Tambaqui (Colossoma macropomum) in RAS Technology: Zootechnical, Hematological, Biochemical and Kn Profiles at Different Stocking Densities During the Initial Grow-Out Phase
Abstract
1. Introduction
2. Materials and Methods
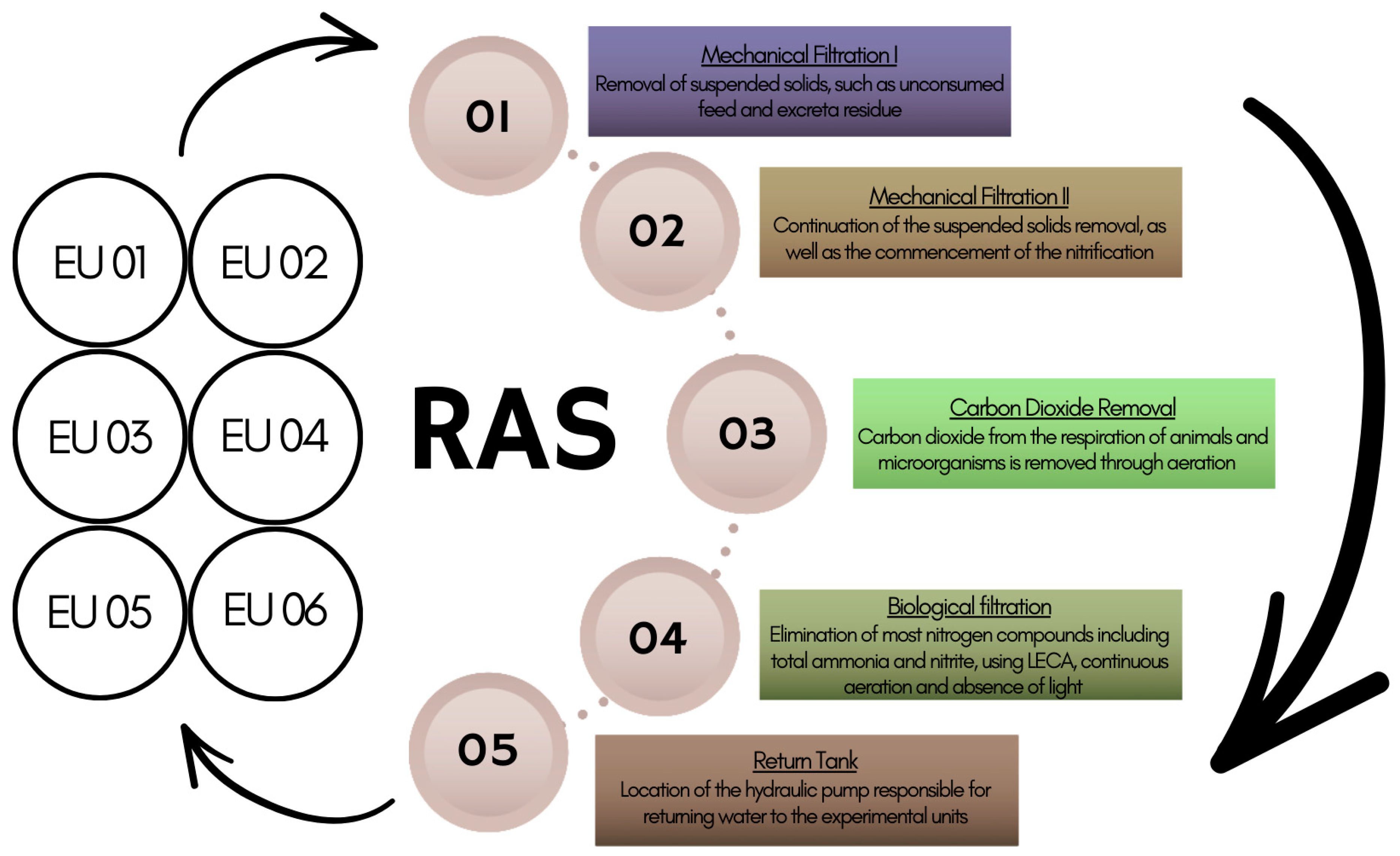
2.1. Fish, Experimental Design and RAS
2.2. Feeding, Biometric Procedures and Growth Performance
2.3. Blood Parameters and Body Condition
2.4. Water Quality
2.5. Statistical Analysis
2.6. Ethical Approval
2.7. Theory/Calculation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Encyclopaedia Britannica. Brazil: History, Map, Culture, Population & Facts. Available online: https://www.britannica.com/place/Brazil (accessed on 6 February 2024).
- Wagner, Y.G.; Coelho, A.B.; Travassos, G.F. Análise do consumo domiciliar de pescados no Brasil utilizando dados da POF 2017–2018. Rev. Econ. Sociol. Rural 2023, 61, e250494. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; Food and Agriculture Organization: Rome, Italy, 2024; ISBN 978-92-5-138763-4. [Google Scholar]
- PeixeBR. Anuário 2027 Peixe BR da Piscicultura. Available online: https://www.peixebr.com.br/anuario/ (accessed on 20 December 2024).
- IBGE. Pesquisa da Pecuária Municipal—PPM 2023. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9107-producao-da-pecuaria-municipal.html?=&t=resultados (accessed on 8 October 2024).
- Rodrigues, A.P.O.; Lima, A.F.; Maciel, P.O.; Santos, P.R.R.; Flores, R.M.V.; Silva, A.P. Stocking density during the initial grow-out phase of tambatinga in net pens. Ciênc. Rural 2016, 46, 163–168. [Google Scholar] [CrossRef]
- Li, L.; Shen, Y.; Yang, W.; Xu, X.; Li, J. Effect of different stocking densities on fish growth performance: A meta-analysis. Aquaculture 2021, 544, 737152. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Liu, H.; Xie, S. Effects of starvation on glucose and lipid metabolism in gibel carp (Carassius auratus gibelio var. CAS III). Aquaculture 2018, 496, 166–175. [Google Scholar] [CrossRef]
- Santos, F.A.C.; Julio, G.S.C.; Luz, R.K. Stocking density in Colossoma macropomum larviculture, a freshwater fish, in recirculating aquaculture system. Aquac. Res. 2021, 52, 1185–1191. [Google Scholar] [CrossRef]
- Castro, D.R.C.; Brabo, M.F.; Rocha, R.M.; Campelo, D.A.V.; Veras, G.C.; Rodrigues, R.P. Custo de produção e rentabilidade da criação de tambaqui Colossoma macropomum no estado do Pará, Amazônia, Brasil. Res. Soc. Dev. 2020, 9, e58996522. [Google Scholar] [CrossRef]
- López, P.; Anzoátegui, D. Engorde de la cachama (Colossoma macropomum, Cuvier, 1816) cultivada en un sistema de recirculación de agua. Zootec. Trop. 2013, 31, 271–277. [Google Scholar]
- Assis, Y.P.A.S.; Porto, L.A.; Melo, N.F.A.C.; Palheta, G.D.A.; Luz, R.K.; Favero, G.C. Feed restriction as a feeding management strategy in Colossoma macropomum juveniles under recirculating aquaculture system (RAS). Aquaculture 2020, 529, 735689. [Google Scholar] [CrossRef]
- Brandão, F.R.; Gomes, L.C.; Chagas, E.C.; Araújo, L.D. Densidade de estocagem de juvenis de tambaqui durante a recria em tanques-rede. Pesqui. Agropecu. Bras. 2004, 39, 357–362. [Google Scholar] [CrossRef]
- Inoue, L.A.K.A.; Bezerra, A.C.; Miranda, W.S.; Muniz, A.W.; Boijink, C.L. Cultivo de tambaqui em gaiolas de baixo volume: Efeito da densidade de estocagem na produção de biomassa. Ciênc. Anim. Bras. 2014, 15, 437–443. [Google Scholar] [CrossRef]
- Silva, C.A.; Fujimoto, R.Y. Crescimento de tambaqui em resposta a densidade de estocagem em tanques-rede. Acta Amazon. 2015, 45, 323–332. [Google Scholar] [CrossRef]
- Costa, J.; Freitas, R.; Gomes, A.L.; Bernadino, G.; Carneiro, D.; Martins, M.I. Effect of stocking density on economic performance for Colossoma macropomum (Cuvier, 1816), juvenile in earthen ponds. Lat. Am. J. Aquat. Res. 2016, 44, 165–170. [Google Scholar] [CrossRef]
- Frisso, R.M.; Matos, F.T.; Moro, G.V.; Mattos, B.O. Stocking density of Amazon fish (Colossoma macropomum) farmed in a continental neotropical reservoir with a net cages system. Aquaculture 2020, 529, 735702. [Google Scholar] [CrossRef]
- Lira, K.F.S.; Sousa, J.B.B.; Batista, B.R.; Procópio, D.P.; Ribeiro, J.S.A.; Hoshiba, M.A. Avaliação econômica da larvicultura de tambatinga (Colossoma macropomum × Piaractus brachypomus) em diferentes densidades de estocagem em sistema de bioflocos. Res. Soc. Dev. 2021, 10, e46210111960. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Fudge, M.; Higgins, V.; Vince, J.; Rajaguru, R. Social acceptability and the development of commercial RAS aquaculture. Aquaculture 2023, 568, 739295. [Google Scholar] [CrossRef]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef]
- Bhujel, R.C. Statistics for Aquaculture; Wiley-Blackwell: Ames, IA, USA, 2008; ISBN 978-0-8138-1587-9/2008. [Google Scholar]
- Inoue, L.A.K.A.; Boijink, C.L.; Ribeiro, P.T.; Silva, A.M.D.; Affonso, E.G. Avaliação de respostas metabólicas do tambaqui exposto ao eugenol em banhos anestésicos. Acta Amazon. 2011, 41, 327–332. [Google Scholar] [CrossRef]
- Wintrobe, M.M. Variations on the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematol. 1934, 51, 32–49. [Google Scholar]
- Gubiani, É.A.; Ruaro, R.; Ribeiro, V.R.; Santa Fé, Ú.M.G. Relative condition factor: Le cren’s legacy for fisheries science. Acta Limnol. Bras. 2020, 32, e3. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Marcon, J.L.; Lemos, J.R.G.; Fim, J.D.I.; Affonso, E.G.; Ono, E.A. Índices de condição corporal em juvenis de Brycon amazonicus (SPIX & AGASSIZ, 1829) e Colossoma macropomum (CUVIER, 1818) na Amazônia. Bol. Inst. Pesca 2008, 34, 197–204. [Google Scholar]
- Lima, J.F.; Montagner, D.; Duarte, S.S.; Yoshioka, E.T.O.; Dias, M.K.R.; Tavares-Dias, M. Recirculating system using biological aerated filters on tambaqui fingerling farming. Pesqui. Agropecu. Bras. 2019, 54, e00294. [Google Scholar] [CrossRef]
- Costa, J.A.S.; Sterzelecki, F.C.; Natividade, J.; Souza, R.J.F.; de Carvalho, T.C.C.; de Melo, N.F.A.C.; Luz, R.K.; Palheta, G.D.A. Residue from açai palm, Euterpe oleracea, as substrate for cilantro, Coriandrum sativum, seedling production in an aquaponic system with tambaqui, Colossoma macropomum. Agriculture 2022, 12, 1555. [Google Scholar] [CrossRef]
- Costa, O.T.F.; Ferreira, D.J.S.; Mendonça, F.L.P.; Fernandes, M.N. Susceptibility of the Amazonian fish, Colossoma macropomum (Serrasalminae), to short-term exposure to nitrite. Aquaculture 2004, 232, 627–636. [Google Scholar] [CrossRef]
- Hurtado, F.B.; Figueiredo, F.M.; Bomfim, S.C.; Queiroz, C.B.; Pontes, W.P. Toxicidade aguda da amônia total (NH3 + NH4+) frente a alevinos de tambaqui. In Ciência Animal e Veterinária: Inovações e Tendências; Rodrigues, N.J.L., Ed.; Editora Científica Digital LTDA: Guarujá, Brazil, 2022; pp. 185–189. ISBN 978-65-5360-123-9. [Google Scholar]
- Silva, W.S.; Ferreira, A.L.; Neves, L.C.; Ferreira, N.S.; Palheta, G.D.A.; Takata, R.; Luz, R.K. Effects of stocking density on survival, growth and stress resistance of juvenile tambaqui (Colossoma macropomum) reared in a recirculating aquaculture system (RAS). Aquac. Int. 2021, 29, 609–621. [Google Scholar] [CrossRef]
- Santos, D.K.M.; Kojima, J.T.; Santana, T.M.; Castro, D.P.; Serra, P.T.; Dantas, N.S.M.; Fonseca, F.A.L.; Mariúba, L.A.M.; Gonçalves, L.U. Farming tambaqui (Colossoma macropomum) in static clear water versus a biofloc system with or without Bacillus subtilis supplementation. Aquac. Int. 2021, 29, 207–218. [Google Scholar] [CrossRef]
- Nascimento, E.T.D.S.; Pereira Junior, R.F.; Reis, V.S.D.; Gomes, B.D.J.F.; Owatari, M.S.; Luz, R.K.; Melo, N.F.A.C.D.; Santos, M.D.L.S.; Palheta, G.D.A.; Sterzelecki, F.C. Production of late seedlings of açai (Euterpe oleraceae) in an aquaponic system with tambaqui (Colossoma macropomum, Cuvier, 1818). Agriculture 2023, 13, 1581. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Sandrim, E.F.S. Características hematológicas de teleósteos brasileiros. I. Série vermelha e dosagens de cortisol e glicose do plasma sangüíneo de espécimes de Colossoma macropomum em condições de cultivo. Acta Sci. 1998, 20, 157–160. [Google Scholar] [CrossRef]
- Costa, O.T.F.; Dias, L.C.; Malmann, C.S.Y.; de Lima Ferreira, C.A.; do Carmo, I.B.; Wischneski, A.G.; de Sousa, R.L.; Cavero, B.A.S.; Lameiras, J.L.V.; Dos-Santos, M.C. The effects of stocking density on the hematology, plasma protein profile and immunoglobulin production of juvenile tambaqui (Colossoma macropomum) farmed in Brazil. Aquaculture 2019, 499, 260–268. [Google Scholar] [CrossRef]
- Tavares-Dias, M. Parâmetros sanguíneos de referência para espécies de peixes cultivados. In Aquicultura No Brasil: Novas Perspectivas; Tavares-Dias, M., Mariano, W.S., Eds.; Pedro & João Editores: São Carlos, Brazil, 2015; pp. 11–30. ISBN 978-85-7993-271-7. [Google Scholar]
- Rocha, M.J.S.; Jerônimo, G.T.; Costa, O.T.F.D.; Malta, J.C.D.O.; Martins, M.L.; Maciel, P.O.; Chagas, E.C. Changes in hematological and biochemical parameters of tambaqui (Colossoma macropomum) parasitized by metazoan species. Rev. Bras. Parasitol. Vet. 2018, 27, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Boaventura, T.P.; da Costa Julio, G.S.; Cortezzi, P.P.; Figueiredo, L.G.; Favero, G.C.; Palheta, G.D.A.; de Melo, N.F.A.C.; Luz, R.K. Growth performance and physiological parameters of Colossoma macropomum in a recirculating aquaculture system (RAS): Importance of stocking density and classification. Aquaculture 2021, 534, 736274. [Google Scholar] [CrossRef]
- Chagas, E.C.; Gomes, L.C.; Martins Júnior, H.; Roubach, R. Produtividade de tambaqui criado em tanque-rede com diferentes taxas de alimentação. Ciênc. Rural 2007, 37, 1109–1115. [Google Scholar] [CrossRef]
- Barros, I.B.A.; Villacorta-Correa, M.A.; Carvalho, T.B. Stocking density and water temperature as modulators of aggressiveness, survival and zootechnical performance in matrinxã larvae, Brycon amazonicus. Aquaculture 2019, 502, 378–383. [Google Scholar] [CrossRef]
- Gomes, L.C.; Chagas, E.C.; Martins-Junior, H.; Roubach, R.; Ono, E.A.; Lourenço, J.N.P. Cage culture of tambaqui (Colossoma macropomum) in a central Amazon floodplain lake. Aquaculture 2006, 253, 374–384. [Google Scholar] [CrossRef]
| Water Quality Parameters | SD1 (140 fish m−3) | SD2 (180 fish m−3) |
|---|---|---|
| pH [H+] | 5.49 ± 0.8 | 5.40 ± 0.8 |
| Dissolved oxygen (mg L−1) | 4.13 ± 0.8 | 4.04 ± 0.9 |
| Temperature (°C) | 28.28 ± 0.8 | 28.28 ± 0.8 |
| Conductivity (µS cm−1) | 576.86 ± 349.8 | 594.24 ± 379.0 |
| Alkalinity (mg CaCO3 L−1) | 16.58 ± 5.2 | 14.13 ± 6.6 |
| Hardness (mg CaCO3 L−1) | 78.33 ± 23.6 | 92.94 ± 47.1 |
| Total ammonia nitrogen (mg L−1) | 0.12 ± 0.1 | 0.15 ± 0.1 |
| Nitrite (mg L−1) | 0.17 ± 0.1 | 0.09 ± 0.1 |
| Growth Performance Parameters | SD1 (140 fish m−3) | SD2 (180 fish m−3) |
|---|---|---|
| Initial weight (g) | 13.4 ± 0.0 | 11.3 ± 0.0 |
| Final weight (g) | 61.7 ± 0.0 | 52.6 ± 0.0 |
| Weight gain (g) | 48.3 ± 0.0 | 41.3 ± 0.0 |
| Initial biomass (kg) | 0.469 ± 0.0 | 0.509 ± 0.1 |
| Final biomass (kg) | 2.16 ± 0.5 | 2.37 ± 0.5 |
| Biomass gain (kg) | 1.69 ± 0.5 | 1.86 ± 0.4 |
| Initial stocking density (kg m3) | 1.88 ± 0.1 | 2.03 ± 0.3 |
| Final density (kg m3) | 8.64 ± 1.9 | 9.46 ± 2.0 |
| Specific growth rate (%) | 3.36 ± 0.34 | 3.40 ± 0.19 |
| Feed conversion ratio | 1.12 ± 0.16 | 1.20 ±0.35 |
| Survival rate (%) | 100.0 | 100.0 |
| Physiological Parameters | SD1 (140 fish m−3) | SD2 (180 fish m−3) |
|---|---|---|
| Hemoglobin (g dL−1) | 4.7 ± 0.5 | 4.5 ± 0.6 |
| Erythrocytes (×106 μL−1) | 1.4 ± 0.3 | 1.4 ± 0.2 |
| Hematocrit (%) | 26.3 ± 2.2 | 25.9 ± 3.5 |
| MCV (fL) | 194.3 ± 33.7 | 194.7 ± 44.6 |
| MCH (pg) | 34.7 ± 6.6 | 33.7 ± 6.3 |
| MCHC (g dL−1) | 17.8 ± 1.2 | 17.6 ± 2.2 |
| Plasma glucose (mg dL−1) | 366.3 ± 123.8 | 353.9 ± 184.5 |
| Total cholesterol (mg dL−1) | 582.2 ± 43.3 | 616.1 ± 37.2 |
| Triglycerides (mg dL−1) | 275.0 ± 158.1 a | 220.1 ± 148.2 b |
| Total proteins (g dL−1) | 3.0 ± 0.6 | 3.1 ± 0.7 |
| Body condition Kn | 1.0 ± 0.1 | 0.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petillo, E.C.; Ferreira, A.d.C.; Oliveira, C.P.F.d.; Brandão, L.V.; Marinho-Pereira, T.; Cavero, B.A.S. Tambaqui (Colossoma macropomum) in RAS Technology: Zootechnical, Hematological, Biochemical and Kn Profiles at Different Stocking Densities During the Initial Grow-Out Phase. Aquac. J. 2025, 5, 1. https://doi.org/10.3390/aquacj5010001
Petillo EC, Ferreira AdC, Oliveira CPFd, Brandão LV, Marinho-Pereira T, Cavero BAS. Tambaqui (Colossoma macropomum) in RAS Technology: Zootechnical, Hematological, Biochemical and Kn Profiles at Different Stocking Densities During the Initial Grow-Out Phase. Aquaculture Journal. 2025; 5(1):1. https://doi.org/10.3390/aquacj5010001
Chicago/Turabian StylePetillo, Emilly Cordeiro, Aline da Cunha Ferreira, Christiane Patrícia Feitosa de Oliveira, Lian Valente Brandão, Thiago Marinho-Pereira, and Bruno Adan Sagratzki Cavero. 2025. "Tambaqui (Colossoma macropomum) in RAS Technology: Zootechnical, Hematological, Biochemical and Kn Profiles at Different Stocking Densities During the Initial Grow-Out Phase" Aquaculture Journal 5, no. 1: 1. https://doi.org/10.3390/aquacj5010001
APA StylePetillo, E. C., Ferreira, A. d. C., Oliveira, C. P. F. d., Brandão, L. V., Marinho-Pereira, T., & Cavero, B. A. S. (2025). Tambaqui (Colossoma macropomum) in RAS Technology: Zootechnical, Hematological, Biochemical and Kn Profiles at Different Stocking Densities During the Initial Grow-Out Phase. Aquaculture Journal, 5(1), 1. https://doi.org/10.3390/aquacj5010001





