The Cardiovascular Physiology of Glucagon-like Peptide-1 Receptor Agonists: From Macro-Level Outcomes to Micro-Level Mechanisms
Abstract
1. Introduction
2. A New Standard of Care: Evidence from Cardiovascular Outcome Trials
2.1. Overview of Landmark CVOTs and MACE Reduction
2.2. The SELECT Trial: Expanding the Paradigm Beyond Diabetes
2.3. Effects on Secondary and Exploratory Endpoints
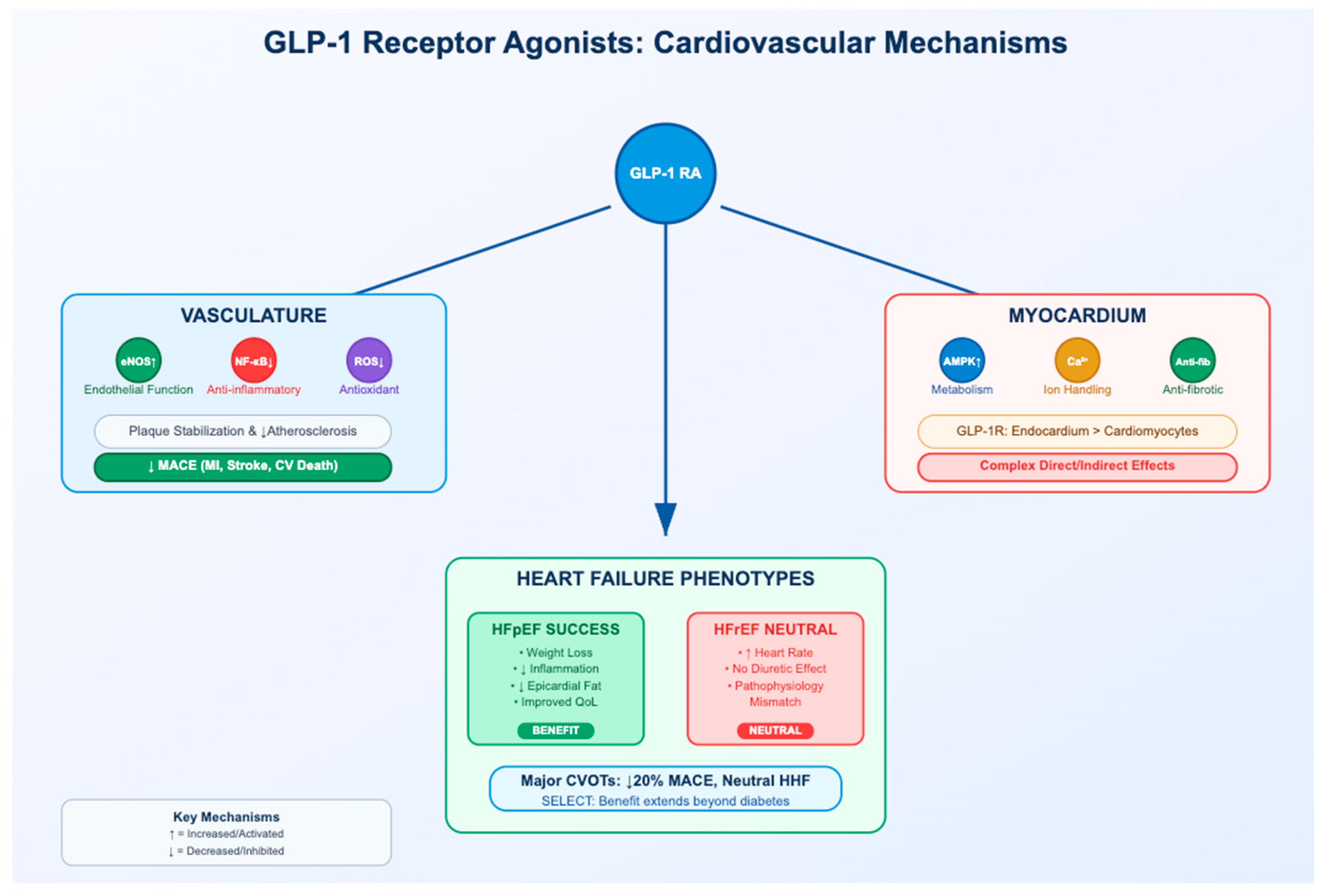
3. The Vasculature as a Primary Target: Mechanisms in Atherosclerosis
3.1. Improving Endothelial Function and NO Bioavailability
3.2. Attenuating Vascular Inflammation and Oxidative Stress
3.3. Modulating Plaque Composition and Stability
4. The Myocardium: A Complex Interplay of Direct and Indirect Effects
4.1. The GLP-1 Receptor in the Human Heart: A Contentious Presence
4.2. Modulation of Myocardial Metabolism and Mitochondrial Function
4.3. Direct Effects on Cardiomyocyte Ion Homeostasis and Contractility
4.4. Attenuation of Cardiac Fibrosis and Adverse Remodeling
5. GLP-1 Receptor Agonists in Heart Failure: A Tale of Two Phenotypes
5.1. Clear Benefits in Heart Failure with Preserved Ejection Fraction (HFpEF)
- Targeting Adiposity: GLP-1 RAs induce significant weight loss, which reduces the overall hemodynamic burden on the heart. Critically, they also appear to reduce visceral and epicardial adipose tissue (EAT), the metabolically active and pro-inflammatory fat depots that are strongly implicated in promoting myocardial stiffness and diastolic dysfunction [48].
- Targeting Inflammation: HFpEF is increasingly viewed as an inflammatory disease. GLP-1 RAs directly counter this by exerting potent systemic and local anti-inflammatory effects, reducing levels of inflammatory markers like C-reactive protein and inhibiting pro-inflammatory signaling pathways [4].
- Targeting Vascular Dysfunction: Many patients with HFpEF suffer from coronary microvascular dysfunction. By improving endothelial function and NO bioavailability, GLP-1 RAs can address this component of the disease, improving myocardial perfusion [33].
5.2. Neutrality and Caution in Heart Failure with Reduced Ejection Fraction (HFrEF)
- Adverse Chronotropic Effects: A consistent physiological effect of GLP-1 RA therapy is a modest but persistent increase in heart rate of 3–5 beats per minute [52]. In individuals without heart failure, the modest rise in heart rate is clinically negligible; however, in HFrEF patients it may be detrimental. However, in the context of HFrEF, where the heart is already failing and under high sympathetic stress, elevated heart rate is a well-established negative prognostic factor. The increased myocardial oxygen demand associated with a faster heart rate could be detrimental, potentially negating or overriding any other potential benefits of the drug [53].
- Lack of Favorable Hemodynamic Effects: A key mechanism of benefit for other successful HFrEF therapies, such as SGLT2 inhibitors, is their ability to induce osmotic diuresis and reduce plasma volume, thereby decreasing cardiac preload and congestion. GLP-1 RAs do not appear to share these robust hemodynamic effects, limiting their utility in the volume-overloaded state typical of HFrEF.
- Pathophysiological Mismatch: The primary drivers of HFrEF progression often involve extensive myocyte loss, adverse remodeling, and profound neurohormonal activation. The primary mechanisms of GLP-1 RAs—metabolic optimization and inflammation reduction—may be less effective at targeting these core features of HFrEF compared to their effectiveness against the metabolic drivers of HFpEF.
6. Unanswered Questions and Future Directions
6.1. Definitive Localization and Function of the Cardiac GLP-1R
6.2. Disentangling Direct vs. Indirect Effects
6.3. The Next Frontier: Dual and Tri-Agonists
6.4. Long-Term Effects in Broader Populations
6.5. Elucidating the HFrEF Paradox
6.6. Next-Generation Agents and Expanding Heart Failure Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA Carboxylase |
| AMPK | 5′ Adenosine Monophosphate-activated Protein Kinase |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| cAMP | Cyclic Adenosine Monophosphate |
| CVOT | Cardiovascular Outcome Trial |
| DPP-4 | Dipeptidyl Peptidase-4 |
| EAT | Epicardial Adipose Tissue |
| eNOS | Endothelial Nitric Oxide Synthase |
| ERK1/2 | Extracellular Signal-regulated Kinase 1/2 |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLP-1R | Glucagon-Like Peptide-1 Receptor |
| GLP-1RA | Glucagon-Like Peptide-1 Receptor Agonist |
| HF | Heart Failure |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| MACE | Major Adverse Cardiovascular Events |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MI | Myocardial Infarction |
| MMP | Matrix Metalloproteinase |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B cells |
| NO | Nitric Oxide |
| PI3K | Phosphoinositide 3-Kinase |
| PKA | Protein Kinase A |
| ROS | Reactive Oxygen Species |
| SGLT2i | Sodium-Glucose Cotransporter-2 Inhibitor |
| SMC | Smooth Muscle Cell |
| T2DM | Type 2 Diabetes Mellitus |
| TNF-α | Tumor Necrosis Factor-alpha |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VSMC | Vascular Smooth Muscle Cell |
References
- Accioli, R.; Salvini, V.; Xiao, J.; Lazzerini, P.E.; Roever, L.; Acampa, M. Year in review: Discussions in general cardiovascular medicine. Front. Cardiovasc. Med. 2023, 10, 1341650. [Google Scholar] [CrossRef]
- Saraiva, F.K.; Sposito, A.C. Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc. Diabetol. 2014, 13, 142. [Google Scholar] [CrossRef]
- Gupta, N.; Zayyad, Z.; Bhattaram, R.; Tiu, D.; Dau, J.; Guburxani, V.; Kalzuna, S.D.; Shroff, A.R. Beyond blood sugar: A scoping review of GLP-1 receptor agonists in cardiovascular care. Cardiol. Ther. 2025, 14, 351–366. [Google Scholar] [CrossRef]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.S. Cardiovascular disease—Unanswered questions. Circulation 1973, 47, A341–A357. [Google Scholar]
- Barry, A.R.; Boswell, R.; Babadagli, H.E.; Chen, J.W.; Cowley, E.; Eberhardt, T.E.; May, T.A. Review of the top 5 cardiology studies of 2023–24. Can. Pharm. J./Rev. Pharm. Can. 2025, 158, 17151635251339445. [Google Scholar] [CrossRef]
- Le, R.; Nguyen, M.T.; Allahwala, M.A.; Psaltis, J.P.; Marathe, C.S.; Marathe, J.A.; Psaltis, P.J. Cardiovascular protective properties of GLP-1 receptor agonists: More than just diabetic and weight loss drugs. J. Clin. Med. 2024, 13, 4674. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Fonarow, G.C.; McGuire, D.K.; Hernandez, A.F.; Vaduganathan, M.; Rosenstock, J.; Handelsman, Y.; Verma, S.; Anker, S.D.; McMurray, J.J.V.; et al. Glucagon-like peptide 1 receptor agonists and heart failure. Circulation 2020, 142, 1205–1218. [Google Scholar] [CrossRef]
- Shankar, P.S.; Jali, M.V. Standards of care in diabetes—2025. RGUHS J. Med. Sci. 2025, 15, 4–7. [Google Scholar] [CrossRef]
- Muskiet, M.H.; Tonneijck, L.; Huang, Y.; Liu, M.; Saremi, A.; Heerspink, H.J.; van Raalte, D.H. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: An exploratory analysis of the ELIXA trial. Lancet Diabetes Endocrinol. 2018, 6, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE−/− and LDLr−/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Kabir, M.G.; Drucker, D.J. Glucagon-like peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Mol. Metab. 2022, 66, 101641. [Google Scholar] [CrossRef]
- McLean, B.A.; Wong, C.K.; Kaur, K.D.; Seeley, R.J.; Drucker, D.J. Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide. JCI Insight 2021, 6, e153732. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.; Gopal, K.; Greenwell, A.A.; Young, A.; Gill, R.; Aburasayn, H.; Al Batran, R.; Chahade, J.J.; Gandhi, M.; Eaton, F.; et al. The GLP-1 receptor agonist liraglutide increases myocardial glucose oxidation rates and mitigates experimental diabetic cardiomyopathy. Can. J. Cardiol. 2021, 37, 140–150. [Google Scholar] [CrossRef]
- Zinman, B.; Nauck, M.A.; Bosch-Traberg, H.; Frimer-Larsen, H.; Ørsted, D.D.; Buse, J.B.; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Liraglutide and glycaemic outcomes in the LEADER trial. Diabetes Ther. 2018, 9, 2383–2392. [Google Scholar] [CrossRef]
- Mikhail, N. Cardiovascular effects of liraglutide. Curr. Hypertens. Rev. 2019, 15, 64–69. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R. Cardiovascular safety and benefits of semaglutide in patients with type 2 diabetes: Findings from SUSTAIN 6 and PIONEER 6. Front. Endocrinol. 2021, 12, 645566. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riddle, M.C.; Rydén, L.; Xavier, D.; et al. Design and baseline characteristics of participants in the REWIND trial on cardiovascular effects of dulaglutide. Diabetes Obes. Metab. 2018, 20, 42–49. [Google Scholar]
- Gerstein, H.C.; Li, Z.; Ramasundarahettige, C.; Baek, S.; Branch, K.R.; Del Prato, S.; Lam, C.S.; Lopes, R.D.; Pratley, R.; Rosenstock, J.; et al. Exploring the relationship between efpeglenatide dose and cardiovascular outcomes in type 2 diabetes: Insights from the AMPLITUDE-O trial. Circulation 2023, 147, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.; Kristensen, S.L.; Branch, K.R.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.; Lopes, R.D.; McMurray, J.J.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Verma, S.; Poulter, N.R.; Bhatt, D.L.; Bain, S.C.; Buse, J.B.; Leiter, L.A.; Nauck, M.A.; Pratley, R.E.; Zinman, B.; Ørsted, D.D.; et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without history of myocardial infarction or stroke. Circulation 2018, 138, 2884–2894. [Google Scholar] [CrossRef]
- Neuen, B.L.; Fletcher, R.A.; Heath, L.; Perkovic, A.; Vaduganathan, M.; Badve, S.V.; Tuttle, K.R.; Pratley, R.; Gerstein, H.C.; Perkovic, V.; et al. Cardiovascular, kidney, and safety outcomes with GLP-1 receptor agonists alone and in combination with SGLT2 inhibitors in type 2 diabetes: A systematic review and meta-analysis. Circulation 2024, 150, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Deanfield, J.; Kahn, S.E.; Weeke, P.E.; Toplak, H.; Scirica, B.M.; Rydén, L.; Rathor, N.; Plutzky, J.; Morales, C.; et al. Semaglutide and cardiovascular outcomes by baseline HbA1c and change in HbA1c in people with overweight or obesity but without diabetes in SELECT. Diabetes Care 2024, 47, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Liu, M.; Xu, Y.; Ling, Q.; Lin, W.; Zhang, J.; Yan, Z.; Ma, J.; Li, W.; et al. Clinical outcomes with GLP-1 receptor agonists in patients with heart failure: A systematic review and meta-analysis of randomized controlled trials. Drugs 2023, 83, 1293–1307. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. New insights into the use of liraglutide—Impact on cardiovascular risk and microvascular outcomes. Biomedicines 2023, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; White, J.; Pagidipati, N.J.; Lokhnygina, Y.; Wainstein, J.; Murin, J.; Iqbal, N.; Öhman, P.; Lopes, R.D.; Reicher, B.; et al. Effect of once-weekly exenatide in patients with type 2 diabetes mellitus with and without heart failure: Insights from EXSCEL. Circulation 2019, 140, 1613–1622. [Google Scholar] [CrossRef]
- Park, B.; Bakbak, E.; Teoh, H.; Krishnaraj, A.; Dennis, F.; Quan, A.; Rotstein, O.D.; Butler, J.; Hess, D.A.; Verma, S. GLP-1 receptor agonists and atherosclerosis protection: The vascular endothelium takes center stage. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H1159–H1176. [Google Scholar] [CrossRef]
- Ferhatbegović, L.; Mršić, D.; Macić-Džanković, A. The benefits of GLP-1 receptors in cardiovascular diseases. Front. Clin. Diabetes Healthc. 2023, 4, 1293926. [Google Scholar] [CrossRef]
- Ravassa, S.; Zudaire, A.; Diez, J. GLP-1 and cardioprotection: From bench to bedside. Cardiovasc. Res. 2012, 94, 316–324. [Google Scholar] [CrossRef]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 receptor expression within the human heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, T.; Welungoda, I.; Widdop, R.E.; Simpson, R.W.; Dear, A.E. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE−/− mouse model. Diabetes Vasc. Dis. Res. 2013, 10, 353–360. [Google Scholar] [CrossRef]
- García-Vega, D.; Sánchez-López, D.; Rodríguez-Carnero, G.; Villar-Taibo, R.; Viñuela, J.E.; Lestegás-Soto, A.; Seoane-Blanco, A.; Moure-González, M.; Bravo, S.B.; Fernández, Á.L.; et al. Semaglutide modulates prothrombotic and atherosclerotic mechanisms. Cardiovasc. Diabetol. 2024, 23, 1. [Google Scholar] [CrossRef]
- Badve, S.V.; Bilal, A.; Lee, M.M.; Sattar, N.; Gerstein, H.C.; Ruff, C.T.; McMurray, J.J.; Rossing, P.; Bakris, G.; Mahaffey, K.W.; et al. Effects of GLP-1 receptor agonists on kidney and cardiovascular disease outcomes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2025, 13, 15–28. [Google Scholar] [CrossRef]
- Ang, R.; Mastitskaya, S.; Hosford, P.S.; Basalay, M.; Specterman, M.; Aziz, Q.; Li, Y.; Orini, M.; Taggart, P.; Lambiase, P.D.; et al. Modulation of cardiac ventricular excitability by GLP-1. Circ. Arrhythm. Electrophysiol. 2018, 11, e006740. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Sharma, A.; Butler, J.; Packer, M.; Zannad, F.; Vasques-Nóvoa, F.; Leite-Moreira, A.; Neves, J.S. Glucagon-like peptide-1 receptor agonists across the spectrum of heart failure. J. Clin. Endocrinol. Metab. 2024, 109, 4–9. [Google Scholar] [CrossRef]
- Rroji, M.; Spahia, N.; Figurek, A.; Spasovski, G. Targeting diabetic atherosclerosis: The role of GLP-1 receptor agonists, SGLT2 inhibitors, and nonsteroidal mineralocorticoid receptor antagonists. Biomedicines 2025, 13, 728. [Google Scholar] [CrossRef]
- Banerjee, M. Epicardial fat paradox and differential effects of GLP-1 receptor agonists across heart failure phenotypes. Circulation 2023, 16, e010966. [Google Scholar] [CrossRef]
- Luna-Marco, C.; Iannantuoni, F.; Hermo-Argibay, A.; Devos, D.; Salazar, J.D.; Víctor, V.M.; Rovira-Llopis, S. Cardiovascular benefits of SGLT2 inhibitors and GLP-1 receptor agonists through effects on mitochondrial function and oxidative stress. Free Radic. Biol. Med. 2024, 213, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Krammer, T.; Baier, M.J.; Hegner, P.; Zschiedrich, T.; Lukas, D.; Wolf, M.; Le Phu, C.; Lutz, V.; Evert, K.; Kozakov, K.; et al. Cardioprotective effects of semaglutide on isolated human ventricular myocardium. Eur. J. Heart Fail. 2025, 27, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Kobara, M.; Toba, H.; Nakata, T. A glucagon-like peptide 1 analog protects mitochondria and attenuates hypoxia–reoxygenation injury in cultured cardiomyocytes. J. Cardiovasc. Pharmacol. 2022, 79, 568–576. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, J.; Diao, S.; Zhang, G.; Xiao, M.; Chang, D. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction. Chem.-Biol. Interact. 2020, 332, 109252. [Google Scholar] [CrossRef]
- Khadke, S.; Kumar, A.; Bhatti, A.; Dani, S.S.; Al-Kindi, S.; Nasir, K.; Virani, S.S.; Upadhyay, J.; Garcia-Banigan, D.C.; Abraham, S.; et al. GLP-1 receptor agonist in nonobese patients with type 2 diabetes mellitus and heart failure with preserved ejection fraction. Journal of Cardiac Failure 2025, 31, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Lubberding, A.F.; Veedfald, S.; Achter, J.S.; Nissen, S.D.; Soattin, L.; Sorrentino, A.; Vega, E.T.; Linz, B.; Eggertsen, C.H.E.; Mulvey, J.; et al. Glucagon-like peptide-1 increases heart rate by a direct action on the sinus node. Cardiovasc. Res. 2024, 120, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Shchendrygina, A.; Rakisheva, A.; Giverts, I.; Rustamova, Y.; Soloveva, A. Effects of GLP-1 receptor agonists on cardiac function, exercise capacity and quality of life. Card. Fail. Rev. 2024, 10, e10. [Google Scholar] [CrossRef]
- Ametov, A.S.; Kamynina, L.L.; Akhmedova, Z.G. Cardioprotective effects of glucagon-like peptide 1 receptor agonists. Kardiologiia 2014, 54, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Dani, S.S.; Makwana, B.; Khadke, S.; Kumar, A.; Jhund, P.; Nasir, K.; Sattar, N.; Al-Kindi, S.; Fonarow, G.; Butler, J.; et al. An observational study of cardiovascular outcomes of tirzepatide vs GLP-1 receptor agonists. JACC Adv. 2025, 4, 101740. [Google Scholar] [CrossRef]
- Manoria, P.C. From research to practice: The future of cardiovascular care. Cureus 2025, 17, e84473. [Google Scholar] [CrossRef]
- Gal, D.; Thijs, B.; Glänzel, W.; Sipido, K.R. Hot topics and trends in cardiovascular research. Eur. Heart J. 2019, 40, 2363–2374. [Google Scholar] [CrossRef]
- Patrascanu, O.S.; Tutunaru, D.; Musat, C.L.; Dragostin, O.M.; Fulga, A.; Nechita, L.; Ciubara, A.B.; Piraianu, A.I.; Stamate, E.; Poalelungi, D.G.; et al. Future horizons: The potential role of artificial intelligence in cardiology. J. Pers. Med. 2024, 14, 656. [Google Scholar] [CrossRef]
- Pahud de Mortanges, A.; Sinaci, E.; Salvador, D., Jr.; Bally, L.; Muka, T.; Wilhelm, M.; Bano, A. GLP-1 receptor agonists and coronary arteries: From mechanisms. Front. Pharmacol. 2022, 13, 856111. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, X.; Zou, Y.; Zhang, M.; Chen, Y.; Zhu, W.; Han, B. Semaglutide attenuates myocardial ischemia-reperfusion injury by inhibiting ferroptosis of cardiomyocytes via activation of PKC-S100A9 axis. Front. Pharmacol. 2025, 16, 1529652. [Google Scholar] [CrossRef]
- Trevisan, M.; Fu, E.L.; Szummer, K.; Norhammar, A.; Lundman, P.; Wanner, C.; Sjölander, A.; Jernberg, T.; Carrero, J.J. Glucagon-like peptide-1 receptor agonists and the risk of cardiovascular events in diabetes patients surviving an acute myocardial infarction. Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 7, 104–111. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Tuttle, K.R.; Rossing, P.; Rasmussen, S.; Perkovic, V.; Nielsen, O.W.; Mann, J.F.; MacIsaac, R.J.; Kosiborod, M.N.; Kamenov, Z.; et al. Effects of semaglutide on heart failure outcomes in diabetes and chronic kidney disease in the FLOW trial. J. Am. Coll. Cardiol. 2024, 84, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.G.; Snipelisky, D.; AbouEzzeddine, O.; Vader, J.; Cooper, L.; Kelley, J.; Perez, A.; Varian, K.; Lala, A.; Shah, M.; et al. Unanswered questions in contemporary heart failure. J. Card. Fail. 2017, 23, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Silversides, C.K. JACC: Advances and the future of cardiology. JACC Adv. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; Abd El Aziz, M.; Drucker, D.J. Cardiovascular actions and clinical outcomes with GLP-1 receptor agonists and DPP-4 inhibitors. Circulation 2017, 136, 028136. [Google Scholar] [CrossRef]
- Karatzia, L.; Aung, N.; Aksentijevic, D. Artificial intelligence in cardiology: Hope for the future and power for the present. Front. Cardiovasc. Med. 2022, 9, 945726. [Google Scholar] [CrossRef]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 2025, 392, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P. Can dual incretin receptor agonists exert better cardiovascular protection than selective GLP-1 receptor agonists? Highlights from SURPASS-CVOT. Diabetes Ther. 2025, 16, 1–6. [Google Scholar] [CrossRef]
- Bonfioli, G.B.; Rodella, L.; Metra, M.; Vizzardi, E. GLP-1 receptor agonists as promising anti-inflammatory agents in HFpEF. Heart Fail. Rev. 2025, 30, 131–136. [Google Scholar] [CrossRef]
- Hage, C. GLP-1 receptor agonists in heart failure: How far to expand use? Lancet 2024, 404, 909–911. [Google Scholar] [CrossRef]
- Ying, Y.; Zhu, H.; Liang, Z.; Ma, X.; Li, S. GLP-1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J. Mol. Endocrinol. 2015, 55, 245–256. [Google Scholar] [CrossRef]
- Svensson, L.G. Commentary: What are the greatest unanswered questions? Toward a unified theory on cardiac surgery treatment. J. Thorac. Cardiovasc. Surg. 2022, 164, 795–796. [Google Scholar] [CrossRef]
- Metra, M.; Carubelli, V.; Ravera, A.; Coats, A.J.S. Heart failure 2016: Still more questions than answers. Int. J. Cardiol. 2017, 227, 766–777. [Google Scholar] [CrossRef]
- Lopez-Jimenez, F.; Attia, Z.; Arruda-Olson, A.M.; Carter, R.; Chareonthaitawee, P.; Jouni, H.; Kapa, S.; Lerman, A.; Luong, C.; Medina-Inojosa, J.R.; et al. Artificial intelligence in cardiology: Present and future. Mayo Clin. Proc. 2020, 95, 1015–1039. [Google Scholar] [CrossRef] [PubMed]
- Nuamnaichati, N.; Mangmool, S.; Chattipakorn, N.; Parichatikanond, W. Stimulation of GLP-1 receptor inhibits methylglyoxal-induced mitochondrial dysfunctions in H9c2 cardiomyoblasts: Potential role of Epac/PI3K/Akt pathway. Front. Pharmacol. 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Sethi, Y.; Patel, N.; Kaka, N.; Kaiwan, O.; Kar, J.; Moinuddin, A.; Goel, A.; Chopra, H.; Cavalu, S. Precision medicine and the future of cardiovascular diseases: A clinically oriented comprehensive review. J. Clin. Med. 2023, 12, 1799. [Google Scholar] [CrossRef] [PubMed]
| Trial Name | Drug | Sample Size | Baseline ASCVD (%) | Follow-Up (Years) | Primary MACE Outcome (HR [95% CI]) | HHF Outcome (HR [95% CI]) |
|---|---|---|---|---|---|---|
| ELIXA [10] | Lixisenatide | 6068 | 100% | 2.1 | 1.02 [0.89–1.17] | 0.96 [0.75–1.23] |
| LEADER [15] | Liraglutide | 9340 | 81% | 3.8 | 0.87 [0.78–0.97] | 0.87 [0.73–1.05] |
| SUSTAIN-6 [17] | Semaglutide (SC) | 3297 | 83% | 2.1 | 0.74 [0.58–0.95] | 1.11 [0.77–1.61] |
| EXSCEL [27] | Exenatide (weekly) | 14,752 | 73% | 3.2 | 0.91 [0.83–1.00] | 0.94 [0.78–1.13] |
| REWIND [18] | Dulaglutide | 9901 | 31% | 5.4 | 0.88 [0.79–0.99] | 0.93 [0.77–1.12] |
| PIONEER 6 [17] | Semaglutide (oral) | 3183 | 85% | 1.3 | 0.79 [0.57–1.11] | 0.86 [0.48–1.55] |
| AMPLITUDE-O [19] | Efpeglenatide | 4076 | 90% | 1.8 | 0.73 [0.58–0.92] | 0.61 [0.38–0.98] |
| SELECT [6] | Semaglutide (SC) | 17,604 | 100% (No Diabetes) | 3.3 | 0.80 [0.72–0.89] | 0.82 [0.71–0.96] |
| HARMONY [20] | Albiglutide | 9463 | 100% | 1.6 | 0.78 [0.68–0.90] | 0.85 [0.70–1.04] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoor, M. The Cardiovascular Physiology of Glucagon-like Peptide-1 Receptor Agonists: From Macro-Level Outcomes to Micro-Level Mechanisms. Physiologia 2025, 5, 34. https://doi.org/10.3390/physiologia5030034
Mansoor M. The Cardiovascular Physiology of Glucagon-like Peptide-1 Receptor Agonists: From Macro-Level Outcomes to Micro-Level Mechanisms. Physiologia. 2025; 5(3):34. https://doi.org/10.3390/physiologia5030034
Chicago/Turabian StyleMansoor, Masab. 2025. "The Cardiovascular Physiology of Glucagon-like Peptide-1 Receptor Agonists: From Macro-Level Outcomes to Micro-Level Mechanisms" Physiologia 5, no. 3: 34. https://doi.org/10.3390/physiologia5030034
APA StyleMansoor, M. (2025). The Cardiovascular Physiology of Glucagon-like Peptide-1 Receptor Agonists: From Macro-Level Outcomes to Micro-Level Mechanisms. Physiologia, 5(3), 34. https://doi.org/10.3390/physiologia5030034






