Abstract
Serotonin (5-hydroxytryptamine—5-HT) is an important neurotransmitter that exerts a remarkably large array of biological roles in the central nervous system and at the body level. It is involved in generating emotions, being a natural mood stabilizer; it reduces depression, anxiety, modulates sleep, and has many other effects. It is also involved in fetal and postnatal brain development. This variety of biological effects, particularly in the central nervous system, with influence on behavior and cognitive functions, relies on a large number of pre- and postsynaptic serotonin receptor (5-HTR) isoforms spread throughout the brain. They can be grouped in seven large families and include over 18 subtypes, identified based on gene sequences, expression patterns, and pharmacological responses. While in vertebrates these receptors have been properly characterized and described, their correspondents in invertebrates have been far less explored, despite the assumption that they may have similar properties to those described in vertebrates. This paper summarizes the current knowledge in several important areas that together define the entire scope of serotonin receptor research, with a particular emphasis on the role of serotonergic central pathways and circuitry in thermoregulation and correlations with neurologic and psychiatric pathology.
1. Overview of Serotonin and Its Functions
The history of serotonin discovery is long and intricate. The substance was first isolated by Vittorio Erspamer at the University of Pavia in 1935 from enterochromaffin cells and named enteramine due to its property to induce intestinal contraction. Later, in 1948, a group of researchers at the Cleveland Clinic (M. Rapport, A. Green, I. Page) identified a substance present in serum with strong vasoconstrictor effects and named it serotonin. The identity of the two substances was established in 1952, and the presence of serotonin in the brain was confirmed in 1953 by Page and B. Twarog [1].
Serotonin is produced by neuronal and non-neuronal cells. Neuronal serotonin is synthesized in serotonergic neurons [2,3], while non-neuronal serotonin is produced by enterochromaffin cells in the intestinal epithelium and a few other places, for example, tactile epithelial cells in the skin (Merkel cells) or enterochromaffin cells in bronchial epithelium (Kulchitsky cells), isolated or in clusters named neuroepithelial bodies. Serotonin can function as a classic neurotransmitter and hormone-like substance. Plasma serotonin is collected by platelets, and can be released upon clot formation, alleviating hemorrhage by instant vasoconstriction [4].
Serotonin is a neurotransmitter that exerts a diversity of biological roles, many of them still poorly understood, acting both in the periphery by mediating vasoconstriction and intestinal motility, as well as in the central nervous system, where it is involved in neurodevelopment, cognition, reward, learning and memory, mood, anxiety and depression, and physiological processes such as the sleep-wake cycle, thermoregulation, sexual function, cardiovascular and respiratory regulation [5]. It has been implicated in a wide range of physiological and behavioral processes in vertebrates, although it does not appear to be essential for the realization of these processes. Serotonin fits well within the definition of central nervous system (CNS) neuromodulators, in contrast to other neurotransmitters such as catecholamines, acetylcholine, histamine, and neuropeptides.
Serotonin is an important chemical mediator that has been conserved throughout evolution. It is synthesized from tryptophan by a couple of enzymes (Figure 1) and is subsequently packaged into vesicles by the vesicular monoamine transporter 2 (VMAT2) [6]. In mammals, serotonin plays an essential role during embryonic development and regulates neuronal connectivity by modulating cell migration and cytoarchitecture. In adulthood, it regulates several types of cognitive behaviors, e.g., reward-based learning and memory, attentional processes, emotional responses involved in anxiety or depression, such as the conditioned fear stress-induced freezing behavior used as a model of anxiety [7]. Abnormal levels of serotonin result in aberrant morphology and function of the nervous system. In particular, changes in neural circuits may be related to inappropriate actions and/or levels of serotonin during key developmental stages, increasing susceptibility to psychiatric disorders [8].
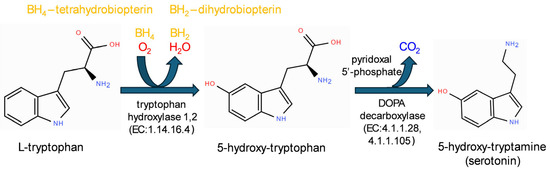
Figure 1.
The metabolic pathway of serotonin synthesis from tryptophan.
Serotonin is recognized as a major neuromodulator of the nervous system in both invertebrates and vertebrates and is thought to impact animal cognition and behavior. Animal behavior has been suggested to vary along five different axes: boldness–shyness, avoidance–exploration, activity, sociability, and aggression [9]. Depending on the individual’s motivational state, serotonin can decrease or increase aggressive behaviour [10]. Serotonin is negatively correlated with anxiety traits [10,11].
Serotonin plays an essential role in several pathophysiological processes, as well as in the etiology and treatment of CNS diseases such as depression, schizophrenia, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, and migraine [6]. It also plays an important role in cancer biology, including tumor initiation, promotion, metastasis, and tumor recurrence [12,13].
2. Neuroanatomy and Neurochemistry of Serotonergic Pathways
2.1. Serotonin Pathways in the Brain
The serotonergic system can reach a surprising level of complexity given its relatively small number of neurons. CNS serotonin is secreted by approximately 200,000 serotonergic neurons of the raphe nuclei [8], belonging to a rostral and a caudal group. However, only 5% of serotonin is secreted in the CNS; the vast majority (95%) is produced by the intestinal wall nervous plexuses and enterochromaffin cells, and this part of serotonin cannot cross the blood–brain barrier, exerting only peripheral effects [6].
The compared anatomy of serotonergic connections is difficult because the system is still insufficiently characterized. Serotonergic neurons and sensory neurons that project to certain areas of the brain have unique projections. These projections are specific to different serotonergic raphe nuclei. These nuclei are located in the median region of the brainstem, named raphe, and they can be evidenced via different methods such as immunohistochemistry, autoradiography, or chemical reactions resulting in fluorescent compounds [14]. They can be broadly classified into a superior (rostral) group, with ascending connections into different brain areas, and an inferior (caudal) group, with descending connections to the spinal cord. The brainstem raphe nuclei are grouped into nine serotonin-fluorescent clusters (B1–B9), the largest ones being nucleus raphe dorsalis (DRN: B6–B7) and nucleus raphe magnus (NRM: B3) [3]. The caudal group (nucleus raphe magnus, pallidus, obscurus) provides descending projections in the medulla and spinal cord, while the rostral group nuclei (dorsalis, median, caudal linear) send projections to virtually all parts of the brain, controlling essential processes of neurodevelopment such as neural plasticity and synaptogenesis during both prenatal life and infancy. The superior group is composed of the nucleus raphe magnus, median raphe nucleus, caudal linear nucleus, dorsal raphe nucleus, and a small group along the superior edge of the medial lemniscus, placed between the superior limit of the inferior olivary nucleus and the red nucleus (supralemniscal group). In some species, such as rats, there is another serotonergic neuron group in the hypothalamic dorsomedial nucleus [3]. The inferior group is composed of nucleus raphe obscurus, nucleus raphe pallidus, the caudal part of nucleus raphe magnus overlapping the dorsal limit of the medial lemniscus and the trapezoid body, extending laterally into the giant cell nucleus reticularis, the ventral lateral medulla, and area postrema (Figure 2).
The dorsal raphe nucleus has been approached by many studies. This structure receives a high percentage of synaptic contacts terminating on the dendrites of neurons. Reciprocal connections involving these synapses mediate the increase in excitation that occurs between the dorsal raphe nucleus and the lateral habenular nucleus (LHb). LHb is a reward-negative center connected to the preoptic area, conveying information from the hippocampus and lateral septum, the ventral pallidum (input from nucleus accumbens and mediodorsal thalamic nucleus), the lateral hypothalamus, and other regions (Figure 3). In the developmental context, stress-induced changes in dorsal raphe nucleus function can begin very early in life.
Serotonin-containing projections of raphe neurons are found in many parts of the brain, including the thalamus, preoptic area, hypothalamus, amygdala, and hippocampus, as well as in the cerebral cortex (Figure 2). The cortical serotonergic fibers are distributed with the highest density in the molecular layer (I) and the internal granular (IV) and internal pyramidal (V) layers. Projections from the dorsal raphe nucleus are called D fibers, and they are fine, sometimes varicose, highly branched, and widely spread in layers I and VA (outer zone of layer V), while those from the median raphe nucleus (M fibers) are thick, tortuous, and with varicosities in some species; for example, in cats [15] or marmosets [16] they form basket terminals in outer layers (I–III), making synapses with both pyramidal and non-pyramidal inhibitory interneurons. A recent study proved that optogenetic activation of dorsal raphe nucleus serotonergic neurons in anesthetized mice produced a wide-scale activation of cortical areas assessed via BOLD (blood oxygenation level-dependent) contrast imaging, an advanced method of fMRI (functional magnetic resonance imaging) [17].
Beyond this ascending serotonergic system, the inferior group of raphe nuclei generates a descending serotonergic system, composed of a ventromedial pathway, which travels, after crossing the pyramidal decussation, along the ventral column, forming the raphe obscurus spinal tract, to the ventral horn motoneurons in Rexed lamina X, and a lateral pathway to the central periependymal gray matter. Fibers from the nucleus raphe magnus also project into the substantia gelatinosa (Rexed lamina II). Other projections from the ventral lateral medulla, which may be myelinated, travel along the spinothalamic tract and spinoolivary tract to the autonomic centers of the intermediate horns [18]. There are also serotonin-secreting neurons located in the peripheral nervous system [19,20].
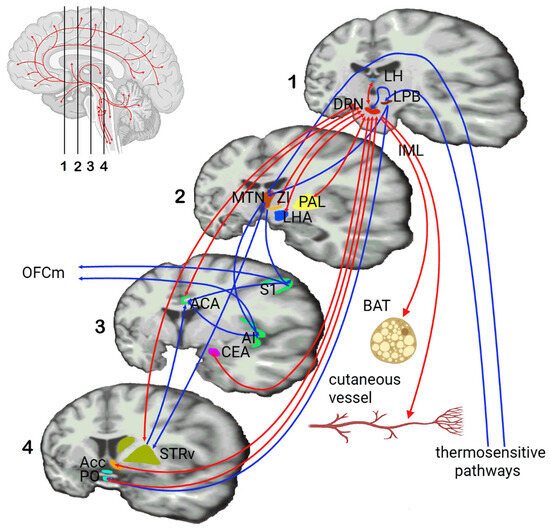
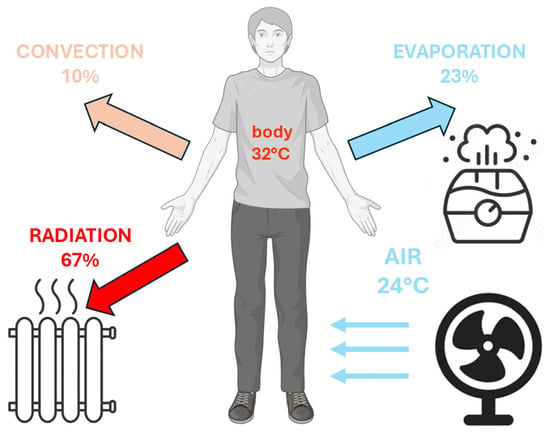

Figure 2.
Functional connections of the serotonergic raphe nuclei with different brain structures, with an emphasis on afferent and efferent thermoregulatory pathways. Legend: DRN—dorsal raphe nucleus, LPB—lateral parabrachial nucleus, MTN—medial thalamic nuclei, S1—primary somatosensory cortical area, AI—agranular insular area, ACA—anterior cingulate area, OFCm—medial orbitofrontal cortex, STRv—ventral striatum, LH—lateral habenula, PAL—globus pallidus, ZI—zona incerta, LHA—lateral hypothalamic area, CEA—central amygdala, Acc—nucleus accumbens, PO—preoptic area, IML—intermediolateral cell column, BAT—brown adipose tissue (adapted from [21,22,23,24,25]).

Figure 3.
Heat balance, including production and different dissipation pathways of the human body.
Advanced methods can be used to specifically probe targets of defined serotonergic projections, allowing the development of receptor agonists and antagonists that selectively modulate groups of serotonin receptors. By targeting specific neural pathways, researchers can fine-tune the modulation of serotonergic activity, leading to the creation of more precise and effective pharmacological interventions.
2.2. Interactions with Other Neurotransmitters
Neurotransmitters are chemical signalling compounds that accumulate in the presynaptic compartment in neurotransmitter vesicles located in axonal synaptic boutons, and are released into the synaptic cleft when depolarization triggered by axonally propagated action potentials reaches these boutons, opening voltage-dependent calcium channels [26]. Paracrine transmission is due to the presence of bioactive substances that are able to modulate or fine-tune neurotransmission, acting not only at synapses, but also in additional compartments. Such areas are represented, for example, by Hoenemann’s beads, small bumps distributed everywhere along a monoaminergic fiber. Some authors have differentiated a classical synaptic “wiring” transmission and a so-called “volume” transmission involving extrasynaptic serotonergic receptors and transporters and serotonin-glia interactions [27].
In serotonergic neurons of the inferior group of raphe nuclei, serotonin co-localizes with neuropeptides such as enkephalins, substance P, and thyrotropin-releasing hormone (TRH) [28,29]. In certain neurons serotonin can also co-localize with non-peptide neurotransmitters, such as norepinephrine, in the area postrema [30] or γ-aminobutyric acid (GABA) in the dorsal raphe nucleus [31].
The serotonergic system has become well established as an essential component in the pathophysiology of anxiety and anxiety-related disorders, the most common group of psychiatric disorders [32]. Serotonergic projections are located in limbic structures such as the septum, hippocampus, amygdala, and frontal cortex, which are areas known to be involved in modulating anxiety-related behaviors. Moreover, chronic treatment with anxiolytic drugs, for example, benzodiazepines, which act on the GABAergic system, leads to a reduction of pre- and postsynaptic serotonergic receptors in the limbic system. The relevance of the serotonergic system in anxiety-related disorders is also supported by the fact that a new generation of drugs used for their treatment, selective serotonin reuptake inhibitors (SSRIs), increase the availability of serotonin in the synaptic cleft and have anxiolytic and antidepressant properties. At the same time, initiation of chronic SSRI treatment enhances the adaptive response of the serotonergic system [6].
An interesting recent finding is that serotonergic dorsal raphe neurons make asymmetric excitatory synapses with dopaminergic neurons of the ventral tegmental area (VTA), which in turn release dopamine in the nucleus accumbens (Acc), promoting reward [33]. Serotonergic neurons of the dorsal raphe nucleus have also been shown to activate noradrenergic neurons of locus coeruleus, with subsequent influence on prefrontal cortex noradrenergic circuits, as demonstrated by noradrenaline level measurements in these regions via dual-probe microdialysis [34]. Systemic or local administration of SSRIs such as citalopram resulted in increased noradrenaline levels in locus coeruleus and decreased levels in the prefrontal cortex; these effects were abolished by specific antagonists of ionotropic serotonergic receptors [35], and intensified by specific agonists [34]. Other agonists of metabotropic serotonergic receptors also increased noradrenaline levels in microdialisates from the prefrontal cortex [36] or hippocampus [37], while the β-adrenoreceptors antagonist propranolol released inhibition of dorsal raphe nucleus serotonergic neurons firing [38]. Specific serotonergic receptor agonists also increased extracellular acetylcholine in the brain cortex and hippocampus [39,40].
Elegant experimental studies using optogenetic methods proved interactions between dopaminergic and serotonergic pathways projecting to the nucleus accumbens (Acc) [41]. Anterograde tracing of both types of fibers with adeno-associated virus vectors carrying fluorescently labeled agonists and targeted knock-out of serotonin or dopamine transporters, in conjunction with fiber-optic fluorometric measurements of activation by dopamine or serotonin of engineered G-protein-coupled activation-based sensors (GRAB sensors) [42], revealed that randomly delivered rewards in an appetitive task enhanced dopamine release and inhibited serotonin release in the Acc. Optogenetic inhibition of both dopaminergic and serotonergic signaling in this experimental model disrupted reinforcement learning. A similar optogenetic-fMRI study in mice revealed differential modulation of serotonin receptor network connectivity upon activation of serotonin release in the DRN; these responses could be modulated by administration of the SSRI fluoxetine [43]. These modern approaches confirm the known interaction between serotonergic and dopaminergic systems in reward-based motivational and reinforcement behaviors [44], implying serotonergic projections from the rostral raphe nuclei to a limbic-cortico-striatal circuit. Self-stimulation of the ventral tegmental area (VTA) and medial forebrain bundle by DRN leads to excitatory or feed-forward inhibitory effects of fibers from the prefrontal cortex and lateral habenula on dopaminergic VTA and DRN neurons [23]. These findings coincide with the results of animal studies that prove the role of the dopaminergic system in encoding reward prediction errors, while the serotonergic system encodes unsigned prediction errors [45]. Positive or negative reward sensitivity can be modulated by acute tryptophan or serotonin depletion or by selective serotonin reuptake inhibitors. Such manipulations in rodents decreased appetitive responses and instrumental reinforcement learning, mimicking effects present in SERT knock-out mice [46], but increased stay–win behavior in reversal learning protocols [47]. The ipsi/contralateral connectivity of limbic system components with serotonergic raphe nuclei can vary for different fiber tracts: thus, the DRN receives bilateral inputs from the prefrontal cortex and lateral habenula, but only ipsilateral inputs from the amygdala and hypothalamic nuclei [48]. Optogenetic stimulation of DRN activated connections to the bed nucleus of the stria terminalis, a structure connected to the amygdala and involved in fear and anxiety [49].
3. Serotonin Metabolic Pathways
5-hydroxy-tryptamine (serotonin) is a monoamine that exerts diverse functions in both the central nervous system and peripheral organs. Serotonin is synthesized from the amino acid tryptophan (Figure 1), which is converted in a two-step reaction into 5-hydroxy-tryptophan (5-HTP) by the rate-limiting enzyme tryptophan hydroxylase (TPH) (EC:1.14.16.4), which has two isoforms in different species, and then into serotonin by the lyase aromatic acid decarboxylase (AAAD or AADC), also known as DOPA-decarboxylase (DDC) (EC:4.1.1.28). In some fungal species, such as Psilocybe cubensis, L-tryptophan can be decarboxylated by a specific L-tryptophan decarboxylase (EC 4.1.1.105), producing L-tryptamine [50,51], which is further metabolized by a kinase and a methyl-transferase into psilocybin, a naturally occurring psychedelic prodrug.
In mammals, most of the serotonin in the periphery is synthesized by the enterochromaffin cells of the intestine. In this context, serotonin acts locally in the gut and enters the circulation, where >95% of it is taken up by platelets [52]. Serotonin uptake by platelets is produced by serotonylation, a binding of serotonin to glutamine residues mediated by transglutaminase. This process occurring on small GTPases like RhoA and Rab4 activates the release of alpha-granule content from platelets, which consists of a mixture of growth factors (e.g., insulin-like growth factor 1) and procoagulant factors (von Willebrand factor, factor 5, fibronectin, thrombospondin). Serotonylation also controls insulin release from pancreatic beta cells [53]. Basal serotonin tonically inhibits secretion of glucagon-like peptide-1 (GLP-1) by a particular type of enteroendocrine cells, L-cells. GLP-1 is a 30 or 31-residue hormonal peptide that stimulates insulin secretion by endocrine pancreatic beta cells [54]. Serotonin is cleared from the circulation by the serotonin transporter (SERT) and then enters the liver, where it is metabolized by monoamine oxidase-A (MAO-A) to 5-hydroxyindoleacetic acid (5-HIAA). Deletion of tryptophan hydroxylase 1, the enzyme that synthesizes peripheral serotonin in the intestine, has recently been found to improve glucose metabolism under high-fat diet (HFD) conditions [55].
Serotonin released by presynaptic boutons of serotonergic neurons is recaptured via SERT (a specific high-affinity transporter), and partly by a low-affinity transporter, plasma membrane monoamine transporter (PMAT) [56]. Serotonin catabolism is complicated because it preferentially involves the MAO-A enzyme in mammals, and this subtype, which differs from MAO-B, is not usually located at the terminals of serotonergic neurons. It would imply that serotonin catabolism is partly associated with other cells. In any case, serotonin and its metabolite 5-HIAA are excreted in the urine. Carcinoid tumors of the digestive tract, along with other neuroendocrine tumors, can cause excessive production of serotonin and therefore 5-HIAA in many species. The amount of serotonin differs depending on the tissue, but generally it follows the density of fibers and cell bodies. In crayfish, the highest amount is found in the cerebral ganglia (about 300 pg/mg), followed by the thoracic chain (120 pg/mg) and the abdominal chain (10 pg/mg). In mammals, the highest amounts of serotonin are found in the substantia nigra (500–1000 pg/mg). In birds, serotonin content is higher in the amygdala compared to the thalamus or striatum [57].
The Effects of Serotonin on Metabolism
Factors that can alter serotonin levels during pregnancy or early childhood have been associated with a higher risk of developing serotonergic disorders [58]: changes in nutrition (e.g., low-tryptophan foods), stress factors (e.g., maternal separation), infections (e.g., flu), and antidepressant drugs that act as serotonin reuptake inhibitors (SSRIs) exert their effects during fetal life and at early postnatal ages.
High serotonergic tone promotes obesity, as shown by studies in rats with altered serotonin homeostasis [59]. At the brain level, biogenic serotonin exerts opposite effects on body weight regulation: increasing brain serotonin activity is expected to decrease body weight, while increasing peripheral serotonin activity will increase body weight and adiposity. In a genetic model of rats with constitutionally high or low serotonin homeostasis (hyperserotonergic/hyposerotonergic rats), differences in endogenous serotonin levels modulate the body’s net energy balance [60]. In hyper/hyposerotonergic rats, researchers assessed physiological characteristics associated with body weight homeostasis and the large-scale expression profile of body weight-regulating genes in the hypothalamus, a major brain region that controls energy balance. The results showed that stress induced by contention in common cages of many individuals for rats with a high level of serotonin, compared to animals with a low level of serotonin, led to an increased body weight (by 12%) maintained throughout life, a higher daily food intake (by 9%), and a different distribution of fat type—more white adipose tissue and less brown adipose tissue. A large number of hypothalamic genes can explain 22% of the occurrence of obesity: mutations in MC4-R—the melanocortin-4 receptor gene, in heterozygous form, in the β3-adrenergic receptor gene or PPARγ2—peroxisome proliferator-activated receptor, a nuclear transcription factor with a key role in adipocyte differentiation [61], are other examples of genes involved in simple obesity that regulate body weight. These genes were analyzed for mRNA expression. Only a few of 84 body weight-regulating hypothalamic genes included in the analysis showed significant differences in mRNA expression between serotonergic animal subtypes (e.g., neuropeptide Y receptor, fibroblast growth factor), but hyperserotonergic animals showed a clear trend to up-regulate mRNA for a number of orexigenic peptides signaling, their receptors, and other molecules with orexigenic activity. Receptors for peripheral signals (leptin, insulin) and downstream signaling molecules were not altered, indicating no change in central insulin/leptin resistance [59].
4. Types of Serotonin Receptors
Serotonin receptors are divided into seven classes (Table 1) and grouped by conserved sequence motifs, second messenger systems, and founding members. Class 1 receptors couple Gαs proteins, while class 2 receptors couple inhibitory Gαo proteins. Class 3 receptors comprise ionotropic receptors, i.e., ligand-gated cation-permeable channels opened directly by serotonin [62]. All serotonin receptors except the ionotropic 5-HT3 receptor are G protein-coupled receptors (GPCRs) [4,63]. Serotonin levels in the synaptic cleft determine the receptors involved in serotonergic neurotransmission. Serotonin activates at least 18 different receptors. Ionotropic receptors (5-HT3) are found in the CNS and peripheral neurons, modulating their activity. These receptors mediate fast excitatory synapses with rapid desensitization [62]. They are involved in learned behaviors that can be modified by selective serotonin reuptake inhibitors, such as anxiety and vomiting. Metabotropic (G protein-coupled) receptors also mediate rapid effects, particularly on some cortical neurons, by activating via Gq/G11 phospholipase C (PLC) and inositol-1, 4, 5-trisphosphate Ca2+ channels (IP3R) to release Ca2+ from stores. However, some of them, such as serotonin receptors of families 4, 6, and 7, act via Gs-mediated stimulation of adenylyl cyclase (AC), resulting in increased levels of cAMP, protein kinase A (PKA), and mitogen-activated protein kinase (MAPK) activity, phosphoinositides, and intracellular calcium mobilization [64]. This causes receptor downregulation or ion channel inhibition that is implicated in reducing long-term tolerance to SSRIs.
The diversity of serotonin-induced effects on cognitive function and behavioral responses is related to its simultaneous effects on a multitude of neural targets and a large number of receptors. Because of these specificities, serotonergic systems can provide fine regulation of behaviors in various situations. The organization of the serotonergic system is completely different between species, from a limited number of Drosophila cells (100 serotonin immunoreactive cells) to several hundreds of thousands of neurons in vertebrates [65].


Table 1.
Families of ionotropic and metabotropic serotonin receptors (adapted from [66]).
Table 1.
Families of ionotropic and metabotropic serotonin receptors (adapted from [66]).
| Family | Potential | Type | Mechanism of Action |
|---|---|---|---|
| 5-HT1 | Inhibitory | Gi/Go protein-coupled | AC inhibition—decreasing intracellular concentration of cAMP |
| 5-HT2 | Excitatory | Gq/11—protein-coupled | PLC activation—increasing intracellular concentration of IP3 and DAG |
| 5-HT3 | Excitatory | Ligand—gated Na+/K+ channel | Depolarization of cell plasma membrane |
| 5-HT4 | Excitatory | Gs—protein-coupled | AC activation—increasing intracellular concentration of cAMP |
| 5-HT5 | Inhibitory | Gi/Go protein-coupled | AC inhibition—decreasing intracellular concentration of cAMP |
| 5-HT6 | Excitatory | Gs—protein-coupled | AC activation—increasing intracellular concentration of cAMP |
| 5-HT7 | Excitatory | Gs—protein-coupled | AC activation—increasing intracellular concentration of cAMP |
Gx—trimeric membrane-anchored G protein with αβγ subunits; AC—adenylate cyclase; PLC—phospholipase C; IP3—inositol 1,4,5-trisphosphate; DAG—diacylglycerol.
Serotonin receptors have been classified as 5-HT1A-F, 5-HT2A-C, 5-HT3, 5-HT4, 5-HT5, 5-HT6 and 5-HT7. Most serotonin receptors are GPCRs and show heterogeneity, with several having both excitatory and inhibitory functions [32]. They are coupled to various G proteins: 5-HT1, 5-HT5 to Gi protein; 5-HT2 to Gq protein; and 5-HT4, 5-HT6, and 5-HT7 to the Gs protein. Immunohistochemical studies show that these receptors are expressed very early during embryonic development, with the 5-HT7 receptor showing the most widespread distribution and the rest being more restricted to the raphe nuclei [67]. All serotonin receptor families, except for 5-HT3R, are expressed by mammalian raphe neurons. During postnatal development, the expression levels of these receptors are dynamically regulated, suggesting that they have an essential role during brain development. Except for 5-HT1B and 1D receptors, which are presynaptic, all the rest can be retrieved at the postsynaptic level. Of these postsynaptic receptors, 5-HT1A receptors play an important role in early neurodevelopment, being present at the dendritic as well as the somatic level [8].
4.1. 5-HT1 Receptors
The 5-HT1 receptor is a phylogenetically old receptor and is present from the lowest of invertebrates to the highest of mammals. The 5-HT1 receptor is the most studied of the serotonin receptors. Apart from animals, S. cerevisiae also possesses 5-HT1 receptors, indicating that 5-HT1 receptors predate the divergence of these two kingdoms. The 5-HT1 receptors were first discovered in rats in 1979 [68], and they were also the first cloned serotonergic receptors [69]. 5-HT1 receptors are Gi-coupled and their activation decreases cAMP production [70]. Moreover, they cause hyperpolarization of neurons by activating inward rectifier K+ (Kir) channels [71]. 5-HT1 receptors are involved in a variety of physiological functions: hormone release, cardiovascular regulation, and locomotor activity, as well as in the pathogenesis and treatment of diseases such as affective disorders, anxiety disorders, schizophrenia, drug abuse, and many others. In the CNS, 5-HT1Rs are expressed in clusters of neurons located in the shaft and midbrain. 5-HT1B and 5-HT1D receptors are widely distributed in the brain, with somewhat lower cortical densities; 5-HT2B receptors feature the highest densities in the basal ganglia and hippocampus-subiculum [72]. 5-HT1A receptors are located both pre- and postsynaptically on raphe neurons, while 5-HT1B receptors are located on the perisomatic membranes of presynaptic varicosities. The mechanisms underlying postsynaptic inhibition through this family of receptors involve inhibition of cAMP formation and activation of big conductance K+ channels. 5-HT1Rs are major pharmacological targets for development of psychoactive compounds. An important aspect of these receptors concerns their differential actions in pathological conditions, which in turn may produce diversity in therapeutic responses. Future studies will also allow better resolution of the mechanisms underlying the paradoxical effects of different ligands at 5-HT1 receptors, particularly in emotional and addictive behaviors [73].
4.2. 5-HT2 Receptors
Five distinct human genes encode 5-HT2 receptors: 5-HT2A, 5-HT2B, and 5-HT2C, and two closely related 5-HT2C receptors present in rat and mouse (5-HT2C2 and 5-HT2C5). 5-HT2A, 5-HT2B, and 5-HT2C receptors are related to each other and share a common ancestor with 5-HT2-like receptors from different taxa, including a cnidarian (Nematostella vectensis), a platyhelminth (Schistosoma mansoni), and an arthropod (Drosophila melanogaster). The function of these receptors is unknown [74]. 5-HT2A and 5-HT2C receptors are present in the CNS, and have multiple and similar functions. All members of this 5-HT2 receptor family are coupled to GαQ/Z proteins. Pre- and post-translational modifications of 5-HT2A receptors are closely related to CNS disorders such as depression, schizophrenia, dementia, and alcohol and nicotine addiction [75].
It is important to consider the therapeutic implications of understanding the role of 5-HT2 receptors on vascular function. Several disorders have been linked to an imbalance of NO and superoxide in the vascular system. Many such conditions are associated with cardiovascular disease, including hypertension, diabetes, and restenosis after angioplasty. Given the presence of 5-HT2 receptors on the endothelium, or very close to it, as in the vascular smooth muscle (VSM), it is reasonable to speculate that selective drugs targeting these locations of the vasculature could be used to increase NO production in disorders related to NO deficiency (arterial hypertension, diabetes, etc.), as well as to reduce NO production in conditions related to excess NO, such as acute inflammation. This would result in the desired vasculoprotective effects of the former, as well as the desired intravascular inhibitory effects of the latter [75].
Currently, there are several selective 5-HT2 ligands that have been used for in vitro mechanistic studies of blood vessel function, such as 5-HT2A antagonists ketanserin and sarpogrelate [76] and 5-HT2B antagonists RS-127445 and VU6047534 [77]. However, none of these selective 5-HT2 antagonist drugs have proven useful in clinical trials. Given that it appears that therapeutic effects could be obtained from blocking or enhancing the action of these 5-HT2 receptors located in or very close to the vasculature, next-generation compounds such as selective antagonists and/or agonists would be extremely useful for the development of new therapies.
4.3. 5-HT3 Receptors
5-HT3 is the only family composed of ionotropic serotonin receptors. 5HT-3Rs are pentameric ligand-gated ion channels similar to nicotinic acetylcholine receptors, GABAA and glycine receptors, each subunit being composed of four transmembrane helices. They are located within both the peripheral nervous system (PNS) and the central nervous system (CNS). 5-HT3 receptors were the first neurotransmitter-gated ion channels to be cloned [78]; therefore, they are the best studied serotonin receptors. There are five subunit types: 5-HT3A, 5-HT3B, 5-HT3C, 5-HT3D, and 5-HT3E, which are co-assembled into heteropentameric receptors.
5-HT3R ligands can be classified into three groups: classical agonists (5-HT, 2-methyl-5-HT, and 1-(m-chlorophenyl)biguanide), partial agonists (2-chloro-5-HT, 5-F-HT, and 2-piperazin-1-yl-5-HT), and agonists with atypical structure (β-carbolines, quipazine, PSB-11) [79]. Partial agonists are thought to act primarily through an indirect mechanism, stabilizing an intermediate state of the receptor. Use of a 5-HT3 receptor mutant lacking a conserved proline in the M2 segment results in altered agonist binding and activation [80,81]. In the CNS, homo- or heteropentameric 5-HT3 receptors are assembled from 5-HT3A and 5-HT3B subunits with distinct pharmacological properties. Maturation of receptor subtypes has a broad impact on the surface expression and gating kinetics of agonist- and antagonist-induced responses [82]. The gating mechanism of 5-HT3A receptor isoforms represents a unique experimental platform to delineate the gating mechanisms of Cys-loop receptors [83] that modulate CNS synapse functions associated with anxiety, stress, addiction, and depression. 5-HT3 receptors operate at nanomolar concentrations of serotonin, which are within the physiological range of concentrations present in the CNS.
5-HT3 receptors mediate fast excitatory neurotransmission, as well as hyperalgesic and antinociceptive effects in systemic diseases such as inflammation, neuropathic pain, and chronic pain induced by tumors and diabetes. They are also involved in the well-known side effects of some antidepressants and in the mechanism of drug addiction and reward processes. Pathological activation of 5-HT3 receptors triggers excessive excitation of nerve cells, leading to action potential bursting and hyperexcitability [32]. It is associated with various neurological and psychiatric disorders, including drug addiction, schizophrenia, anxiety, headaches, depression, epilepsy, motion sickness, and postoperative nausea and vomiting [79]. Receptor agonists activate nociceptive pathways, triggering the onset of visceral pain and increased drive via peripheral 5-HT3 receptors, while antagonists effectively prevent and ameliorate the visceromotor response to colonic irritation.
4.4. 5-HT4 Receptors
5-HT4 receptors are members of the large GPCR superfamily. They exhibit specific coupling to the AC and cAMP pathway in various species, including mammals, birds, amphibians, and teleosts. Major achievements have been made in the field of cardiovascular and neurological disorders by using specific 5-HT4 receptor ligands as therapeutic tools [84]. They are important targets for treating gastrointestinal disorders such as constipation and neurological disorders like Alzheimer’s disease (AD) and ischemic stroke. Post-mortem studies in healthy elderly and Alzheimer’s disease patients have shown an age-related decline of this receptor density in the human cortex [85].
4.5. 5-HT5 Receptors
The 5-HT5 receptor is a largely overlooked member of the serotonin receptor superfamily due to the lack of selective pharmacological tools and limited understanding of its function. Two 5-HT5 genes were cloned in rats and mice in the early 1990s [86]. However, in humans, only 5-HT5A forms functional receptors, the 5-HT5B gene being interrupted by an early stop codon and thus being translated into a non-functional protein [87]. Expression of this type of receptor at the mRNA level is almost absent in the periphery [88], except for embryonic rat dorsal root ganglia, superior cervical ganglion, petrosal ganglion, and carotid body [89], while in the brain, 5-HT5A is present in the cortex, cerebellum, thalamus, hypothalamus, habenula, dorsal raphe nucleus, and spinal cord [86]. Activation of 5-HT5 receptors is propagated via Gi/Go, AC, and the cAMP pathway, resulting in neuromodulatory as well as vasomotor (dilatory or constricting) effects. Although no clinically applicable drug acting specifically on these receptors has been developed to date, a few selective agonists and antagonists are available, such as SB-699551-A, ASP5737, A-843277, A-763079, A-833551, AS2030680, AS2674723, AS3304, AM366 [64,90,91,92,93,94,95]. 5-HT5Rs may represent therapeutic targets in various neuropsychiatric and metabolic diseases, such as anxiety, depression, schizophrenia, and cocaine addiction [64,91,92,95]. Deregulation of the 5-HT5 receptors with weight loss agents could be a significant side effect, since 5-HT5 receptor agonist AS3304 is a candidate for treatment of cancer cachexia [96,97,98,99].
4.6. 5-HT6 Receptors
The 5-HT6R subtype is exclusively located in the brain, being highly expressed in the cortex and some hippocampal regions that are critically involved in cognition and memory. It is also expressed in the limbic system and ventral striatum structures such as the nucleus accumbens septi. Consistent evidence has been gathered concerning the relationship between 5-HT6R and cognition, and a new role has been described in the regulation of arousal [100].
Pharmacological and genetic evidence supporting the important role of 5-HT6R in brain disorders is now rapidly accumulating. 5-HT6R binding of antipsychotics like clozapine or olanzapine, and of antidepressants like amitriptyline, supports a role of these receptors in schizophrenia and depression [101]. 5-HT6R activation, particularly through the cAMP signaling pathway, attenuates many pathophysiological processes involved in these CNS disorders. 5-HT6Rs are expressed and can affect neuronal and non-neuronal cells, including microglia and astrocytes, raising the possibility of targeting cell type-specific 5-HT6R functions in specific brain disorders [102]. In addition to promising results in Alzheimer’s disease, statistically significant effects have been proven on extrapyramidal symptoms (Parkinson-like parameters, agonist behavior), neurotoxicity, cognitive impairment, autonomic nervous system function, affective components, difficulties in self-adhesion, activities of daily living, and locomotor activity (in aged animals) [103].
Substantial evidence from knockout and pharmacological studies indicates that 5-HT6 receptor antagonists represent an attractive strategy for psychotherapeutic drug discovery [101]. For example, the selective 5-HT6R antagonist SB-399885 was capable of alleviating scopolamine-induced cognitive deficits in an object recognition task and the Morris water maze test in aged rats [104]. These receptors could become targets for anxiolytic, antidepressant, antipsychotic, or even antiparkinsonian drugs devoid of the adverse effects of classical psychotropic drugs.
4.7. 5-HT7 Receptors
5-Hydroxytryptamine (serotonin) 7 receptors (5-HT7Rs) were the last of the serotonin receptor family to be identified in 1993 [105]. However, more than three decades later, their functional roles and pathological implications remain incompletely understood [106]. Diversity in receptor functionality is dictated by a complex interplay between the receptor, tissues, and pathophysiological context. In the central nervous system (CNS), 5-HT7Rs are widely expressed and have been implicated in the regulation of a number of processes, including the sleep-wake cycle, body temperature, cognitive function, depression, nociception, and gastrointestinal system motility.
Several studies have identified 5-HT7 receptors in areas of the brain known to be related to mood, behavior, and circadian rhythms, exerting specific functions within them. 5-HT7Rs have been implicated in a number of neuropsychiatric conditions, including anxiety and mood disorders, schizophrenia, and sleep disorders. They also play a role in cognitive processes, being located in areas related to spatial or associative memory formation, including the hippocampus, and are thought to enhance synaptic plasticity. Therefore, 5-HT7Rs may provide a possible target in the treatment of diseases with impaired cognition and memory, such as Alzheimer’s or Parkinson’s disease [106].
5. Serotonin Pathways and Thermoregulation
5.1. Definition and Mechanisms of Thermoregulation
The term “thermoregulation” refers to the physiological ability to maintain body temperature within a certain range. Animal species can be broadly classified into poikilotherms, conditional homeotherms, and homeotherms, i.e., evolved organisms (birds and mammals) able to maintain a constant temperature over a wide range of external conditions. The term “homeothermy” (from the Greek “homeo”—unchangeable, “thermos”—heat) is often used as a synonym for thermoregulation. In this condition, heat conservation or heat dissipation mechanisms are activated to maintain body temperature within a narrow range (normothermia or euthermia—36.1 to 37.2 °C in humans) [107], independent of changes in ambient temperature. The production of heat through metabolic processes, as well as its retention or loss, are the main heat regulation mechanisms (Figure 3) [108].
The most important nervous center that coordinates the processes of heat production and heat loss is the hypothalamus, particularly the thermal control center located in the preoptic area. In addition, temperature-sensitive receptors are spread throughout the body, including muscles and internal organs, and any change in temperature is detected by these receptors. “Cold” and “warm” receptors are located in the skin and other tissues. Information from these receptors reaches the central nervous system, which possesses its own thermosensory mechanisms, and regulatory processes like shivering or non-shivering thermogenesis or thermolysis are activated. An important thermogenic mechanism occurs in the brown adipose tissue [22,97], where thermogenins of the inner mitochondrial membrane, activated by sympathetic stimulation, dissipate the proton gradient, transforming its energy directly into heat, without ATP generation.
The ability to maintain a relatively stable body temperature within a narrow range when being exposed to variable environmental and metabolic conditions is essential for the proper functioning of organs and cells. When body temperature rises, various physiological responses occur to dissipate heat, such as vasodilation, sweating, and, in certain species, panting. Conversely, when body temperature drops, physiological responses aimed at conserving and producing heat are triggered, such as vasoconstriction, piloerection, and shivering [52,71,109,110].
5.2. Experimental Evidence Concerning Involvement of Serotonergic System in Thermoregulation
Serotonin is involved in pathways that affect thermogenesis and vasomotor tone; however, it does not serve as a dedicated thermoregulator. Its effects are context-dependent, indirect, and receptor subtype-specific. Although central serotonergic circuits are involved in thermoregulation, they have no direct connection to febrile responses, the mediators of which are prostaglandins acting on hypothalamic EP3 inhibitory prostanoid receptors [110].
Temperature is one of the most important internal parameters that must be monitored and maintained within a narrow range, and thus thermal sensing and specific homeostatic control mechanisms have been present in various types of organisms since early in phylogenesis. Sophisticated scientific instrumentation was developed for accurate temperature measurement at different levels, from macroscopic to single-cell level, including classical thermal dilation thermometers (e.g., alcohol-filled), thermistors, thermocouples, thermoresistive glass pipette tips, infrared radiation detectors and cameras, and thermosensitive fluorescent dyes [111,112]. These tools have contributed to investigation of molecular mechanisms involved in thermogenesis and thermolysis in different tissues and organs, as well as the nervous and endocrine control of these processes, defining the field of thermal biology [113].
Pioneering experiments of Charles Richet and Aronsohn and Sachs proved the existence of a thermoregulatory “warming center” in the brain based on mechanical lesion of the hypothalamus and surrounding areas [114,115]. Subsequent studies used carotid blood warming to induce thermoregulatory responses [116,117]. In 1912 Henry Barbour used thin water-filled metal tubes named thermodes implanted in the preoptic area [118], and later Magoun et al. restricted the location of thermoregulatory centers to the anterior hypothalamus based on restricted radio-frequency heating in the cat brain [119]. Subsequent research established that thermal sensory information from the periphery and to a lesser extent from central organs is conveyed, beyond specific primary somatosensory projection areas, to the preoptic area, recognized as a thermoregulatory center, which controls via efferent signals heat production or dissipation mechanisms [21], and these controls remain active also in unconscious animals [120,121]. Temperature changes in the preoptic area activate peripheral compensatory mechanisms in many species [122].
The molecular sensors of peripheral or central thermoreception have been better understood with the cloning of several thermosensitive ion channels, some of them belonging to the transient receptor potential (TRP) family, activated in the non-noxious and noxious range of temperatures [123,124,125,126]. Afferent thermal sensitivity pathways are composed of three neurons: a primary sensory neuron in the dorsal root or trigeminal ganglia, a secondary neuron in Rexed laminae I (zona marginalis) and II (substantia gelatinosa) of the dorsal horn, and a third central neuron. Somatosensory neurons of the dorsal horn can be subdivided into a nociceptive group responding to specific noxious stimuli, a polymodal nociceptive group, and a non-nociceptive thermosensitive group, responding gradually to heating or cooling stimuli [127]. The third central neuron is located in the main thalamic nuclei for the spinothalamic pathway, or in the dorsal part of the lateral parabrachial nucleus for the spinoparabrachial pathway, further projecting in the preoptic area or the dorsal raphe nucleus, activating serotonergic pathways to the ventral striatum and nucleus accumbens [24].
The two spinal pathways convey different types of information in the case of noxious stimuli: the spinothalamic pathway provides the central projection leading to the pain sensation, while the spinoparabrachial pathway contributes to the affective dimension of pain, producing secondary effects such as anxiety or depression [128]. Further projections of the spinothalamic thermosensitive pathway are in the primary somatosensory projection cortex and insular cortex, while projections of the spinoparabrachial pathway are in centers of the preoptic area (median and medial preoptic nuclei), and oxytocin-secreting neurons of the hypothalamic paraventricular nucleus. The dorsal lateral parabrachial nucleus projects to serotonergic raphe nuclei, particularly the interfascicular zone of the dorsal raphe nucleus, which in turn projects to areas involved in interpreting the affective nature of thermosensation, such as the ventral striatum and further to the medial orbitofrontal cortex and the pregenual cingulate cortex. These areas also receive connections from the cortical projection areas (primary somatosensory and insular cortex) and from the ventrobasal thalamus via the ventral striatum. Meanwhile, the lateral orbitofrontal cortex integrates and translates the unpleasant character of thermosensory inputs. These thermosensitive projection regions of the brain may function abnormally in mood disorders like depression or mania [129,130]. The activity of the ventral striatum, an area involved in pleasant sensations evoked by thermal stimuli, is decreased in depression and increased in bipolar disorders [131,132]. Another thermosensitive projection region, the pregenual anterior cingulate cortex, shifts from negative to positive affective tonus during treatment with antidepressants [133].
Studies of thermoregulatory responses in different animal species upon temperature changes in the anterior hypothalamus and preoptic area have been performed, particularly by the group of Jack Boulant [122]. Warming of these central thermoregulatory centers evoked peripheral thermolysis responses such as increased skin blood flow and sweating, while central cooling activates thermogenesis mechanisms, such as adrenergic activation of the brown adipose tissue, reduced peripheral heat loss via decreased blood flow, piloerection, and, in extreme cases, shivering (Figure 4). When the preoptic area is lesioned, pyrogen injection is no longer capable of inducing fever responses [134]. Moreover, maneuvers like carotid blood warming or warm saline irrigation of the third ventricle could induce thermolysis responses such as peripheral vasodilation and even panting [116,117,118,135,136]. Subsequent studies using thermodes and microelectrode impalement in the preoptic area and anterior hypothalamus led to identification of specific heat-sensing neurons, with increases in firing rates up to 0.8 AP/°C; similar experiments were later performed in brain slices [134]. Approximately 30% of preoptic area neurons were classified as warm-sensitive, and <5% as cold-sensitive, receiving inhibitory inputs from warm-sensitive neurons (Figure 4). Electrophysiology and mathematical modeling studies suggested that thermosensitivity in these central neurons could be achieved via temperature-dependent inhibition of background leak two-pore domain weak inward rectifier (TWIK) K+ channels, such as TASK-1 (TWIK-related acid-sensitive K+ channel) and TRAAK (TWIK-related arachidonic acid-stimulated K+ channel), and possible activation of TRPV4 channels [137]. Similar electrophysiology and expression studies on brain slices identified thermosensitive dopaminergic neurons in the substantia nigra pars compacta with temperature-dependent changes in firing rate driven by TRPV3 and TRPV4, two ion channels active in the physiological range of temperatures [138].
Numerous classical studies have also established a relationship between depressive mood disorders and impaired thermoregulation [139,140,141]. Thus, depressed patients feature increased overnight body temperature, while antidepressant therapy reduces nocturnal core temperature [142,143]. Given the intricate relationship between mood disorders and the serotonergic system and the effects of psychoactive compounds on serotonergic receptors, a corollary of these studies is the involvement of serotonergic pathways and responses in central thermoregulation [144], although circuits involved in regulation of mood and behavior are distinct from those involved in core thermoregulation. The seminal studies of Bligh identified the anterior hypothalamus as a site of action for projections of serotonergic raphe nuclei [145], while numerous serotonergic receptors in blood vessels are involved in vasomotricity and peripheral temperature control [146]. Early neurochemistry studies evidenced high concentrations of monoamines such as noradrenaline and serotonin in the hypothalamus [147,148], leading to the hypothesis formulated by von Euler in 1961 that monoamine neurotransmitters may participate in core temperature regulation [149]. Briefly thereafter, experiments performed by Feldberg and Myers found that catecholamines injected in lateral cerebral ventricles of the cat decreased temperature, while serotonin injected in the same region increased it [150]. Interestingly, the thermogenetic effect of intracerebroventricular serotonin lasted more than 18 h, but subsequent studies in cat and dog showed sustained release of prostaglandins from the hypothalamus in these conditions, which are likely mediators of these effects [151,152]. Similar experiments with injection in cerebral ventricles or anterior hypothalamus of monoamines, acetylcholine, or monoamine oxidase (MAO) inhibitors like tranylcypromine produced different thermoregulatory effects in various species (fishes, amphibians, reptiles, birds, and mammals), depending on the external temperature [153,154,155,156]. Moreover, serotonin receptor partial agonists and inhibitors on peripheral 5-HTRs such as lysergic acid dyetylamide (LSD-25) [157] or methysergide were able to counteract increased heat loss and fall in core temperature induced by serotonin or its agonist, norfenfluramine [145]. In fact, LSD effects on thermoregulation upon subcutaneous administration in male C57Bl/6J mice have been proven to be complex and biphasic, 5-HTR activation resembling an inverted U-shape due to receptor internalization at high doses, resulting in hypothermia and hypolocomotion by effects on 5-HT1AR [158].
During the following two decades central serotonergic modulation studies on thermoregulation have been focused on rodent animal models, but they used increasingly extended sets of agonists and antagonists specific for different types of serotonin receptors [159,160,161]. 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT) was identified as selective agonist of 5-HT1A receptors, while the butyrophenone spiperone (spiroperidol) and ketanserin were classified as selective antagonists of 5-HT2A receptors. 5-HT2R activation by 5-OH-tryptophan induced specific behavioral responses like head twitches, “wet dog” shaking, or lordosis behavior in mice and rats, while 5-HT1R activation resulted in rotational movements in rats with dorsal raphe nucleus lesions or myoclonus in guinea pigs [162]. Related to thermal responses, systemic administration of 5-HT2R agonists like 6-chloro-2-(1-piperazinyl)pyrazine (CPP or MK-212), mCPP, or quipazine and the serotonin-releasing agent fenfluramine resulted in hyperthermia in rats [163], while intrahypothalamic injection of serotonin or 8-OH-DPAT decreased core temperatures [164,165]. Using selective 5-HTR antagonists, Gudelsky et al. proved that central hypothalamic activation of 5-HT1AR dose-dependently induces hypothermia, while central specific activation of 5-HT2R has the opposite effect [162]. The hyperthermic response to mCPP injection in rats was alleviated by 5-HT2 or mixed 5-HT2/1 antagonists such as ritanserin, mesulergine, or metergoline, but not by 5-HT1A/B and β-adrenergic receptors inhibitors like propranolol or pindolol, or 5-HT3 antagonists ondansetron and tropisetron [166,167,168]. In normal human subjects, intravenous administration of 0.08 mg/kg body weight mCPP led to a biphasic response consisting of mild initial hypothermia (−0.04 °C at 12 min) followed by progressive hyperthermia (+0.17 °C at 90 min) accompanied by increased plasma norepinephrine levels [144]. Other studies assessed the effects of intravenous administration in rats of 5-HT1AR agonist 8-OH-DPAT, 5-HT1A/BR agonist RU 24969, 5-HT2C/2A receptors agonists ±1-2,5-dimethoxy-iodophenyl-2-aminopropane (DOI) and mCPP, and 5-HT3R agonist 2-methyl-5-HT (2ME): all agonists increased oxytocin mRNA levels in the paraventricular nucleus and all except for 2ME in the supraoptic nucleus, while only DOI and mCPP increased vasopressin mRNA levels in the paraventricular nucleus and no agonist changed its mRNA expression in the supraoptic nucleus; plasma oxytocin levels were increased by all agonists and plasma vasopressin levels only by DOI and mCPP [169]. Subsequent studies of Henrik Stig Jørgensen showed that unilateral lesion of the dorsal raphe nucleus or paraventricular nucleus via stereotaxic injection of the serotonergic neuron toxin 5,7-dihydroxytryptamine (5,7-DHT) reduced arginine vasopressin and adrenocorticotropic hormone (ACTH) responses to stress, and also that serotonin stress-induced prolactin secretion is mediated by 5-HT2Rs and 5-HT3Rs, while serotonin stress-induced ACTH secretion is mediated by 5-HT1A/2A/2C receptors in a manner that is only partially dependent on corticotropin-releasing hormone (CRH) [170]. Thus, serotonin can influence secretion of CRH and ACTH at multiple levels: hypothalamic, at the hypothalamo-hypophyseal portal system (Gr.T. Popa-Fielding), at the adenohypophyseal and cortical adrenal level. This interference between serotonin and CRH/ACTH may lead to important consequences in thermoregulation responses due to the fact that corticotropin is a major component of stress responses. It was shown that 21 days of chronic unpredictable stress in rats led to increases in basal body temperature and hypothermic instead of thermogenetic responses to cold, while treatment with corticosterone synthesis inhibitor metyrapone during stress partly restored normal thermoregulation [171]. In turn, oxytocin and vasopressin released during exposure to warm temperatures may induce thermoregulatory cooling [172]. Body temperature, as well as circulating levels of corticosterone and other hormones, are subjected to interrelated circadian rhythms, which become dampened with an increase in age [173].
An elegant demonstration of the role of sympathetic autonomic regulation of body temperature via increased brown adipose tissue (BAT) activity induced by skin cooling in rats under general anesthesia was performed by Nakamura and Morrison [22]. The authors monitored several physiological parameters such as cutaneous, BAT, rectal and brain temperature, heart rate, arterial blood pressure, CO2 concentration in expired air, and electric activity of sympathetic nerve fibers distributed in the BAT, showing that skin cooling induced by a jet of air results in sympathetic stimulation that increases heart rate, basal metabolism reflected in CO2 concentration, and via efferent sympathetic fibers BAT thermogenesis, but without major changes in rectal or brain temparature. These adaptive responses could be inhibited by bilateral stereotaxic injections of different compounds in specific brain regions: GABAA antagonist bicuculline in the preoptic area, 5-HT1AR agonist 8-OH-DPAT or non-selective ionotropic glutamate receptor inhibitor kynurenic acid or glycine or GABAA agonist agarin (muscimol) in the rostral nucleus raphe pallidus (a serotonergic system center), by agarin injection in the dorsomedial hypothalamus nucleus; however, bilateral agarin microinjection in the dorso- or ventro-lateral caudal periaqueductal gray failed to inhibit thermogenetic responses. These responses define a complex neuronal circuit with serotonergic and GABAergic involvement in the raphe-hypothalamus-preoptic area, connected to peripheral thermoreceptors via ascending pathways and to the BAT via efferent sympathetic pathways. Numerous other studies showed activation of raphe serotonergic neurons by non-noxious warming or cooling afferent signals propagated via the spinoparabrachial pathway [174,175,176]. In another animal model study male adult rats were exposed to a protocol with random stress-inducing procedures, including immersion into cold water, for three consecutive weeks, then tested after another 4 days by exposure to cold temperature (10 °C) for 30 to 60 min, with body temperature recordings initially and after 6 h. In these conditions stressed rats were unable to increase body temperature after cold room exposure, as did the non-stressed control animals. Treatment with corticosterone synthesis inhibitor metyrapone 50 mg/kg i.p. during the stress-induction period improved cold-activated thermogenesis. In other experiments, one day after the stress-inducing procedures, some groups were administered 250 nL of 5-HT2A/C receptors agonist DOI at 10 nM concentration or artificial cerebrospinal fluid (ACSF) in the medial preoptic area bilaterally. In these conditions the DOI-injected stressed animals developed higher temperature increases triggered by cold exposure compared to no stress-DOI-injected and stressed-ACSF groups, proving that specific activation of these serotonergic receptors in the preoptic area is capable of triggering thermogenesis [171]. Immunohistochemistry studies on rats exposed to either room temperature (23 °C) or warm temperature (37 °C) for 105 min proved in the warm-exposed group of animals activation of the interfascicular dorsal raphe nucleus serotonergic neurons, increasing expression at protein level of the transcription factor protooncogene c-Fos, an immediate-early gene activated by growth factors, cytokines, tumor promoters or UV irradiation, and of tryptophan hydroxylase [174].
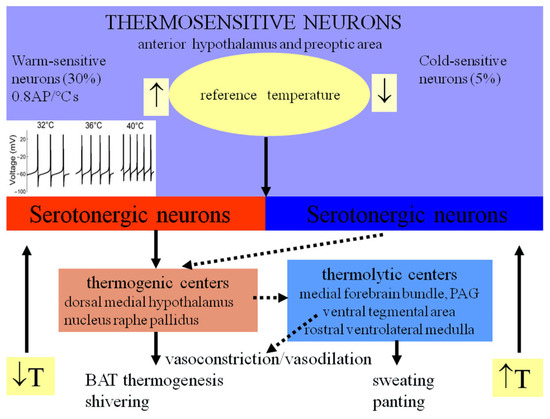

Figure 4.
Central and peripheral mechanisms of thermoregulation (adapted from [21,22,137,162]). (T—temperature, AP—action potential, PAG—periaqueductal gray, BAT—brown adipose tissue).
Another interesting study was performed on normal vs. serotonin transporter (SERT) double-knockout rats. In SERT−/− rats stress-induced hyperthermia was almost absent. A similar effect could be obtained in normal (SERT+/+) rats by administering the 5-HT1AR agonist flesinoxan 10 mg/kg i.p., and this effect was reversed by co-administration of the 5-HT1AR antagonist WAY100635 1 mg/kg i.p. WAY100635 alone induced hyperthermia in SERT−/− but not in SERT+/+ rats, proving that stress-induced hyperthermia and hypothermia are regulated by distinct populations of 5HT1A receptors [177]. Summarizing, central and peripheral mechanisms of thermoregulation involve multiple types of serotonergic receptors, with 5-HT1AR, 5-HT3R, and 5-HT7R reducing core body temperature, the first type via inhibition of both non-shivering and shivering thermogenesis and increasing heat dissipation by activation of peripheral blood flow. 5-HT2R activation exerts antagonistic effects on 5-HT1AR [71]. Ionotropic serotonergic receptors (5-HT3R) activation by agonist mCPBG (m-chlorophenylbiguanide) induced a slight hyperthermic effect in rats (+0.5 °C), which was inhibited by 5-HT3R antagonist MDL72222 [178]. However, data on 5-HT3R involvement in thermoregulation are still limited and inconsistent. All these studies created a detailed view of the central mechanisms involved in temperature sensing and thermoregulation, as well as their connections to psychiatric disorders. However, experimental results in rodents with pharmacological modulators of different serotonergic receptors represent only indirect proofs, and their translation to human physiology and clinical implications should be performed with caution.
6. Clinical and Therapeutic Implications of the Serotonergic System and Thermoregulation
The serotonergic system modulates a variety of neurotransmitters and neuromodulators, including monoamines (norepinephrine, epinephrine, dopamine), GABA, ACh, hormones like substance P, oxytocin, vasopressin, cortisol, prolactine [6]. Thus, serotonin is involved in control of diverse brain functions and psychic processes, such as memory and learning, pain, emotional reactions, and the dopamine-based reward system, activity patterns, circadian rhythms, sleep-wake cycles, thermoregulation, food intake, sexual activity, aggressiveness, locomotion; therefore, it represents a special target for therapy of neurologic and psychiatric disorders [101].
The monoamines dopamine, norepinephrine, and serotonin are modulatory neurotransmitters that produce a fine-tuning of the CNS, particularly the systems involved in emotions, mood, and affectivity [41]. Selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine and zimelidine, are used to treat depression and anxiety disorders. They are thought to help relieve the symptoms of these disorders by increasing the synaptic availability of serotonin upon blocking the serotonin transporter (SERT) [179]. In contrast to SSRIs, some 5-HT1AR inhibitors are used as anxiolytics and some 5-HT3R inhibitors act as effective antiemetics used to alleviate nausea induced by cancer chemotherapy [72]. In addition, psychotropic and hallucinogenic drugs such as cocaine, amphetamine, 3,4-methylenedioxymethamphetamine (MDMA, commonly known as ecstasy), mescaline, psylocybin, lysergide (LSD), metisergide, and others are selective 5-HT2R agonists, similar to DOI/DOB/DOM and other substituted phenethylamine derivatives [180]. These compounds can be classified into three categories: tryptamines, phenethylamines (amphetamines), and lysergamides. LSD belongs to both tryptamine and lysergamide classes. Tryptamines include, obviously, serotonin, tryptamine, melatonin, and psychedelic alkaloids such as DMT (N,N-dimethyltryptamine), psylocybin, Nω-methylserotonin (norbufotenin), bufotenine, bufotenidine, N-acetylserotonin, baeocystin, aeruginascin, lespedamine, acetriptyne, and the major antidepressant zalsupindole. Phenetylamines also include a large number of compounds with diverse effects: CNS stimulants (amphetamine, dopamine, adrenaline, noradrenaline), recreational drugs (MDMA, MDPV—monkey dust, metamphetamine, cathinone), decongestants and bronchodilators (ephedrine and pseudoephedrine, phenylephrine, isoprenaline, salbutamol), antidepressants (phenelzine, bupropion), and drugs used in Parkinson’s disease such as selegiline [181]. Lysergamides (also named ergoamides) are also psychedelic drugs such as LSD, methysergide, amesergide, ergotamine, ergometrine (used in the treatment of migraine), cabergoline, etc, active on different serotonin and dopamine receptors [182].
As shown in the previous section, many of these serotonergic receptor agonists and antagonists, including serotonin itself [183], induced temperature changes and modulated thermoregulatory responses when administered in animal studies ([162,164,167,178], reviewed in [71]) as well as in humans [144]. The long and intricate story of discovery of central thermoregulatory neuronal circuits and mechanisms, briefly exposed in chapter 5.2, resulted in identification of the anterior hypothalamus and preoptic area as the main command centers for thermogenetic and thermolytic peripheral responses, and these structures are controlled directly and indirectly by the upper group of serotonergic brainstem raphe nuclei, in particular DRN, while the lower group and specifically NRPa send thermoregulatory commands to the periphery (Figure 2) [21]. Given the tight connection between thermoregulation and the serotonergic system, and the involvement of this system in modulation of cognitive behaviors, physiological regulatory responses, and psychic functions such as mood, affectivity, aggressiveness, anxiety, and depression, it is reasonable to conclude that emotional and affective states are associated with thermoregulatory responses via central serotonergic circuits. A number of clinical studies assessed the relationship between circadian changes in body temperature and depression and found reduced variation compared to normal subjects [143,184,185,186,187], a fact that can be correlated with the control exerted by serotonergic circuits on the sleep–wake circadian cycle and to antidepressive effects of SSRIs via increase of synaptic concentrations of serotonin. These connections could also provide a scientific explanation for the widely used empirical classical therapy applied for patients with psychiatric disorders, consisting of daily cold showers: cold exposure could activate the central serotonergic system. In an exhaustive review on the relationships between thermosensory and central serotonergic systems and behavior, Charles Raison et al. propose an opposite approach, namely activation of warm thermosensory pathways as a potential cure in affective disorders such as major depressive disorder via activation of brain serotonergic neurons [24]. Raison played a key role in promoting novel treatments for major depressive disorders based on manoeuvres that activate the central serotonergic system, such as whole-body hyperthermia [188] or psychedelic medicines [189].
The complex central serotonergic networks explain the effectiveness of therapies targeting serotonergic components in various affectivity and mood disorders, including anxiety disorders, depression, obsessive-compulsive disorders, and related conditions such as attention deficit and hyperactivity disorder (ADHD), autism spectrum disorders (ASDs), schizophrenia, addiction, and migraine. The most commonly used drugs in the treatment of mood disorders are selective serotonin reuptake inhibitors (SSRIs) and monoamine oxidase inhibitors (MAOIs) [190]. These compounds act by increasing serotonin levels in the brain, but special diets rich in tryptophan, such as chicken, soy, grains, tuna, nuts, and bananas, can also exert beneficial effects [191]. SSRIs include a variety of compounds widely used for clinical applications, such as sertraline, fluoxetine, paroxetine, fluvoxamine, and escitalopram. Although SSRIs are not more effective than tricyclic antidepressants (TCAs), which inhibit both serotonin and noradrenaline reuptake [179], they are considered safer antidepressants because of milder side effects and higher toxic doses [192]. Other serotonin and norepinephrine reuptake inhibitors (SNRIs) that act in a dose-dependent manner include drugs such as venlafaxine and duloxetine, which are very effective as antidepressants in SSRI-resistant cases. There is a wide spectrum of psychiatric disorders amenable to SSRI therapy: major depressions, anxiety disorders, panic disorders, obsessive-compulsive disorders (OCDs), eating disorders such as bulimia nervosa but not anorexia nervosa, premature ejaculation, as well as migraine and other pain syndromes [6]. Installation of clinical effects occurs after a latency of 6–8 weeks; therefore, co-administration of antidepressants with faster onset of action, such as ketamine, may be beneficial [179]. SSRIs produce various side effects, including irritability and anxiety worsening during first weeks of therapy, emotional blunting, sexual dysfunction, increased suicidal risk, akathisia, bruxism, worsening of secondary (closed-angle) glaucoma, arrhythmogenic risk via QT interval prolongation, increased gastrointestinal or intracranial bleeding risk due to depletion of platelet serotonin levels, and increased fracture risk. Serotonin syndrome may occur due to activation of CNS 5-HT1A/2C receptors, leading to tachycardia, fever, shivering, sweating, mydriasis, hyperreflexia, and myoclonus [193]. Given these adverse effects, SSRI therapy for patients with psychiatric disorders could be ingeniously complemented by thermal modulation, via either cooling or exposure to hyperthermia (followed by post-exposure cooling) to naturally increase CNS synaptic serotonin levels.
A major psychiatric disease that may benefit from pharmacological modulation of the serotonergic system is schizophrenia. Antagonists or agonists of different types of serotonergic receptors may be more effective in these patients than major antipsychotics of first and second generation, which are plagued by numerous side effects, and generally act only on positive symptoms, not on negative symptoms and cognitive deficits [90]. The most promising and potentially useful compound is the 5-HT6R antagonist AVN-211 [194], followed by the 5-HT1A/2A agonist, 5-HT2B/6/7 antagonist, and dopamine D1/D2 agonist brilaroxazine (RP5063) [195], and the less promising 5-HT2AR and dopamine D1/D2R antagonist LuAF-35700 [196,197]. Even classical antipsychotics such as 5-HT2AR antagonists olanzapine and risperidone show some therapeutic efficiency, increasing dopamine release in the striatum by alleviating the inhibitory effects of serotonergic pathways [198].
Serotonergic receptors agonists and antagonists can also be effective in different neurodegenerative disorders, by enhancing synaptic neurotransmission, alleviating neuronal degradation (neurotrophic or neurodynamic effects), and counteracting mood and emotional disturbances encountered in these conditions. For example, advanced stages of Alzheimer’s disease are accompanied by a significant reduction of the central serotonergic network originating in brainstem raphe nuclei, explaining the effectiveness of 5-HT2AR and 5-HT4R agonists in improving reinforcement learning and memory. Neurofibrillary tangles can degrade serotonergic raphe nuclei neurons, and decreased CNS serotonergic activity may worsen beta-amyloid-induced neuroinflammation [199]. The histaminergic H1 receptor competitive antagonist loratadine, which also acts as a selective 5-HT2R antagonist, may counteract microglia involvement in neuroinflammation and promote amyloid clearance, preventing amyloid plaque formation [200,201]. The main symptomatic/pathogenic therapy in Alzheimer’s disease is based on acetylcholinestarease inhibitors such as donepezil or NMDAR non-competitive antagonists like memantine. However, 5-HT6R antagonists (e.g., idalopiridine) and 5-HT4R agonists may improve cholinergic neurotransmission in this condition by enhancing synaptic ACh release, while 5-HT2AR antagonists like brexipiprazole or pimavanserine modulate multiple neurotransmitter pathways, and may also counterbalance reduction in 5-HT1AR and 5-HT2AR levels in the hippocampus and prefrontal cortex in frontotemporal dementia [85], being effective in Alzheimer’s disease with psychotic symptoms such as hallucinations and delusions [202]. The neuroprotective effects of these agents are also retrieved for metabotropic glutamate receptors mGluR5 antagonists such as 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl]-pyridine (MTEP) and its predecessor 2-methyl-6-(phenylethynyl)-pyridine (MPEP), anxiolytic and antidepressant compounds [203,204] that reduce addictive effects of alcohol, nicotine, morphine, cocaine and metamphetamine, counteracting their withdrawal symptomatology [205,206,207,208].
Similarly, in Parkinson’s disease, a condition associated with dopaminergic neuron loss in the substantia nigra pars compacta and alpha-synuclein aggregation with Lewy body formation, the main therapy with levodopa can be supplemented with 5-HT1AR antagonists or 5-HT2AR inverse agonists like pimavanserine to reduce L-DOPA-induced dyskinesia [209,210]. Like in other neurodegenerative diseases, 5-HTR agonists may reduce neurodegradation, oxidative stress, and neuronal inflammation. In addition, SSRIs may improve psychiatric symptoms, while 5-HT3R inhibitors in the spinal cord dorsal horn exert analgesic effects [211,212]. A similar algesic pathology occurs in amyotrophic lateral sclerosis, involving 5-HT1R, 5-HT2R, 5-HT3R, and 5-HT7R; therefore, their inhibition may exert analgesic effects [213]. Serotonergic neuron degradation may also induce muscle spasms, which can be approached with 5-HT2B/CR inverse agonists [214]. Moreover, increased platelet serotonin levels have been associated with prolonged survival [215], while 5-HT2BR deletion aggravates spinal cord disease progression in mice, prompting for beneficial effects of agonists of these receptors, which can slow down disease progression [216,217]. In Huntington’s disease, a genetic disease associated with chorea due to neuronal accumulation and aggregation of huntingtin, modulation of the serotonergic system may provide neuroprotective effects, also improving motor and cognitive deficits and alleviating psychiatric symptoms. 5-HT2AR antagonist and 5-HT1AR agonist perospirone improved motor and psychiatric disturbances [218]. Multiple sclerosis patients experience sensory and motor pathology similar to that of amyotrophic lateral sclerosis, including widespread neuropathic pain and muscular spasms, which can be treated with SSRIs like fluoxetine or 5-HT2B/CR agonists. Fluoxetine-triggered activation of these receptors exerts anti-inflammatory effects, like serotonin itself [219]. 5-HT7Rs on T helper lymphocytes may be involved in interleukin-10 release with subsequent immunosuppressive effects in multiple sclerosis patients treated with α4-integrin monoclonal antibody natalizumab, thus agonists of 5-HT7Rs may represent an interesting therapeutic alternative [220].
7. Conclusions
The serotonergic system is a phylogenetically old monoamine signaling system present in all organisms with a central nervous system. In vertebrates, serotonergic pathways originating in a network of brainstem raphe nuclei and providing a wide central and peripheral distribution of fibers are involved in a large variety of behavioral responses, including reward-based decision making and reinforcement learning, attention, mood, aggressiveness, and vegetative responses such as food intake (appetitive reward) and thermoregulation. Pioneering immunohistochemistry and physiology studies revealed a strong connection between serotonergic circuits, psychotropic compounds such as lysergide (LSD) and other psychedelic drugs, and antipsychotics such as reserpine. Subsequent in-depth research resulted in identification and characterization of seven families of serotonergic receptors, most of them metabotropic receptors with seven transmembrane domains, except for 5-HT3Rs, which are pentameric ligand-gated ion channels, and of an impressive panel of more or less specific agonists and antagonists. Many of them are clinically used in psychiatric disorders or neurodegenerative diseases. A long line of scientific research originating in the second half of the nineteenth century revealed involvement of serotonergic nuclei and pathways in complex central thermoregulatory circuits that can be specifically modulated by pharmacological agents acting on different types of serotonergic receptors. In spite of this massive accumulation of knowledge, there are still many aspects of these complex regulatory responses and underlying cellular and molecular mechanisms awaiting further elucidation. Novel scientific methods, such as in vivo fluorophore-based tracking of serotonergic synaptic transmission in individual neurons, targeted knock-out, and optogenetics, provide an opportunity to uncover new details of the functioning of neuronal circuits underlying these essential behavioral responses, possibly leading to new therapeutic approaches. An almost entirely unexplored area is that of pharmacogenomics of serotonergic receptors, involving characterization of response to agonists and antagonists of serotonergic receptors with point mutations, an avenue of investigation leading to personalized medicine approaches and better characterization of the so-called secondary pharmacology of many new drugs.
Author Contributions
Conceptualization, A.-I.N., B.A., D.F.M. and C.B.; methodology, A.-I.N., B.A., D.F.M. and C.B.; investigation, A.-I.N., B.A., D.F.M. and C.B.; formal analysis, A.-I.N., B.A. and C.B.; validation, A.-I.N., B.A., D.F.M. and C.B.; data curation, A.-I.N. and B.A.; resources, A.-I.N., B.A., D.F.M. and C.B.; supervision, B.A., D.F.M. and C.B.; project administration, B.A. and D.F.M.; writing—original draft, A.-I.N. and B.A.; writing—review and editing, A.-I.N., B.A., D.F.M. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge their colleagues from the Department of Biophysics & Physiology, Faculty of Biology, University of Bucharest, for their help and technical assistance in performing this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAAD/AADC/DDC | aromatic acid decarboxylase/DOPA-decarboxylase |
| AAV | adeno-associated virus |
| AC | adenylate cyclase |
| ACA | anterior cingulate area |
| Acc | nucleus accumbens |
| ACSF | artificial cerebrospinal fluid |
| ACTH | adrenocorticotropic hormone |
| ACh | acetylcholine |
| ADHD | attention deficit and hyperactivity disorder |
| AI | agranular insular area |
| AP | action potential |
| ASD | autism spectrum disorder |
| ATP | adenosine-5′-triphosphate |
| BAT | brown adipose tissue |
| BOLD | blood oxygenation level-dependent [fMRI] |
| cAMP | 3′,5′-cyclic adenosine monophosphate |
| CEA | central amygdala |
| c-Fos | protooncogene discovered in rat fibroblasts as product of the transforming gene of FBJ MSV (Finkel–Biskis–Jinkins murine osteogenic sarcoma virus), part of Fos family of transcription factors |
| CNS | central nervous system |
| CRH | corticotropin-releasing hormone |
| DAG | diacylglycerol |
| 5,7-DHT | 5,7-dihydroxytryptamine |
| DMT | N,N-dimethyltryptamine |
| DOB | dimethoxybromoamphetamine |
| DOI | 1-2,5-dimethoxy-iodophenyl-2-aminopropane |
| DOM | 2,5-dimethoxy-4-methylamphetamine |
| DRN | dorsal raphe nucleus |
| EGFP | enhanced green fluorescent protein |
| fMRI | functional magnetic resonance imaging |
| GABA | γ-aminobutyric acid |
| GLP-1 | glucagon-like peptide-1 |
| GRAB | G-protein-coupled activation-based [sensor] |
| GPCR | G protein-coupled receptor |
| Gi/Go | α subunit of inhibitory G protein |
| Gq/11 | Gq/11 G protein family |
| Gs | stimulatory G protein |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HTP | 5-hydroxy-tryptophan |
| 5-HT | 5-hydroxy-tryptamine (serotonin) |
| 5-HT[1 to 7][A to F] | serotonin receptor with specified family number [1–7] and type [A–F] |
| 5-HTR | serotonin receptor |
| IML | intermediolateral cell column |
| IP3 | inositol 1,4,5-trisphosphate |
| Kir | inward rectifier K+ channel |
| L-DOPA | levo-3,4-dihydroxyphenylalanine |
| LH | lateral habenula |
| LHA | lateral hypothalamic area |
| LHb | lateral habenular nucleus |
| LPB | lateral parabrachial nucleus |
| LSD | lysergic acid diethylamide (lysergide) |
| MAOI | monoamine oxidase inhibitor |
| MAPK | mitogen-activated protein kinase |
| MC4-R | melanocortin-4 receptor |
| mCPBG | m-chlorophenylbiguanide |
| mCPP | meta-chlorophenylpiperazine |
| 2ME | 2-methyl-5-HT |
| MDMA | 3,4-methylenedioxymethamphetamine |
| MDPV | Methylenedioxypyrovalerone (monkey dust) |
| mGluR | metabotropic glutamate receptor |
| MPEP | 2-methyl-6-(phenylethynyl)-pyridine |
| MTEP | 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl]-pyridine |
| MTN | medial thalamic nuclei |
| NO | nitric oxide |
| NRM | nucleus raphe magnus |
| NRPa | nucleus raphe pallidus anterior |
| mRNA | messenger ribonucleic acid |
| NMDAR | N-methyl-D-aspartate receptor |
| OCD | obsessive-compulsive disorder |
| OFCm | medial orbitofrontal cortex |
| 8-OH-DPAT | 8-hydroxy-2-(di-n-propylamino)-tetralin |
| PAL | globus pallidus |
| PBS | phosphate-buffered saline |
| PKA | protein kinase A |
| PMAT | plasma membrane monoamine transporter |
| PNS | peripheral nervous system |
| PO/POA | preoptic area |
| PPARγ2 | peroxisome proliferator-activated receptor |
| Pir | piriform cortex |
| S1 | primary somatosensory cortical area |
| SERT | serotonin transporter |
| SNRI | serotonin and norepinephrine reuptake inhibitor |
| SSRI | selective serotonin reuptake inhibitor |
| STRv | ventral striatum |
| TASK | TWIK-related acid-sensitive K+ channel |
| TCA | tricyclic antidepressant |
| TPH | tryptophan hydroxylase |
| TRAAK | TWIK-related arachidonic acid-stimulated K+ channel |
| TRPV | transient receptor potential ion channel, vanilloid subgroup |
| TWIK | two-pore domain weak inward rectifier K+ channel |
| VMAT2 | vesicular monoamine transporter 2 |
| VSM | vascular smooth muscle |
| VTA | ventral tegmental area |
| ZI | zona incerta |
References
- Whitaker-Azmitia, P.M. The discovery of serotonin and its role in neuroscience. Neuropsychopharmacology 1999, 21, 2S–8S. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol. Scand. Suppl. 1964, 232, 231–255. [Google Scholar]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Sharma, V.K.; Loh, Y.P. The discovery, structure, and function of 5-HTR1E serotonin receptor. Cell Commun. Signal. 2023, 21, 235. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Buhot, M.C. Serotonin receptors in cognitive behaviors. Curr. Opin. Neurobiol. 1997, 7, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.S.; Fiedler, J.L. What Do We Really Know About 5-HT(1A) Receptor Signaling in Neuronal Cells? Front. Cell. Neurosci. 2016, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, F. The Role of Serotonin in Animal Personality. Available online: https://liu.diva-portal.org/smash/record.jsf?pid=diva2%3A1108010&dswid=-7306 (accessed on 4 July 2025).
- Lucki, I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 1998, 44, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Quah, S.K.L.; McIver, L.; Roberts, A.C.; Santangelo, A.M. Trait Anxiety Mediated by Amygdala Serotonin Transporter in the Common Marmoset. J. Neurosci. 2020, 40, 4739–4749. [Google Scholar] [CrossRef]
- Balakrishna, P.; George, S.; Hatoum, H.; Mukherjee, S. Serotonin Pathway in Cancer. Int. J. Mol. Sci. 2021, 22, 1268. [Google Scholar] [CrossRef]
- Chen, L.; Huang, S.; Wu, X.; He, W.; Song, M. Serotonin signalling in cancer: Emerging mechanisms and therapeutic opportunities. Clin. Transl. Med. 2024, 14, e1750. [Google Scholar] [CrossRef]
- Carlsson, A. Perspectives on the discovery of central monoaminergic neurotransmission. Annu. Rev. Neurosci. 1987, 10, 19–40. [Google Scholar] [CrossRef]
- Mulligan, K.A.; Törk, I. Serotoninergic innervation of the cat cerebral cortex. J. Comp. Neurol. 1988, 270, 86–110. [Google Scholar] [CrossRef]
- Hornung, J.P.; Fritschy, J.M.; Törk, I. Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J. Comp. Neurol. 1990, 297, 165–181. [Google Scholar] [CrossRef]
- Hamada, H.T.; Abe, Y.; Takata, N.; Taira, M.; Tanaka, K.F.; Doya, K. Optogenetic activation of dorsal raphe serotonin neurons induces brain-wide activation. Nat. Commun. 2024, 15, 4152. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Young, B.S. Projections of nucleus paragigantocellularis lateralis to locus coeruleus and other structures in rat. Brain Res. 1987, 406, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Dreyfus, C.F.; Pickel, V.M.; Joh, T.H.; Reis, D.J. Serotonergic neurons in the peripheral nervous system: Identification in gut by immunohistochemical localization of tryptophan hydroxylase. Proc. Natl. Acad. Sci. USA 1977, 74, 3086–3089. [Google Scholar] [PubMed]
- Zhang, H.; Leitner, D.R.; Hasegawa, Y.; Waldor, M.K. Peripheral serotonergic neurons regulate gut motility and anxiety-like behavior. Curr. Biol. 2024, 34, R133–R134. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Nakai, S.; Tanaka, M.; Kanosue, K. Neuronal circuitries involved in thermoregulation. Auton. Neurosci. 2000, 85, 18–25. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R127–R136. [Google Scholar] [CrossRef]
- Pollak Dorocic, I.; Fürth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlén, M.; Meletis, K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 2014, 83, 663–678. [Google Scholar] [CrossRef]
- Raison, C.L.; Hale, M.W.; Williams, L.E.; Wager, T.D.; Lowry, C.A. Somatic influences on subjective well-being and affective disorders: The convergence of thermosensory and central serotonergic systems. Front. Psychol. 2014, 5, 1580. [Google Scholar] [CrossRef] [PubMed]
- Rorden, C. Neuroanatomy Atlas: Coronal Slices. Available online: https://people.cas.sc.edu/rorden/anatomy/na_coro.html (accessed on 10 April 2025).
- Quentin, E.; Belmer, A.; Maroteaux, L. Somato-Dendritic Regulation of Raphe Serotonin Neurons; A Key to Antidepressant Action. Front. Neurosci. 2018, 12, 982. [Google Scholar] [CrossRef]
- Gianni, G.; Pasqualetti, M. Wiring and Volume Transmission: An Overview of the Dual Modality for Serotonin Neurotransmission. ACS Chem. Neurosci. 2023, 14, 4093–4104. [Google Scholar] [CrossRef]
- Azmitia, E.C. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology 1999, 21, 33S–45S. [Google Scholar] [CrossRef]
- Bowker, R.M.; Westlund, K.N.; Sullivan, M.C.; Wilber, J.F.; Coulter, J.D. Descending serotonergic, peptidergic and cholinergic pathways from the raphe nuclei: A multiple transmitter complex. Brain Res. 1983, 288, 33–48. [Google Scholar] [CrossRef]
- Miceli, M.O.; Post, C.A.; van der Kooy, D. Catecholamine and serotonin colocalization in projection neurons of the area postrema. Brain Res. 1987, 412, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Nanopoulos, D.; Belin, M.F.; Maitre, M.; Vincendon, G.; Pujol, J.F. Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 1982, 232, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Chilmonczyk, Z.; Bojarski, A.J.; Pilc, A.; Sylte, I. Functional Selectivity and Antidepressant Activity of Serotonin 1A Receptor Ligands. Int. J. Mol. Sci. 2015, 16, 18474–18506. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, S.; Qi, J.; Wang, H.; Cachope, R.; Mejias-Aponte, C.A.; Gomez, J.A.; Mateo-Semidey, G.E.; Beaudoin, G.M.J.; Paladini, C.A.; et al. Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons. Cell Rep. 2019, 26, 1128-1142.e7. [Google Scholar] [CrossRef]
- Ortega, J.E.; Mendiguren, A.; Pineda, J.; Meana, J.J. Regulation of central noradrenergic activity by 5-HT(3) receptors located in the locus coeruleus of the rat. Neuropharmacology 2012, 62, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pastor, B.; Ortega, J.E.; Meana, J.J. Involvement of serotonin 5-HT3 receptors in the modulation of noradrenergic transmission by serotonin reuptake inhibitors: A microdialysis study in rat brain. Psychopharmacology 2013, 229, 331–344. [Google Scholar] [CrossRef]
- Gobert, A.; Millan, M.J. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology 1999, 38, 315–317. [Google Scholar] [CrossRef]
- Done, C.J.; Sharp, T. Biochemical evidence for the regulation of central noradrenergic activity by 5-HT1A and 5-HT2 receptors: Microdialysis studies in the awake and anaesthetized rat. Neuropharmacology 1994, 33, 411–421. [Google Scholar] [CrossRef]
- Sprouse, J.S.; Aghajanian, G.K. (−)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur. J. Pharmacol. 1986, 128, 295–298. [Google Scholar] [CrossRef]
- Consolo, S.; Ramponi, S.; Ladinsky, H.; Baldi, G. A critical role for D1 receptors in the 5-HT1A-mediated facilitation of in vivo acetylcholine release in rat frontal cortex. Brain Res. 1996, 707, 320–323. [Google Scholar] [CrossRef]
- Izumi, J.; Washizuka, M.; Miura, N.; Hiraga, Y.; Ikeda, Y. Hippocampal serotonin 5-HT1A receptor enhances acetylcholine release in conscious rats. J. Neurochem. 1994, 62, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Cardozo Pinto, D.F.; Pomrenze, M.B.; Guo, M.Y.; Touponse, G.C.; Chen, A.P.F.; Bentzley, B.S.; Eshel, N.; Malenka, R.C. Opponent control of reinforcement by striatal dopamine and serotonin. Nature 2025, 639, 143–152. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.; Dai, B.; Qian, T.; Zeng, J.; Li, X.; Zhuo, Y.; Zhang, Y.; Wang, Y.; Qian, C.; et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 2020, 17, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Salvan, P.; Fonseca, M.; Winkler, A.M.; Beauchamp, A.; Lerch, J.P.; Johansen-Berg, H. Serotonin regulation of behavior via large-scale neuromodulation of serotonin receptor networks. Nat. Neurosci. 2023, 26, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.Y.; Amoroso, M.W.; Uchida, N. Serotonergic neurons signal reward and punishment on multiple timescales. elife 2015, 4, 06346. [Google Scholar] [CrossRef]
- Fischer, A.G.; Ullsperger, M. An Update on the Role of Serotonin and its Interplay with Dopamine for Reward. Front. Hum. Neurosci. 2017, 11, 484. [Google Scholar] [CrossRef]
- Izquierdo, A.; Carlos, K.; Ostrander, S.; Rodriguez, D.; McCall-Craddolph, A.; Yagnik, G.; Zhou, F. Impaired reward learning and intact motivation after serotonin depletion in rats. Behav. Brain Res. 2012, 233, 494–499. [Google Scholar] [CrossRef]
- Brown, H.D.; Amodeo, D.A.; Sweeney, J.A.; Ragozzino, M.E. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. J. Psychopharmacol. 2012, 26, 1443–1455. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, M.Z.; Li, Q.; Deng, J.; Mu, D.; Sun, Y.G. Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus. Cell Rep. 2017, 18, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewcz, C.A.; Mazzone, C.M.; D’Agostino, G.; Halladay, L.R.; Hardaway, J.A.; DiBerto, J.F.; Navarro, M.; Burnham, N.; Cristiano, C.; Dorrier, C.E.; et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 2016, 537, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, T.; Kramer, K.; Werten, S.; Rupp, B.; Hoffmeister, D. Characterization of the Gateway Decarboxylase for Psilocybin Biosynthesis. Chembiochem 2022, 23, e202200551. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Liu, C.T.; Pluskal, T.; Chung, Y.K.; Weng, J.K. Monoamine Biosynthesis via a Noncanonical Calcium-Activatable Aromatic Amino Acid Decarboxylase in Psilocybin Mushroom. ACS Chem. Biol. 2018, 13, 3343–3353. [Google Scholar] [CrossRef]
- Moon, J.H.; Oh, C.M.; Kim, H. Serotonin in the regulation of systemic energy metabolism. J. Diabetes Investig. 2022, 13, 1639–1645. [Google Scholar] [CrossRef]
- Paulmann, N.; Grohmann, M.; Voigt, J.P.; Bert, B.; Vowinckel, J.; Bader, M.; Skelin, M.; Jevsek, M.; Fink, H.; Rupnik, M.; et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009, 7, e1000229. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.E.; Brubaker, P.L. Glucagon-Like Peptide 1 Secretion by the L-Cell. Diabetes 2006, 55, S70–S77. [Google Scholar] [CrossRef]
- Crane, J.D.; Palanivel, R.; Mottillo, E.P.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef]
- Zhou, M.; Engel, K.; Wang, J. Evidence for significant contribution of a newly identified monoamine transporter (PMAT) to serotonin uptake in the human brain. Biochem. Pharmacol. 2007, 73, 147–154. [Google Scholar] [CrossRef]
- Fritsch, M.; Richter, S. The formation of the nervous system during larval development in Triops cancriformis (Bosc) (crustacea, Branchiopoda): An immunohistochemical survey. J. Morphol. 2010, 271, 1457–1481. [Google Scholar] [CrossRef]
- Hanswijk, S.I.; Spoelder, M.; Shan, L.; Verheij, M.M.M.; Muilwijk, O.G.; Li, W.; Liu, C.; Kolk, S.M.; Homberg, J.R. Gestational Factors throughout Fetal Neurodevelopment: The Serotonin Link. Int. J. Mol. Sci. 2020, 21, 5850. [Google Scholar] [CrossRef]
- Kesić, M.; Baković, P.; Horvatiček, M.; Proust, B.L.J.; Štefulj, J.; Čičin-Šain, L. Constitutionally high serotonin tone favors obesity: Study on rat sublines with altered serotonin homeostasis. Front. Neurosci. 2020, 14, 219. [Google Scholar] [CrossRef]
- Kesić, M.; Baković, P.; Farkaš, V.; Bagarić, R.; Kolarić, D.; Štefulj, J.; Čičin-Šain, L. Constitutive Serotonin Tone as a Modulator of Brown Adipose Tissue Thermogenesis: A Rat Study. Life 2023, 13, 1436. [Google Scholar] [CrossRef]
- Jagannathan, L.; Socks, E.; Balasubramanian, P.; McGowan, R.; Herdt, T.M.; Kianian, R.; MohanKumar, S.M.J.; MohanKumar, P.S. Oleic acid stimulates monoamine efflux through PPAR-α: Differential effects in diet-induced obesity. Life Sci. 2020, 255, 117867. [Google Scholar] [CrossRef]
- Lummis, S.C. 5-HT(3) receptors. J. Biol. Chem. 2012, 287, 40239–40245. [Google Scholar] [CrossRef] [PubMed]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef]
- Volk, B.; Nagy, B.J.; Vas, S.; Kostyalik, D.; Simig, G.; Bagdy, G. Medicinal chemistry of 5-HT5A receptor ligands: A receptor subtype with unique therapeutical potential. Curr. Top. Med. Chem. 2010, 10, 554–578. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Giovanni, G.D.; Cattaert, D.; De Deurwaerdère, P. Molecular Sciences Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Vleugels, R.; Verlinden, H.; Broeck, J.V. Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2015, 2, 314. [Google Scholar] [CrossRef]
- Frolova, V.S.; Nikishina, Y.O.; Shmukler, Y.B.; Nikishin, D.A. Serotonin Signaling in Mouse Preimplantation Development: Insights from Transcriptomic and Structural-Functional Analyses. Int. J. Mol. Sci. 2024, 25, 12954. [Google Scholar] [CrossRef] [PubMed]
- Peroutka, S.J.; Snyder, S.H. Multiple serotonin receptors: Differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol. Pharmacol. 1979, 16, 687–699. [Google Scholar] [CrossRef]
- Albert, P.R.; Zhou, Q.Y.; Van Tol, H.H.; Bunzow, J.R.; Civelli, O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J. Biol. Chem. 1990, 265, 5825–5832. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Voronova, I.P. 5-HT Receptors and Temperature Homeostasis. Biomolecules 2021, 11, 1914. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 2013, 7, 25. [Google Scholar] [CrossRef]
- Kaufman, J.; DeLorenzo, C.; Choudhury, S.; Parsey, R.V. The 5-HT1A receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2016, 26, 397–410. [Google Scholar] [CrossRef]
- Jensen, N.H.; Cremers, T.I.; Sotty, F. Therapeutic potential of 5-HT2C receptor ligands. Sci. World J. 2010, 10, 1870–1885. [Google Scholar] [CrossRef]
- Moravčíková, L.; Csatlósová, K.; Ďurišová, B.; Ondáčová, K.; Pavlovičová, M.; Lacinová, L.; Dremencov, E. Role of serotonin-2A receptors in pathophysiology and treatment of depression. In 5-HT2A Receptors in the Central Nervous System; Guiard, B.P., Di Giovanni, G., Eds.; Humana Press: Cham, Switzerland, 2018; pp. 205–230. [Google Scholar]
- Nagatomo, T.; Rashid, M.; Abul Muntasir, H.; Komiyama, T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol. Ther. 2004, 104, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.S.; Bender, A.M.; Shay, S.; Paffenroth, K.C.; Gladson, S.; Dickerson, J.W.; Watson, K.J.; Kapolka, N.J.; Boutaud, O.; Rook, J.M.; et al. Development of a Peripherally Restricted 5-HT(2B) Partial Agonist for Treatment of Pulmonary Arterial Hypertension. JACC Basic Transl. Sci. 2023, 8, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Mochizuki, S.; Takemoto, Y.; Akuzawa, S. Molecular cloning of human 5-hydroxytryptamine3 receptor: Heterogeneity in distribution and function among species. Mol. Pharmacol. 1995, 48, 407–416. [Google Scholar] [CrossRef]
- Bétry, C.; Etiévant, A.; Oosterhof, C.; Ebert, B.; Sanchez, C.; Haddjeri, N. Role of 5-HT3 Receptors in the Antidepressant Response. Pharmaceuticals 2011, 4, 603–629. [Google Scholar] [CrossRef]
- Lummis, S.C.; Beene, D.L.; Lee, L.W.; Lester, H.A.; Broadhurst, R.W.; Dougherty, D.A. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature 2005, 438, 248–252. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lummis, S.C. The 5-HT3 receptor as a therapeutic target. Expert. Opin. Ther. Targets 2007, 11, 527–540. [Google Scholar] [CrossRef]
- Basak, S.; Gicheru, Y.; Samanta, A.; Molugu, S.K.; Huang, W.; Fuente, M.; Hughes, T.; Taylor, D.J.; Nieman, M.T.; Moiseenkova-Bell, V.; et al. Cryo-EM structure of 5-HT(3A) receptor in its resting conformation. Nat. Commun. 2018, 9, 514. [Google Scholar] [CrossRef]
- Yuan, S.; Filipek, S.; Vogel, H. A Gating Mechanism of the Serotonin 5-HT3 Receptor. Structure 2016, 24, 816–825. [Google Scholar] [CrossRef]
- Kitayama, E.; Kimura, M.; Ouchi, T.; Furusawa, M.; Shibukawa, Y. Functional Expression of IP, 5-HT(4), D(1), A(2A), and VIP Receptors in Human Odontoblast Cell Line. Biomolecules 2023, 13, 879. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Jakaria, M.; Jo, S.-H.; Kim, I.-S.; Choi, D.-K. G-Protein-Coupled Receptors in CNS: A Potential Therapeutic Target for Intervention in Neurodegenerative Disorders and Associated Cognitive Deficits. Cells 2020, 9, 506. [Google Scholar] [CrossRef]
- Matthes, H.; Boschert, U.; Amlaiky, N.; Grailhe, R.; Plassat, J.L.; Muscatelli, F.; Mattei, M.G.; Hen, R. Mouse 5-hydroxytryptamine5A and 5-hydroxytryptamine5B receptors define a new family of serotonin receptors: Cloning, functional expression, and chromosomal localization. Mol. Pharmacol. 1993, 43, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R. 5-ht5A receptors as a therapeutic target. Pharmacol. Ther. 2006, 111, 707–714. [Google Scholar] [CrossRef]
- Nelson, D.L. 5-HT5 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Keith, I.M.; Beckman, M.J.; Brownfield, M.S.; Vidruk, E.H.; Bisgard, G.E. 5-HT5a receptors in the carotid body chemoreception pathway of rat. Neurosci. Lett. 2000, 278, 9–12. [Google Scholar] [CrossRef]
- Capuzzi, E.; Caldiroli, A.; Ciscato, V.; Russo, S.; Buoli, M. Experimental Serotonergic Agents for the Treatment of Schizophrenia. J. Exp. Pharmacol. 2021, 13, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Corbett, D.F.; Heightman, T.D.; Moss, S.F.; Bromidge, S.M.; Coggon, S.A.; Longley, M.J.; Roa, A.M.; Williams, J.A.; Thomas, D.R. Discovery of a potent and selective 5-ht5A receptor antagonist by high-throughput chemistry. Bioorg Med. Chem. Lett. 2005, 15, 4014–4018. [Google Scholar] [CrossRef]
- Cyrano, E.; Popik, P. Assessing the effects of 5-HT(2A) and 5-HT(5A) receptor antagonists on DOI-induced head-twitch response in male rats using marker-less deep learning algorithms. Pharmacol. Rep. 2025, 77, 135–144. [Google Scholar] [CrossRef]
- Gwynne, W.D.; Shakeel, M.S.; Girgis-Gabardo, A.; Kim, K.H.; Ford, E.; Dvorkin-Gheva, A.; Aarts, C.; Isaac, M.; Al-Awar, R.; Hassell, J.A. Antagonists of the serotonin receptor 5A target human breast tumor initiating cells. BMC Cancer 2020, 20, 724. [Google Scholar] [CrossRef]
- Thomas, D.R.; Soffin, E.M.; Roberts, C.; Kew, J.N.; de la Flor, R.M.; Dawson, L.A.; Fry, V.A.; Coggon, S.A.; Faedo, S.; Hayes, P.D.; et al. SB-699551-A (3-cyclopentyl-N-[2-(dimethylamino)ethyl]-N-[(4’-{[(2-phenylethyl)amino]methyl}-4-biphenylyl)methyl]propanamide dihydrochloride), a novel 5-ht5A receptor-selective antagonist, enhances 5-HT neuronal function: Evidence for an autoreceptor role for the 5-ht5A receptor in guinea pig brain. Neuropharmacology 2006, 51, 566–577. [Google Scholar] [PubMed]
- Yamazaki, M.; Okabe, M.; Yamamoto, N.; Yarimizu, J.; Harada, K. Novel 5-HT5A receptor antagonists ameliorate scopolamine-induced working memory deficit in mice and reference memory impairment in aged rats. J. Pharmacol. Sci. 2015, 127, 362–369. [Google Scholar] [CrossRef]
- Jamu, I.M.; Okamoto, H. Recent advances in understanding adverse effects associated with drugs targeting the serotonin receptor, 5-HT GPCR. Front. Glob. Womens Health 2022, 3, 1012463. [Google Scholar] [CrossRef]
- Oh, C.M.; Park, S.; Kim, H. Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes Metab. J. 2016, 40, 89–98. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S.M. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J. Clin. Med. Res. 2009, 1, 72–80. [Google Scholar] [CrossRef]
- Sargent, B.J.; Henderson, A.J. Targeting 5-HT receptors for the treatment of obesity. Curr. Opin. Pharmacol. 2011, 11, 52–58. [Google Scholar] [CrossRef]
- Yun, H.M.; Rhim, H. 5-HT 6 receptor ligands, EMD386088 and SB258585, differentially regulate 5-HT 6 receptor-independent events. Toxicol. Vitr. 2011, 25, 2035–2040. [Google Scholar] [CrossRef]
- Terry, A.V., Jr.; Buccafusco, J.J.; Wilson, C. Cognitive dysfunction in neuropsychiatric disorders: Selected serotonin receptor subtypes as therapeutic targets. Behav. Brain Res. 2008, 195, 30–38. [Google Scholar] [CrossRef]
- Chagraoui, A.; Thibaut, F.; De Deurwaerdère, P. 5-HT6 receptors: Contemporary views on their neurobiological and pharmacological relevance in neuropsychiatric disorders. Dialogues Clin. Neurosci. 2025, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Park, K.R.; Kim, E.C.; Kim, S.; Hong, J.T. Serotonin 6 receptor controls Alzheimer’s disease and depression. Oncotarget 2015, 6, 26716–26728. [Google Scholar] [CrossRef] [PubMed]
- Hirst, W.D.; Stean, T.O.; Rogers, D.C.; Sunter, D.; Pugh, P.; Moss, S.F.; Bromidge, S.M.; Riley, G.; Smith, D.R.; Bartlett, S.; et al. SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur. J. Pharmacol. 2006, 553, 109–119. [Google Scholar] [CrossRef]
- Pittala, V.; Pittala, D. Latest advances towards the discovery of 5-HT(7) receptor ligands. Mini Rev. Med. Chem. 2011, 11, 1108–1121. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdes-Ferrer, S.I. Central nervous system effects of 5-HT(7) receptors: A potential target for neurodegenerative diseases. Mol. Med. 2022, 28, 70. [Google Scholar] [CrossRef]
- Mitchell, K.; Rosenbaum, L. What Is a Normal Body Temperature? Available online: https://www.webmd.com/first-aid/normal-body-temperature (accessed on 4 August 2025).
- Morrison, S.F. Regulation of body temperature. In Medical Physiology, 3rd ed.; Boron, W.F., Boulpaep, E.L., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 1193–1203. [Google Scholar]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, G.A. Hyperthermia and Serotonin: The Quest for a “Better Cyproheptadine”. Int. J. Mol. Sci. 2022, 23, 3365. [Google Scholar] [CrossRef]
- Chapman, C.F.; Liu, Y.; Sonek, G.J.; Tromberg, B.J. The use of exogenous fluorescent probes for temperature measurements in single living cells. Photochem. Photobiol. 1995, 62, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Zohar, O.; Ikeda, M.; Shinagawa, H.; Inoue, H.; Nakamura, H.; Elbaum, D.; Alkon, D.L.; Yoshioka, T. Thermal imaging of receptor-activated heat production in single cells. Biophys. J. 1998, 74, 82–89. [Google Scholar] [CrossRef]
- Okabe, K.; Uchiyama, S. Intracellular thermometry uncovers spontaneous thermogenesis and associated thermal signaling. Commun. Biol. 2021, 4, 1377. [Google Scholar] [CrossRef]
- Aronsohn, E.; Sachs, J. Die Beziehungen des Gehirns zur Körperwärme und zum Fieber. Arch. Für Die Gesamte Physiol. Des Menschen Und Der Tiere 1885, 37, 232–301. [Google Scholar] [CrossRef]
- Richet, C. De l’influence des lesions du cerveau sur la temperature. Acad. Des Sci. 1884, 98, 295. [Google Scholar]
- Kahn, R.H. Über die erwärmung des carotidenblutes. Arch. Anat. Physiol. (Physiol. Abstr. Suppl. Band) 1904, 1904, 81–134. [Google Scholar]
- Moorhouse, V.H.K. Effect of increased temperature of the carotid blood. Am. J. Physiol. 1911, 28, 223–234. [Google Scholar] [CrossRef][Green Version]
- Barbour, H.G. Die wirkung unmittelbarer erwärmung und abkühlung der wärmezentra auf die körpertemperatur. Arch. Exp. Path Pharmakother. 1912, 70, 1–26. [Google Scholar] [CrossRef]
- Magoun, H.W.; Harrison, F.; Brobeck, J.R.; Ranson, S.W. Activation of heat loss mechanisms by local heating of the brain. J. Neurophysiol. 1938, 1, 101. [Google Scholar] [CrossRef]
- Jessen, C. Independent clamps of peripheral and central temperatures and their effects on heat production in the goat. J. Physiol. 1981, 311, 11–22. [Google Scholar] [CrossRef]
- Sakurada, S.; Shido, O.; Fujikake, K.; Nagasaka, T. Relationship between body core and peripheral temperatures at the onset of thermoregulatory responses in rats. Jpn. J. Physiol. 1993, 43, 659–667. [Google Scholar] [CrossRef]
- Boulant, J.A.; Dean, J.B. Temperature receptors in the central nervous system. Annu. Rev. Physiol. 1986, 48, 639–654. [Google Scholar] [CrossRef]
- Buijs, T.J.; McNaughton, P.A. The Role of Cold-Sensitive Ion Channels in Peripheral Thermosensation. Front. Cell. Neurosci. 2020, 14, 262. [Google Scholar] [CrossRef]
- Cesare, P.; Moriondo, A.; Vellani, V.; McNaughton, P.A. Ion channels gated by heat. Proc. Natl. Acad. Sci. USA 1999, 96, 7658–7663. [Google Scholar] [CrossRef]
- Siemens, J.; Kamm, G.B. Cellular populations and thermosensing mechanisms of the hypothalamic thermoregulatory center. Pflug. Arch. 2018, 470, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef]
- Craig, A.D.; Krout, K.; Andrew, D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J. Neurophysiol. 2001, 86, 1459–1480. [Google Scholar] [CrossRef]
- Hunt, S.P.; Mantyh, P.W. The molecular dynamics of pain control. Nat. Rev. Neurosci. 2001, 2, 83–91. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Hulvershorn, L.A.; Karne, H.; Gunn, A.D.; Hartwick, S.L.; Wang, Y.; Hummer, T.A.; Anand, A. Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biol. Psychiatry 2012, 71, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, H.P.; Martin, A.; Kaufman, J.; Leung, H.C.; Skudlarski, P.; Lacadie, C.; Fulbright, R.K.; Gore, J.C.; Charney, D.S.; Krystal, J.H.; et al. Frontostriatal abnormalities in adolescents with bipolar disorder: Preliminary observations from functional MRI. Am. J. Psychiatry 2003, 160, 1345–1347. [Google Scholar] [CrossRef]
- Marchand, W.R.; Yurgelun-Todd, D. Striatal structure and function in mood disorders: A comprehensive review. Bipolar Disord. 2010, 12, 764–785. [Google Scholar] [CrossRef]
- Victor, T.A.; Furey, M.L.; Fromm, S.J.; Öhman, A.; Drevets, W.C. Changes in the neural correlates of implicit emotional face processing during antidepressant treatment in major depressive disorder. Int. J. Neuropsychopharmacol. 2013, 16, 2195–2208. [Google Scholar] [CrossRef]
- Boulant, J.A. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 2000, 31 (Suppl. 5), S157–S161. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M. The central and reflex mechanism of panting. J. Physiol. 1933, 77, 319–336. [Google Scholar] [CrossRef]
- Hasama, B. Pharmakologische und physiologische studien über die scheisszentren; über den einfluss der direkten mechanisch elektrischen reizung auf die schweiss-sowie wärmezentren. Arch. Exp. Pathol. 1929, 146, 129. [Google Scholar] [CrossRef]
- Wechselberger, M.; Wright, C.L.; Bishop, G.A.; Boulant, J.A. Ionic channels and conductance-based models for hypothalamic neuronal thermosensitivity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R518–R529. [Google Scholar] [CrossRef]
- Guatteo, E.; Chung, K.K.; Bowala, T.K.; Bernardi, G.; Mercuri, N.B.; Lipski, J. Temperature sensitivity of dopaminergic neurons of the substantia nigra pars compacta: Involvement of transient receptor potential channels. J. Neurophysiol. 2005, 94, 3069–3080. [Google Scholar] [CrossRef]
- Avery, D.H.; Wildschiødtz, G.; Rafaelsen, O.J. Nocturnal temperature in affective disorder. J. Affect. Disord. 1982, 4, 61–71. [Google Scholar] [CrossRef]
- Elsenga, S.; Van den Hoofdakker, R.H. Body core temperature and depression during total sleep deprivation in depressives. Biol. Psychiatry 1988, 24, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Wehr, T.A. Sleep loss: A preventable cause of mania and other excited states. J. Clin. Psychiatry 1989, 50 (Suppl. 8–16). discussion 45–47. [Google Scholar]
- Lund, R.; Kammerloher, A.; Dirlich, G. Body temperature in endogenously depressed patients during depression and remission. In Circadian Rhythms in Psychiatry; Wehr, T.A., Goodwin, F.K., Eds.; Boxwood Press: Pacific Grove, CA, USA, 1983; pp. 77–88. [Google Scholar]
- von Zerssen, D.; Barthelmes, H.; Dirlich, G.; Doerr, P.; Emrich, H.M.; von Lindern, L.; Lund, R.; Pirke, K.M. Circadian rhythms in endogenous depression. Psychiatry Res. 1985, 16, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Wehr, T.A.; Rosenthal, N.E.; Bartko, J.J.; Oren, D.A.; Luetke, C.; Murphy, D.L. Serotonin and thermoregulation. Physiologic and pharmacologic aspects of control revealed by intravenous m-CPP in normal human subjects. Neuropsychopharmacology 1995, 13, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bligh, J. The central neurology of mammalian thermoregulation. Neuroscience 1979, 4, 1213–1216. [Google Scholar] [CrossRef]
- Fozard, J.R. Neuronal 5-HT receptors in the periphery. Neuropharmacology 1984, 23, 1473–1486. [Google Scholar] [CrossRef]
- Amin, A.H.; Crawford, T.B.; Gaddum, J.H. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J. Physiol. 1954, 126, 596–618. [Google Scholar] [CrossRef]
- Vogt, M. The concentration of sympathin in different parts of the central nervous system under normal conditions and after the administration of drugs. J. Physiol. 1954, 123, 451–481. [Google Scholar] [CrossRef]
- von Euler, C.P. Physiology and pharmacology of temperature regulation. Pharmacol. Rev. 1961, 13, 361–398. [Google Scholar] [CrossRef]
- Feldberg, W.; Myers, R.D. Effects on Temperature of Amines Injected into the Cerebral Ventricles. A New Concept of Temperature Regulation. J. Physiol. 1964, 173, 226–231. [Google Scholar] [CrossRef]
- Feldberg, W.; Myers, R.D. Appearance of 5-hydroxytryptamine and an unidentified pharmacologically active lipid acid in effluent from perfused cerebral ventricles. J. Physiol. 1966, 184, 837–855. [Google Scholar] [CrossRef]
- Holmes, S.W. The spontaneous release of prostaglandins into the cerebral ventricles of the dog and the effect of external factors on this release. Br. J. Pharmacol. 1970, 38, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, H.A.; Kluger, M.J. Endogenous pyrogen-like substance produced by reptiles. J. Physiol. 1977, 267, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Bligh, J.; Cottle, W.H.; Maskrey, M. Influence of ambient temperature on the thermoregulatory responses to 5-hydroxytryptamine, noradrenaline and acetylcholine injected into the lateral cerebral ventricles of sheep, goats and rabbits. J. Physiol. 1971, 212, 377–392. [Google Scholar] [CrossRef]
- Findlay, J.D.; Thompson, G.E. The effect of intraventricular injections of noradrenaline, 5-hydroxytryptamine, acetylcholine and tranylcypromine on the ox (Bos taurus) at different environmental temperatures. J. Physiol. 1968, 194, 809–816. [Google Scholar] [CrossRef]
- Hellon, R.F. Monoamines, pyrogens and cations: Their actions on central control of bodytemperature. Pharmacol. Rev. 1974, 26, 289–321. [Google Scholar] [CrossRef] [PubMed]
- Gaddum, J.H. Antagonism between lysergic acid diethylamide and 5-hydroxytryptamine. J. Physiol. 1953, 121, 15p. [Google Scholar]
- Glatfelter, G.C.; Pottie, E.; Partilla, J.S.; Stove, C.P.; Baumann, M.H. Comparative Pharmacological Effects of Lisuride and Lysergic Acid Diethylamide Revisited. ACS Pharmacol. Transl. Sci. 2024, 7, 641–653. [Google Scholar] [CrossRef]
- Middlemiss, D.N.; Fozard, J.R. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur. J. Pharmacol. 1983, 90, 151–153. [Google Scholar] [CrossRef]
- Nelson, D.L.; Pedigo, N.W.; Yamamura, H.I. Multiple 3H-5-hydroxytryptamine binding sites in rat brain. J Physiol 1981, 77, 369–372. [Google Scholar]
- Peroutka, S.J. Selective labeling of 5-HT1A and 5-HT1B binding sites in bovine brain. Brain Res. 1985, 344, 167–171. [Google Scholar] [CrossRef]
- Gudelsky, G.A.; Koenig, J.I.; Meltzer, H.Y. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology 1986, 25, 1307–1313. [Google Scholar] [CrossRef]
- Pawłowski, L. Amitriptyline and femoxetine, but not clomipramine or citalopram, antagonize hyperthermia induced by directly acting 5-hydroxytryptamine-like drugs in heat adapted rats. J. Pharm. Pharmacol. 1984, 36, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Gudelsky, G.A.; Koenig, J.I.; Meltzer, H.Y. Altered responses to serotonergic agents in Fawn-Hooded rats. Pharmacol. Biochem. Behav. 1985, 22, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J. Neural Transm. 1985, 61, 131–135. [Google Scholar] [CrossRef]
- Kłodzińska, A.; Chojnacka-Wójcik, E. Hyperthermia induced by m-trifluoromethylphenylpiperazine (TFMPP) or m-chlorophenylpiperazine (m-CPP) in heat-adapted rats. Psychopharmacology 1992, 109, 466–472. [Google Scholar] [CrossRef]
- Mazzola-Pomietto, P.; Aulakh, C.S.; Tolliver, T.; Murphy, D.L. Functional subsensitivity of 5-HT2A and 5-HT2C receptors mediating hyperthermia following acute and chronic treatment with 5-HT2A/2C receptor antagonists. Psychopharmacology 1997, 130, 144–151. [Google Scholar] [PubMed][Green Version]
- Wozniak, K.M.; Aulakh, C.S.; Hill, J.L.; Murphy, D.L. Hyperthermia induced by m-CPP in the rat and its modification by antidepressant treatments. Psychopharmacology 1989, 97, 269–274. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kjaer, A.; Knigge, U.; Møller, M.; Warberg, J. Serotonin stimulates hypothalamic mRNA expression and local release of neurohypophysial peptides. J. Neuroendocrinol. 2003, 15, 564–571. [Google Scholar] [CrossRef]
- Jørgensen, H.S. Studies on the neuroendocrine role of serotonin. Dan. Med. Bull. 2007, 54, 266–288. [Google Scholar]
- Natarajan, R.; Northrop, N.A.; Yamamoto, B.K. Protracted effects of chronic stress on serotonin-dependent thermoregulation. Stress. 2015, 18, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.; Ramos, L.; Reekie, T.; Misagh, G.H.; Narlawar, R.; Kassiou, M.; McGregor, I.S. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: A biotelemetry study in rats. Br. J. Pharmacol. 2014, 171, 2868–2887. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, A.; Benstaali, C.; Bogdan, A.; Auzéby, A.; Touitou, Y. Body temperature and locomotor activity as marker rhythms of aging of the circadian system in rodents. Exp. Gerontol. 1999, 34, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.W.; Dady, K.F.; Evans, A.K.; Lowry, C.A. Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Exp. Neurol. 2011, 227, 264–278. [Google Scholar] [CrossRef]
- Kelly, K.J.; Donner, N.C.; Hale, M.W.; Lowry, C.A. Swim stress activates serotonergic and nonserotonergic neurons in specific subdivisions of the rat dorsal raphe nucleus in a temperature-dependent manner. Neuroscience 2011, 197, 251–268. [Google Scholar] [CrossRef][Green Version]
- Lee, H.S.; Kim, M.A.; Valentino, R.J.; Waterhouse, B.D. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003, 963, 57–71. [Google Scholar] [CrossRef]
- Olivier, J.D.; Cools, A.R.; Olivier, B.; Homberg, J.R.; Cuppen, E.; Ellenbroek, B.A. Stress-induced hyperthermia and basal body temperature are mediated by different 5-HT(1A) receptor populations: A study in SERT knockout rats. Eur. J. Pharmacol. 2008, 590, 190–197. [Google Scholar] [CrossRef]
- Mazzola-Pomietto, P.; Aulakh, C.S.; Murphy, D.L. Temperature, food intake, and locomotor activity effects of a 5-HT3 receptor agonist and two 5-HT3 receptor antagonists in rats. Psychopharmacology 1995, 121, 488–493. [Google Scholar] [CrossRef]
- Millan, M.J. Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: Novel concepts, new drugs. Neurotherapeutics 2009, 6, 53–77. [Google Scholar] [CrossRef]
- Roth, B.L.; Hanizavareh, S.M.; Blum, A.E. Serotonin receptors represent highly favorable molecular targets for cognitive enhancement in schizophrenia and other disorders. Psychopharmacology 2004, 174, 17–24. [Google Scholar] [CrossRef]
- Inan, F.; Brunt, T.M.; Contrucci, R.R.; Hondebrink, L.; Franssen, E.J.F. Novel Phenethylamines and Their Potential Interactions with Prescription Drugs: A Systematic Critical Review. Ther. Drug Monit. 2020, 42, 271–281. [Google Scholar] [CrossRef]
- Schiff, P.L. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 98. [Google Scholar] [CrossRef]
- Feldberg, W.; Myers, R.D. A New Concept of Temperature Regulation by Amines in the Hypothalamus. Nature 1963, 200, 1325. [Google Scholar] [CrossRef]
- Shin, M.; Carpenter, J.S.; Park, S.H.; Janiszewski, C.; Tonini, E.; McKenna, S.; Hindmarsh, G.; Iorfino, F.; Nichles, A.; Zmicerevska, N.; et al. Twenty-four-hour Skin Temperature Rhythms in Young People with Emerging Mood Disorders: Relationships with Illness Subtypes and Clinical Stage. J. Biol. Rhythm. 2025, 40, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Souetre, E.; Pringuey, D.; Salvati, E.; Robert, P. [Circadian rhythms and depression]. Ann. Med. Psychol. 1985, 143, 845–870. [Google Scholar]
- Souetre, E.; Pringuey, D.; Salvati, E.; Robert, P.; Darcourt, G. [Circadian rhythms of the central temperature and blood cortisol in endogenous depression]. Encephale 1985, 11, 185–198. [Google Scholar]
- von Zerssen, D.; Dirlich, G.; Doerr, P.; Emrich, H.M.; Lund, R.; Ploog, D. Are biological rhythms disturbed in depression? Acta Psychiatr. Belg. 1985, 85, 624–635. [Google Scholar] [PubMed]
- Janssen, C.W.; Lowry, C.A.; Mehl, M.R.; Allen, J.J.; Kelly, K.L.; Gartner, D.E.; Medrano, A.; Begay, T.K.; Rentscher, K.; White, J.J.; et al. Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 789–795. [Google Scholar] [CrossRef]
- Raison, C.L.; Sanacora, G.; Woolley, J.; Heinzerling, K.; Dunlop, B.W.; Brown, R.T.; Kakar, R.; Hassman, M.; Trivedi, R.P.; Robison, R.; et al. Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial. JAMA 2023, 330, 843–853. [Google Scholar] [CrossRef]
- Matsui, F.; Hecht, P.; Yoshimoto, K.; Watanabe, Y.; Morimoto, M.; Fritsche, K.; Will, M.; Beversdorf, D. DHA Mitigates Autistic Behaviors Accompanied by Dopaminergic Change in a Gene/Prenatal Stress Mouse Model. Neuroscience 2018, 371, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Hulsken, S.; Märtin, A.; Mohajeri, M.H.; Homberg, J.R. Food-derived serotonergic modulators: Effects on mood and cognition. Nutr. Res. Rev. 2013, 26, 223–234. [Google Scholar] [CrossRef]
- Anderson, I.M. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: A meta-analysis of efficacy and tolerability. J. Affect. Disord. 2000, 58, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N. Engl. J. Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Lavrovsky, Y.; Ivanenkov, Y.A. AVN-211, Novel and Highly Selective 5-HT6 Receptor Small Molecule Antagonist, for the Treatment of Alzheimer’s Disease. Mol. Pharm. 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Cantillon, M.; Ings, R.; Bhat, L. Initial Clinical Experience of RP5063 Following Single Doses in Normal Healthy Volunteers and Multiple Doses in Patients with Stable Schizophrenia. Clin. Transl. Sci. 2018, 11, 387–396. [Google Scholar] [CrossRef]
- Kane, J.M.; Kinon, B.J.; Forray, C.; Such, P.; Mittoux, A.; Lemming, O.M.; Hertel, P.; Howes, O.D. Efficacy and safety of Lu AF35700 in treatment-resistant schizophrenia: A randomized, active-controlled trial with open-label extension. Schizophr. Res. 2022, 248, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.S.; Paz, V.; Didriksen, M.; Celada, P.; Artigas, F. Lu AF35700 reverses the phencyclidine-induced disruption of thalamo-cortical activity by blocking dopamine D(1) and D(2) receptors. Eur. J. Pharmacol. 2023, 953, 175802. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Ledo, J.H.; Azevedo, E.P.; Beckman, D.; Ribeiro, F.C.; Santos, L.E.; Razolli, D.S.; Kincheski, G.C.; Melo, H.M.; Bellio, M.; Teixeira, A.L.; et al. Cross Talk Between Brain Innate Immunity and Serotonin Signaling Underlies Depressive-Like Behavior Induced by Alzheimer’s Amyloid-β Oligomers in Mice. J. Neurosci. 2016, 36, 12106–12116. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Shahidi, S.; Rohani, A.H.; Soleimani Asl, S.; Komaki, A. Protective effects of 5-HT(1A) receptor antagonist and 5-HT(2A) receptor agonist on the biochemical and histological features in a rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2019, 96, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, C.; Lv, J.; Zhu, X.; Jiang, X.; Lu, W.; Lu, Y.; Tang, Z.; Wang, J.; Shen, X. Antiallergic drug desloratadine as a selective antagonist of 5HT(2A) receptor ameliorates pathology of Alzheimer’s disease model mice by improving microglial dysfunction. Aging Cell 2021, 20, e13286. [Google Scholar] [CrossRef]
- Xing, C.; Chen, H.; Bi, W.; Lei, T.; Hang, Z.; Du, H. Targeting 5-HT Is a Potential Therapeutic Strategy for Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 13446. [Google Scholar] [CrossRef]
- Cosford, N.D.; Tehrani, L.; Roppe, J.; Schweiger, E.; Smith, N.D.; Anderson, J.; Bristow, L.; Brodkin, J.; Jiang, X.; McDonald, I.; et al. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: A potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003, 46, 204–206. [Google Scholar] [CrossRef]
- Lea, P.M.t.; Movsesyan, V.A.; Faden, A.I. Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors. Br. J. Pharmacol. 2005, 145, 527–534. [Google Scholar] [CrossRef]
- Dravolina, O.A.; Danysz, W.; Bespalov, A.Y. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology 2006, 187, 397–404. [Google Scholar] [CrossRef]
- Gass, J.T.; Osborne, M.P.; Watson, N.L.; Brown, J.L.; Olive, M.F. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 2009, 34, 820–833. [Google Scholar] [CrossRef]
- Kotlinska, J.; Bochenski, M. The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats. Eur. J. Pharmacol. 2008, 598, 57–63. [Google Scholar] [CrossRef]
- Palmatier, M.I.; Liu, X.; Donny, E.C.; Caggiula, A.R.; Sved, A.F. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology 2008, 33, 2139–2147. [Google Scholar] [CrossRef][Green Version]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef]
- Meloni, M.; Puligheddu, M.; Sanna, F.; Cannas, A.; Farris, R.; Tronci, E.; Figorilli, M.; Defazio, G.; Carta, M. Efficacy and safety of 5-Hydroxytryptophan on levodopa-induced motor complications in Parkinson’s disease: A preliminary finding. J. Neurol. Sci. 2020, 415, 116869. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Reyes-Long, S.; Bandala, C.; Morraz-Varela, A.; Bonilla-Jaime, H.; Alfaro-Rodriguez, A. Neuropathic Pain in Parkinson’s Disease. Neurol. India 2022, 70, 1879–1886. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, L.G.; Liu, L.B.; An, M.Q.; Dong, L.G.; Gu, H.Y.; Dai, Y.P.; Wang, F.; Mao, C.J.; Liu, C.F. Inhibition of Spinal 5-HT3 Receptor and Spinal Dorsal Horn Neuronal Excitability Alleviates Hyperalgesia in a Rat Model of Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 7253–7264. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Yao, X.X.; Gao, S.H.; Li, R.; Li, B.J.; Yang, W.; Cui, R.J. Role of 5-HT receptors in neuropathic pain: Potential therapeutic implications. Pharmacol. Res. 2020, 159, 104949. [Google Scholar] [CrossRef] [PubMed]
- Dentel, C.; Palamiuc, L.; Henriques, A.; Lannes, B.; Spreux-Varoquaux, O.; Gutknecht, L.; René, F.; Echaniz-Laguna, A.; Gonzalez de Aguilar, J.L.; Lesch, K.P.; et al. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: A link to spasticity. Brain 2013, 136, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.; Spreux-Varoquaux, O.; Bensimon, G.; Jullien, P.; Lacomblez, L.; Salachas, F.; Bruneteau, G.; Pradat, P.F.; Loeffler, J.P.; Meininger, V. Platelet serotonin level predicts survival in amyotrophic lateral sclerosis. PLoS ONE 2010, 5, e13346. [Google Scholar] [CrossRef]
- Arnoux, A.; Ayme-Dietrich, E.; Dieterle, S.; Goy, M.A.; Schann, S.; Frauli, M.; Monassier, L.; Dupuis, L. Evaluation of a 5-HT(2B) receptor agonist in a murine model of amyotrophic lateral sclerosis. Sci. Rep. 2021, 11, 23582. [Google Scholar] [CrossRef]
- El Oussini, H.; Bayer, H.; Scekic-Zahirovic, J.; Vercruysse, P.; Sinniger, J.; Dirrig-Grosch, S.; Dieterlé, S.; Echaniz-Laguna, A.; Larmet, Y.; Müller, K.; et al. Serotonin 2B receptor slows disease progression and prevents degeneration of spinal cord mononuclear phagocytes in amyotrophic lateral sclerosis. Acta Neuropathol. 2016, 131, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Roppongi, T.; Togo, T.; Nakamura, S.; Asami, T.; Yoshimi, A.; Shiozaki, K.; Kato, D.; Kawanishi, C.; Hirayasu, Y. Perospirone in treatment of Huntington’s disease: A first case report. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Sviridova, A.; Rogovskii, V.; Kudrin, V.; Pashenkov, M.; Boyko, A.; Melnikov, M. The role of 5-HT(2B)-receptors in fluoxetine-mediated modulation of Th17- and Th1-cells in multiple sclerosis. J. Neuroimmunol. 2021, 356, 577608. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, F.; Guillard, C.; Mollet, L.; Auzou, P.; Gosset, D.; Madouri, F.; Valéry, A.; Menuet, A.; Ozsancak, C.; Pallix-Guyot, M.; et al. T Lymphocyte Serotonin 5-HT(7) Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients. Biomedicines 2022, 10, 2418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).