Synergistic Microbial Interactions Between Algae and Bacteria Augment Growth and Immune Performance in Red Tilapia (Oreochromis sp.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Monitoring of Water Quality Parameters
2.3. Measurement of Growth Parameters
2.4. Chlorophyll and Carotenoid Estimation in Water
2.5. Carotenoid Estimation in Tissues
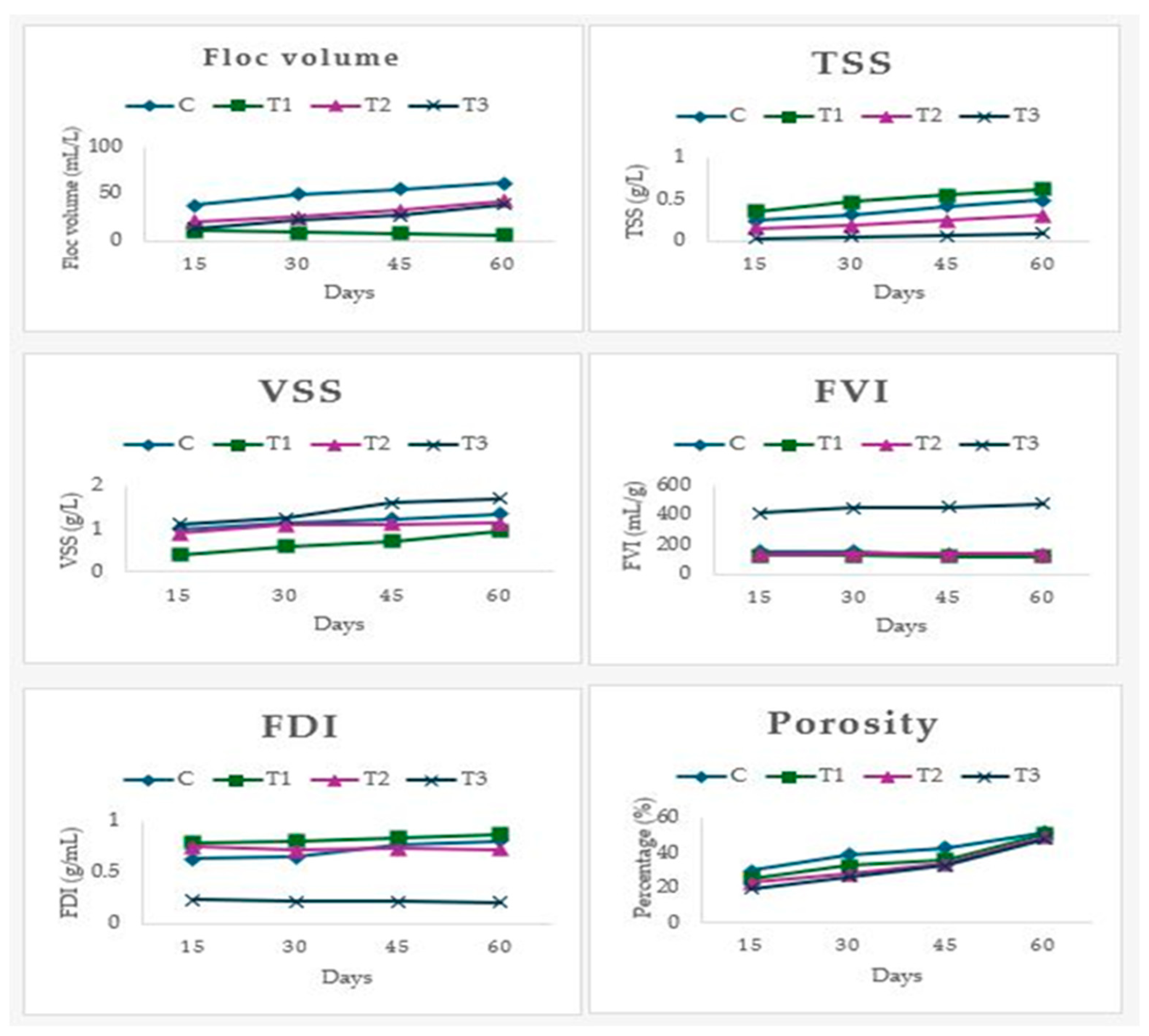
2.6. Measurement of Floc and Sludge Parameters
2.7. Total Bacterial Count and Algal Count
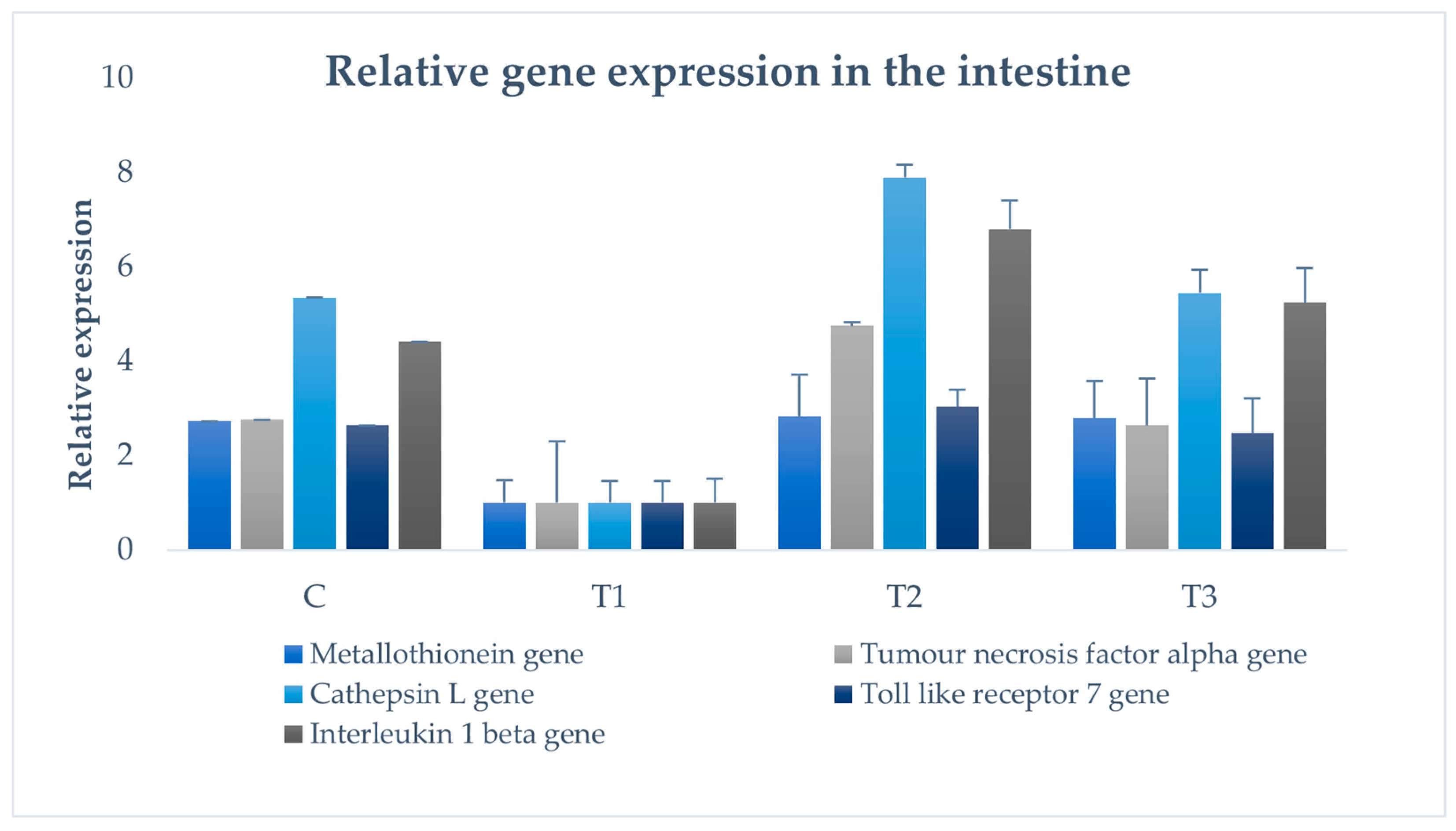
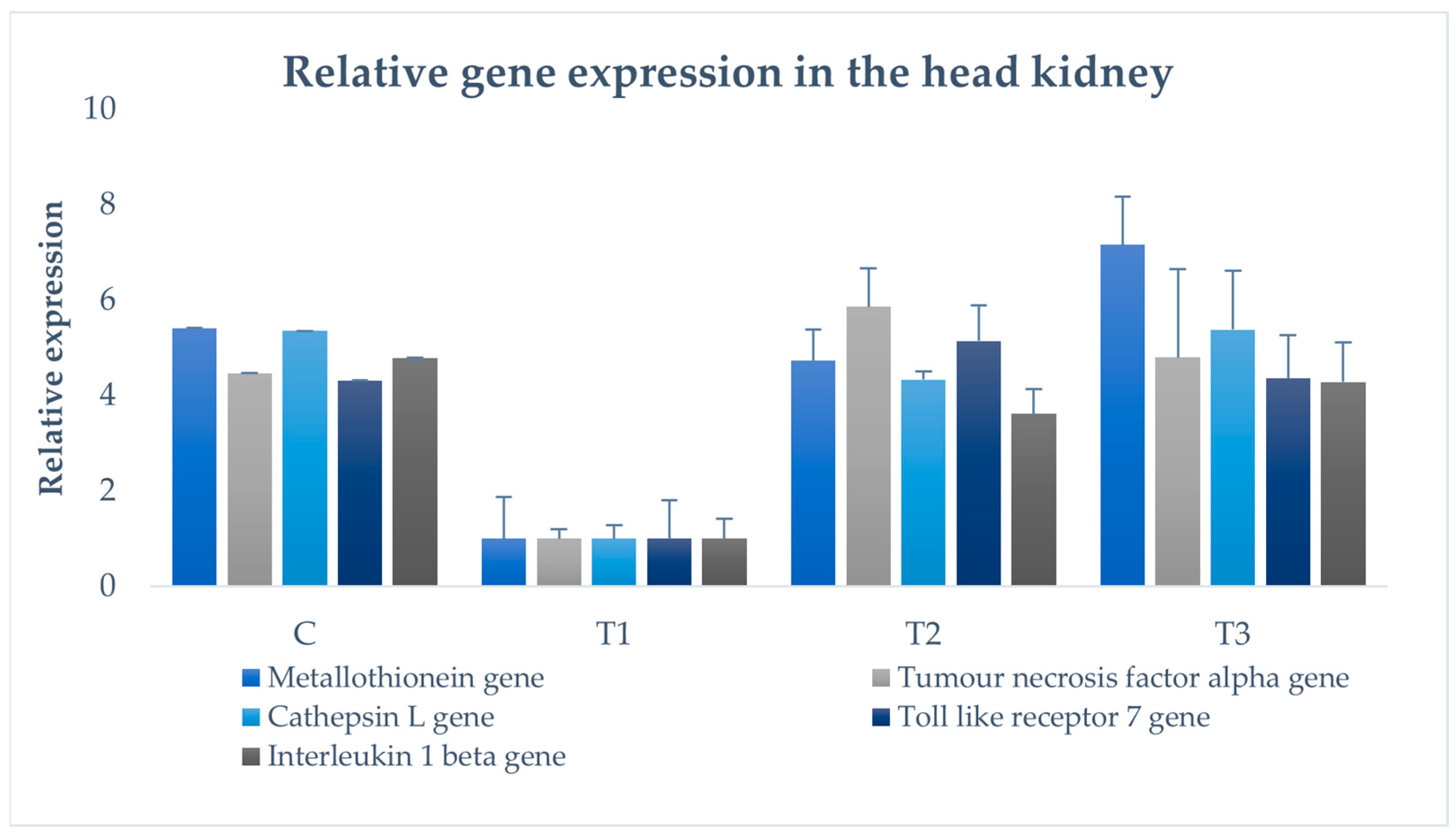
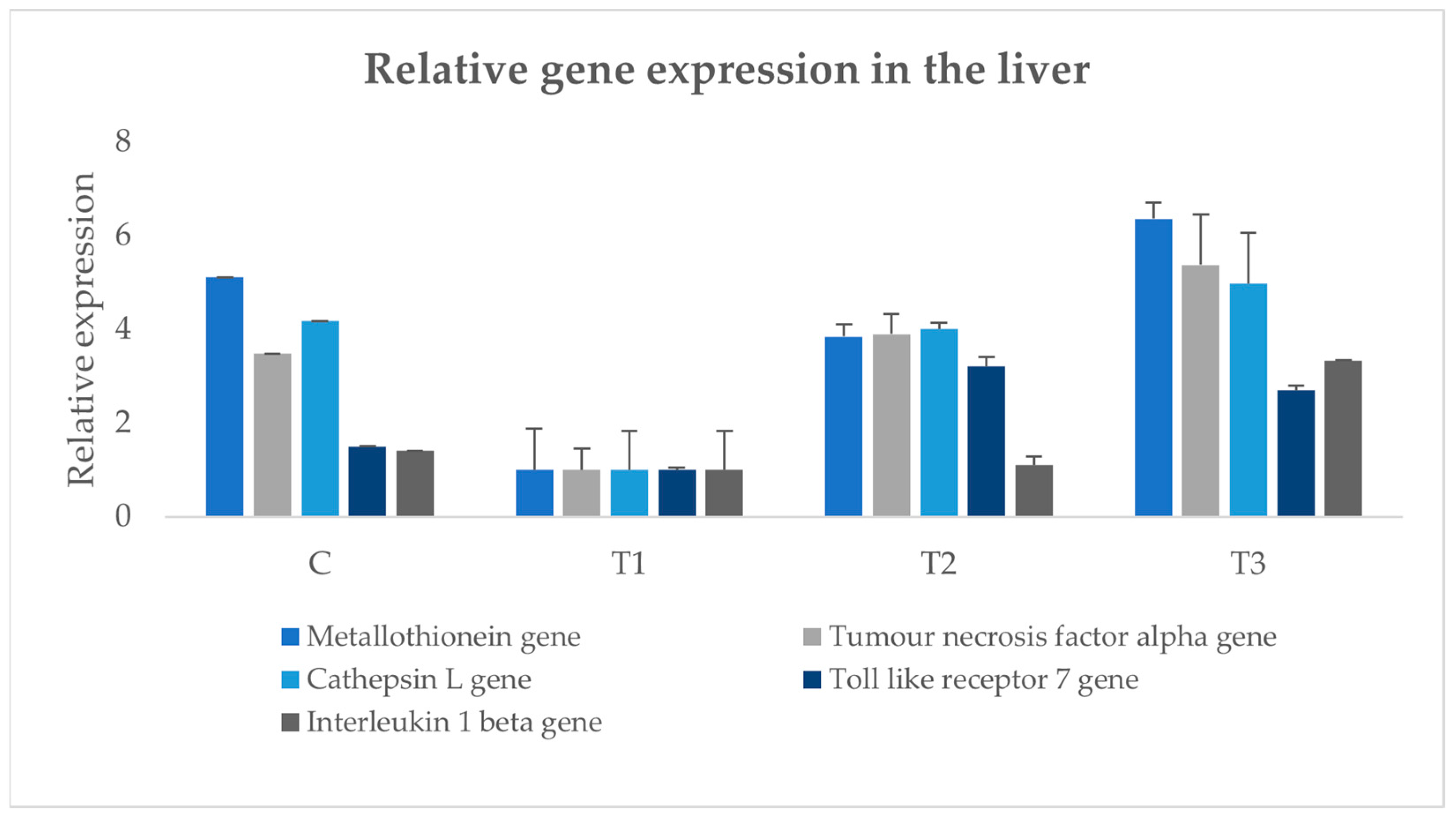
2.8. Examination of Immune Gene Expression
2.9. Pathogen Challenge of Experimental Fish Against Aeromonas Hydrophila
2.10. Statistical Analysis
3. Results
3.1. Water Quality Parameters
3.2. Growth Parameters
3.3. Chlorophyll and Carotenoid Level
3.4. Floc Characteristics
3.5. Sludge Characteristics
3.6. Total Bacterial Count
3.7. Total Algal Count
3.8. Immune Gene Expression
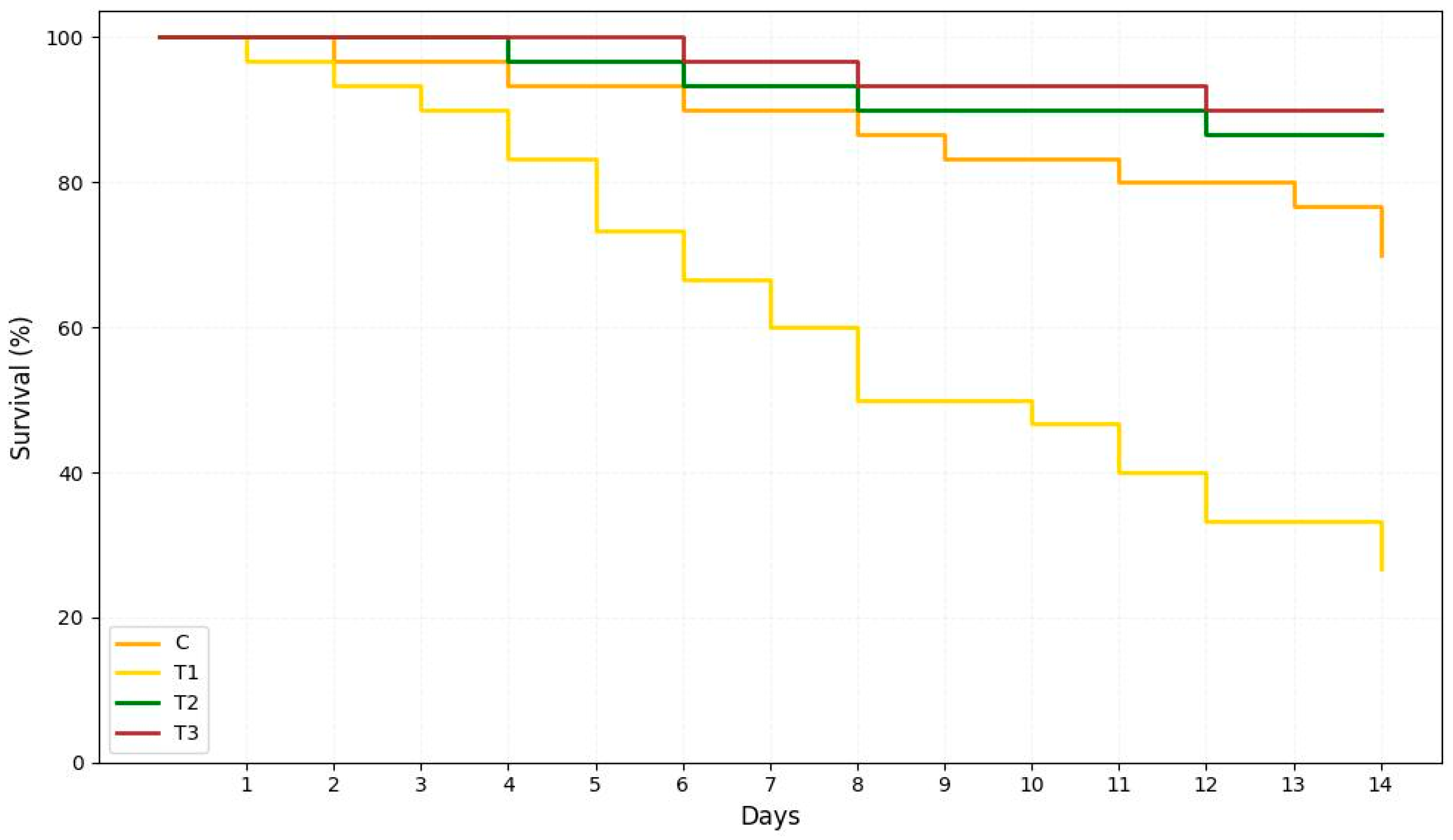
3.9. Challenge of the Experimental Fish Against Aeromonas Hydrophila
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BFT | Biofloc Technology |
| FRP | Fiberglass Reinforced Plastic |
| FCR | Food Coversion Ratio |
| SGR | Specific Growth Rate |
| ICAR-CIBA | Indian Council of Agricultural Research—Central Institute of Brackishwater Aquaculture |
| RPS | Relative Percentage Survival |
| CFU | Colony-forming Units |
| ANOVA | Analysis of Variance |
References
- Avnimelech, Y.; Kochba, M. Evaluation of nitrogen uptake and excretion by tilapia in biofloc tanks, using 15N tracing. Aquaculture 2009, 278, 163–168. [Google Scholar] [CrossRef]
- DeLong, D.C.; Losordo, T.M. Recirculating Aquaculture Systems; NRAC Publication: Washington, DC, USA, 2012; No. 401. [Google Scholar]
- Piedrahita, R.H. Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 2003, 226, 35–44. [Google Scholar] [CrossRef]
- Pillay, T.V.R.; Kutty, M.N. Aquaculture: Principles and Practices, 2nd ed.; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar]
- Hargreaves, J.A.; Tucker, C.S. Managing Ammonia in Fish Ponds; SRAC Publication: Stoneville, MS, USA, 2004; No. 4603. [Google Scholar]
- Aliabad, H.S.; Naji, A.; Mortezaei, S.R.S.; Sourinejad, I.; Akbarzadeh, A. Effects of restricted feeding levels and stocking densities on water quality, growth performance, body composition and mucosal innate immunity of Nile tilapia (Oreochromis niloticus) fry in a biofloc system. Aquaculture 2022, 546, 737320. [Google Scholar] [CrossRef]
- Avnimelech, Y. Bio-filters: The need for an new comprehensive approach. Aquac. Eng. 2006, 34, 172–178. [Google Scholar] [CrossRef]
- Taufik, M.; Ismail, T.I.T.; Manan, H.; Ikhwanuddin, M.; Salam, A.I.A.; Rahim, A.I.A.; Ishak, A.N.; Kamaruzzan, A.S.; Draman, A.S.; Kasan, N.A. Synergistic effects of Recirculating Aquaculture System (RAS) with combination of clear water, probiotic and biofloc technology: A review. Aquac. Fish. 2023, 9, 883–892. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El-Sayed, A.I.; Ahmed, H.A. Effects of dietary supplementation of heat-killed Lactobacillus plantarum on growth performance, immune responses and stress resistance of juvenile red sea bream, Pagrus major. Aquaculture 2015, 442, 29–36. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Lawrence, A.L.; Boardman, G.D.; Patnaik, S.; Marsh, L.; Flick, G.J., Jr. Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2009, 296, 51–57. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mohammadi, A.; Emerenciano, M.G.C. Microorganisms in biofloc aquaculture system. Aquac. Rep. 2022, 26, 101300. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Ogello, E.O.; Outa, N.O.; Obiero, K.O.; Kyule, D.N.; Munguti, J.M. The prospects of biofloc technology (BFT) for sustainable aquaculture development. Sci. Afr. 2021, 14, e01053. [Google Scholar] [CrossRef]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Lee, K.M.; Kim, J.H. Biofloc technology in fish aquaculture: A review. Antioxidants 2023, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Ekasari, J.; Maryam, S. Evaluation of biofloc technology application on water quality and production performance of red tilapia Oreochromis sp. cultured at different stocking densities. Hayati J. Biosci. 2012, 19, 73–80. [Google Scholar] [CrossRef]
- Burford, M.A.; Thompson, P.J.; McIntosh, R.P.; Bauman, R.H.; Pearson, D.C. The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture 2004, 232, 525–537. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquac. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q. Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture 2012, 356, 147–152. [Google Scholar] [CrossRef]
- Mansour, A.T.; Esteban, M.Á. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 64, 202–209. [Google Scholar] [CrossRef]
- Azimi, A.; Shekarabi, S.P.H.; Paknejad, H.; Harsij, M.; Khorshidi, Z.; Zolfaghari, M.; Hatami, A.S.; Dawood, M.A.; Mazloumi, N.; Zakariaee, H. Various carbon/nitrogen ratios in a biofloc-based rearing system of common carp (Cyprinus carpio) fingerlings: Effect on growth performance, immune response, and serum biochemistry. Aquaculture 2022, 548, 737622. [Google Scholar] [CrossRef]
- Cotner, J.B.; Biddanda, B.A. Small Players, Large Role: Microbial Influence on Biogeochemical Processes in Pelagic Aquatic Ecosystems. Ecosystems 2002, 5, 105–121. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotech. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Pekkoh, J.; Chaichana, C.; Thurakit, T.; Phinyo, K.; Lomakool, S.; Ruangrit, K.; Duangjan, K.; Suwannarach, N.; Kumla, J.; Cheirsilp, B.; et al. Dual-bioaugmentation strategy to enhance the formation of algal-bacteria symbiosis biofloc in aquaculture wastewater supplemented with agricultural wastes as an alternative nutrient sources and biomass support materials. Bioresour. Technol. 2022, 359, 127469. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.J. Interactions between bacteria and algae in aquatic ecosystems. Ann. Rev. Ecol. System. 1982, 13, 291–314. Available online: https://www.jstor.org/stable/2097070 (accessed on 12 December 2024). [CrossRef]
- Joint, I.; Tait, K.; Wheeler, G. Cross-kingdom signalling: Exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1223–1233. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.B.; Smith, A.G. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr. Opin. Plant Biol. 2015, 26, 147–153. [Google Scholar] [CrossRef]
- Coveney, M.F.; Wetzel, R.G. Bacterial metabolism of algal extracellular carbon. Hydrobiologia 1989, 173, 141–149. [Google Scholar] [CrossRef]
- Armstrong, E.; Rogerson, A.; Leftley, J.W. The abundance of heterotrophic protists associated with intertidal seaweeds. Estuar. Coast. Shelf Sci. 2000, 50, 415–424. [Google Scholar] [CrossRef]
- Rowe, G.T.; Deming, J.W. The Role of Bacteria in the Turnover of Organic Carbon in Deep-Sea Sediments. 1985. Available online: https://elischolar.library.yale.edu/journal_of_marine_research/1804/ (accessed on 17 December 2024).
- Muñoz, R.; Guieysse, B.; Mattiasson, B. Phenanthrene biodegradation by an algal-bacterial consortium in two-phase partitioning bioreactors. Appl. Microbiol. Biotechnol. 2003, 61, 261–267. [Google Scholar] [CrossRef]
- Kim, B.H.; Ramanan, R.; Cho, D.H.; Oh, H.M.; Kim, H.S. Role of Rhizobium, a plant growth promoting bacterium, in enhancing algal biomass through mutualistic interaction. Biomass Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Teplitski, M.; Rajamani, S. Signal and nutrient exchange in the interactions between soil algae and bacteria. In Biocommunication in Soil Microorganisms; Springer: Berlin/Heidelberg, Germany, 2010; pp. 413–426. [Google Scholar] [CrossRef]
- Kazamia, E.; Czesnick, H.; Nguyen, T.T.V.; Croft, M.T.; Sherwood, E.; Sasso, S.; Hodson, S.J.; Warren, M.J.; Smith, A.G. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 2012, 14, 1466–1476. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef]
- Sanmukh, S.; Bruno, B.; Ramakrishnan, U.; Khairnar, K.; Swaminathan, S.; Paunikar, W. Bioactive compounds derived from microalgae showing antimicrobial activities. J. Aquac. Res. Dev. 2014, 5, 224. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20143284901 (accessed on 24 March 2025). [CrossRef]
- Galal, A.A.; Reda, R.M.; Mohamed, A.A.R. Influences of Chlorella vulgaris dietary supplementation on growth performance, hematology, immune response and disease resistance in Oreochromis niloticus exposed to sub-lethal concentrations of penoxsulam herbicide. Fish Shellfish Immunol. 2018, 77, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.D.; Galián, C.; Fernández, V.; Chaves-Pozo, E.; de la Serrana, D.G.; Sáez, M.I.; Galafaz Díaz, A.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Influence of low dietary inclusion of the microalga Nannochloropsis gaditana (Lubián 1982) on performance, fish morphology, and muscle growth in juvenile gilthead seabream (Sparus aurata). Animals 2020, 10, 2270. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, S.; Huang, Y.; Chang, K.; Zhao, X. Addition of Chlorella sorokiniana meal in the diet of juvenile rainbow trout (Oncorhynchus mykiss): Influence on fish growth, gut histology, oxidative stress, immune response, and disease resistance against Aeromonas salmonicida. Fish Shellfish Immunol. 2022, 129, 243–250. [Google Scholar] [CrossRef]
- Huang, K.; Liu, X.; Ma, R.; Wang, B.; Ho, S.H.; Chen, J.; Xie, Y. Effects of substituting fish meal with Chlorella meal on growth performance, whole-body composition, pigmentation, and physiological health of marbled eel (Anguilla marmorata). Algal Res. 2024, 80, 103523. [Google Scholar] [CrossRef]
- Silva, V.F.; Pereira, P.K.; Martins, M.A.; Lorenzo, M.A.D.; Cella, H.; Lopes, R.G.; Derner, R.B.; Magallón-Servín, P.; Vieira, F.D.N. Effects of microalgae addition and fish feed supplementation in the integrated rearing of Pacific white shrimp and Nile tilapia using biofloc technology. Animals 2022, 12, 1527. [Google Scholar] [CrossRef]
- Taw, N. Future of biofloc technology in Asia. In Proceedings of the Roundtables Aquaculture Series 2012, Phuket, Thailand, 15–16 August 2012; pp. 1–30. [Google Scholar]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 2231, 606X. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20153044304 (accessed on 26 March 2025).
- Olson, J.A.; Lakshman, M.R. Carotenoid converstions. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 189, pp. 425–432. [Google Scholar]
- Li, D.H.; Ganczarczyk, J. Flow through activated sludge flocs. Water Res. 1988, 22, 789–792. [Google Scholar] [CrossRef]
- Beutler, M.; Wiltshire, K.H.; Meyer, B.; Moldaenke, C.; Lüring, C.; Meyerhöfer, M.; Hansen, U.P.; Dau, H. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 2002, 72, 39–53. [Google Scholar] [CrossRef]
- Mohlman, F.W. The sludge index. Sew. Work. J. 1934, 6, 119–122. Available online: https://www.jstor.org/stable/25028375 (accessed on 17 March 2025).
- Bergey, D.H. Bergey’s Manual of Determinative Bacteriology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994; pp. 1–695. [Google Scholar]
- Holt, P.G. Alveolar macrophages. I. A simple technique for the preparation of high numbers of viable alveolar macrophages from small laboratory animals. J. Immunol. Methods 1979, 27, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Yardımcı, B.; Aydın, Y. Pathological findings of experimental Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Ank. Üniversitesi Vet. Fakültesi Derg. 2011, 58, 47–54. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Evans, J.J. In ovo methods for utilizing the modified live Edwardsiella ictaluri vaccine against enteric septicemia in channel catfish. Aquaculture 2002, 203, 221–227. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; World Aquaculture Society: Baton Rouge, LA, USA, 2009; p. 182. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20113266301 (accessed on 15 February 2025).
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, M.; Zhang, J.; Jiang, X.; Zheng, Z. The interactions of algae-bacteria symbiotic system and its effects on nutrients removal from synthetic wastewater. Bioresour. Technol. 2018, 247, 44–50. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Marimuthu, C.; Arun, J.; Subathra, M.; Priyadharsini, P.; Nirmala, N.; Sarojadevi, S. Microalgae for Treating Wastewater. In Sustainable Industrial Wastewater Treatment and Pollution Control; Springer Nature: Singapore, 2023; pp. 1–12. [Google Scholar] [CrossRef]
- Qv, M.; Dai, D.; Liu, D.; Wu, Q.; Tang, C.; Li, S.; Zhu, L. Towards advanced nutrient removal by microalgae-bacteria symbiosis system for wastewater treatment. Bioresour. Technol. 2023, 370, 128574. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Wei, H.; Zhu, X.; Han, D.; Jin, J.; Yang, Y.; Xie, S. Biofloc formation improves water quality and fish yield in a freshwater pond aquaculture system. Aquaculture 2019, 506, 256–269. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Cabeza, C.; van Lier, J.B.; van der Steen, P. Effects of thermal and enzymatic pre-treatments on the solubilisation of extracellular polymeric substances (EPS) and subsequent anaerobic digestion of microalgae-bacterial biomass. Algal Res. 2023, 72, 103130. [Google Scholar] [CrossRef]
- Nakanishi, K.; Deuchi, K. Culture of a high-chlorophyll-producing and halotolerant Chlorella vulgaris. J. Biosci. Bioeng. 2014, 117, 617–619. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.N.; Oyler, G.A.; Wilkinson, L.; Betenbaugh, M.J. A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotech. 2008, 19, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.R.; Sherazi, T.A.; Hassan, S.U.; Shahzad, S.A.; Faheem, Z. Anti-inflammatory, anti-infectious and anti-cancer potential of marine algae and sponge: A review. Eur. J. Inflam. 2022, 20, 20587392221075514. [Google Scholar] [CrossRef]
- Zhang, P.; Peng, R.; Jiang, X.; Jiang, M.; Zeng, G. Effects of Nannochloropsis oculata and Thalassiosira pseudonana monocultures on growth performance and nutrient composition of Litopenaeus vannamei. Algal Res. 2022, 66, 102769. [Google Scholar] [CrossRef]
- Kumaresan, V.; Bhatt, P.; Palanisamy, R.; Gnanam, A.; Pasupuleti, M.; Arockiaraj, J. A murrel cysteine protease, cathepsin L: Bioinformatics characterization, gene expression and proteolytic activity. Biologia 2014, 69, 395–406. [Google Scholar] [CrossRef]
- Wang, R.; Song, L.; Su, B.; Zhao, H.; Zhang, D.; Peatman, E.; Li, C. Mucosal expression signatures of two Cathepsin L in channel catfish (Ictalurus punctatus) following bacterial challenge. Fish Shellfish Immunol. 2015, 47, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.R.; Wang, L.C.; Lin, H.T.; Lin, J.H.Y. Bioactivity of orange-spotted grouper (Epinephelus coioides) cathepsin L: Proteolysis of bacteria and regulation of the innate immune response. Fish Shellfish Immunol. 2022, 122, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Feng, Y.; Fan, W.; Duan, J.; Duan, Y.; Xiong, G.; Wang, K.; Deng, Y.; Geng, Y.; Ouyang, P.; et al. Potential ability for metallothionein and vitamin E protection against cadmium immunotoxicity in head kidney and spleen of grass carp (Ctenopharyngodon idellus). Ecotoxicol. Environ. Saf. 2019, 170, 246–252. [Google Scholar] [CrossRef]
- Menaga, M.; Felix, S.; Charulatha, M.; Gopalakannan, A.; Panigrahi, A. Effect of in-situ and ex-situ biofloc on immune response of Genetically Improved Farmed Tilapia. Fish Shellfish Immunol. 2019, 92, 698–705. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.; Mamoon, A.; Abdelghany, M.F.; Abdel-Hamid, E.A.; Abdel-Razek, N.; Ali, F.S.; Shady, S.H.; Gewida, A.G. Dietary Chlorella vulgaris modulates the performance, antioxidant capacity, innate immunity, and disease resistance capability of Nile tilapia fingerlings fed on plant-based diets. Anim. Feed. Sci. Technol. 2022, 283, 115181. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Abdelrahman, H.A. Effects of dietary Lactobacillus plantarum (KC426951) in biofloc and stagnant-renewal culture systems on growth performance, mucosal parameters, and serum innate responses of Nile tilapia Oreochromis niloticus. Fish Physiol. Biochem. 2020, 46, 1167–1181. [Google Scholar] [CrossRef]
- do Carmo Alves, A.P.; Peconick, A.P.; da Silva Cerozi, B.; Possebon Cyrino, J.E. Role of probiotics on the immunity of Nile tilapia Oreochromis niloticus: A review. Aquac. Int. 2022, 30, 1905–1929. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Zanuzzo, F.S.; Koch, J.F.A.; de Oliveira, C.A.F.; Sima, P.; Vetvicka, V. Development of fish immunity and the role of β-glucan in immune responses. Molecules 2020, 25, 5378. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Ramos-Vega, A.; Angulo, C.; Monreal-Escalante, E.; Guardiola, F.A. Microalgae with immunomodulatory effects on fish. Rev. Aquac. 2023, 15, 1522–1539. [Google Scholar] [CrossRef]
- Menaga, M.; Mboya, J.; Sugantham, F.; Panigrahi, A.; Subramanian, S.; Chia, S.; Beesigamukama, D.; Yossa, R.; Tanga, C. Algae-bacteria synergy leads to improved growth and immune defense in red tilapia (Oreochromis sp.). In AFRAQ 2024: Blue Farming—New Horizons for Economic Growth, Book of Abstracts, Proceedings of the African Chapter of the World Aquaculture Society, Hammamet, Tunisia, 19–22 November 2024; World Aquaculture Society (WAS) African Chapter: Midrand, South Africa, 2024; p. 146. [Google Scholar]
| Gene Name | Accession Number | Primers | Base Pair |
|---|---|---|---|
| Tumor necrosis factor-alpha (tnf-a) | XM_003438427.5 | GCTACGACTCCCAGCACTTTG (FP) GCGGTACTGCTCGGATCTCT (RP) | 72 |
| Metallothionein (mt) | XM_003447045.5 | GCCACTCCTACACCGTCATTC (FP) CTGGCGTTGCTCTTGTCTCTT (RP) | 63 |
| Toll-like receptor 7 (tlr7) | XM_019352834.2 | CCTATTTTGGCAACTGGCATCT (FP) CACTTCACTCCCATTGTTGATCTC (RP) | 78 |
| Cathepsin L (ctsl) | XM_003444107.5 | TGTCTTGCTCGTGGGCTATG (FP) CAGCTATTTTTCACCAGCCAGTAG (RP) | 63 |
| Interleukin-1 beta (il-1b) | KF747686.1 | TGTCGCTCTGGGCATCAA (FP) GGCTTGTCGTCATCCTTGTGA (RP) | 63 |
| β-actin | EU887951.1 | CCACACAGTGCCCATCTACGA (FP) CCACGCTCTGTCAGGATCTTCA (RP) | 120 |
| 18S rRNA | XR_003216134 | GTGCATGGCCGTTCTTAGTT (FP) CTCAATCTCGTGTGGCTGAA (RP) | 150 |
| Parameter | C | T1 | T2 | T3 |
|---|---|---|---|---|
| pH | 7.40 ± 0.03 a | 6.71 ± 0.04 a | 7.65 ± 0.05 a | 7.45 ± 0.02 a |
| Ammonia (NH4-N; mg/L) | 0.014 ± 0.003 a | 0.031 ± 0.005 a | 0.009 ± 0.002 a | 0.015 ± 0.004 a |
| Temperature (°C) | 30.0 ± 0.15 a | 29.0 ± 0.12 a | 29.0 ± 0.08 a | 29.0 ± 0.10 a |
| Nitrite (NO2-N; mg/L) | 0.0365 ± 0.002 a | 0.0225 ± 0.001 a | 0.0245 ± 0.001a | 0.0215 ± 0.001 a |
| Calcium (mg/L) | 88.5 ± 1.2 b | 70.5 ± 1.5 ba | 102.5 ± 1.1 bc | 84.5 ± 1.4 ba |
| Magnesium (mg/L) | 90.5 ± 1.0 a | 52.55 ± 0.60 b | 69.65 ± 0.75 c | 70.85 ± 0.65 c |
| Alkalinity (mg/L) | 111.5 ± 1.1 a | 94.5 ± 1.0 b | 88.5 ± 1.2 c | 90.5 ± 1.3 b |
| DO (mg/L) | 3.05 ± 0.10 a | 3.05 ± 0.12 a | 5.05 ± 0.11 a | 4.15 ± 0.09 a |
| Parameter | C | T1 | T2 | T3 |
|---|---|---|---|---|
| Initial weight (g) | 85 ± 0.5 a | 85 ± 0.5 a | 85 ± 0.5 a | 85 ± 0.5 a |
| Final weight (g) | 222 ± 0.7 a | 212 ± 0.7 a | 227 ± 0.7 a | 211 ± 0.7 a |
| Weight gain (g) | 137.5 ± 0.7 a | 127.5 ± 0.7 a | 142 ± 0.7 a | 126.5 ± 0.7 a |
| SGR | 1.57 ± 0.007 a | 1.50 ± 0.007 a | 1.61 ± 0.02 a | 1.49 ± 0.01 a |
| FCR | 1.85 ± 0.017 a | 2.00 ± 0.012 a | 1.79 ± 0.009 b | 2.01 ± 0.004 a |
| Survival (%) | 97 a | 99 a | 99 a | 99 a |
| Parameter | C | T1 | T2 | T3 |
|---|---|---|---|---|
| Chlorophyll a (%) | 0.45 ± 0.007 a | 0.5 ± 0.007 a | 0.62 ± 0.007 b | 0.68 ± 0.007 b |
| Chlorophyll b (%) | 1.21 ± 0.03 a | 1.34 ± 0.03 a | 1.58 ± 0.03 b | 1.75± 0.03 b |
| Carotenoid in water (%) | 0.26 ± 0.01 a | 0.18 ± 0.02 a | 0.4 ± 0.01 a | 0.36 ± 0.01 a |
| Carotenoid in tissue (µg/g) | 31.7 ± 0.98 a | 33.6 ± 0.56 a | 37.6 ± 0.56 a | 30.5 ± 0.70 a |
| Parameter | C | T1 | T2 | T3 |
|---|---|---|---|---|
| SV (mL/L) | 58 ± 2.82 a | 15.7 ± 0.103 b | 24.5 ± 0.707 b | 19.5 ± 0.707 b |
| SVI (mL/g) | 319 ± 9.89 a | 148 ± 9.71 b | 160 ± 11.31 b | 100 ± 10.60 c |
| SDI (g/mL) | 0.031 ± 0.001 a | 0.104 ± 0.006 b | 0.06 ± 0.004 c | 0.099 ± 0.001 b |
| Porosity (%) | 60 ± 0.002 a | 38 ± 0.005 b | 24 ± 0.005 c | 19 ± 0.007 c |
| TSS (g/L) | 0.07 ± 0.014 a | 0.45 ± 0.011 | 0.025 ± 0.007 b | 0.31 ± 0.014 c |
| VSS (g/L) | 1.2 ± 0.07 a | 0.6 ± 0.01 b | 1.77 ± 0.04 a | 1.49 ± 0.35 a |
| Days | Treatments | |||
|---|---|---|---|---|
| C | T1 | T2 | T3 | |
| 7th Day | 260 ± 6.63 a | 220 ± 8.85 a | 490 ± 14.41 b | 546 ± 9.33 b |
| 14th Day | 495 ± 6.63 a | 615 ± 16.18 b | 640 ± 43.40 b | 876 ± 79.19 c |
| 21st Day | 133 ± 18.89 a | 213.5 ± 18.89 b | 105 ± 8.99 a | 715 ± 24.48 c |
| 28th Day | 1057.5 ± 18.89 a | 174 ± 8.48 b | 960 ± 48.52 a | 410 ± 38.83 c |
| 35th Day | 925 ± 49.49 a | 698 ± 77.78 a | 665 ± 17.77 a | 710 ± 25.26 a |
| 42nd Day | 462.5 ± 24.74 a | 172.5 ± 36.06 b | 470 ± 31.11 a | 290.5 ± 18.79 b |
| 49th Day | 123 ± 15.55 a | 195.5 ± 36.06 a | 366 ± 14.65 b | 512 ± 12.45 c |
| 56th Day | 220 ± 55.68 a | 305 ± 20.60 b,c | 340 ± 29.84 c | 260 ± 12.79 a,b |
| 60th Day | 340 ± 45.8 a | 115 ± 25.60 b | 320 ± 21.4 a | 215 ± 22.9 c |
| Days | Treatments | |||
|---|---|---|---|---|
| C | T1 | T2 | T3 | |
| 7th day | 35 ± 6.61 a | 270 ± 28.28 b | 17.5 ± 3.53 a | 90 ± 56.56 c |
| 14th day | 150 ± 7.03 a | 16,625 ± 219.75 b | 134 ± 5.65 a | 19,150 ± 118.08 b |
| 21st day | 587 ± 56.82 a | 21,500 ± 202.08 b | 675 ± 148.49 a | 890 ± 42.42 c |
| 28th day | 1980 ± 31.27 a | 6750 ± 114.21 b | 19,000 ± 141.42 c | 2800 ± 48.52 d |
| 35th day | 15,468 ± 68.06 a | 11,688 ± 441.94 a | 21,875 ± 17.67 b | 39,375 ± 88.38 c |
| 42nd day | 3694 ± 36.56 a | 13,500 ± 141.421 b | 3900 ± 141.421 a | 6250 ± 70.71 c |
| 49th day | 5821 ± 52.19 a | 16,200 ± 282.84 b | 6850 ± 0.71 a | 8250 ± 70.71 c |
| 56th day | 6729 ± 63.23 a | 18,750 ± 70.71 b | 1550 ± 70.71 c | 9350 ± 70.71 a |
| 60th day | 7158 ± 28.61 a | 19,750 ± 60.71 b | 1457 ± 64.1 c | 9565 ± 34.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meenakshisundaram, M.; Mboya, J.B.; Sugantham, F.; Panigrahi, A.; Gamba, J.L.; Subramanian, S.; Chia, S.Y.; Beesigamukama, D.; Munguti, J.; Ogello, E.; et al. Synergistic Microbial Interactions Between Algae and Bacteria Augment Growth and Immune Performance in Red Tilapia (Oreochromis sp.). Aquac. J. 2025, 5, 12. https://doi.org/10.3390/aquacj5030012
Meenakshisundaram M, Mboya JB, Sugantham F, Panigrahi A, Gamba JL, Subramanian S, Chia SY, Beesigamukama D, Munguti J, Ogello E, et al. Synergistic Microbial Interactions Between Algae and Bacteria Augment Growth and Immune Performance in Red Tilapia (Oreochromis sp.). Aquaculture Journal. 2025; 5(3):12. https://doi.org/10.3390/aquacj5030012
Chicago/Turabian StyleMeenakshisundaram, Menaga, Jimmy B. Mboya, Felix Sugantham, Akshaya Panigrahi, Juliana L. Gamba, Sevgan Subramanian, Shaphan Y. Chia, Dennis Beesigamukama, Jonathan Munguti, Erick Ogello, and et al. 2025. "Synergistic Microbial Interactions Between Algae and Bacteria Augment Growth and Immune Performance in Red Tilapia (Oreochromis sp.)" Aquaculture Journal 5, no. 3: 12. https://doi.org/10.3390/aquacj5030012
APA StyleMeenakshisundaram, M., Mboya, J. B., Sugantham, F., Panigrahi, A., Gamba, J. L., Subramanian, S., Chia, S. Y., Beesigamukama, D., Munguti, J., Ogello, E., Yossa, R., & Tanga, C. M. (2025). Synergistic Microbial Interactions Between Algae and Bacteria Augment Growth and Immune Performance in Red Tilapia (Oreochromis sp.). Aquaculture Journal, 5(3), 12. https://doi.org/10.3390/aquacj5030012





