Exploring the Impact of Selenium Nanoparticles on Growth and Gonadal Development in Asian Seabass (Lates calcarifer): A Systematic Review and Meta-Analysis
Abstract
1. Introduction
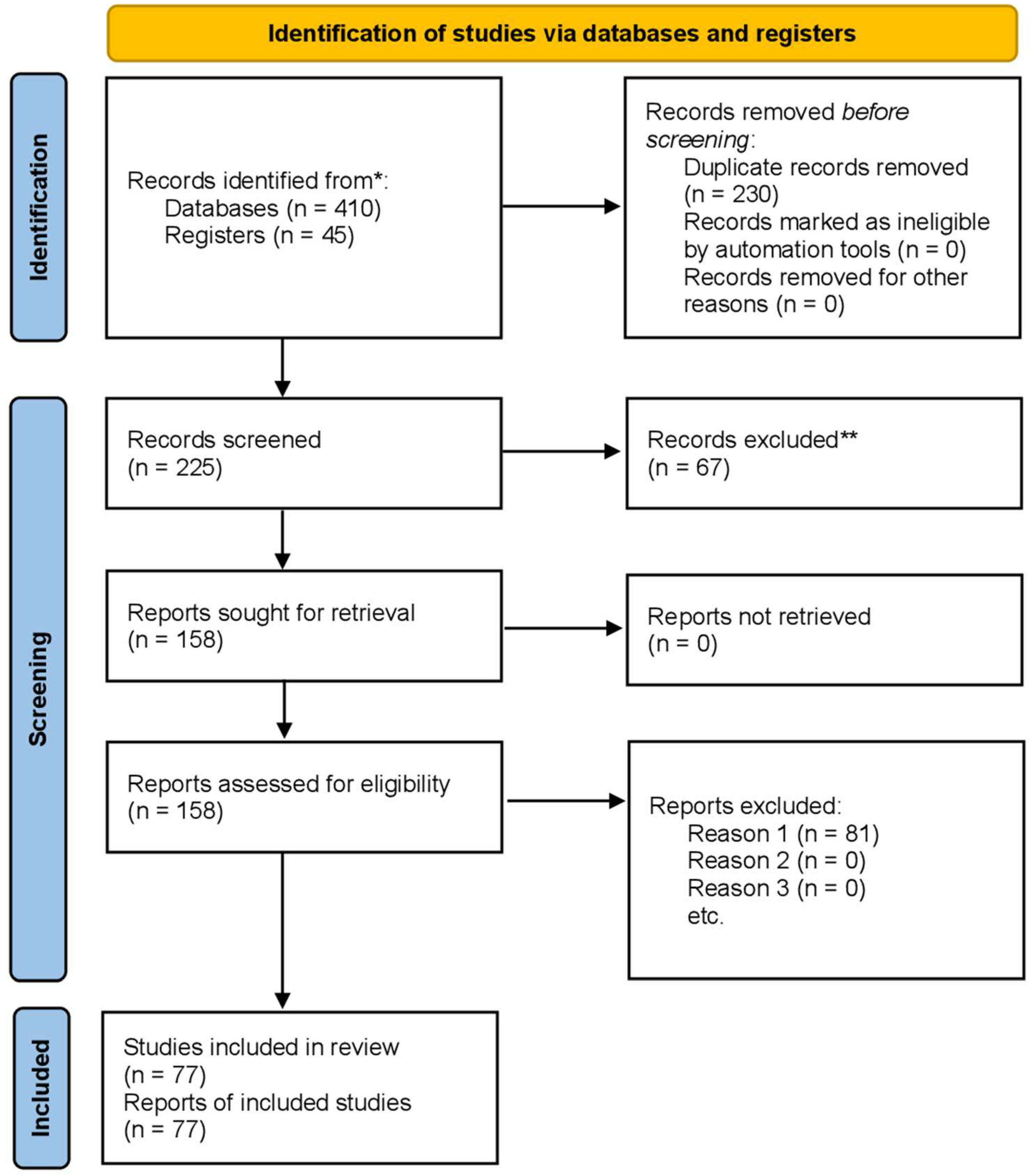
2. Materials and Methods
2.1. Data Collection and Extraction
2.2. Inclusion and Exclusion Criteria
2.3. Risk of Bias and Publication Bias Assessment
2.4. Data Calculation and Statistical Analysis
- Small Effect Size = 0.2 ≤ |d| ≤ 0.5;
- Medium Effect Size = 0.5 ≤ |d| ≤ 0.8;
- Large Effect Size = d ≥ 0.8.
3. Results and Discussion
3.1. Physicochemical Properties and Characterization of Selenium Nanoparticles
3.2. Effects of Selenium Nanoparticles on Biological Systems
3.3. Effect of Selenium Nanoparticles (SeNPs) on the Growth Performance of Asian Seabass
3.4. Effect of Selenium Nanoparticles (SeNPs) on Gonadal Development and Reproductive Performance of Asian Seabass
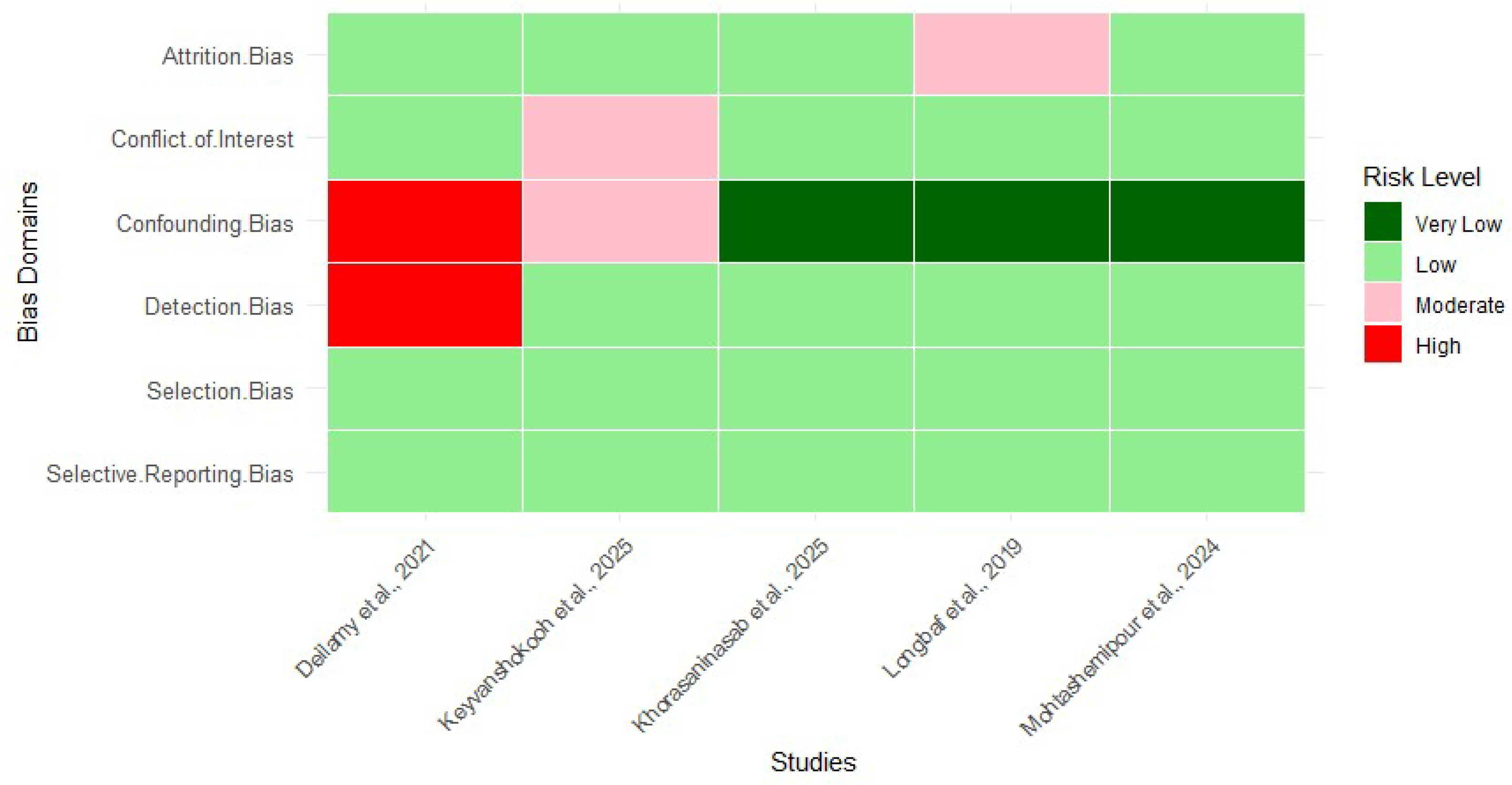
3.5. Risk of Bias Assessment of Included Studies
3.6. Potential Risks and Limitations of Selenium Nanoparticles (SeNPs) in Aquaculture
4. Conclusions
List of Abbreviations
| Abbreviation | Full Form |
| SD | Standard Deviation |
| SE | Standard Error |
| MD | Mean Difference |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| OSF | Open Science Framework |
| SGR | Specific Growth Rate |
| FCR | Feed Conversion Ratio |
| CI | Confidence Interval |
| SeNPs | Selenium Nanoparticles |
| CTS-SeNPs | Chitosan-Selenium Nanoparticles |
| AgNPs | Silver Nanoparticles |
| ZnONPs | Zinc Oxide Nanoparticles |
| GSI | Gonadosomatic Index |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilham, I.; Siddik, M.A.B.; Fotedar, R. Effects of organic selenium supplementation on growth, accumulation, haematology and histopathology of juvenile barramundi (Lates calcarifer) fed high soybean meal diets. Biol. Trace Elem. Res. 2016, 174, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Irmawati, I.; Umar, M.T.; Husain, A.A.A.; Malina, A.C.; Kadir, N.N.; Alimuddin, A. Distribution and characteristics of Asian seabass (Lates calcarifer Bloch, 1790) in South Sulawesi. IOP Conf. Ser. Earth Environ. Sci. 2020, 564, 012011. [Google Scholar] [CrossRef]
- Grey, D.L. An overview of Lates calcarifer in Australia and Asia. Manag. Wild Cult. Sea Bass/Barramundi 1987, 20, 15–21. [Google Scholar]
- Food and Agriculture Organization (FAO). Cultured Aquatic Species Information Programme, Aquaculture Topics and Activities; FAO Fisheries and Aquaculture Department: Rome, Italy, 2016. [Google Scholar]
- Siddik, M.A.B.; Islam, M.A.; Hanif, M.A.; Chaklader, M.R.; Kleindienst, R. Barramundi, Lates calcarifer (Bloch, 1790): A new dimension to fish farming in coastal Bangladesh. J. Aquac. Res. Dev. 2016, 7, 12. [Google Scholar]
- Vijayaram, S.; Ghafarifarsani, H.; Vuppala, S.; Nedaei, S.; Mahendran, K.; Murugappan, R.; Chou, C.-C. Selenium nanoparticles: Revolutionizing nutrient enhancement in aquaculture–A review. Biol. Trace Elem. Res. 2025, 203, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Khan, K.U.; Zuberi, A.; Nazir, S.; Fernandes, J.B.K.; Jamil, Z.; Sarwar, H. Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk. J. Zool. 2016, 40, 704–712. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Hakkaku, N.; Iwamoto, R.; Suzuki, J.; Suzuki, T.; Tajima, Y.; Konishi, K.; Minami, S.; Ichinose, S.; Ishizaka, K.; et al. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 2009, 284, 32522–32532. [Google Scholar] [CrossRef] [PubMed]
- Boitani, C.; Puglisi, R. Selenium, a key element in spermatogenesis and male fertility. Adv. Exp. Med. Biol. 2008, 636, 65–73. [Google Scholar] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Labunsky, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Penglase, S.; Nordgreen, A.; Van der Meeren, T.; Olsvik, P.A.; Sæle, Ø.; Sweetman, J.W.; Baeverfjord, G.; Helland, S.; Hamre, K. Increasing the level of selenium in rotifers (Brachionus plicatilis ‘Cayman’) enhances the mRNA expression and activity of glutathione peroxidase in cod (Gadus morhua L.) larvae. Aquaculture 2010, 306, 259–269. [Google Scholar] [CrossRef]
- Chris, U.O.; Singh, N.B.; Agarwal, A. Nanoparticles as feed supplement on growth behaviour of cultured catfish (Clarias gariepinus) fingerlings. Mater. Today: Proc. 2018, 5, 9076–9081. [Google Scholar] [CrossRef]
- Rathore, S.S.; Murthy, H.S.; Mamun, M.A.-A.; Nasren, S.; Rakesh, K.; Kumar, B.T.N.; Abhiman, P.B.; Khandagale, A.S. Nano-selenium supplementation to ameliorate nutrition physiology, immune response, antioxidant system and disease resistance against Aeromonas hydrophila in monosex Nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2021, 199, 3073–3088. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.S.; Maulu, S.; Verdegem, M.; Abdel-Tawwab, M. Embracing nanotechnology for selenium application in aquafeeds. Rev. Aquac. 2023, 15, 112–129. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; El-gendy, G.M.; Ahmed, A.I.; Elharoun, E.R.; Hassaan, M.S. Nanoselenium versus bulk selenium as a dietary supplement: Effects on growth, feed efficiency, intestinal histology, haemato-biochemical and oxidative stress biomarkers in Nile tilapia (Oreochromis niloticus Linnaeus, 1758) fingerlings. Aquac. Res. 2021, 52, 5642–5655. [Google Scholar] [CrossRef]
- Naderi, M.; Keyvanshokooh, S.; Ghaedi, A.; Salati, A.P. Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture 2017, 474, 40–47. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H.; Mozanzadeh, M.T. Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol. Biochem. 2018, 44, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, M.F.; Fath El-Bab, A.F.; Abd-Elghany, M.F.; Abdel-Warith, A.-W.A.; Younis, E.M.; Dawood, M.A.O. Selenium nanoparticles act potentially on the growth performance, hemato-biochemical indices, antioxidative, and immune-related genes of European seabass (Dicentrarchus labrax). Biol. Trace Elem. Res. 2021, 199, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Deilamy Pour, H.; Mousavi, S.M.; Zakeri, M.; Keyvanshokooh, S.; Kochanian, P. Synergistic effects of selenium and magnesium nanoparticles on growth, digestive enzymes, some serum biochemical parameters and immunity of Asian sea bass (Lates calcarifer). Biol. Trace Elem. Res. 2021, 199, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Longbaf Dezfouli, M.; Ghaedtaheri, A.; Keyvanshokooh, S.; Salati, A.P.; Mousavi, S.M.; Pasha-Zanoosi, H. Combined or individual effects of dietary magnesium and selenium nanoparticles on growth performance, immunity, blood biochemistry, and antioxidant status of Asian seabass (Lates calcarifer) reared in freshwater. Aquac. Nutr. 2019, 25, 1422–1430. [Google Scholar] [CrossRef]
- Khademzade, O.; Kochanian, P.; Zakeri, M.; Alavi, S.M.; Mozanzadeh, M.T. Oxidative Stress-Related Semen Quality and Fertility in the Male Arabian Yellowfin Sea Bream (Acanthopagrus arabicus) Fed a Selenium Nanoparticle-Supplemented Plant Protein-Rich Diet. Aquac. Nutr. 2022, 2022, 3979203. [Google Scholar] [CrossRef]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar] [PubMed]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Assessment of toxicity of selenium and cadmium selenium quantum dots: A review. Chemosphere 2017, 188, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Hageman, S.P.; van der Weijden, R.D.; Stams, A.J.; Buisman, C.J. Bio-production of selenium nanoparticles with diverse physical properties for recovery from water. Int. J. Miner. Process. 2017, 169, 7–15. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple, and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; Bouwmeester, H.; Gottardo, S.; Amenta, V.; Arena, M.; Brandhoff, P.; Marvin, H.J.; Mech, A.; Moniz, F.B.; Pesudo, L.Q.; et al. Nanomaterials for products and application in agriculture, feed, and food. Trends Food Sci. Technol. 2016, 54, 155–164. [Google Scholar] [CrossRef]
- Vera, P.; Echegoyen, Y.; Canellas, E.; Nerin, C.; Palomo, M.; Madrid, Y.; Cámara, C. Nano selenium as an antioxidant agent in a multilayer food packaging material. Anal. Bioanal. Chem. 2016, 408, 6659–6670. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Patel, H.; Patel, T.; Patel, K.; Selvaraj, K. Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf. B Biointerfaces 2013, 103, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A summary of new findings on the biological effects of selenium in selected animal species—A critical review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.G.; Cuencas Barrientos, M.E.; Mozzi, F.; Pescuma, M. Survival of selenium-enriched lactic acid bacteria in a fermented drink under storage and simulated gastrointestinal digestion. Food Res. Int. 2019, 123, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, X.; Chen, Q.; Yu, Q.; Sun, D.; Liu, J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.D.; Vardhanabhuti, B.; Lin, M.; Mustapha, A. Antibacterial properties of selenium nanoparticles and their toxicity to Caco-2 cells. Food Control 2017, 77, 17–24. [Google Scholar] [CrossRef]
- Khosravi, S.; Khabbazi, S.; Yoon, G.; Park, G.; Lee, B.; Bai, S.C. Effects of dietary supplementation with selenium, silver, and zinc oxide nanoparticles on the growth performance, meat quality, and immune response in fish. Aquac. Res. 2017, 48, 1236–1247. [Google Scholar]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental selenium and human health: An update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Fasil, D.M.; Hamdi, H.; Al-Barty, A.; Zaid, A.A.; Parashar, S.K.S.; Das, B. Selenium and zinc oxide multinutrient supplementation enhanced growth performance in zebra fish by modulating oxidative stress and growth-related gene expression. Front. Bioeng. Biotechnol. 2021, 9, 721717. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Zommara, M.; Eweedah, N.M.; Helal, A.I.; Aboel-Darag, M.A. The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2020, 27, 9843–9852. [Google Scholar] [CrossRef] [PubMed]
- Ashouri, S.; Keyvanshokooh, S.; Salati, A.P.; Johari, S.A.; Pasha-Zanoosi, H. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles, and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29. [Google Scholar] [CrossRef]
- Swain, P.; Das, R.; Das, A.; Padhi, S.K.; Das, K.C.; Mishra, S.S. Effects of dietary zinc oxide and selenium nanoparticles on growth performance, immune responses, and enzyme activity in rohu (Labeo rohita, Hamilton). Aquac. Nutr. 2019, 25, 486–494. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with selenomethionine in mice. Free. Radic. Biol. Med. 2001, 31, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2005, 23, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Yu, H.; Wu, Z.; Zhang, X. The comparative study of selenium nanoparticles with selenite on the reduction of selenite toxicity in zebrafish embryos. Nanotechnology 2007, 18, 325101. [Google Scholar]
- Hu, C.H.; Li, Y.L.; Xiong, L.; Zhang, Y.M.; Ren, Q.; Yang, Z.J. The role of selenium nanoparticles decorated with fluorescent tag in cellular imaging and therapy of cancers. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 83–91. [Google Scholar]
- Jia, X.; Wang, W.; Zhang, L.; Shi, C.; Zhang, X.; Chen, Z. A novel nano-selenium alleviates oxidative stress, hepatotoxicity, and nephrotoxicity induced by cisplatin in rats. Metallomics 2005, 7, 1544–1554. [Google Scholar]
- Mozanzadeh, M.T.; Safari, O.; Oosooli, R.; Mehrjooyan, S.; Najafabadi, M.Z.; Hoseini, S.J.; Saghavi, H.; Monem, J. The effect of salinity on growth performance, digestive and antioxidant enzymes, humoral immunity, and stress indices in two euryhaline fish species: Yellowfin seabream (Acanthopagrus latus) and Asian seabass (Lates calcarifer). Aquaculture 2021, 534, 736329. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Vatsos, I.N.; Rahman, M.A.; Pham, H.D. Selenium-enriched spirulina (SeE-SP) enhances antioxidant response, immunity, and disease resistance in juvenile Asian seabass (Lates calcarifer). Antioxidants 2022, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, M.F.; Fath El-Bab, A.F.; Shoukry, M. Evaluating the possible feeding strategies of selenium nanoparticles on the growth rate and wellbeing of European seabass (Dicentrarchus labrax). Aquac. Rep. 2020, 18, 100539. [Google Scholar] [CrossRef]
- Mohtashemipour, H.; Mohammadian, T.; Torfi Mozanzadeh, M.; Mesbah, M.; Jangaran Nejad, A. Dietary selenium nanoparticles improved growth and health indices in Asian seabass (Lates calcarifer) juveniles reared in high saline water. Aquac. Nutr. 2024, 2024, 7480824. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.G.; Yang, R.J.; Yue, W.B.; Xun, W.J.; Zhang, C.X.; Ren, Y.X.; Shi, L.; Lei, F.-L. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim. Reprod. Sci. 2010, 118, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Khademzade, O.; Kochanian, P.; Moini, S.; Zeynali, S. Selenium nanoparticle-supplemented plant protein-rich diet enhances selenium retention in liver, testes, and semen of male fish. Aquac. Nutr. 2021, 27, 1022–1033. [Google Scholar]
- Keyvanshokooh, S.; Salati, A.P.; Ghasemi, A.; Nazemroaya, S.; Houshmand, H.; Mozanzadeh, M.T. Reproductive Benefits of Dietary Selenium Nanoparticles (SeNPs) in Asian Seabass (Lates calcarifer) Male Broodstock. Mar. Biotechnol. 2025, 27, 45. [Google Scholar] [CrossRef] [PubMed]
- Khorasaninasab, S.A.; Keyvanshokooh, S.; Mozanzadeh, M.T.; Ghasemi, A.; Naderi, M.; Nazemroaya, S. Maternal supplementation with selenium nanoparticles enhances reproductive outcomes and larval quality in Asian seabass (Lates calcarifer) by influencing gene expression and physiological parameters. Aquac. Rep. 2025, 43, 102933. [Google Scholar] [CrossRef]
- Wu, L. Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicol. Environ. Saf. 2004, 57, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Lens, P.N. The essential toxin: The changing perception of selenium in environmental sciences. Sci. Total Environ. 2009, 407, 3620–3633. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, W.T.; Amrhein, C.; Fan, T.W.; Flaschi, D.; Glater, J.; Kartinen, E.; Kovac, K.; Lee, E.; Ohlendorf, H.; Owens, L.; et al. Advanced treatment technologies in the remediation of seleniferous drainage waters and sediments. Irrig. Drain. Syst. 2004, 18, 19–42. [Google Scholar] [CrossRef]
- Luoma, S.N.; Presser, T.S. Emerging opportunities in management of selenium contamination. Environ. Sci. Technol. 2009, 43, 8483–8487. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Krishnani, K.K.; Singh, N.P. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. 2018, 25, 8914–8927. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef] [PubMed]
- Dirican, S. A review on effects of selenium in the aquatic environment. Int. J. Agric. Sci. Nat. Resour. 2018, 5, 21–24. [Google Scholar]
- Bano, I.; Skalickova, S.; Arbab, S.; Urbankova, L.; Horky, P. Toxicological effects of nanoselenium in animals. J. Anim. Sci. Biotechnol. 2022, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- El-Sharawy, M.E.; Hamouda, M.; Soliman, A.A.; Amer, A.A.; El-Zayat, A.M.; Sewilam, H.; Younis, E.M.; Abdel-Warith, A.-W.A.; Dawood, M.A.O. Selenium nanoparticles are required for the optimum growth behavior, antioxidative capacity, and liver wellbeing of striped catfish (Pangasianodon hypophthalmus). Saudi J. Biol. Sci. 2021, 28, 7241–7247. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-M.; Wang, X.-L.; Jin, X.-M.; Huang, J.-Q.; Wang, L.-S. The effect of selenium on antioxidant system in aquaculture animals. Front. Physiol. 2023, 14, 1153511. [Google Scholar] [CrossRef] [PubMed]
- Mal, J.; Veneman, W.J.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Peijnenburg, W.J.; Vijver, M.G.; Lens, P.N.L. A comparison of fate and toxicity of selenite, biogenically, and chemically synthesized selenium nanoparticles to zebrafish (Danio rerio) embryogenesis. Nanotoxicology 2017, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Bhattacharjee, S.; Daware, A.; Tribedi, P.; Krishnani, K.K.; Minhas, P.S. Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res. Lett. 2015, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, L.; Little, E.E.; Buckler, D.R.; Wiedmeyer, R.H. Toxicity and bioaccumulation of waterborne and dietary selenium in juvenile bluegill (Lepomis macrochirus). Aquat. Toxicol. 1993, 27, 265–279. [Google Scholar] [CrossRef]
- Lemly, A.D. Toxic effects of selenium in fish. In Selenium Assessment in Aquatic Ecosystems: A Guide for Hazard Evaluation and Water Quality Criteria; Springer: New York, NY, USA, 2002; pp. 39–58. [Google Scholar]
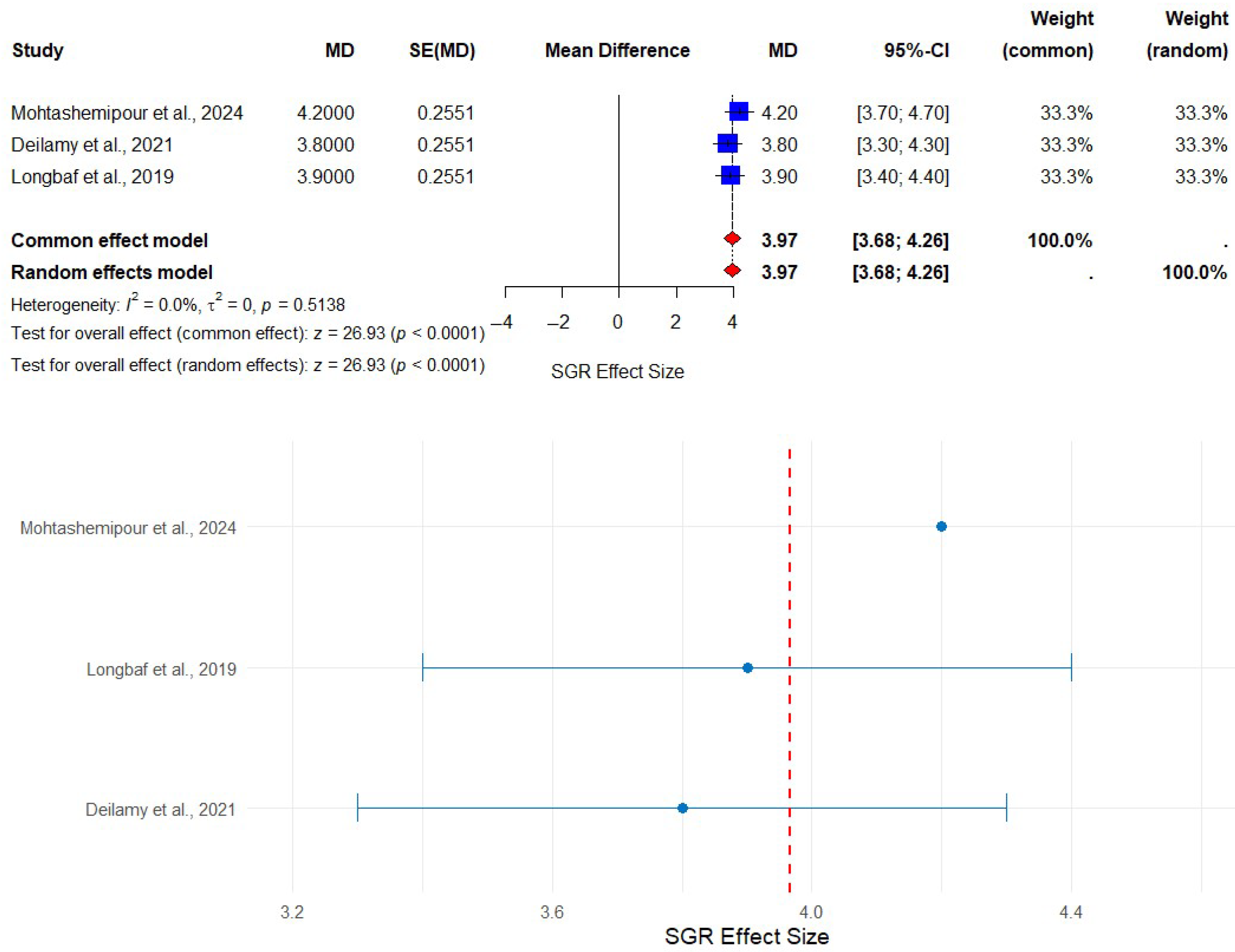
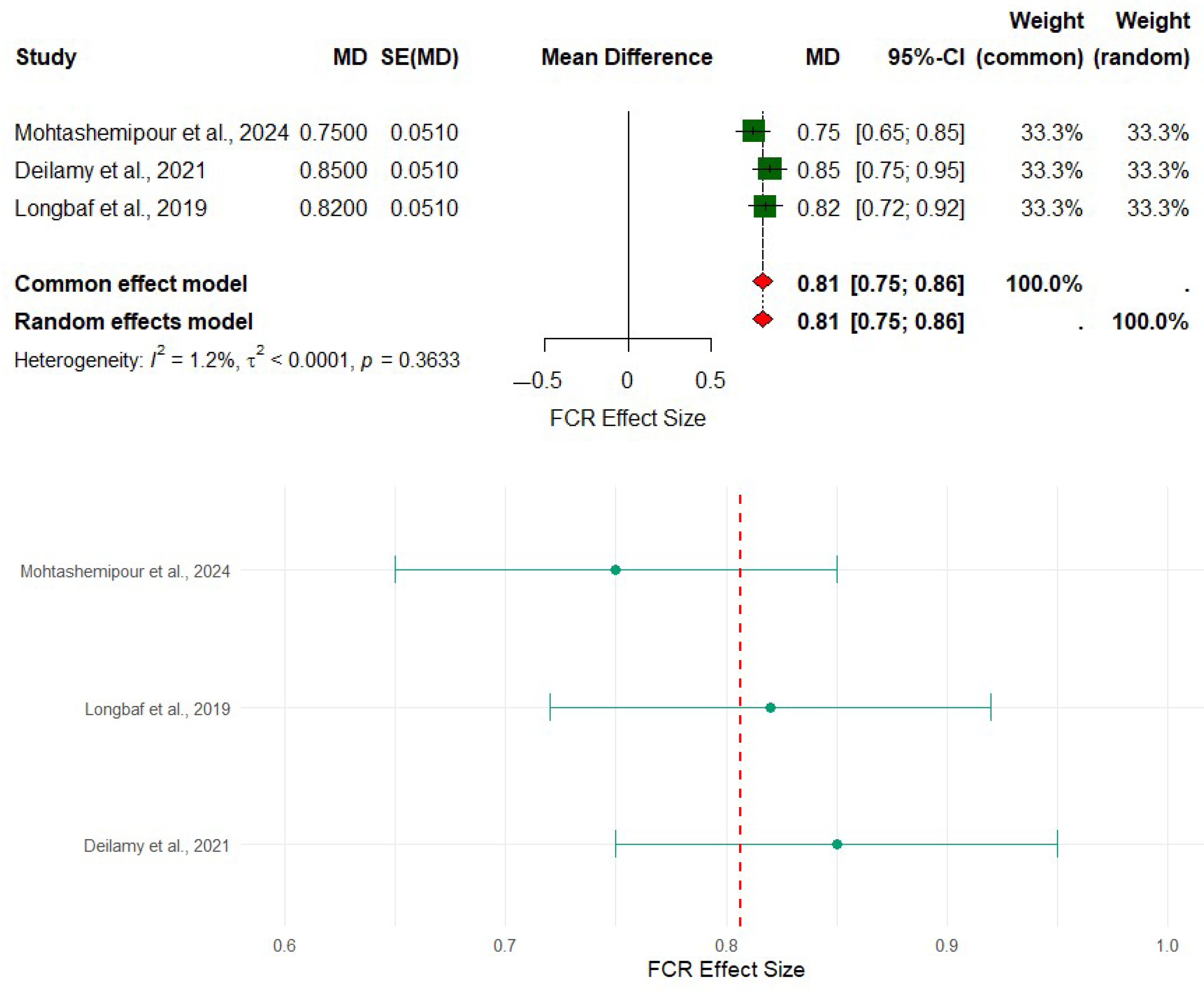
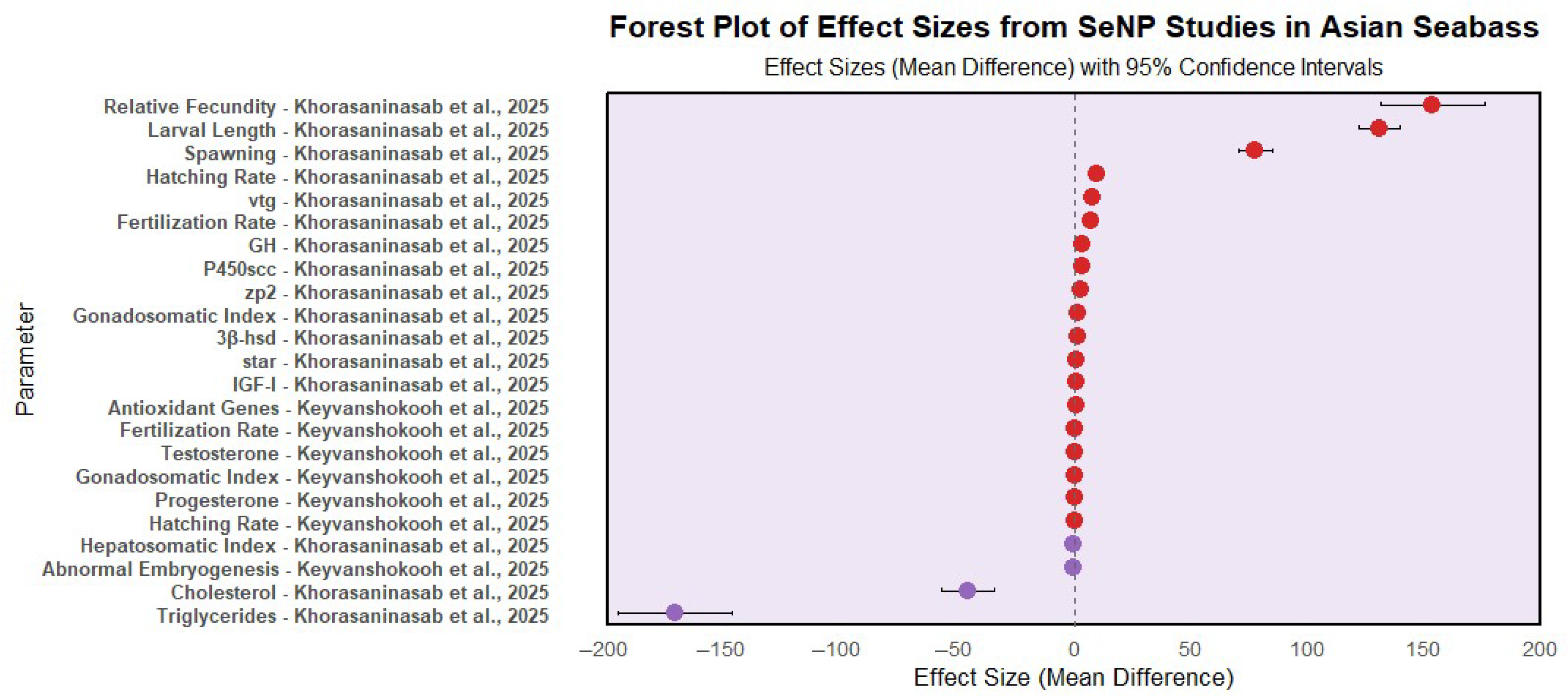
| Aquaculture Species | Administration Period | Inclusion Level (SeNPs) | Growth Performance | Digestive Enzymes | SGR | FCR | References |
|---|---|---|---|---|---|---|---|
| Asian seabass (Lates calcarifer) | 60 days | 0, 0.5, 1.0, 2, and 4 mg/kg | Linear and quadratic growth increase with SeNPs supplementation (p < 0.05) | Increased total protease, trypsin, chymotrypsin, ALP, lipase, and α-amylase in SeNPs4 (p < 0.05) | Highest SGR in SeNPs4 group | Best FCR observed in SeNPs4 group | [59] |
| 42 days | 4 mg/kg | Highest body weight gain, highest specific growth rate | Significant differences in digestive enzymes (except amylase) | Improved in combined treatment | Improved in combined treatment | [22] | |
| 42 days | 4 mg/kg diet | Improved weight gain, specific growth rate, and feed intake | Enhanced immune response, lower malondialdehyde | Higher in SeNPs and Combination groups | Reduced in SeNPs and MgNPs groups | [23] |
| Category | Parameter | Control Diet (CD) | SeNP-Supplemented Diet | Significance (p < 0.05) | Key Findings | Reference(s) |
|---|---|---|---|---|---|---|
| Reproductive Performance | Gonadosomatic Index (GSI) | Lower | Higher ↑ | ✅ | Enhanced reproductive potential | [62] |
| Spawning frequency | Lower | Higher | ✅ | SeNP-fed broodfish had increased spawning frequency | [63] | |
| Relative fecundity | Lower | Higher | ✅ | Higher egg production in SeNP group | ||
| Fertilization rate (%) | Lower | Higher ↑ | ✅ | Improved fertilization success and sperm quality | [62,63] | |
| Hatching rate (%) | Lower | Higher ↑ | ✅ | Boosted larval production and hatchability | ||
| Abnormal embryogenesis (%) | Higher ↑ | Lower | ✅ | Reduced developmental defects | [62] | |
| Antioxidant Defense | GPx activity | Lower | Higher | ✅ | Enhanced antioxidant capacity | [63] |
| Reduced glutathione (GSH) | Lower | Higher | ✅ | Better redox status | ||
| Malondialdehyde (MDA) | Higher | Lower | ✅ | Reduced oxidative stress | ||
| sod (Superoxide Dismutase) | Lower | Higher ↑ | ✅ | Increased ROS detoxification | [62] | |
| cat (Catalase) | Lower | Higher ↑ | ✅ | Strengthened oxidative stress response | ||
| gst (Glutathione-S-Transferase) | Lower | Higher ↑ | ✅ | Enhanced antioxidant activity | ||
| selenop (Liver) | Lower | Higher ↑ | ✅ | Selenium transport and metabolism | ||
| Hormonal Profile | Testosterone | Similar | Similar | ✘ | No significant change in T levels | [63] |
| Estradiol | Similar | Similar | ✘ | No significant change in E2 levels | ||
| Progesterone | Higher | Lower | ✅ | Decreased progesterone in SeNP-fed fish | ||
| ar (Androgen Receptor, Testis) | Lower | Higher ↑ | ✅ | Testosterone regulation | [62] | |
| p450scc (Steroidogenesis, Testis) | Lower | Higher ↑ | ✅ | Enhanced hormone synthesis | [62,63] | |
| cdk1 (Cell Cycle, Testis) | Lower | Higher ↑ | ✅ | Improved cell division | [62] | |
| Serum cholesterol and triglycerides | Higher | Lower | ✅ | Better lipid metabolism | [63] | |
| Gene Expression | Steroidogenic genes (star, P450scc, 3β-hsd) | Lower expression | Upregulated | ✅ | Promoted steroidogenesis | [62,63] |
| Vitellogenesis genes (zp2, vtg) | Lower expression | Upregulated | ✅ | Improved vitellogenesis | [63] | |
| Larval Quality | Growth-promoting genes (GH, IGF-I) | Lower expression | Higher expression | ✅ | Better larval growth gene expression | |
| Body size and developmental traits | Smaller/less developed | Larger/better developed | ✅ | Enhanced larval morphology | ||
| Se Deposition | Se in liver, ovary, and larvae | Lower | Higher | ✅ | Improved Se bioavailability and tissue deposition | |
| Safety and Toxicity | Adverse effects observed? | No | No | – | Safe for dietary SeNP supplementation | [62,63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.; Siddique, M.A.B.; Hasan, S.J.; Haque, M.M.; Hasan, M.M.; Ahammad, A.K.S. Exploring the Impact of Selenium Nanoparticles on Growth and Gonadal Development in Asian Seabass (Lates calcarifer): A Systematic Review and Meta-Analysis. Aquac. J. 2025, 5, 11. https://doi.org/10.3390/aquacj5030011
Ahmed I, Siddique MAB, Hasan SJ, Haque MM, Hasan MM, Ahammad AKS. Exploring the Impact of Selenium Nanoparticles on Growth and Gonadal Development in Asian Seabass (Lates calcarifer): A Systematic Review and Meta-Analysis. Aquaculture Journal. 2025; 5(3):11. https://doi.org/10.3390/aquacj5030011
Chicago/Turabian StyleAhmed, Ilias, Mohammad Abu Baker Siddique, Shanur Jahedul Hasan, Mohammad Mahfujul Haque, Md. Mahmudul Hasan, and A. K. Shakur Ahammad. 2025. "Exploring the Impact of Selenium Nanoparticles on Growth and Gonadal Development in Asian Seabass (Lates calcarifer): A Systematic Review and Meta-Analysis" Aquaculture Journal 5, no. 3: 11. https://doi.org/10.3390/aquacj5030011
APA StyleAhmed, I., Siddique, M. A. B., Hasan, S. J., Haque, M. M., Hasan, M. M., & Ahammad, A. K. S. (2025). Exploring the Impact of Selenium Nanoparticles on Growth and Gonadal Development in Asian Seabass (Lates calcarifer): A Systematic Review and Meta-Analysis. Aquaculture Journal, 5(3), 11. https://doi.org/10.3390/aquacj5030011






