Individual Shrimp Rearing Increases the Power of Experimental Trials
Abstract
1. Introduction
- Calculated differential survival rates;
- Evaluated growth performance parameters (i.e., final body weight and length);
- Determined welfare parameters (i.e., final antenna length and frequency of cataracts).
2. Materials and Methods
2.1. Population Studied and Pre-Treatment
2.2. Experimental Design of Group-Size Trial
2.3. Statistical Analyses
- -
- Survival rate (%) = 100 × final number of shrimp/initial number of shrimp;
- -
- Final body weight (g);
- -
- Final body length (cm);
- -
- Total antenna length (cm) = length left antenna + length right antenna;
- -
- Cataract rate (%) = 100 × number of shrimp/(number of cataracts in left eye + number of cataracts in right eye).
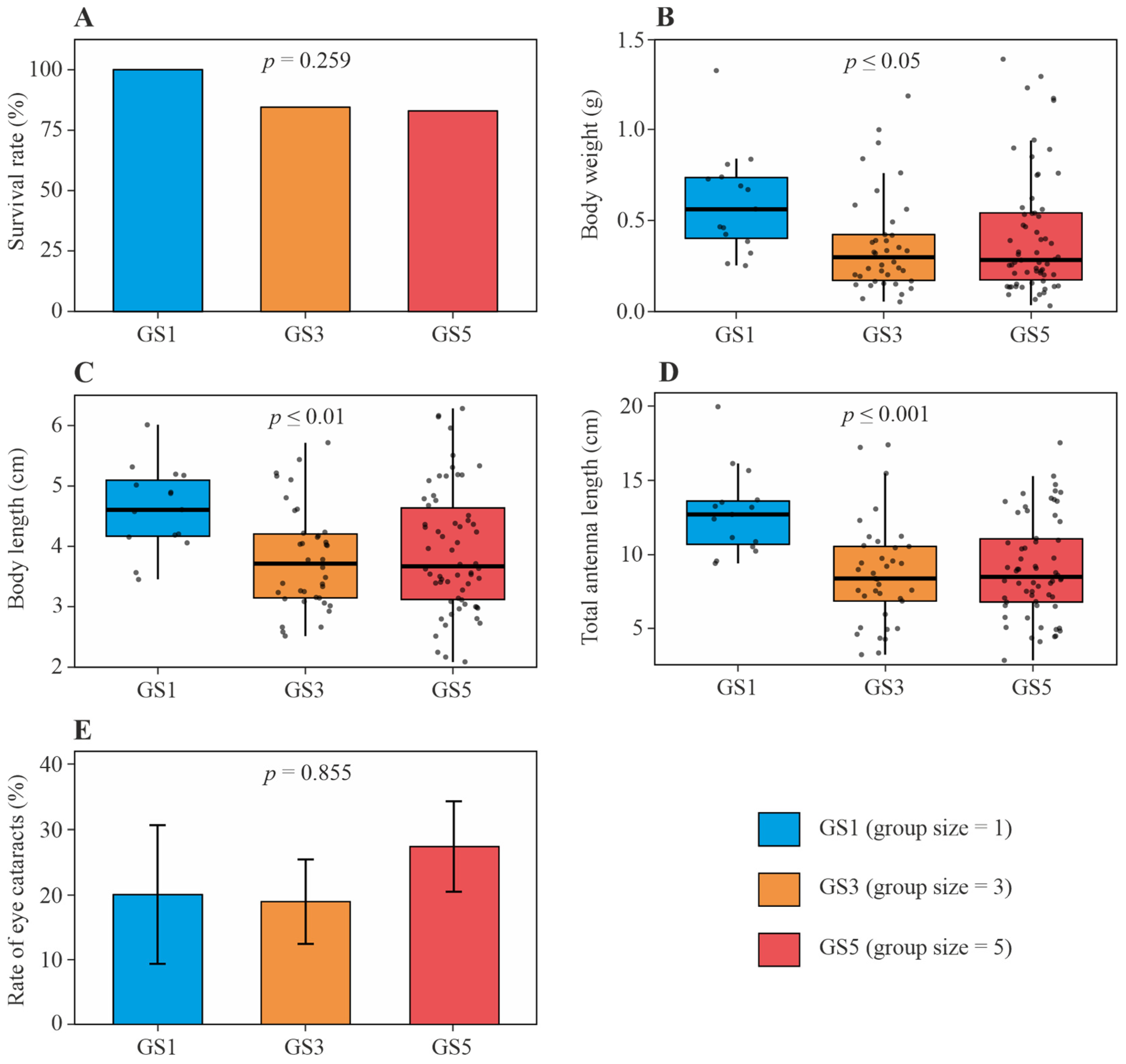
3. Results
3.1. PWS Survival Rate
3.2. PWS Growth Parameters
3.3. PWS Welfare Parameters
4. Discussion
4.1. High Survival Rate of PWS in Single-Shrimp System
4.2. Increased Growth Performance of PWS in Single-Shrimp System
4.3. No Reduced Welfare of PWS in Single-Shrimp System
4.4. Rearing PWS in Single-Shrimp Systems Does Not Bias Experimental Trials
4.5. Limitations and Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2021 (FishStatJ); FAO Fisheries and Aquaculture Division: Rome, Italy, 2023. [Google Scholar]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Cock, J.; Gitterle, T.; Salazar, M.; Rye, M. Breeding for disease resistance of Penaeid shrimps. Aquaculture 2009, 286, 1–11. [Google Scholar] [CrossRef]
- Cummins, V.C.; Rawles, S.D.; Thompson, K.R.; Velasquez, A.; Kobayashi, Y.; Hager, J.; Webster, C.D. Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2017, 473, 337–344. [Google Scholar] [CrossRef]
- Röthig, T.; Barth, A.; Tschirner, M.; Schubert, P.; Wenning, M.; Billion, A.; Wilke, T.; Vilcinskas, A. Insect feed in sustainable crustacean aquaculture. J. Insects Food Feed. 2023, 9, 1115–1138. [Google Scholar] [CrossRef]
- Albalat, A.; Zacarias, S.; Coates, C.J.; Neil, D.M.; Planellas, S.R. Welfare in farmed decapod crustaceans, with particular reference to Penaeus vannamei. Front. Mar. Sci. 2022, 9, 886024. [Google Scholar] [CrossRef]
- Pedrazzani, A.S.; Cozer, N.; Quintiliano, M.H.; Tavares, C.P.d.S.; da Silva, U.d.A.T.; Ostrensky, A. Non-Invasive Methods for Assessing the Welfare of Farmed White-Leg Shrimp (Penaeus vannamei). Animals 2023, 13, 807. [Google Scholar] [CrossRef]
- Ren, S.; Mather, P.B.; Tang, B.; Hurwood, D.A. Insight into selective breeding for robustness based on field survival records: New genetic evaluation of survival traits in pacific white shrimp (Penaeus vannamei) breeding line. Front. Genet. 2022, 13, 1018568. [Google Scholar] [CrossRef]
- Samocha, T.M.; Hamper, L.; Emberson, C.R.; Davis, A.D.; McIntosh, D.; Lawrence, A.L.; Van Wyk, P.M. Review of some recent developments in sustainable shrimp farming practices in Texas, Arizona, and Florida. J. Appl. Aquac. 2002, 12, 1–42. [Google Scholar] [CrossRef]
- Barth, A.; Stelbrink, B.; Klüber, P.; Schubert, P.; Bendag, S.; Wilke, T. Broad acceptance of sustainable insect-based shrimp feeds requires reproducible and comparable research. Aquac. Int. 2025, 33, 101. [Google Scholar] [CrossRef]
- Neal, R.A. Methods of marking shrimp. In Proceedings of the World Scientific Conference on the Biology and Culture of Shrimps and Prawns; Mistakidis, M.N., Ed.; FAO Fisheries Reports; FAO: Rome, Italy, 1969; Volume 57, pp. 589–1165. [Google Scholar]
- Jepsen, N.; Thorstad, E.B.; Havn, T.; Lucas, M.C. The use of external electronic tags on fish: An evaluation of tag retention and tagging effects. Anim. Biotelemetry 2015, 3, 49. [Google Scholar] [CrossRef]
- Arce, S.M.; Argue, B.J.; Thompson, D.A.; Moss, S.M. Evaluation of a fluorescent, alphanumeric tagging system for penaeid shrimp and its application in selective breeding programs. Aquaculture 2003, 228, 267–278. [Google Scholar] [CrossRef]
- George, M.J. Mark-recovery experiments in crustaceans. In Proceedings of the Symposium on Crustacea, Ernakulam, India, 12–15 January 1965; pp. 1284–1295. [Google Scholar]
- Bardera, G.; Owen, M.A.G.; Façanha, F.N.; Alcaraz-Calero, J.M.; Alexander, M.E.; Sloman, K.A. The influence of density and dominance on Pacific white shrimp (Litopenaeus vannamei) feeding behaviour. Aquaculture 2021, 531, 735949. [Google Scholar] [CrossRef]
- Foote, A.R.; Stratford, C.N.; Coman, G.J. Passive integrated transponder (PIT) tagging black tiger shrimp, Penaeus monodon: Applications for breeding programs. Aquaculture 2018, 491, 321–324. [Google Scholar] [CrossRef]
- Brown, J.H.; McCauley, S.; Ross, B.; Taylor, A.; Huntingford, F. A test of two methods for marking larvae and postlarvae of the giant freshwater prawn, Macrobrachium rosenbergii. Aquac. Res. 2003, 34, 49–54. [Google Scholar] [CrossRef]
- Arce, S.M.; Moss, S.M. Tagging Technology Helps Monitor Shrimp Performance in Research, Genetic Improvement. Global Seafood Alliance. 2002. Available online: https://www.globalseafood.org/advocate/tagging-technology-helps-monitor-shrimp-performance-in-research-genetic-improvement/ (accessed on 24 November 2024).
- Gibbons, W.J.; Andrews, K.M. PIT tagging: Simple technology at its best. BioScience 2004, 54, 447–454. [Google Scholar] [CrossRef]
- da Costa, F.P.; Gomes, B.S.F.d.F.; Pereira, S.D.d.N.A.; de Fátima Arruda, M. Influence of stocking density on the behaviour of juvenile Litopenaeus vannamei (Boone, 1931). Aquac. Res. 2016, 47, 912–924. [Google Scholar] [CrossRef]
- Bardera, G.; Usman, N.; Owen, M.; Pountney, D.; Sloman, K.A.; Alexander, M.E. The importance of behaviour in improving the production of shrimp in aquaculture. Rev. Aquac. 2019, 11, 1104–1132. [Google Scholar] [CrossRef]
- Hultgren, K.; Emmett Duffy, J.; Rubenstein, D.R. Sociality in Shrimps. In Comparative Social Evolution; Rubenstein, D.R., Abbot, P., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 224–250. [Google Scholar]
- Studer, B.H. Pacific Whiteleg Shrimp Litopenaeus vannamei. FishEthoBase. 2015. Available online: https://www.researchgate.net/publication/295255663 (accessed on 24 November 2024).
- Dugassa, H.; Gaetan, D. Biology of White leg shrimp, Penaeus vannamei: Review. World J. Fish Mar. Sci. 2018, 10, 5–17. [Google Scholar]
- Bayot, B.; Rodríguez, J.; Arguello, W.; Cornejo-Rodríguez, M.H.; Sonnenholzner, S. An evaluation of intraspecific competition for Pacific white shrimp Penaeus vannamei (Boone) in extensive/semi-intensive ponds. Aquac. Int. 2014, 22, 1025–1039. [Google Scholar] [CrossRef]
- Shrimp Welfare Project. Shrimp Welfare Report. 2024. Available online: https://www.shrimpwelfareproject.org/shrimp-welfare-report (accessed on 24 November 2024).
- Compassion in World Farming International. Whiteleg Shrimp—Corporate Ask. 2024. Available online: https://www.compassioninfoodbusiness.com/resources/fish/whiteleg-shrimp-corporate-ask/ (accessed on 24 November 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 24 November 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Obaldo, L.G.; Masuda, R. Effect of diet size on feeding behavior and growth of Pacific white shrimp, Litopenaeus vannamei. J. Appl. Aquac. 2006, 18, 101–110. [Google Scholar] [CrossRef]
- Sanchez, D.; Fox, J.M.; Lawrence, A.; Castille, F.; Dunsford, B. A methodology for evaluation of dietary feeding stimulants for the Pacific white shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2005, 36, 14–23. [Google Scholar] [CrossRef]
- Stallings, C.D.; Dingeldein, A.L. Intraspecific cooperation facilitates synergistic predation. Bull. Mar. Sci. 2012, 88, 317–318. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Shayo, F.A.; Nevejan, N.; Van Hoa, N. Effects of stocking densities and feeding rates on water quality, feed efficiency, and performance of white leg shrimp Litopenaeus vannamei in an integrated system with sea grape Caulerpa lentillifera. J. Appl. Phycol. 2021, 33, 3331–3345. [Google Scholar] [CrossRef]
- Frommen, J.G. Aggressive communication in aquatic environments. Funct. Ecol. 2020, 34, 364–380. [Google Scholar] [CrossRef]
- Thiel, M.; Breithaupt, T. Chemical communication in crustaceans: Research challenges for the twenty-first century. In Chemical Communication in Crustaceans; Breithaupt, T., Thiel, M., Eds.; Springer: New York, NY, USA, 2010; pp. 3–22. [Google Scholar]

| Characteristic | PWS |
|---|---|
| Species name | Pacific white shrimp, Penaeus vannamei Boone, 1931 |
| Origin | Suburban Seafood Germany UG (Nebelschütz, Germany) |
| Genetic strain | For DNA barcoding information (COI) on the strain used see GenBank accession numbers PQ465998 and PQ465999 |
| Life stage | Juvenile specimens (71 days old) |
| Weight | 0.10 ± 0.07 g |
| Length | 2.48 ± 0.46 cm |
| Characteristic | PWS |
|---|---|
| Duration of experiment | 30 days (20 July 2023–18 August 2023) |
| Rearing system | Indoor recirculation clear water system with plastic tanks (capacity 9.2 L each) |
| Group size | 1, 3, or 5 shrimp per tank |
| Replications | 15 replications each for group sizes of 1, 3 and 5 shrimp |
| Feed | Daily at 8.00 a.m., 12:00 a.m., 4:00 p.m., and 8:00 p.m.: 0.003 g per shrimp “Vanna Starter PL3” (Le Gouessant Aquaculture, Lamballe, France) Daily at 8:00 p.m.: 0.004 g (days 1–18) or 0.008 g (days 19–30) per shrimp Spirulina (Spirulina Flakes, Ocean Nutrition, Essen, Belgium) |
| Water filtration | Vlies Dreambox 3.0 with fleece filter and protein skimmer (Royal Exclusiv Christian Walter GmbH & Co. KG, Wesseling, Germany), and a flow-through biofilter with media (Aqua-Light GmbH, Bramsche, Germany) |
| Lightning | LED light (ca. 12 h light/12 h dark) |
| Water parameters | |
| Salinity | 26.8 ± 0.53 |
| Temperature (°C) | 27.5 ± 0.36 |
| pH | 8.0 ± 0.1 |
| Dissolved oxygen content (mg L−1) | 6.30 ± 0.05 |
| Nitrite content (mg L−1) | 2–10 |
| Nitrate content (mg L−1) | 25–50 |
| Phosphate content (mg L−1) | 0.2–0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilke, T.; Bendag, S.; Barth, A.; Reinold, T.; Schubert, P. Individual Shrimp Rearing Increases the Power of Experimental Trials. Aquac. J. 2025, 5, 2. https://doi.org/10.3390/aquacj5010002
Wilke T, Bendag S, Barth A, Reinold T, Schubert P. Individual Shrimp Rearing Increases the Power of Experimental Trials. Aquaculture Journal. 2025; 5(1):2. https://doi.org/10.3390/aquacj5010002
Chicago/Turabian StyleWilke, Thomas, Slim Bendag, Annalena Barth, Tim Reinold, and Patrick Schubert. 2025. "Individual Shrimp Rearing Increases the Power of Experimental Trials" Aquaculture Journal 5, no. 1: 2. https://doi.org/10.3390/aquacj5010002
APA StyleWilke, T., Bendag, S., Barth, A., Reinold, T., & Schubert, P. (2025). Individual Shrimp Rearing Increases the Power of Experimental Trials. Aquaculture Journal, 5(1), 2. https://doi.org/10.3390/aquacj5010002





