Tears and Saliva as Biological Matrices for Vitamin D and Glucose Assessment: A Pilot Study
Abstract
1. Introduction
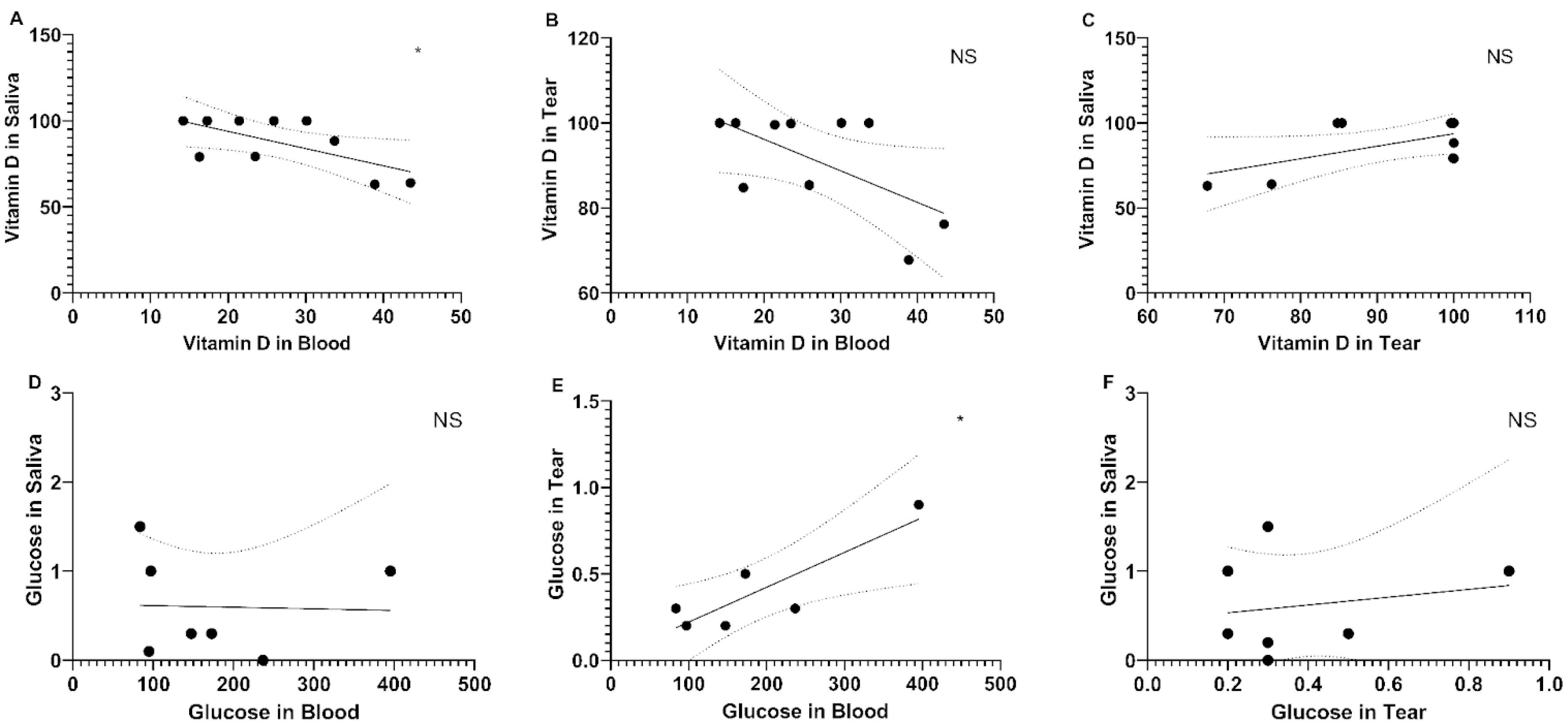
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Institute of Medicine (US). Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Grant, W.B.; Strange, R.C.; Garland, C.F. Sunshine is good medicine. The health benefits of ultraviolet-B induced vitamin D production. J. Cosmet. Dermatol. 2003, 2, 86–98. [Google Scholar] [CrossRef]
- Lu, X.; Elizondo, R.A.; Nielsen, R.; Christensen, E.I.; Yang, J.; Hammock, B.D.; Watsky, M.A. Vitamin D in tear fluid. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5880–5887. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Jain, R.; Singh, S. Vitamin D deficiency in patients with diabetes and COVID-19 infection. Diabetes Metab. Syndr. 2020, 14, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.K.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bahrami, L.S.; Ranjbar, G.; Norouzy, A.; Arabi, S.M. Vitamin D supplementation effects on the clinical outcomes of patients with coronary artery disease: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12923. [Google Scholar] [CrossRef]
- Kienreich, K.; Tomaschitz, A.; Verheyen, N.; Pieber, T.; Gaksch, M.; Grübler, M.R.; Pilz, S. Vitamin D and cardiovascular disease. Nutrients 2013, 5, 3005–3021. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef]
- Hayes, C.E.; Cantorna, M.T.; DeLuca, H.F. Vitamin D and multiple sclerosis. Proc. Soc. Exp. Biol. Med. 1997, 216, 21–27. [Google Scholar] [CrossRef]
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.A.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010, 33, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.C.; Shipman, K.E.; Ramachandran, S. Metabolic syndrome: A review of the role of vitamin D in mediating susceptibility and outcome. World J. Diabetes 2015, 6, 896–911. [Google Scholar] [CrossRef]
- Machado, M.R.C.; Gomes Junior, S.C.; Marinheiro, L.P.F. Vitamina D e diabetes mellitus, suas epidemias e o envelhecimento. O que há de novo? Reprod. Clim. 2014, 29, 54–59. [Google Scholar] [CrossRef][Green Version]
- Razdan, K.; Singh, K.; Singh, D. Vitamin D levels and COVID-19 susceptibility: Is there any correlation? Med. Drug Discov. 2020, 7, 100051. [Google Scholar] [CrossRef] [PubMed]
- Pines, A. Vitamin D and health issues—Questioned benefits. Climacteric 2014, 17, 657–659. [Google Scholar] [CrossRef]
- Mitri, J.; Pittas, A.G. Vitamin D and diabetes. Endocrinol. Metab. Clin. N. Am. 2014, 43, 205–232. [Google Scholar] [CrossRef]
- Valipour, G.; Saneei, P.; Esmaillzadeh, A. Serum vitamin D levels in relation to schizophrenia: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3863–3872. [Google Scholar] [CrossRef]
- Yazar, S.; Hewitt, A.W.; Black, L.J.; McKnight, C.M.; Mountain, J.A.; Sherwin, J.C.; Oddy, W.H.; Coroneo, M.T.; Lucas, R.M.; Mackey, D.A. Myopia is associated with lower vitamin D status in young adults. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Jee, D.; Kang, S.; Yuan, C.; Cho, E.; Arroyo, J.G.; Epidemiologic Survey Committee of the Korean Ophthalmologic Society. Serum 25-Hydroxyvitamin D levels and dry eye syndrome: Differential effects of vitamin D on ocular diseases. PLoS ONE 2016, 11, e0149294. [Google Scholar] [CrossRef]
- Yildirim, P.; Garip, Y.; Karci, A.A.; Guler, T. Dry eye in vitamin D deficiency: More than an incidental association. Int. J. Rheum. Dis. 2016, 19, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.F.; Ray, R.; Watson, D.G.; Delille, C.; Rimler, E.; Cleveland, J.; Lynn, M.J.; Tangpricha, V.; Srivastava, S.K. Vitamin D insufficiency in diabetic retinopathy. Endocr. Pract. 2012, 18, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Vick, S.; Chen, Z.; Chen, J.; Watsky, M.A. Effects of vitamin D receptor knockout and vitamin D deficiency on corneal epithelial wound healing and nerve density in diabetic mice. Diabetes 2020, 69, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Bahramian, A.; Falsafi, P.; Abbasi, T.; Ghanizadeh, M.; Abedini, M.; Kavoosi, F.; Kouhsoltani, M.; Noorbakhsh, F.; Dabbaghi Tabriz, F.; Rajaeih, S.; et al. Comparing serum and salivary levels of vitamin D in patients with recurrent aphthous stomatitis and healthy individuals. J. Dent. 2018, 19, 295–300. [Google Scholar]
- Gholizadeh, N.; Pirzadeh, F.; Mirzaii-Dizgah, I.; Sheykhbahaei, N. Relationship between salivary vitamin D deficiency and oral lichen planus. Photodermatol. Photoimmunol. Photomed. 2020, 36, 384–386. [Google Scholar] [CrossRef]
- Abdolsamadi, H.; Vahedi, M.; Borzouei, S.; Soltanian, A.; Hosseini, A.; Avval, M.Z. Evaluation the relationship between serum and salivary levels of 25(OH)Vit.D with type II diabetes in newly diagnosed diabetics. J. Mol. Biol. Res. 2018, 8, 108–113. [Google Scholar] [CrossRef]
- Sethu, S.; Shetty, R.; Deshpande, K.; Pahuja, N.; Chinnappaiah, N.; Agarwal, A.; Sharma, A.; Ghosh, A. Correlation between tear fluid and serum vitamin D levels. Eye Vis. 2016, 3, 22. [Google Scholar] [CrossRef]
- Lai, Y.T.; Cerquinho, R.G.; Perez, M.M.; Alves, B.D.C.A.; Pereira, E.C.; Azzalis, L.A.; Junqueira, V.B.C.; Soares, L.R.; Fonseca, F.L.A. Determination of vitamin D in tears of healthy individuals by the electrochemiluminescence method. J. Clin. Lab. Anal. 2019, 33, e22830. [Google Scholar] [CrossRef]
- Ting, D.S.; Cheung, G.C.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- Usluogullari, C.A.; Balkan, F.; Caner, S.; Ucler, R.; Kaya, C.; Ersoy, R.; Cakir, B. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr. Disord. 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Kubota, N.; Minami, T.; Shirakawa, R.; Sakurai, Y.; Hayashi, T.; Iwamoto, M.; Takamoto, I.; Kubota, T.; Suzuki, R.; et al. Association between tear and blood glucose concentrations: Random intercept model adjusted with confounders in tear samples negative for occult blood. J. Diabetes Investig. 2021, 12, 266–276. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Kaufman, E.; Lamster, I.B. The diagnostic applications of saliva—A review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Arya, A.; Seth, A. Salivary glucose levels: Can they be a reliable marker in monitoring diabetes mellitus? J. Oral Maxillofac. Pathol. 2014, 18, 214–219. [Google Scholar] [CrossRef]
| n | % | |
|---|---|---|
| Total volunteers | 14 | 100 |
| Female volunteers | 9 | 64.3 |
| Male volunteers | 5 | 35.7 |
| Age (years) | ||
| ≤30 | 2 | 14.3 |
| 31–40 | 4 | 28.6 |
| 41–49 | 2 | 14.3 |
| 50–61 | 6 | 42.8 |
| Mean age (years) | 45.35 | – |
| Diabetes status | ||
| Type II diabetes | 6 | 42.8 |
| Non-diabetic | 8 | 57.2 |
| Vitamin D supplementation | ||
| Diabetic volunteers | 3 | 42.8 * |
| Non-diabetic volunteers | 0 | 0 |
| Group | Serum VitD (ng/mL) | Tear VitD (ng/mL) | Saliva VitD (ng/mL) | Serum Glucose (mg/dL) | Tear Glucose (mg/dL) | Saliva Glucose (mg/dL) |
|---|---|---|---|---|---|---|
| DM | 32.93 ± 15.60 | 92.10 ± 11.01 | 86.32 ± 15.21 | 189.83 ± 114.45 | 0.475 ± 0.31 | 0.40 ± 0.424 |
| Control | 27.92 ± 9.85 | 90.64 ± 14.24 | 88.44 ± 16.81 | 89.95 ± 4.75 | 0.267 ± 0.058 | 0.70 ± 0.668 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, P.H.A.; Jorge, G.K.; Pereira, E.C.; Tsun, L.Y.; Gascón, T.M.; da C. A. Alves, B.; da Veiga, G.L.; de Carvalho, S.S.; Leça, R.G.C.; Lima, V.L.; et al. Tears and Saliva as Biological Matrices for Vitamin D and Glucose Assessment: A Pilot Study. Physiologia 2025, 5, 28. https://doi.org/10.3390/physiologia5030028
Reis PHA, Jorge GK, Pereira EC, Tsun LY, Gascón TM, da C. A. Alves B, da Veiga GL, de Carvalho SS, Leça RGC, Lima VL, et al. Tears and Saliva as Biological Matrices for Vitamin D and Glucose Assessment: A Pilot Study. Physiologia. 2025; 5(3):28. https://doi.org/10.3390/physiologia5030028
Chicago/Turabian StyleReis, Pedro Henrique A., Giovanna K. Jorge, Edimar C. Pereira, Lai Yu Tsun, Thais M. Gascón, Beatriz da C. A. Alves, Glaucia L. da Veiga, Samantha S. de Carvalho, Renato G. Cerquinho Leça, Vagner L. Lima, and et al. 2025. "Tears and Saliva as Biological Matrices for Vitamin D and Glucose Assessment: A Pilot Study" Physiologia 5, no. 3: 28. https://doi.org/10.3390/physiologia5030028
APA StyleReis, P. H. A., Jorge, G. K., Pereira, E. C., Tsun, L. Y., Gascón, T. M., da C. A. Alves, B., da Veiga, G. L., de Carvalho, S. S., Leça, R. G. C., Lima, V. L., & Fonseca, F. L. A. (2025). Tears and Saliva as Biological Matrices for Vitamin D and Glucose Assessment: A Pilot Study. Physiologia, 5(3), 28. https://doi.org/10.3390/physiologia5030028





