The Effects of Creatine Monohydrate and/or Whey Protein on the Muscle Protein Synthesis and Anabolic Signaling Responses in Non-Stressed C2C12 Murine Myotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blotting
2.3. Creatine Assays
2.4. Cytology of 24-h Treatments
2.5. Statistics
3. Results
3.1. Cellular Creatine Levels
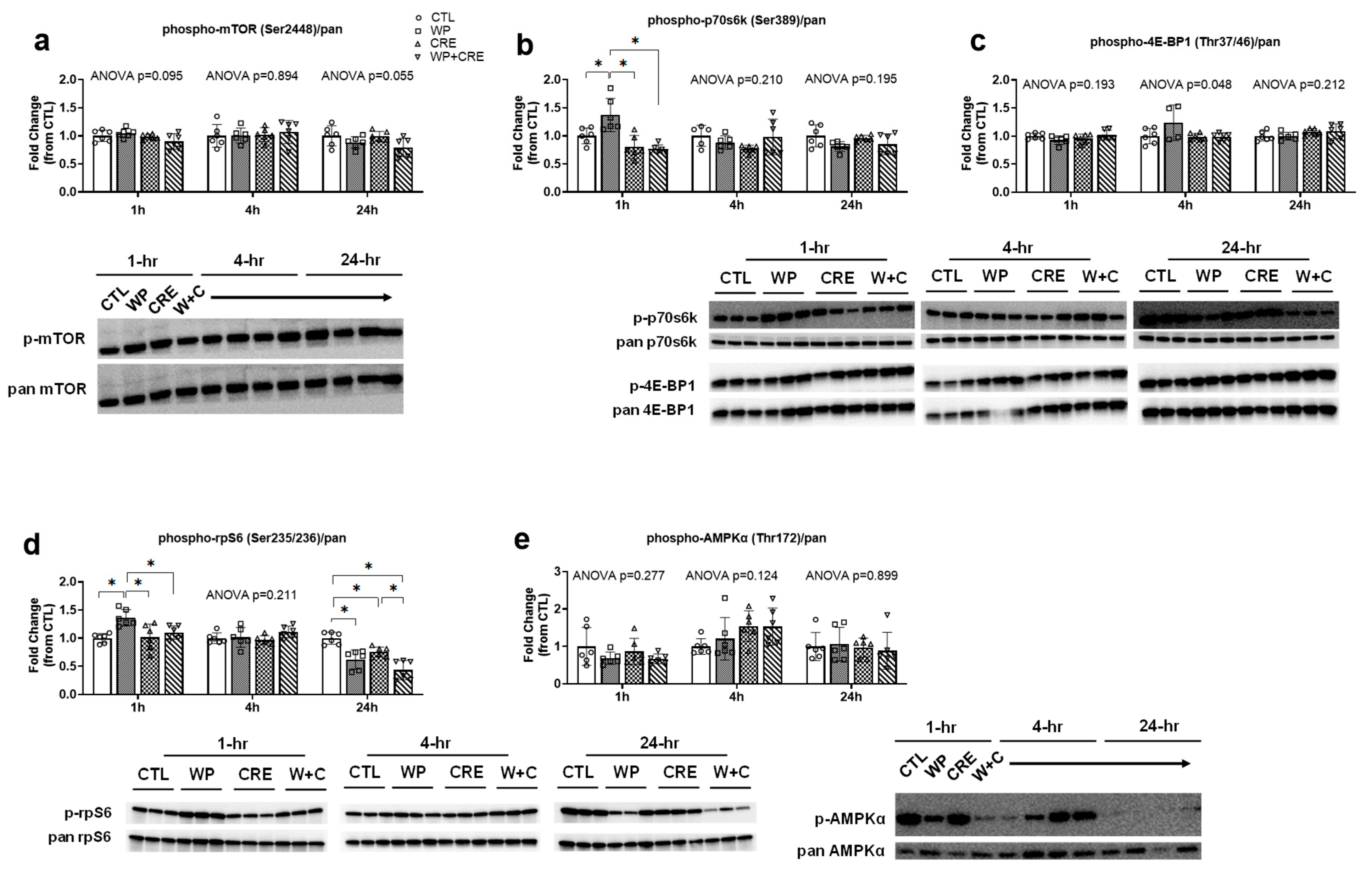
3.2. mTORC1 Pathway and AMPKα Phosphorylation Status
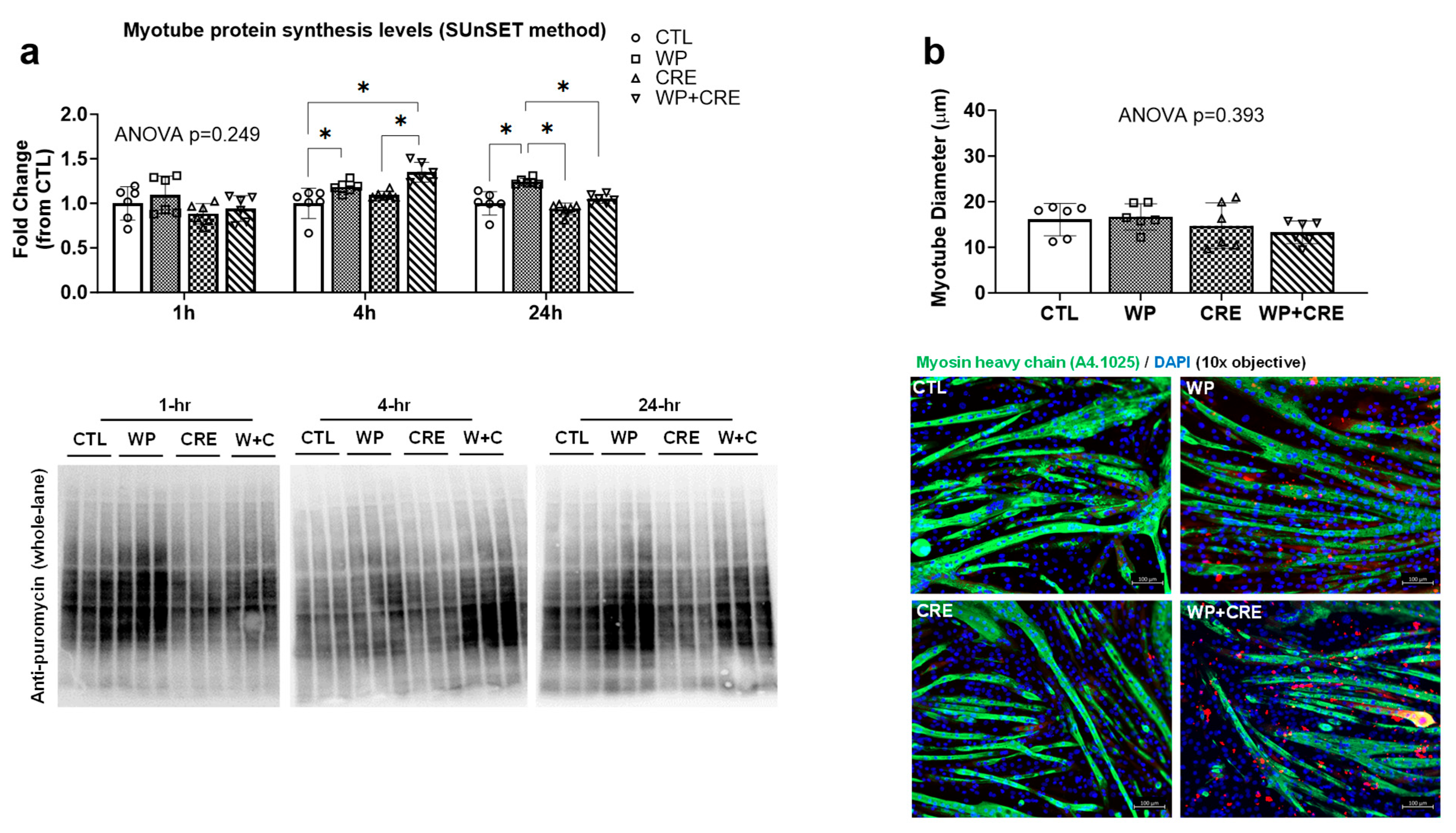
3.3. Myotube Protein Synthesis and Myotube Diameter
4. Discussion
Limitations and Practical Significance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Proud, C.G. The mTOR pathway in the control of protein synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; McCarthy, J.J.; Hornberger, T.A.; Phillips, S.M.; Mackey, A.L.; Nader, G.A.; Boppart, M.D.; Kavazis, A.N.; Reidy, P.T.; Ogasawara, R.; et al. Mechanisms of mechanical overload-induced skeletal muscle hypertrophy: Current understanding and future directions. Physiol. Rev. 2023, 103, 2679–2757. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Interactions between Growth of Muscle and Stature: Mechanisms Involved and Their Nutritional Sensitivity to Dietary Protein: The Protein-Stat Revisited. Nutrients 2021, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- White, J.P. Amino Acid Trafficking and Skeletal Muscle Protein Synthesis: A Case of Supply and Demand. Front. Cell Dev. Biol. 2021, 9, 656604. [Google Scholar] [CrossRef]
- Michel, J.M.; Lievense, K.K.; Norton, S.C.; Costa, J.V.; Alphin, K.H.; Bailey, L.A.; Miller, G.D. The Effects of Graded Protein Intake in Conjunction with Progressive Resistance Training on Skeletal Muscle Outcomes in Older Adults: A Preliminary Trial. Nutrients 2022, 14, 2739. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Deutz, N.E.; Memelink, R.G.; Verlaan, S.; Wolfe, R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial. Nutr. J. 2014, 13, 9. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Lockwood, C.M.; Stout, J.R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr. Metab. 2010, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Cribb, P.J.; Williams, A.D.; Stathis, C.G.; Carey, M.F.; Hayes, A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med. Sci. Sports Exerc. 2007, 39, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Parise, G.; Mihic, S.; MacLennan, D.; Yarasheski, K.E.; Tarnopolsky, M.A. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J. Appl. Physiol. 2001, 91, 1041–1047. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Rosene, J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med. Sci. Sports Exerc. 2001, 33, 1674–1681. [Google Scholar] [CrossRef]
- Olsen, S.; Aagaard, P.; Kadi, F.; Tufekovic, G.; Verney, J.; Olesen, J.L.; Suetta, C.; Kjaer, M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J. Physiol. 2006, 573, 525–534. [Google Scholar] [CrossRef]
- Deldicque, L.; Atherton, P.; Patel, R.; Theisen, D.; Nielens, H.; Rennie, M.J.; Francaux, M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J. Appl. Physiol. 2008, 104, 371–378. [Google Scholar] [CrossRef]
- Deldicque, L.; Theisen, D.; Bertrand, L.; Hespel, P.; Hue, L.; Francaux, M. Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am. J. Physiol. Cell Physiol. 2007, 293, C1263–C1271. [Google Scholar] [CrossRef]
- Louis, M.; Van Beneden, R.; Dehoux, M.; Thissen, J.P.; Francaux, M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C(2)C(12) cells. FEBS Lett. 2004, 557, 243–247. [Google Scholar] [CrossRef]
- Mobley, C.B.; Fox, C.D.; Ferguson, B.S.; Amin, R.H.; Dalbo, V.J.; Baier, S.; Rathmacher, J.A.; Wilson, J.M.; Roberts, M.D. L-leucine, beta-hydroxy-beta-methylbutyric acid (HMB) and creatine monohydrate prevent myostatin-induced Akirin-1/Mighty mRNA down-regulation and myotube atrophy. J. Int. Soc. Sports Nutr. 2014, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Phillips, B.E.; Hill, I.; Greenhaff, P.; Lund, J.N.; Williams, J.P.; Rankin, D.; Wilkinson, D.J.; Smith, K.; Atherton, P.J. Human skeletal muscle is refractory to the anabolic effects of leucine during the postprandial muscle-full period in older men. Clin. Sci. 2017, 131, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Jackman, S.R.; Breen, L.; Smith, K.; Selby, A.; Tipton, K.D. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 2014, 99, 86–95. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Steeds, C.M.; Wiseman, R.W.; Pavlath, G.K. Phosphocreatine as an energy source for actin cytoskeletal rearrangements during myoblast fusion. J. Physiol. 2008, 586, 2841–2853. [Google Scholar] [CrossRef]
- Mobley, C.B.; Mumford, P.W.; McCarthy, J.J.; Miller, M.E.; Young, K.C.; Martin, J.S.; Beck, D.T.; Lockwood, C.M.; Roberts, M.D. Whey protein-derived exosomes increase protein synthesis and hypertrophy in C(2-)C(12) myotubes. J. Dairy Sci. 2017, 100, 48–64. [Google Scholar] [CrossRef]
- Michel, J.M.; Godwin, J.S.; Plotkin, D.L.; Mesquita, P.H.C.; McIntosh, M.C.; Ruple, B.A.; Libardi, C.A.; Mobley, C.B.; Kavazis, A.N.; Roberts, M.D. Proteolytic markers associated with a gain and loss of leg muscle mass with resistance training followed by high-intensity interval training. Exp. Physiol. 2023, 108, 1268–1281. [Google Scholar] [CrossRef]
- Osburn, S.C.; Vann, C.G.; Church, D.D.; Ferrando, A.A.; Roberts, M.D. Proteasome- and Calpain-Mediated Proteolysis, but Not Autophagy, Is Required for Leucine-Induced Protein Synthesis in C2C12 Myotubes. Physiologia 2021, 1, 22–33. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Marworth, J.F.; Figueiredo, V.C.; Della Gatta, P.A.; Petersen, A.C.; Mitchell, C.J.; Cameron-Smith, D. Dose-dependent increases in p70S6K phosphorylation and intramuscular branched-chain amino acids in older men following resistance exercise and protein intake. Physiol. Rep. 2014, 2, e12112. [Google Scholar] [CrossRef]
- Mobley, C.B.; Fox, C.D.; Thompson, R.M.; Healy, J.C.; Santucci, V.; Kephart, W.C.; McCloskey, A.E.; Kim, M.; Pascoe, D.D.; Martin, J.S.; et al. Comparative effects of whey protein versus L-leucine on skeletal muscle protein synthesis and markers of ribosome biogenesis following resistance exercise. Amino Acids 2016, 48, 733–750. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Outlaw, J.J.; Mumford, P.W.; Urbina, S.L.; Hayward, S.; Roberts, M.D.; Taylor, L.W.; Foster, C.A. A Pilot Study Examining the Effects of 8-Week Whey Protein versus Whey Protein Plus Creatine Supplementation on Body Composition and Performance Variables in Resistance-Trained Women. Ann. Nutr. Metab. 2016, 69, 190–199. [Google Scholar] [CrossRef]
- Bemben, M.G.; Witten, M.S.; Carter, J.M.; Eliot, K.A.; Knehans, A.W.; Bemben, D.A. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J. Nutr. Health Aging 2010, 14, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Eliot, K.A.; Knehans, A.W.; Bemben, D.A.; Witten, M.S.; Carter, J.; Bemben, M.G. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J. Nutr. Health Aging 2008, 12, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Vierck, J.L.; Icenoggle, D.L.; Bucci, L.; Dodson, M.V. The effects of ergogenic compounds on myogenic satellite cells. Med. Sci. Sports Exerc. 2003, 35, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lin, D.; Huang, C. NMR-based metabolomic analysis for the effects of creatine supplementation on mouse myoblast cell line C2C12. Acta Biochim. Biophys. Sin. 2017, 49, 617–627. [Google Scholar] [CrossRef]
- Duan, Y.; Zhong, Y.; Song, B.; Zheng, C.; Xu, K.; Kong, X.; Li, F. Suppression of protein degradation by leucine requires its conversion to beta-hydroxy-beta-methyl butyrate in C2C12 myotubes. Aging 2019, 11, 11922–11936. [Google Scholar] [CrossRef]
- Areta, J.L.; Hawley, J.A.; Ye, J.M.; Chan, M.H.; Coffey, V.G. Increasing leucine concentration stimulates mechanistic target of rapamycin signaling and cell growth in C2C12 skeletal muscle cells. Nutr. Res. 2014, 34, 1000–1007. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef]
- Deldicque, L.; Sanchez Canedo, C.; Horman, S.; De Potter, I.; Bertrand, L.; Hue, L.; Francaux, M. Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids 2008, 35, 147–155. [Google Scholar] [CrossRef]
- Williamson, D.L.; Kimball, S.R.; Jefferson, L.S. Acute treatment with TNF-alpha attenuates insulin-stimulated protein synthesis in cultures of C2C12 myotubes through a MEK1-sensitive mechanism. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E95–E104. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Wu, S.H.; Chen, K.L.; Hsu, C.; Chen, H.C.; Chen, J.Y.; Yu, S.Y.; Shiu, Y.J. Creatine Supplementation for Muscle Growth: A Scoping Review of Randomized Clinical Trials from 2012 to 2021. Nutrients 2022, 14, 1255. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jiao, H.; Zhao, J.; Wang, X.; Li, H.; Zhou, Y.; Lin, H. Research Note: Creatine monohydrate alleviates protein breakdown induced by corticosterone via inhibiting ubiquitin proteasome pathway in chicken myotubes. Poult. Sci. 2022, 101, 102177. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Barbieri, E.; Martinelli, C.; Battistelli, M.; Guescini, M.; Vallorani, L.; Casadei, L.; D’Emilio, A.; Falcieri, E.; Piccoli, G.; et al. Creatine supplementation prevents the inhibition of myogenic differentiation in oxidatively injured C2C12 murine myoblasts. Mol. Nutr. Food Res. 2009, 53, 1187–1204. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontos, N.J.; Godwin, J.S.; Agyin-Birikorang, A.; Candow, D.G.; Lockwood, C.M.; Roberts, M.D.; Mobley, C.B. The Effects of Creatine Monohydrate and/or Whey Protein on the Muscle Protein Synthesis and Anabolic Signaling Responses in Non-Stressed C2C12 Murine Myotubes. Physiologia 2025, 5, 17. https://doi.org/10.3390/physiologia5020017
Kontos NJ, Godwin JS, Agyin-Birikorang A, Candow DG, Lockwood CM, Roberts MD, Mobley CB. The Effects of Creatine Monohydrate and/or Whey Protein on the Muscle Protein Synthesis and Anabolic Signaling Responses in Non-Stressed C2C12 Murine Myotubes. Physiologia. 2025; 5(2):17. https://doi.org/10.3390/physiologia5020017
Chicago/Turabian StyleKontos, Nicholas J., Joshua S. Godwin, Anthony Agyin-Birikorang, Darren G. Candow, Christopher M. Lockwood, Michael D. Roberts, and Christopher B. Mobley. 2025. "The Effects of Creatine Monohydrate and/or Whey Protein on the Muscle Protein Synthesis and Anabolic Signaling Responses in Non-Stressed C2C12 Murine Myotubes" Physiologia 5, no. 2: 17. https://doi.org/10.3390/physiologia5020017
APA StyleKontos, N. J., Godwin, J. S., Agyin-Birikorang, A., Candow, D. G., Lockwood, C. M., Roberts, M. D., & Mobley, C. B. (2025). The Effects of Creatine Monohydrate and/or Whey Protein on the Muscle Protein Synthesis and Anabolic Signaling Responses in Non-Stressed C2C12 Murine Myotubes. Physiologia, 5(2), 17. https://doi.org/10.3390/physiologia5020017







