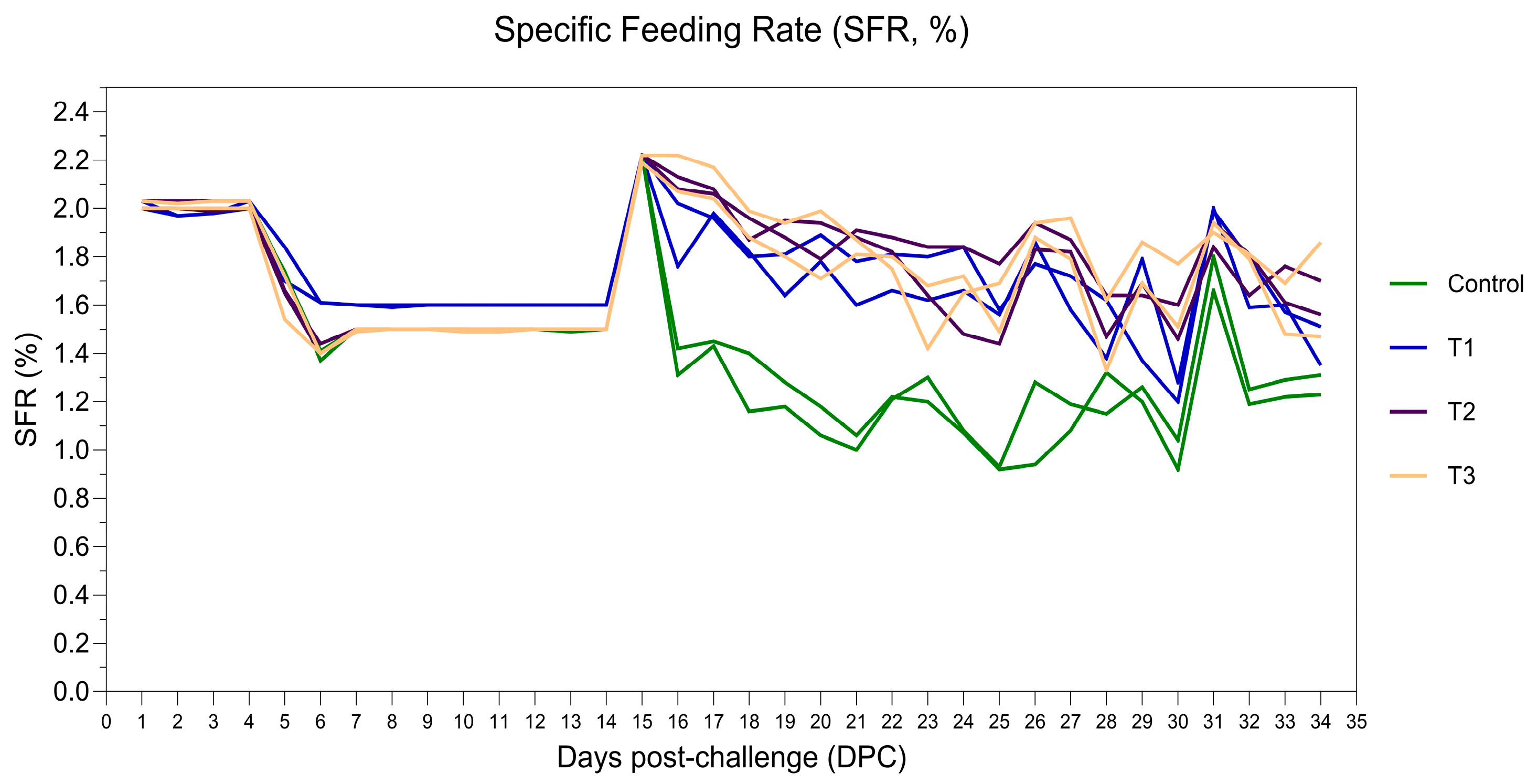
3.4. Treatment Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Frequency of Findings (SRS Score) in Surviving Fish (End of Challenge) | T1 | T2 | T3 | ||||
| Duplicate tank | 1 | 2 | 1 | 2 | 1 | 2 | |
| Dose of FFC (mg/kg BW/day) | 5 | 5 | 7.5 | 7.5 | 10 | 10 | |
| Weight (g) | 436 | 454 | 477 | 471 | 464 | 510 | |
| SD (g) | 62 | 82 | 76 | 71 | 87 | 79 | |
| Externa | Gills pale/hemorrhagic | 2/10 | 1/10 | 0/10 | 2/10 | 0/10 | 3/10 |
| Eyes hemorrhagic | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Eyes exophthalmia | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Skin wounds/ulcers | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Internal | Ascites | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Liver yellow/pale | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | |
| Liver node/hemorrhagic | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Liver inflammation | 2/10 | 2/10 | 3/10 | 2/10 | 3/10 | 5/10 | |
| Spleen pale/nodes | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Splenomegaly | 6/10 | 8/10 | 5/10 | 10/10 | 6/10 | 8/10 | |
| Pyloric caeca congestive/hemorrhagic | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Kidney pale | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Kidney swollen | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Visceral fat | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Feed | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | |
References
- Sernapesca 2024. Fiscalización en Pesca y Acuicultura—SIFA 2023, May 2024. Available online: https://www.sernapesca.cl/app/uploads/2024/03/ifpa_2023_v20240327.pdf (accessed on 4 August 2025).
- Sernapesca 2024. Informe Con Antecedentes Sanitario de Agua Dulce Y Mar—Año 2024. Subdirección De Acuicultura Departamento De Salud Animal Servicio Nacional De Pesca Y Acuicultura, Julio 2025. Available online: https://www.sernapesca.cl/app/uploads/2025/07/Informe-Situacion-Sanitaria-Salmonicultura-Ano-2024.pdf (accessed on 4 August 2025).
- Sernapesca 2025. Informe Sobre Uso de Antimicrobianos Y Antiparasitarios en la Salmonicultura Nacional año 2024. Subdirección De Acuicultura Departamento De Salud Animal Servicio Nacional De Pesca Y Acuicultura, Julio 2025. Available online: https://www.sernapesca.cl/app/uploads/2025/07/Informe-Uso-Antimicrobianos-y-Antiparasitarios-Ano-2024.pdf (accessed on 4 August 2025).
- Luthman, O.; Robb, D.H.F.; Henriksson, P.J.G.; Jørgensen, P.S.; Troell, M. Global overview of national regulations for antibiotic use in aquaculture production. Aquacult. Int. 2024, 32, 9253–9270. [Google Scholar] [CrossRef]
- Fryer, J.L.; Lannan, C.N.; Garcés, L.H.; Larenas, J.J.; Smith, P.A. Isolation of a rickettsiales-like organism from diseased coho salmon (Oncorhynchus kisutch) in Chile. Fish Pathol. 1990, 25, 107–114. [Google Scholar] [CrossRef]
- Fryer, J.L.; Lannan, C.N.; Giovannoni, S.J.; Wood, N.D. Piscirickettsia salmonis gen. Nov., sp. nov., the causative agent of an epizootic disease in salmonid fishes. Int. J. Syst. Bacteriol. 1992, 42, 120–126. [Google Scholar] [CrossRef]
- Schäfer, J.W.; Alvarado, V.; Enriques, R.; Monras, M. The “Coho salmon syndrome” (CSS): A new disease in Chilean salmon, reared in sea water. Bull. Eur. Assoc. Fish Pathol. 1990, 10, 130. [Google Scholar]
- Avendaño-Herrera, R.; Mancilla, M.; Miranda, C.D. Use of antimicrobials in Chilean Salmon farming: Facts, myths and perspectives. Rev. Aquac. 2023, 15, 89–111. [Google Scholar] [CrossRef]
- Olsen, A.B.; Melby, H.P.; Spilberg, L.; Evensen, Ø.; Håstein, T. Piscirickettsia salmonis infection in Atlantic salmon Salmo salar in Norway—Epidemiological, pathological and microbiological findings. Dis. Aquat. Organ. 1997, 31, 35–48. [Google Scholar] [CrossRef]
- Rozas, M.; Enríquez, R. Piscirickettsiosis and Piscirickettsia salmonis in fish: A review. Fish Dis. J. 2014, 37, 163–188. [Google Scholar] [CrossRef]
- Evensen, Ø. Immunization Strategies against Piscirickettsia salmonis Infections: Review of Vaccination Approaches and Modalities and Their Associated Immune Response Profiles. Front. Immunol. 2016, 7, 482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schober, I.; Bunk, B.; Carril, G.; Freese, H.M.; Ojeda, N.; Riedel, T.; Meier-Kolthoff, J.P.; Göker, M.; Spröer, C.; A Flores-Herrera, P.; et al. Ongoing diversification of the global fish pathogen Piscirickettsia salmonis through genetic isolation and transposition bursts. ISME J. 2023, 17, 2247–2258. [Google Scholar] [CrossRef]
- Jones, S.R.M. Piscirickettsiosis. In ICES Identification Leaflets for Diseases and Parasites of Fish and Shellfish; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2019; 5p. [Google Scholar] [CrossRef]
- Figueroa, J.; Cárcamo, J.; Yañez, A.; Olavarria, V.; Ruiz, P.; Manríquez, R.; Muñoz, C.; Romero, A.; Avendaño-Herrera, R. Addressing viral and bacterial threats to salmon farming in Chile: Historical contexts and perspectives for management and control. Rev. Aquac. 2019, 11, 299–324. [Google Scholar] [CrossRef]
- Mauel, M.J.; Giovannoni, S.J.; Fryer, J.L. Phylogenetic analysis of Piscirickettsia salmonis by 16S, internal transcribed spacer (ITS) and 23S ribosomal DNA sequencing. Dis. Aquat. Organ. 1999, 35, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.; Obreque, C.J.; Gaggero, A.; Landskron, E.; Sandino, G.A.; Jashes, M.M. Electrophoretic analysis of ITS from Piscirickettsia salmonis Chilean isolates. FEMS Microbiol. Lett. 2003, 225, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Otterlei, A.; Brevik, Ø.J.; Jensen, D.; Duesund, H.; Sommerset, I.; Frost, P.; Mendoza, J.; McKenzie, P.; Nylund, A.; Apablaza, P. Phenotypic and genetic characterization of Piscirickettsia salmonis from Chilean and Canadian salmonids. BMC Vet. Res. 2016, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Nourdin-Galindo, G.; Sánchez, P.; Molina, C.F.; Espinoza-Rojas, D.A.; Oliver, C.; Ruiz, P.; Vargas-Chacoff, L.; Cárcamo, J.G.; Figueroa, J.E.; Mancilla, M.; et al. Comparative pan-genome analysis of Piscirickettsia salmonis reveals genomic divergences within genogroups. Front. Cell. Infect. Microbiol. 2017, 7, 459. [Google Scholar] [CrossRef]
- Bustos, P. General Manager at ADL diagnostics Chile Personal communication. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.youtube.com/watch%3Fv%3DK1409gYaj7c&ved=2ahUKEwiVkemdx6WPAxWfoa8BHTdVN0oQtwJ6BAgNEAI&usg=AOvVaw1GOxiHZDhLVMIMMFovJ9uf (accessed on 30 November 2024).
- Bustos, P. Presentation at MEVEA Workshop. In Proceedings of the Talk title: “GENETIC VARIANTS OF Piscirickettsia salmonis: Could They Influence the Efficacy of Therapies, the Protection of Vaccines and Genetic Selection? Puerto Varas, Chile, 7 October 2022. [Google Scholar]
- Nota Técnica N°1, 28. Febrero 2023, ADL Diagnostics Chile. Available online: https://adldiagnostic.cl/images/2023/Nota_tecnica_1_-_Genogrupo__genov_ariantes_P_salmonis_270223__V2.pdf (accessed on 30 November 2024).
- SAG 2024. Productos Biológicos Inmunológicos Con Registro Provisional Uso en Salmónidos, Julio 2024. Available online: https://www.sag.gob.cl/sites/default/files/lista_salmonidos_registro_provisional_02072024.pdf (accessed on 4 August 2025).
- Valenzuela-Aviles, P.; Torrealba, D.; Figueroa, C.; Mercado, L.; Dixon, B.; Conejeros, P.; Gallardo-Matus, J. Why vaccines fail against Piscirickettsiosis in farmed salmon and trout and how to avoid it: A review. Front. Immunol. 2022, 13, 1019404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Project Pincoy. Available online: https://proyectopincoy.com (accessed on 4 August 2025).
- InfoSALMON 2024. Proyecto Yelcho: Una iniciativa que Apunta a la Prevención de Enfermedades y Bienestar de los Peces. Available online: https://infosalmon.cl/proyecto-yelcho-una-iniciativa-que-apunta-a-la-prevencion-de-enfermedades-y-bienestar-de-los-peces/ (accessed on 4 August 2025).
- CSARP 2024. Available online: https://www.csarp.cl (accessed on 4 August 2025).
- Rozas-Serri, M.; Peña, A.; Gardner, I.; Peñaloza, E.; Maldonado, L.; Muñoz, A.; Mardones, F.O.; Rodríguez, C.; Ildefonso, R.; Senn, C.; et al. Co-Infection by LF-89-Like and EM-90-Like Genogroups of Piscirickettsia Salmonis in Farmed Atlantic Salmon in Chile: Implications for Surveillance and Control of Piscirickettsiosis. Pathogens 2023, 12, 450. [Google Scholar] [CrossRef]
- Henrıquez, P.; Kaiser, M.; Bohle, H.; Bustos, P.; Mancilla, M. Comprehensive antibiotic susceptibility profiling of Chilean Piscirickettsia salmonis field isolates. Fish Dis. J. 2016, 39, 441–448. [Google Scholar] [CrossRef]
- National Agency for Research and Development (ANID) to Uphold Ethical Standards in Animal Research. Available online: https://anid.cl/etica-de-la-investigacion-post/ (accessed on 4 August 2025).
- San Martin, B.; Fredsno, M.; Cornejo, J.; Godoy, M.; Ibarra, R.; Vidal, R.; Araneda, M.; Anadón, A.; Lapierre, L.; Chang, Y.-F. Optimization of florfenicol dose against Piscirickettsia salmonis in Salmo salar through PK/PD studies. PLoS ONE 2019, 14, e0215174. [Google Scholar] [CrossRef]
- House, M.L.; Bartholomew, J.R.; Winston, J.R.; Fryer, J.L. Relative virulence of three isolates of Piscirickettsia salmonis for coho salmon Oncorhynchus kisutch. Dis. Aquat. Organ. 1999, 35, 107–113. [Google Scholar] [CrossRef]
- Smith, P.A.; Pizarro, P.; Ojeda, P.; Contreras, J.; Oyanedel, S.; Larenas, L. Routes of entry of Piscirickettsia salmonis in rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1999, 37, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rozas-Serri, M.; Ildefonso, R.; Peña, A.; Enríquez, R.; Barrientos, S.; Maldonado, L. Comparative Pathogenesis of Piscirickettsiosis in Atlantic Salmon (Salmo salar L.) Post-Smolt Experimentally Challenged with Lf-89-Like and Em-90- Like Piscirickettsia Salmonis Isolates. Fish Dis. J. 2017, 40, 1451–1472. [Google Scholar] [CrossRef]
- Carill, G.; Winther-Larsen, H.C.; Løvoll, M.; Sørum, H. Cohabitation of Piscirickettsia salmonis genogroups (LF-89 and EM-90): Synergistic effect on growth dynamics. Front. Cell. Infect. Microbiol. 2023, 13, 1253577. [Google Scholar] [CrossRef]
- Contreras-Lynch, S.; Smith, P.; Olmos, P.; Loy, M.E.; Finnegan, W. A Novel and Validated Protocol for Performing MIC Tests to Determine the Susceptibility of Piscirickettsia salmonis Isolates to Florfenicol and Oxytetracycline. Front. Microbiol. 2017, 8, 1255. [Google Scholar] [CrossRef]
- Happold, J.; Mayer, A.; Sadler, R.J. Effectiveness of antimicrobial treatment of salmonid rickettsial septicaemia in commercial salmon and trout farms in Chile. Aquaculture 2020, 525, 735323. [Google Scholar] [CrossRef]
- Price, D.; Stryhn, H.; Sánchez, J.; Ibarra, R.; Tello, A.; St-Hilaire, S. Retrospective analysis of antibiotic treatments against piscirickettsiosis in farmed Atlantic salmon Salmo salar in Chile. Dis. Aquat. Org. 2016, 118, 227–235. [Google Scholar] [CrossRef] [PubMed]

| Florfenicol | Florfenicol | |||
|---|---|---|---|---|
| Treatment Groups | Duflosan Feed Dose (g/kg) | Florfenicol Feed Dose (g/kg) | SFR (%) | Target Therapeutic Dose mg/kg BW/day |
| Control | 0.000 | 0.000 | 1.5 | 0 |
| T1 | 0.667 | 0.333 | 1.5 | 5 |
| T2 | 1.000 | 0.500 | 1.5 | 7.5 |
| T3 | 1.333 | 0.667 | 1.5 | 10 |
| Challenge Inoculum | Bacterial Count (CFU mL−1) |
|---|---|
| 1 | 8.30 × 107 |
| 2 | 2.90 × 107 |
| 3 | 1.13 × 107 |
| 4 | 4.86 × 106 |
| Florfenicol | Florfenicol | |||
|---|---|---|---|---|
| Treatments | Target Level in Feeds (g/kg) | Recovery Level in Feeds (g/kg) | Adjusted SFR (%) | Actual Therapeutic Dose mg/kg BW/day |
| Control | 0.000 | 0 | 1.5 | 0.00 |
| T1 | 0.340 | 0.317 | 1.6 | 5.07 |
| T2 | 0.510 | 0.505 | 1.5 | 7.58 |
| T3 | 0.680 | 0.668 | 1.5 | 10.02 |
| Frequency of Findings (SRS Score) in Mortality Group | Control Group | ||
|---|---|---|---|
| Duplicate tank | 1 | 2 | |
| Dose of FFC (mg/kg BW/day) | 0 | 0 | |
| Weight (g) | 295 | 277 | |
| SD (g) | 29 | 29 | |
| External | Gills pale/hemorrhagic | 16/16 | 13/13 |
| Eyes hemorrhagic | 2/16 | 4/13 | |
| Eyes exophthalmia | 0/16 | 0/13 | |
| Skin wounds/ulcers | 0/16 | 0/13 | |
| Internal | Ascites | 1/16 | 0/13 |
| Liver yellow/pale | 16/16 | 13/13 | |
| Liver node/hemorrhagic | 1/16 | 0/13 | |
| Liver inflammation | 13/16 | 7/13 | |
| Spleen pale/nodes | 12/16 | 9/13 | |
| Splenomegaly | 16/16 | 13/13 | |
| Pyloric caeca congestive/hemorrhagic | 16/16 | 13/13 | |
| Kidney pale | 2/16 | 2/13 | |
| Kidney swollen | 12/16 | 6/13 | |
| Visceral fat | 16/16 | 13/13 | |
| Feed | 16/16 | 13/13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lie, C.I.; Zarza, C.; Småge, S.B.; Ibieta, P.; Ibarra, P.; Jensen, L.B. Evaluation of the Effect of Three Florfenicol Doses Against Salmonid Rickettsial Septicemia (SRS) in Atlantic Salmon (Salmo salar Linnaeus) Challenged by Intraperitoneal Injection. Aquac. J. 2025, 5, 13. https://doi.org/10.3390/aquacj5030013
Lie CI, Zarza C, Småge SB, Ibieta P, Ibarra P, Jensen LB. Evaluation of the Effect of Three Florfenicol Doses Against Salmonid Rickettsial Septicemia (SRS) in Atlantic Salmon (Salmo salar Linnaeus) Challenged by Intraperitoneal Injection. Aquaculture Journal. 2025; 5(3):13. https://doi.org/10.3390/aquacj5030013
Chicago/Turabian StyleLie, Cecilie I., Carlos Zarza, Sverre B. Småge, Pablo Ibieta, Pablo Ibarra, and Linda B. Jensen. 2025. "Evaluation of the Effect of Three Florfenicol Doses Against Salmonid Rickettsial Septicemia (SRS) in Atlantic Salmon (Salmo salar Linnaeus) Challenged by Intraperitoneal Injection" Aquaculture Journal 5, no. 3: 13. https://doi.org/10.3390/aquacj5030013
APA StyleLie, C. I., Zarza, C., Småge, S. B., Ibieta, P., Ibarra, P., & Jensen, L. B. (2025). Evaluation of the Effect of Three Florfenicol Doses Against Salmonid Rickettsial Septicemia (SRS) in Atlantic Salmon (Salmo salar Linnaeus) Challenged by Intraperitoneal Injection. Aquaculture Journal, 5(3), 13. https://doi.org/10.3390/aquacj5030013





