A Practical and Effective Artemia Hatching Method to Eliminate Covert Mortality Nodavirus (CMNV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Artemia Cysts and the Detection of CMNV
2.1.1. Artemia Cysts
2.1.2. RNA Extraction
2.1.3. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.1.4. Result Determination
2.2. Treatments of CMNV+ Artemia Cysts
2.3. CMNV Detection and Viral Load Quantification
3. Results
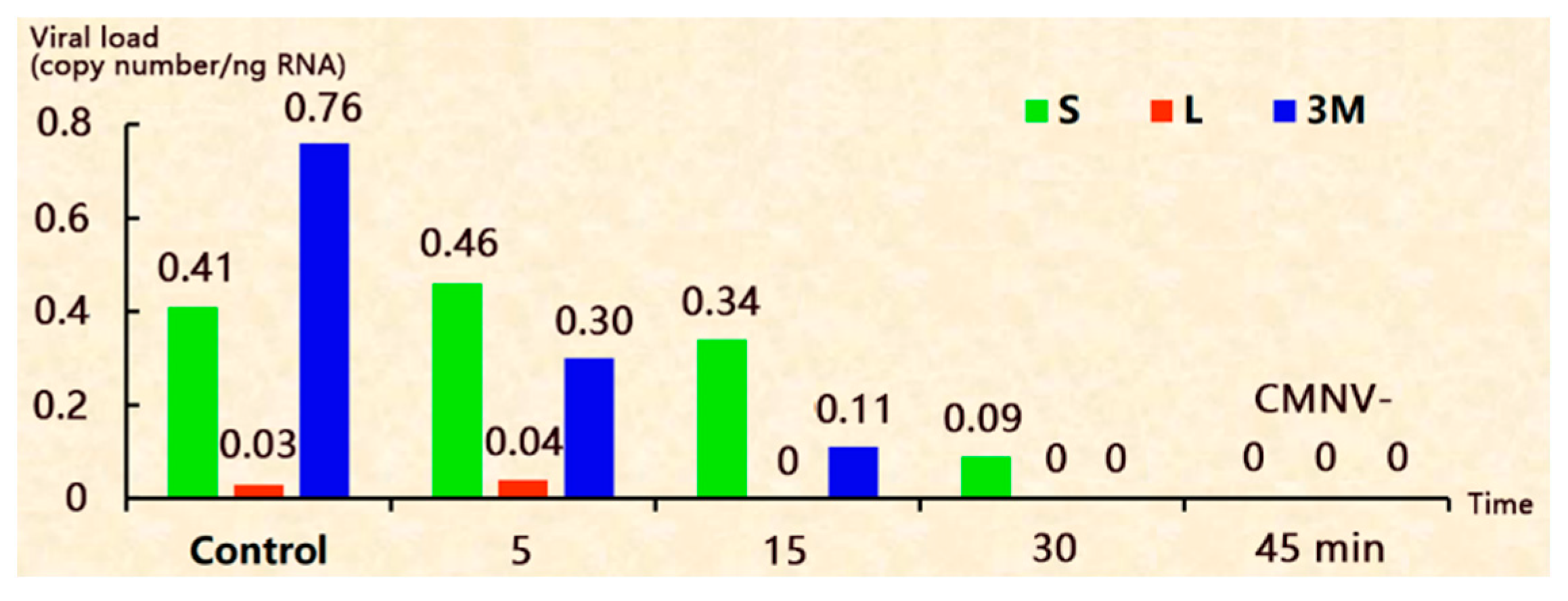
3.1. No Treatment (The Control Group)
3.2. Decapsulation with Na2CO3-NaClO
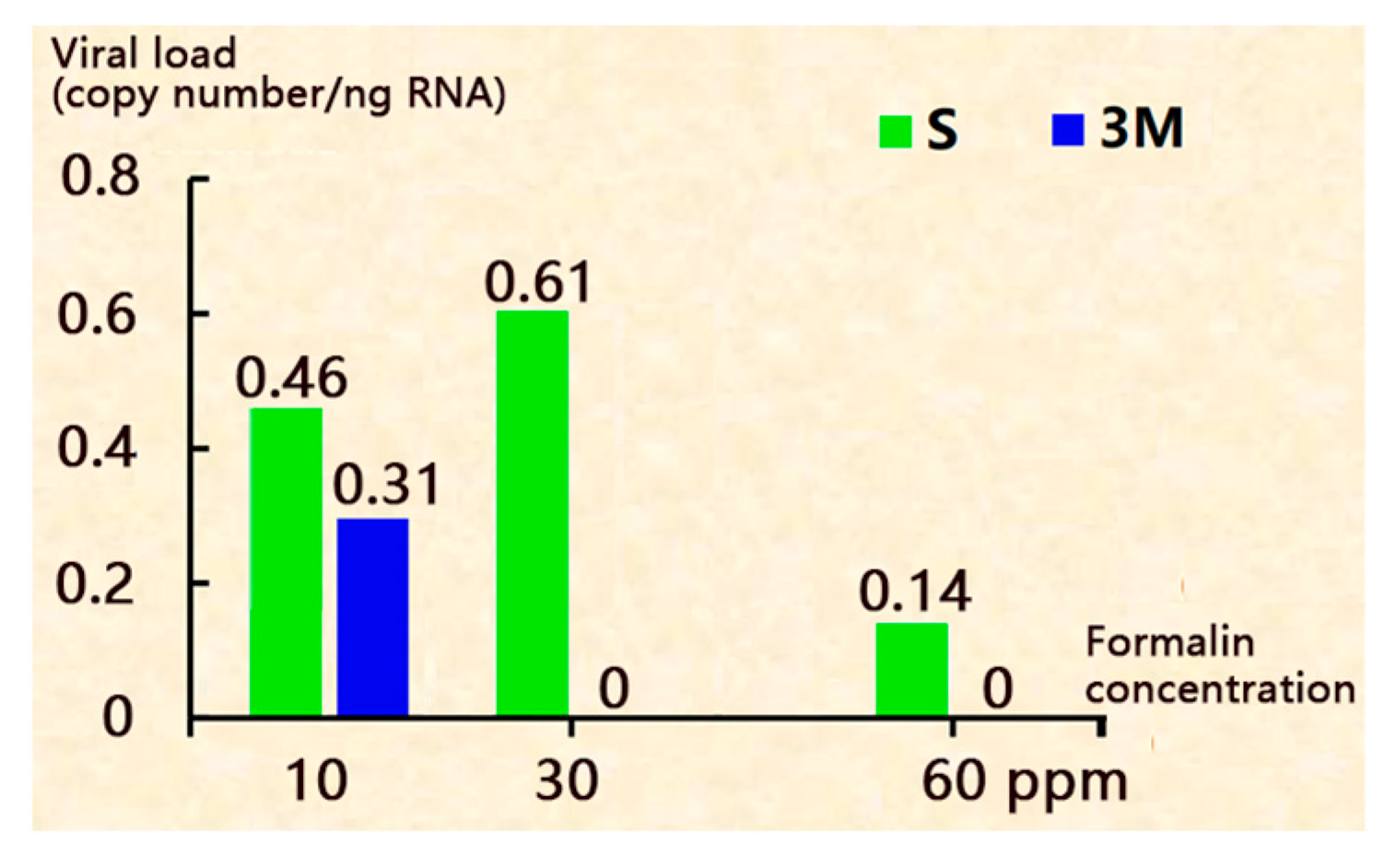
3.3. Adding Formalin in the Hatching Water
3.4. Combination of Decapsulation and Adding Formalin in the Hatching Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Ji, F. Comprehensive control of the covert mortality disease of Pacific white shrimp. Fish Guide to be Rich 2009, 1, 60. [Google Scholar]
- Zhang, Q.; Liu, Q.; Liu, S.; Yang, H.; Liu, S.; Zhu, L.; Yang, B.; Jin, J.; Ding, L.; Wang, X. A new nodavirus is associated with covert mortality disease of shrimp. J. Gen. Virol. 2014, 95, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Y.; Yeap, S.K.; Omar, A.R.; Tan, W.S. Advances in the study of nodavirus. PeerJ 2017, 5, e3841. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S. Chapter 38—Nodaviruses of crustaceans. In Aquaculture Virology, 2nd ed.; Kibenge, F.S.B., Godoy, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 621–641. [Google Scholar]
- Zhang, Q.; Xu, T.; Wan, X.; Liu, S.; Wang, X.; Li, X.; Dong, X.; Yang, B.; Huang, J. Prevalence and distribution of covert mortality nodavirus (CMNV) in cultured crustacean. Virus Res. 2017, 233, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, C.; Yao, L.; Wang, W.; Zhao, W.; Jia, T.; Yu, X.; Yang, G.; Zhang, Q. Investigation on natural infection of covert mortality nodavirus in farmed giant freshwater prawn (Macrobrachium rosenbergii). Animals 2022, 12, 1370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.X.; Wan, X.Y.; Xia, J.T.; Yao, L.; Xu, R.D.; Wang, W.; Yu, X.T.; Zhang, Q.L. Investigation of the prevalence of covert mortality nodavirus (CMNV) in shrimp from 2021 to 2022. Prog. Fish. Sci. 2024, 45, 195–203. [Google Scholar]
- Liu, S.; Wang, X.; Xu, T.; Li, X.; Du, L.; Zhang, Q. Vectors and reservoir hosts of covert mortality nodavirus (CMNV) in shrimp ponds. J. Invertebr. Pathol. 2018, 154, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lavens, P.; Sorgeloos, P. The history, present status and prospects of the availability of Artemia cysts for aquaculture. Aquaculture 2000, 181, 397–403. [Google Scholar] [CrossRef]
- Sorgeloos, P.; Bossuyt, E.; Laviña, E.; Baeza-Mesa, M.; Persoone, G. Decapsulation of Artemia cysts: A simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture 1977, 12, 311–315. [Google Scholar] [CrossRef]
- Xu, T.; Liu, S.; Li, X.; Zhang, Q. Genomic characterization of covert mortality nodavirus from farming shrimp: Evidence for a new species within the family Nodaviridae. Virus Res. 2020, 286, 198092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Yang, H.; Zhu, L.; Wan, X.; Li, X.; Huang, J. Reverse transcription loop-mediated isothermal amplification for rapid and quantitative assay of covert mortality nodavirus in shrimp. J. Invertebr. Pathol. 2017, 150, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zeng, C.; Xia, J.; Liu, Q.; Fang, J.; Zhang, Q. Disinfection of Perinereis aibuhitensis eggs with peroxymonosulfate to eliminate covert mortality nodavirus (CMNV). Aquaculture 2023, 572, 739539. [Google Scholar] [CrossRef]
- Millamena, O.M.; Bombeo, R.F.; Jumalon, N.A.; Simpson, K. Effects of various diets on the nutritional value of Artemia sp. as food for the prawn Penaeus monodon. Mar. Biol. 1988, 98, 217–221. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Sui, L.; Ye, S.; Dong, X.; Huang, J. Research progress on the risk of Artemia acting as a kind of live feed to spread shrimp pathogens. China Anim. Health Inspect 2020, 37, 61–67. [Google Scholar]
- Defoirdt, T.; Crab, R.; Wood, T.K.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Quorum sensing-disrupting brominated furanones protect the gnotobiotic brine shrimp Artemia franciscana from pathogenic Vibrio harveyi, Vibrio campbellii, and Vibrio parahaemolyticus isolates. Appl. Environ. Microbiol. 2006, 72, 6419–6423. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Sudhakaran, R. Exploring the potentiality of Artemia salina to act as a reservoir for microsporidian Enterocytozoon hepatopenaei of penaeid shrimp. Biocatal. Agric. Biotechnol. 2020, 25, 101607. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, L.; Dong, W.; Tian, L.; Cao, C.; Li, J.; Yan, C. Assessment of the role of brine shrimp Artemia in white spot syndrome virus (WSSV) transmission. Vet. Res. Commun. 2010, 34, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, R.; Yoganandhan, K.; Ahmed, V.I.; Hameed, A.S. Artemia as a possible vector for Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) transmissionto Macrobrachium rosenbergii post-larvae. Dis. Aquat. Org. 2006, 70, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sanz, V.A. Specific pathogen free (SPF), specific pathogen resistant (SPR) and specific pathogen tolerant (SPT) as part of the biosecurity strategy for whiteleg shrimp (Penaeus vannamei Boone 1931). Asian Fish Soc. 2018, 31, 112–120. [Google Scholar]
- Eswaran, S. Specific pathogen free (SPF) shrimps in aquaculture. In Advances in Fisheries Biotechnology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 465–470. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Wang, J.; Jiang, D.; Wang, Y.; Jiang, H.G. A Practical and Effective Artemia Hatching Method to Eliminate Covert Mortality Nodavirus (CMNV). Aquac. J. 2025, 5, 5. https://doi.org/10.3390/aquacj5010005
Lu Z, Wang J, Jiang D, Wang Y, Jiang HG. A Practical and Effective Artemia Hatching Method to Eliminate Covert Mortality Nodavirus (CMNV). Aquaculture Journal. 2025; 5(1):5. https://doi.org/10.3390/aquacj5010005
Chicago/Turabian StyleLu, Zhangwang, Jun Wang, Donghuo Jiang, Yan Wang, and Hui G. Jiang. 2025. "A Practical and Effective Artemia Hatching Method to Eliminate Covert Mortality Nodavirus (CMNV)" Aquaculture Journal 5, no. 1: 5. https://doi.org/10.3390/aquacj5010005
APA StyleLu, Z., Wang, J., Jiang, D., Wang, Y., & Jiang, H. G. (2025). A Practical and Effective Artemia Hatching Method to Eliminate Covert Mortality Nodavirus (CMNV). Aquaculture Journal, 5(1), 5. https://doi.org/10.3390/aquacj5010005




