Crude Polysaccharide Extract from the Microalga Porphyridium cruentum Improved Nonspecific Immune Responses and Resistance in Penaeus vannamei Exposed to Vibrio alginolyticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Crude Extract from the Microalga Porphyridium cruentum
2.2. Dietary Preparations
2.3. Feeding Assay
2.4. Hemolymph Sampling for Serum Preparation
2.5. Hemato-Immunological Parameter Evaluation
2.6. Vibrio alginolyticus Experimental Challenge
2.7. Statistical Analysis
3. Results
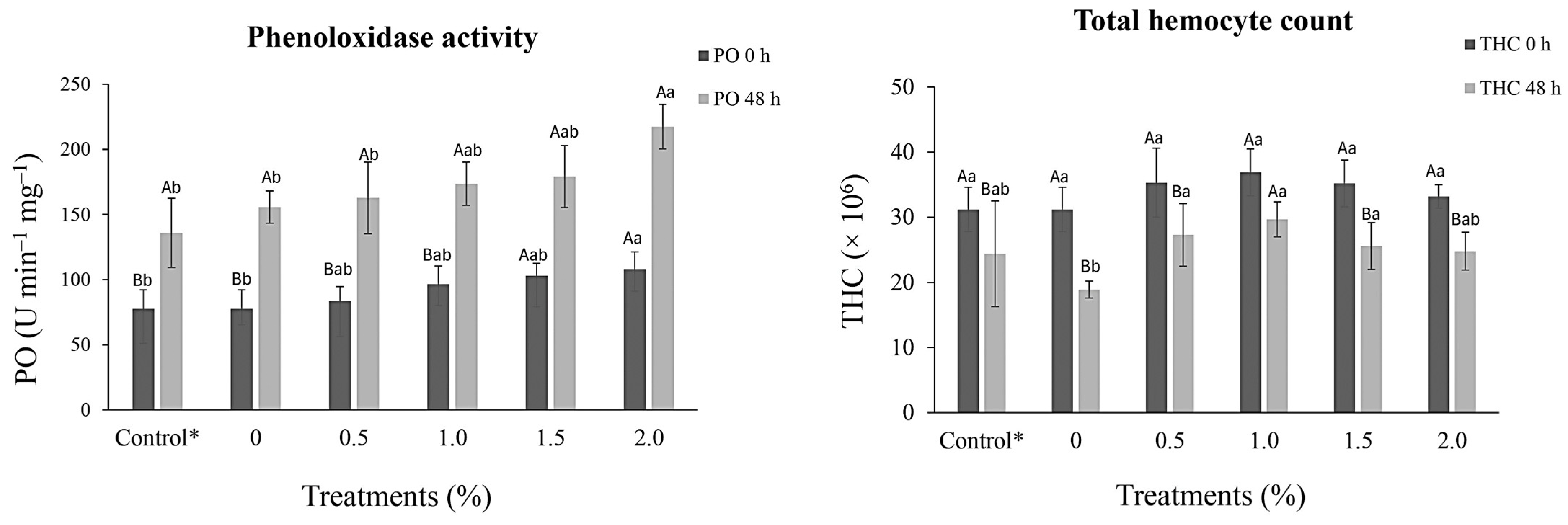
3.1. Hemato-Immunological Parameters
3.2. Experimental Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. In Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Barreto, A.; Peixoto, D.; Fajardo, C.; Pinto, W.; Rocha, R.J.; Conceição, L.E.; Costas, B. Health-promoting additives supplemented in inert microdiets for whiteleg shrimp (Penaeus vannamei) post-larvae: Effects on growth, survival, and health status. Animals 2023, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Abakari, G.; Tan, H.; Wenchang, L.I.U.; Luo, G. Effects of different probiotics (Bacillus subtilis) addition strategies on a culture of Litopenaeus vannamei in biofloc technology (BFT) aquaculture system. Aquaculture 2023, 566, 739216. [Google Scholar] [CrossRef]
- Schleder, D.D.; Peruch, L.G.B.; Poli, M.A.; Ferreira, T.H.; Silva, C.P.; Andreatta, E.R.; Hayashi, L.; Vieira, F.D.N. Effect of brown seaweeds on Pacific white shrimp growth performance, gut morphology, digestive enzymes activity and resistance to white spot virus. Aquaculture 2018, 495, 359–365. [Google Scholar] [CrossRef]
- Filho, L.G.A.D.S.; Diniz, F.M.; Pereira, A.M. Immunostimulants derived from plants and algae to increase resistance of pacific white shrimp (Litopenaeus vannamei) against vibriosis. Stud. Nat. Prod. Chem. 2023, 77, 297–337. [Google Scholar] [CrossRef]
- Le Moullac, G.; Haffner, P. Environmental factors affecting immune responses in Crustacea. Aquaculture 2000, 191, 121–131. [Google Scholar] [CrossRef]
- Amatul-Samahah, M.A.; Omar, W.H.H.W.; Ikhsan, N.F.M.; Azmai, M.N.A.; Zamri-Saad, M.; Ina-Salwany, M.Y. Vaccination trials against vibriosis in shrimp: A review. Aquac. Rep. 2020, 18, 100471. [Google Scholar] [CrossRef]
- Schleder, D.D.; Da Rosa, J.R.; Guimarães, A.M.; Ramlov, F.; Maraschin, M.; Seiffert, W.Q.; Vieira, F.D.N.; Hayashi, L.; Andreatta, E.R. Brown seaweeds as feed additive for white-leg shrimp: Effects on thermal stress resistance, midgut microbiology, and immunology. J. Appl. Phycol. 2017, 29, 2471–2477. [Google Scholar] [CrossRef]
- Cantelli, L.; Goncalves, P.; Guertler, C.; Kayser, M.; Pilotto, M.R.; Barracco, M.A.; Perazzolo, L.M. Dietary supplementation with sulfated polysaccharides from Gracilaria birdiae promotes a delayed immunostimulation in marine shrimp challenged by the white spot syndrome virus. Aquac. Int. 2019, 27, 349–367. [Google Scholar] [CrossRef]
- Kulkarni, A.; Krishnan, S.; Anand, D.; Kokkattunivarthil Uthaman, S.; Otta, S.K.; Karunasagar, I.; Kooloth Valappil, R. Immune responses and immunoprotection in crustaceans with special reference to shrimp. Rev. Aquac. 2021, 13, 431–459. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Söderhäll, K. The proPO and clotting system in crustaceans. Aquaculture 2000, 191, 53–69. [Google Scholar] [CrossRef]
- Liu, F.; Shao, G.Y.; Tian, Q.Q.; Cheng, B.X.; Shen, C.; Wang, A.M.; Zhang, J.H.; Tian, H.Y.; Yang, W.P.; Yu, Y.B. Enhanced growth performance, immune responses, immune-related gene expression and disease resistance of red swamp crayfish (Procambarus clarkii) fed dietary glycyrrhizic acid. Aquaculture 2021, 533, 736202. [Google Scholar] [CrossRef]
- Ringø, E.; Song, S.K. Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquac. Nutr. 2016, 22, 4–24. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Zhang, Q.; Zhang, X.; Yuan, J.; Huang, H.; Xiang, J.; Li, F. A novel candidate gene associated with body weight in the Pacific white shrimp Litopenaeus vannamei. Front. Genet. 2019, 10, 434956. [Google Scholar] [CrossRef] [PubMed]
- Feller, R.; Matos, Â.P.; Mazzutti, S.; Moecke, E.H.; Tres, M.V.; Derner, R.B.; Oliveira, J.V.; Junior, A.F. Polyunsaturated ω-3 and ω-6 fatty acids, total carotenoids and antioxidant activity of three marine microalgae extracts obtained by supercritical CO2 and subcritical n-butane. J. Supercrit. Fluids 2018, 133, 437–443. [Google Scholar] [CrossRef]
- Dantas, D.M.D.M.; Oliveira, C.Y.B.; Costa, R.M.P.B.; Carneiro-da-Cunha, M.D.G.; Gálvez, A.O.; Bezerra, R.D.S. Evaluation of antioxidant and antibacterial capacity of green microalgae Scenedesmus subspicatus. Food Sci. Technol. Int. 2019, 25, 318–326. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Charoonnart, P.; Worakajit, N.; Zedler, J.A.; Meetam, M.; Robinson, C.; Saksmerprome, V. Generation of microalga Chlamydomonas reinhardtii expressing shrimp antiviral dsRNA without supplementation of antibiotics. Sci. Rep. 2019, 9, 3164. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef]
- Arad, S.M.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; Arrojo-Agudo, M.D.L.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, antioxidant activity and cytotoxic effect of sulfated polysaccharides from Porphyridium cruentum. (sf Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Parra-Riofrio, G.; Moreno, P.; García-Rosado, E.; Alonso, M.C.; Uribe-Tapia, E.; Abdala-Diaz, R.T.; Bejar, J. Tetraselmis suecica and Porphyridium cruentum exopolysaccharides show anti-VHSV activity on RTG-2 cells. Aquac. Int. 2023, 31, 3145–3157. [Google Scholar] [CrossRef]
- Risjani, Y.; Mutmainnah, N.; Manurung, P.; Wulan, S.N.; Yunianta. Exopolysaccharide from Porphyridium cruentum (purpureum) is not toxic and stimulates immune response against vibriosis: The assessment using zebrafish and white shrimp Litopenaeus vannamei. Mar. Drugs 2021, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Farias, W.R.; Valente, A.P.; Pereira, M.S.; Mourão, P.A. Structure and anticoagulant activity of sulfated galactans: Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J. Biol. Chem. 2000, 275, 29299–29307. [Google Scholar] [CrossRef] [PubMed]
- Ozório, R.Á.; Lopes, R.G.; Góes, B.; Silva, A.C.P.; Derner, R.B.; Fracalossi, D.M. Growth and enzymatic profile of the pacific white shrimp fed with Porphyridium cruentum extract. Bol. Inst. Pesca 2015, 41, 123–131. [Google Scholar]
- Söderhäll, K.; Häll, L. Lipopolysaccharide-induced activation of prophenoloxidase activating system in crayfish haemocyte lysate. Biochim. Biophys. Acta (BBA) Gen. Subj. 1984, 797, 99–104. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, J.X. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2013, 34, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, C.; Lopes, R.; Derner, R.; Vinatea, L. Using residual water from a marine shrimp farming BFT system. Part I: Nutrient removal and marine microalgae biomass production. Aquac. Res. 2016, 47, 2435–2443. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chew, P.F.; Soh, B.S.; Tham, L.Y. Enhancing phagocytic activity of hemocytes and disease resistance in the prawn Penaeus merguiensis by feeding Spirulina platensis. J. Appl. Phycol. 2003, 15, 279–287. [Google Scholar] [CrossRef]
- Pilotto, M.R.; Milanez, S.; Moreira, R.T.; Rosa, R.D.; Perazzolo, L.M. Potential immunomodulatory and protective effects of the Arthrospira-based dietary supplement on shrimp intestinal immune defenses. Fish Shellfish Immunol. 2019, 88, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, S.; Akbarzadeh, A.; Sajjadi, M.M.; Hajimoradloo, A.; Noori, F. Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2018, 24, 594–604. [Google Scholar] [CrossRef]
- Gross, P.S.; Bartlett, T.C.; Browdy, C.L.; Chapman, R.W.; Warr, G.W. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific White Shrimp, Litopenaeus vannamei, and the Atlantic White Shrimp, L. Setiferus. Dev. Comp. Immunol. 2001, 25, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Millar, D.A.; Ratcliffe, N.A. Invertebrates. In Immunology: A Comparative Approach; Turner, R.J., Ed.; Wiley: Chichester, UK, 1994; pp. 29–68. [Google Scholar]
- Huang, X.; Zhou, H.; Zhang, H. The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. 2006, 20, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Suganya, A.M.; Sanjivkumar, M.; Chandran, M.N.; Palavesam, A.; Immanuel, G. Pharmacological importance of sulphated polysaccharide carrageenan from red seaweed Kappaphycus alvarezii in comparison with commercial carrageenan. Biomed. Pharmacother. 2016, 84, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Chotigeat, W.; Tongsupa, S.; Supamataya, K.; Phongdara, A. Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture 2004, 233, 23–30. [Google Scholar] [CrossRef]
- Yeh, S.T.; Lee, C.S.; Chen, J.C. Administration of hot-water extract of brown seaweed Sargassum duplicatum via immersion and injection enhances the immune resistance of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2006, 20, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Abbas, E.M.; Al-Souti, A.S.; Sharawy, Z.Z.; El-Haroun, E.; Ashour, M. Impact of dietary administration of seaweed polysaccharide on growth, microbial abundance, and growth and immune-related genes expression of the pacific whiteleg shrimp (Litopenaeus vannamei). Life 2023, 13, 344. [Google Scholar] [CrossRef]
- Yuvaraj, N.; Arul, V. Immunomodulatory effects of seagrass Halophila ovalis polysaccharide mixed feed in adult black tiger shrimp Penaeus monodon and its protective efficacy against white spot syndrome virus infection. Iran. J. Fish. Sci. 2017, 16, 993–1007. Available online: http://hdl.handle.net/1834/12261 (accessed on 20 January 2024).
- Van de Braak, C.B.T.; Botterblom, M.H.A.; Huisman, E.A.; Rombout, J.H.W.M.; Van der Knaap, W.P.W. Preliminary study on haemocyte response to white spot syndrome virus infection in black tiger shrimp Penaeus monodon. Dis. Aquat. Org. 2002, 51, 149–155. [Google Scholar] [CrossRef]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Bakky, M.A.H.; Tran, N.T.; Zhang, Y.; Li, S. Utilization of marine macroalgae-derived sulphated polysaccharides as dynamic nutraceutical components in the feed of aquatic animals: A review. Aquac. Res. 2022, 53, 5787–5808. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.W.; Keyser, P.; Sritunyalucksana, K.; Söderhäll, K. Crustacean haemocytes and haematopoiesis. Aquaculture 2000, 191, 45–52. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]

| Sample | Moisture (%) | Crude Protein (%) | Ether Extract (%) | Ash (%) | Fiber (%) |
|---|---|---|---|---|---|
| 0% | 5.59 | 39.20 | 9.56 | 13.59 | 4.53 |
| 0.5% | 6.16 | 38.83 | 9.76 | 13.80 | 3.04 |
| 1.0% | 5.67 | 39.72 | 9.58 | 14.17 | 2.9 |
| 1.5% | 5.72 | 38.38 | 9.63 | 14.27 | 2.98 |
| 2.0% | 5.67 | 37.76 | 9.59 | 14.38 | 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozório, R.Á.; Lopes, R.G.; Vieira, F.d.N.; Bolívar-Ramírez, N.C.; Oliveira, C.Y.B.d.; Barracco, M.A.A.M.; Owatari, M.S.; Fracalossi, D.M.; Derner, R.B. Crude Polysaccharide Extract from the Microalga Porphyridium cruentum Improved Nonspecific Immune Responses and Resistance in Penaeus vannamei Exposed to Vibrio alginolyticus. Aquac. J. 2024, 4, 104-113. https://doi.org/10.3390/aquacj4030008
Ozório RÁ, Lopes RG, Vieira FdN, Bolívar-Ramírez NC, Oliveira CYBd, Barracco MAAM, Owatari MS, Fracalossi DM, Derner RB. Crude Polysaccharide Extract from the Microalga Porphyridium cruentum Improved Nonspecific Immune Responses and Resistance in Penaeus vannamei Exposed to Vibrio alginolyticus. Aquaculture Journal. 2024; 4(3):104-113. https://doi.org/10.3390/aquacj4030008
Chicago/Turabian StyleOzório, Renata Ávila, Rafael Garcia Lopes, Felipe do Nascimento Vieira, Norha Constanza Bolívar-Ramírez, Carlos Yure Barbosa de Oliveira, Margherita Anna Antonia Maria Barracco, Marco Shizuo Owatari, Debora Machado Fracalossi, and Roberto Bianchini Derner. 2024. "Crude Polysaccharide Extract from the Microalga Porphyridium cruentum Improved Nonspecific Immune Responses and Resistance in Penaeus vannamei Exposed to Vibrio alginolyticus" Aquaculture Journal 4, no. 3: 104-113. https://doi.org/10.3390/aquacj4030008
APA StyleOzório, R. Á., Lopes, R. G., Vieira, F. d. N., Bolívar-Ramírez, N. C., Oliveira, C. Y. B. d., Barracco, M. A. A. M., Owatari, M. S., Fracalossi, D. M., & Derner, R. B. (2024). Crude Polysaccharide Extract from the Microalga Porphyridium cruentum Improved Nonspecific Immune Responses and Resistance in Penaeus vannamei Exposed to Vibrio alginolyticus. Aquaculture Journal, 4(3), 104-113. https://doi.org/10.3390/aquacj4030008









