Abstract
Background/Objectives: Volleyball training camps are known to reduce salivary secretory immunoglobulin A (s-SIgA); however, when it begins to decrease is unclear. The validity of a simple device for quantifying s-SIgA is lacking; hence, this study aimed to observe detailed s-SIgA changes during a volleyball training camp after moving to a high altitude and to investigate the difference in s-SIgA response between the two quantification methods, namely, the enzyme-linked immunosorbent assay (ELISA) and lateral flow device (LFD). Methods: Twenty-four male university volleyball players participated in the observational study. Measurements were collected at three points of the training camp (days 1, 4, and 7). The s-SIgA was quantified using conventional ELISA and the new LFD method. Results: The s-SIgA concentrations quantified using the two methods decreased significantly by day 4 (p < 0.05) and continued to decrease until day 7 (p < 0.05). A significant positive correlation was found between the s-SIgA concentrations quantified using the LFD and ELISA (p < 0.05, rs = 0.319). Conclusions: These results indicate that a high-altitude volleyball training camp may suppress oral immune function by day 4 and that the evaluation of s-SIgA concentration using the LFD method is beneficial. A faster and easier method for assessing s-SIgA could contribute to athletes’ condition management strategies.
1. Introduction
Athletes work hard to improve their performance. To perform well in games, it is necessary to pay more attention to daily physical condition management. Infections can result in poor performance and can be transferred to other players and staff on the team. Thus, it is important to maintain the immune function in conditioning athletes. Individuals who perform high-intensity exercise, such as athletes, are at an increased risk of developing upper respiratory tract infections (URTIs) [1].
Salivary secretory immunoglobulin A (s-SIgA) is a type of immune antibody that prevents the invasion of mucous membranes by specifically binding to pathogens that have invaded the body [2]. The s-SIgA plays an important role in the body’s first-line defense [3]. Decreased s-SIgA is reported to be associated with the development of URTIs [4]. High training loads in various sports are shown to cause a decrease in athletes’ s-SIgA [5,6,7]. Thus, it is effective to monitor s-SIgA over time to prevent URTIs.
Sports and training at high-altitude are known to increase the incidence of infection [8]. Additionally, the occurrence of URTI symptoms has been reported between 8 and 14 days after movement to high-altitude [9]. One possible mechanism is the involvement of glutamine. It has been reported that the rate of glutamine synthesis decreases chronically during high-altitude stays, and it has been pointed out that that may lead to bacterial translocation and weakened immune status [10]. Thus, moving to high altitudes may reduce immune function. A previous study of volleyball players investigating the effect of high-altitude training camps on s-SIgA responses demonstrated that the s-SIgA secretion rate and saliva flow rates decreased after the training camps [11]. The s-SIgA secretion rate was corrected by multiplying the s-SIgA concentration by the saliva flow rate. Another previous study reported that a high-altitude training camp reduced the saliva secretion rate, which indicates the saliva flow rate [12]. Only measurements after the volleyball training camp were conducted at the campsite’s high altitudes [11]. Therefore, it is unclear whether the decrease in s-SIgA secretion rates of volleyball players after the training camp was due to training or staying at a high altitude. Additionally, variations in s-SIgA during training camp periods shorter than the 8–14 days when URTI symptoms have been reported in previous studies are unclear.
There has also been an issue with the method of quantifying s-SIgA. s-SIgA quantification by the enzyme-linked immunosorbent assay (ELISA), as reported in many studies, takes about a day and a half to obtain results [13,14]; however, prompt feedback is required in an athlete’s condition management. By using the lateral flow device (LFD)—a novel method for quantifying s-SIgA concentration—it has become possible to obtain the analysis results earlier (within 30 min after collecting saliva) [15,16]. However, the difference in s-SIgA concentrations between the conventional ELISA method and the novel LFD method has not been well understood.
Therefore, the present study aimed to set a baseline after moving to a high-altitude training camp and aimed to observe the effect of continuing volleyball training on changes in s-SIgA quantified using two methods (ELISA and LFD). This study hypothesized that the s-SIgA, which was the baseline value after moving to the high altitude, would show a decline during the training camp period. It was also hypothesized that the s-SIgA measured by the two quantitative methods would have the same pattern of change trends.
2. Results
Table 1 summarizes the changes in the subjective scales, physical strength, and saliva components from days 1 to 7. Based on the subjective scales, no significant changes were observed throughout the training camp, except for physical stress, upper-body incompatibility, and appetite. Physical stress and upper-body incompatibility significantly decreased on day 4 compared to day 1, indicating that athletes condition worsened. Appetite significantly decreased on days 4 and 7 compared to day 1, indicating that appetite continued to decline until late into the training camp. The vertical jump did not change significantly, but the handgrip strength significantly decreased on days 4 and 7 compared to day 1. Comparing the saliva components on day 1, the saliva flow rate increased significantly on day 4, and the s-SIgA secretion rates decreased significantly on day 7.

Table 1.
Changes in measurements during the high-altitude volleyball training camp.
Figure 1 shows the changes in s-SIgA concentrations quantified using the ELISA and LFD. Both the ELISA and LFD s-SIgA concentrations were significantly lower on days 4 and 7 than on day 1.
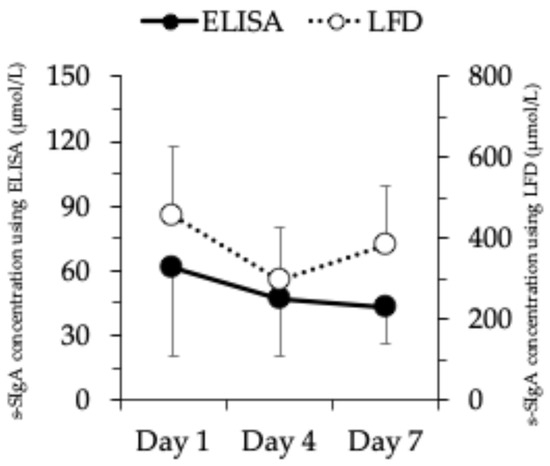
Figure 1.
Changes in s-SIgA concentrations were quantified using ELISA and LFD. Values are represented as mean ± standard deviation. s-SIgA, salivary secretory immunoglobulin A; ELISA, enzyme-linked immunosorbent assay; LFD, lateral flow device.
Figure 2 shows the results of the univariate correlation between the s-SIgA concentrations determined by ELISA and s-SIgA concentrations determined by the LFD and saliva flow rate. The s-SIgA determined using the ELISA was positively correlated with s-SIgA by the LFD (rs = 0.319, p < 0.01) (Figure 2A) but was negatively correlated with the saliva flow rate (rs = −0.445, p < 0.01) (Figure 2B).
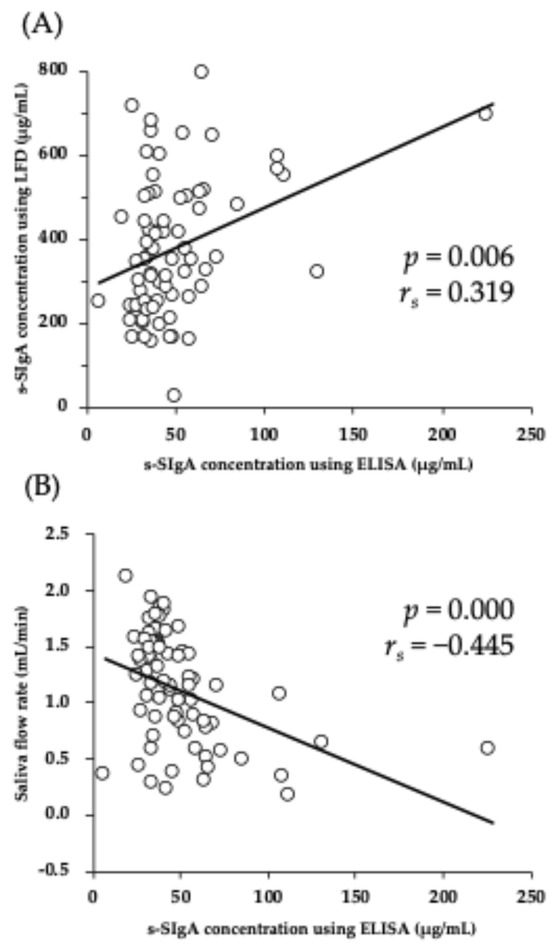
Figure 2.
Univariate correlations between s-SIgA concentration determined via ELISA and s-SIgA concentration determined using (A) LFD and (B) saliva flow rate. ELISA: enzyme-linked immunosorbent assay; LFD: lateral flow device; s-SIgA: salivary secretory immunoglobulin A.
Figure 3 shows the results of the Bland–Altman plot. The average difference in s-SIgA concentrations between the ELISA and LFD method was −327 µg/mL, and the limit of agreement ranged from −630 to −23 µg/mL. The 95% confidence interval ranged from −363 to −290 µg/mL, with a fixed error (p < 0.01) and proportional error (p < 0.01) in s-SIgA concentrations determined by the ELISA and LFD.
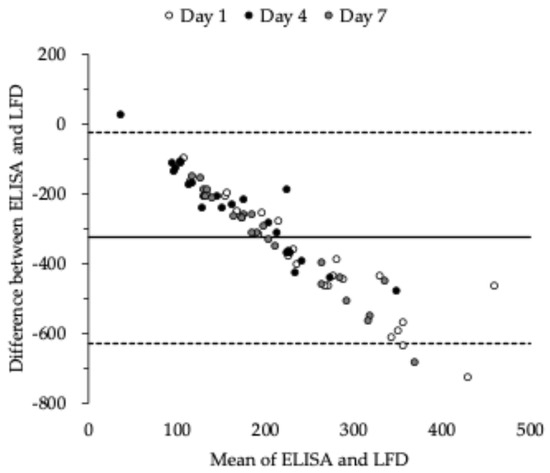
Figure 3.
Bland–Altman plot for s-SIgA using ELISA and LFD. ELISA: enzyme-linked immunosorbent assay; LFD: lateral flow device; s-SIgA: salivary secretory immunoglobulin A.
3. Discussion
In this study, we observed and examined the changes in s-SIgA concentrations during a training camp for volleyball players after moving to a high altitude by quantifying them using the ELISA and LFD. The main findings of this study were as follows. First, s-SIgA concentrations decreased during the volleyball training camp, even after moving to a high altitude. Second, s-SIgA concentrations quantified by the ELISA and LFD showed similar patterns of change. These results suggest that s-SIgA is affected by volleyball training regardless of the altitude and that s-SIgA evaluation using the LFD method can be useful in managing the physical condition of athletes.
A previous study has shown that s-SIgA decreased after an 11-day volleyball training camp, suggesting that this change may be due to a decrease in the saliva flow rate [11]. This is because the altitude before and after the training camp differed by nearly 1000 m. Previous studies have reported that high-altitude training decreases the saliva flow rate and may activate brain natriuretic peptides [12,17]; hence, moving to a high altitude may lead to a decrease in the saliva flow rate. Therefore, it has become difficult to understand whether the decrease in s-SIgA secretion rate after the training camp in the previous study was due to moving to a high-altitude location or the effect of volleyball training. The results of the present study showed that s-SIgA concentration decreased even after moving to a high altitude, indicating that volleyball training affected the s-SIgA response. Figure 1 shows that the s-SIgA concentration, quantified by either the ELISA or LFD, decreased on day 4 and remained low until day 7. In volleyball training camps, it is necessary to proactively rest from the beginning after moving to the training camp because oral immune function declines by day 4.
One of the novelties of this study is that s-SIgA was evaluated longitudinally using two methods. It was demonstrated that s-SIgA quantified by the ELISA and LFD during the training camp showed a similar pattern of change (Figure 1). In addition, there was a positive correlation observed between the s-SIgA concentrations quantified using each method (Figure 2). These findings support those of a previous study that found a strong positive correlation between the s-SIgA concentrations in the two quantification methods [18]. Thus, these results indicate that s-SIgA can be quantified with high accuracy using the LFD method in situations where a higher physical load is applied, such as in a training camp conducted by an athlete. However, there are important points to note regarding the interpretation of s-SIgA levels quantified using the LFD method, in particular, regarding the saliva flow rate (saliva volume). In many studies, the s-SIgA secretion rate calculated from the product of the s-SIgA concentration and saliva flow rate has been used to evaluate s-SIgA [12,19,20,21]. This is because even if the s-SIgA concentration shows an apparently high value due to condensation, the absolute amount of s-SIgA may not necessarily increase when corrected by the amount of saliva per unit of time. In this study, a significant negative correlation was also observed between the s-SIgA concentration measured by the ELISA and the saliva flow rate (Figure 2). Therefore, it is appropriate to evaluate the s-SIgA secretion rate, especially when quantified by the ELISA. However, in this study, the saliva flow rate could not be evaluated when using the LFD method; hence, the relationship between the saliva flow rate and s-SIgA concentration using the LFD method should be examined in future studies.
This study has several limitations. First, the mechanisms by which the pattern changes in the subjective and objective scales differed during the training camp are unclear. Objective scale s-SIgA concentration and handgrip strength decreased on day 4 and remained low on day 7. However, the subjective scale, fatigue, and physical stress using the VAS worsened on day 4 but not on day 7. On day 7, the athlete’s mood towards the end of the training camp may have positively affected the subjective scale. In other words, immune function may be weakened without the athlete’s knowledge. Therefore, the relationship between psychological status and subjective scales, including psychological investigations, should be examined more carefully in the future. Second, the Bland–Altman test confirmed that there was a fixed error and a proportional error. This indicates that s-SIgA quantified by the ELISA tends to have a lower value and that the difference between the two methods increases as the measured value increases. Hence, it should be considered that the s-SIgA quantified by the LFD method may be overinterpreted.
In this study, s-SIgA levels decreased by day 4 after the start of the volleyball camp. This indicates that oral immune function was suppressed from day 4 of intensive training. The persistent suppression of immune function weakens athletes’ performance. Volleyball players, coaches, and support staff should be aware of a more positive recovery during training camps in which higher-intensity exercises are continued compared to normal training periods. To assess s-SIgA without standard values, it is important to observe changes within an individual. It would be effective to monitor changes in s-SIgA for each individual using the LFD method to evaluate the status of oral immune function in athletes more easily. Appropriate evaluation using objective indicators will contribute to the implementation of effective training.
4. Materials and Methods
4.1. Ethics Approval
This study was approved by the Institutional Review Board of our University (Issue Number: 019-H080). The study was conducted in accordance with the principles outlined in the Declaration of Helsinki, and all participants provided written informed consent before participating.
4.2. Experimental Approach
To investigate s-SIgA fluctuations during high-altitude (approximately 1000 m) volleyball training camps, we monitored 24 male collegiate volleyball players during their summer training camp. The target team is a top-level team from a Japanese university that made it to the finals at the national competition of university teams. Observations were made at three different points of the training camp (days 1, 4, and 7). Table 2 shows the training schedule for the volleyball camp. Technical training included practicing basic volleyball techniques such as receiving, serving, spiking, tossing, and blocking. Tactical training includes practicing a series of offence patterns, from receiving and tossing to spiking. Match training is a type of practice that simulates a match. s-SIgA concentrations, physical strength, and subjective fatigue were assessed. To evaluate athletes’ physical condition, saliva samples were collected. Participant samples included biological markers whose collection satisfied the following criteria: (1) no medical insurance was required, (2) sampling procedure was non-invasive and did not cause pain, and (3) continuous collection was possible. Two methods (ELISA and LFD) were used for saliva collection to quantify s-SIgA concentrations.

Table 2.
Training schedule during the high-altitude volleyball training camp.
4.3. Participants
Twenty-four male volleyball players participated in this study (mean age, 19.7 ± 1.3 years; mean height, 183.7 ± 9.2 cm; mean weight, 78.2 ± 10.9 kg; mean volleyball experience, 10.5 ± 2.7 years). None of the participants had any diseases or injuries, and none were smokers. The target team in this study is elite-level university students who are highly ranked in inter-collegiate championships in the target country. Since the study period was during a long university vacation, training sessions per week ranged from 6 to 12.
4.4. Saliva Sampling and Analysis
Considering diurnal changes, saliva samples using the two methods (ELISA and LFD) were collected at the same time (7:00 a.m.) at all measurement points. Saliva samples for ELISA were collected with chewing based on a previous study [13]. Saliva samples for LFD were collected without masticatory stimuli based on a previous study [22]. The Oral Fluid Collector (OFC; IPRO Interactive, Oxfordshire, UK) was used for saliva collection for LFD. The OFC has a volume indicator that changes color from white to dark blue when it contains 0.5 mL of saliva. The s-SIgA concentration was analyzed using a real-time LFD according to the manufacturer’s guidelines (IPRO Interactive, Oxfordshire, UK). The analysis of s-SIgA concentration using the LFD method was performed within 1 h of saliva collection. Considering the effect of masticatory stimulation, saliva for LFD was collected first. The s-SIgA secretion rate was calculated as follows: s-SIgA secretion rate (µg/min) = saliva flow rate (mL/min) * s-SIgA concentration (µg/mL) using ELISA.
4.5. Physical Strength
As an indicator of muscular strength, handgrip strength was measured using a Takei dynamometer (T.K.K. 5401; Takei Kiki Kogyo, Niigata, Japan) and the vertical jump using a Takei jump meter (T.K.K. 5406; Takei Kiki Kogyo, Niigata, Japan). Vertical jump was measured by attaching a measuring device to the waist and allowing arm swing. These parameters were measured twice, and the one with better records was used as data.
4.6. Subjective Evaluation of Questionnaire
All athletes completed a questionnaire regarding their psychophysiological status and performance. The questionnaire was administered on a subjective scale using a visual analog scale (VAS). The contents of the questionnaire included total condition, fatigue, psychological stress, physical stress, upper-body incompatibility, lower-body incompatibility, performance from the previous day, and appetite. Athletes wrote down where their feelings fit on the 100 mm line between the best and the worst for each item. A high value indicates good condition, and a low value indicates poor condition.
4.7. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics software (version 24.0; IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was used to evaluate the normality of distributions. Time-dependent changes in the measurements were assessed using repeated-measures analysis of variance (ANOVA). Dunnett’s post hoc test was used when a significant difference was obtained from ANOVA. The relationships among ELISA s-SIgA concentration, saliva flow rate, and LFD s-SIgA concentration were assessed using Spearman’s rank correlation coefficients (rs), since there was a non-normally distributed variable. The Bland–Altman test [23] was performed to confirm the validity of the ELISA and LFD methods for quantifying s-SIgA concentration. Statistical significance was set at p < 0.05.
5. Conclusions
The high-altitude volleyball training camp significantly reduced s-SIgA by day 4 and persisted until day 7. This result suggests that volleyball training rather than a higher altitude location may affect the s-SIgA concentration. Additionally, s-SIgA levels quantified using the ELISA and LFD showed similar patterns of change. This suggests that the LFD method is an effective quantitative method. However, the following points must be noted when using the LFD method: 1. Difficulty quantifying saliva flow rate; 2. The quantitative value is high; 3. The measurement error increases as the quantitative value is high.
Author Contributions
Conceptualization, R.S. and K.O.; Formal Analysis, R.S.; Investigation, R.S., K.Y., H.G. and K.O.; Methodology, H.G.; Supervision, K.Y., S.T. and K.O.; Writing—Original Draft, R.S.; Writing—Review and Editing, S.T. and K.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of our University (Issue Number: 019-H080). The study was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Informed Consent Statement
All participants provided written informed consent before participating.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank all volleyball players and team staff involved in this study; the staff for helping us with the measurements and analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nieman, D.C. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sports Exerc. 1994, 26, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Lamm, M.E. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 1997, 51, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Mazanec, M.B.; Nedrud, J.G.; Kaetzel, C.S.; Lamm, M.E. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 1993, 14, 430–435. [Google Scholar] [CrossRef]
- Neville, V.; Gleeson, M.; Folland, J.P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med. Sci. Sports Exerc. 2008, 40, 1228–1236. [Google Scholar] [CrossRef]
- Fahlman, M.M.; Engels, H.J. Mucosal IgA and URTI in American college football players: A year longitudinal study. Med. Sci. Sports Exerc. 2005, 37, 374–380. [Google Scholar] [CrossRef]
- Moreira, A.; De Moura, N.R.; Coutts, A.; Costa, E.C.; Kempton, T.; Aoki, M.S. Monitoring internal training load and mucosal immune responses in futsal athletes. J. Strength Cond. Res. 2013, 27, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Novas, A.M.; Rowbottom, D.G.; Jenkins, D.G. Tennis, Incidence of URTI and salivary IgA. Int. J. Sports Med. 2003, 24, 223–229. [Google Scholar] [CrossRef]
- Demel, U.; Domej, W.; Tilz, G.P. Function and repertoire of the immune system in body’s defense. Wien. Med. Wochenschr. 2000, 150, 175–177. [Google Scholar] [PubMed]
- Bailey, D.M.; Davies, B.; Castell, L.M.; Collier, D.J.; Milledge, J.S.; Hulin, D.A.; Seddon, P.S.; Young, L.S. Symptoms of infection and acute mountain sickness; associated metabolic sequelae and problems in differential diagnosis. High Alt. Med. Biol. 2003, 4, 319–331. [Google Scholar] [CrossRef]
- Wagenmakers, A.J. Amino acid metabolism, muscular fatigue and muscle wasting. Speculations on adaptations at high altitude. Int. J. Sports Med. 1992, 13, S110–S113. [Google Scholar] [CrossRef] [PubMed]
- Sone, R.; Yamamoto, K.; Ohishi, K. Effect of pre-season training camp on oral immune functions in elite collegiate volleyball players. J. Phys. Fit. Sports Med. 2021, 10, 39–44. [Google Scholar] [CrossRef]
- Watanabe, K.; Jesmin, S.; Murase, Y.; Takeda, T.; Shiraki, T.; Sengoku, Y. Effects of Repetitive Altitude Training on Salivary Immunoglobulin A Secretion in Collegiate Swimmers. J. Clin. Med. Res. 2019, 11, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Kumai, Y.; Akama, T.; Hayashi, E.; Murakami, H.; Soma, R.; Kuno, S.; Kono, I. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br. J. Sports Med. 2003, 37, 76–79. [Google Scholar] [CrossRef]
- Murase, Y.; Shimizu, K.; Tanimura, Y.; Hanaoka, Y.; Watanabe, K.; Kono, I.; Miyakawa, S. Salivary extracellular heat shock protein 70 (eHSP70) levels increase after 59 min of intense exercise and correlate with resting salivary secretory immunoglobulin A (SIgA) levels at rest. Cell Stress Chaperones 2016, 21, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Coad, S.; Gray, B.; Wehbe, G.; McLellan, C. Physical demands and salivary immunoglobulin A responses of elite Australian rules football athletes to match play. Int. J. Sports Physiol. Perform. 2015, 10, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.L.; Wong, D.P.; Dunlop, G.; Groussard, C.; Kebsi, W.; Dellal, A.; Morgans, R.; Zouhal, H. High-intensity training and salivary immunoglobulin A responses in professional top-level soccer players: Effect of training intensity. J. Strength Cond. Res. 2016, 30, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Jesmin, S.; Takeda, T.; Shiraki, T. Influence of Altitude Training on Brain Natriuretic Peptide and Atrial Natriuretic Peptide in Japanese Collegiate Swimmers. Int. J. Sport Exerc. Health Res. 2019, 3, 14–18. [Google Scholar] [CrossRef]
- Coad, S.; Mclellan, C.; Whitehouse, T.; Gray, B. Validity and reliability of a novel salivary immunoassay for individual profiling in applied sports science. Res. Sports Med. 2015, 23, 140–150. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Mortatti, A.L.; Arruda, A.F.S.; Freitas, C.G.; de Arruda, M.; Aoki, M.S. Salivary IgA response and upper respiratory tract infection symptoms during a 21-week competitive season in young soccer players. J. Strength Cond. Res. 2014, 28, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Sato, H.; Suga, Y.; Yamahira, S.; Toba, M.; Hamuro, K.; Kakumoto, K.; Kohda, N.; Akama, T.; Kono, I.; et al. The effects of Lactobacillus pentosus strain b240 and appropriate physical training on salivary secretory IgA levels in elderly adults with low physical fitness: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2014, 54, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Morgans, R.; Owen, A.; Doran, D.; Drust, B.; Morton, J.P. Prematch salivary secretory immunoglobulin A in soccer players from the 2014 World Cup qualifying campaign. Int. J. Sports Physiol. Perform. 2015, 10, 401–403. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).