Impact of High-Intensity Exercise on BDNF Levels and Its Implications in High-Performance Sport: A Systematic Review
Abstract
:1. Introduction
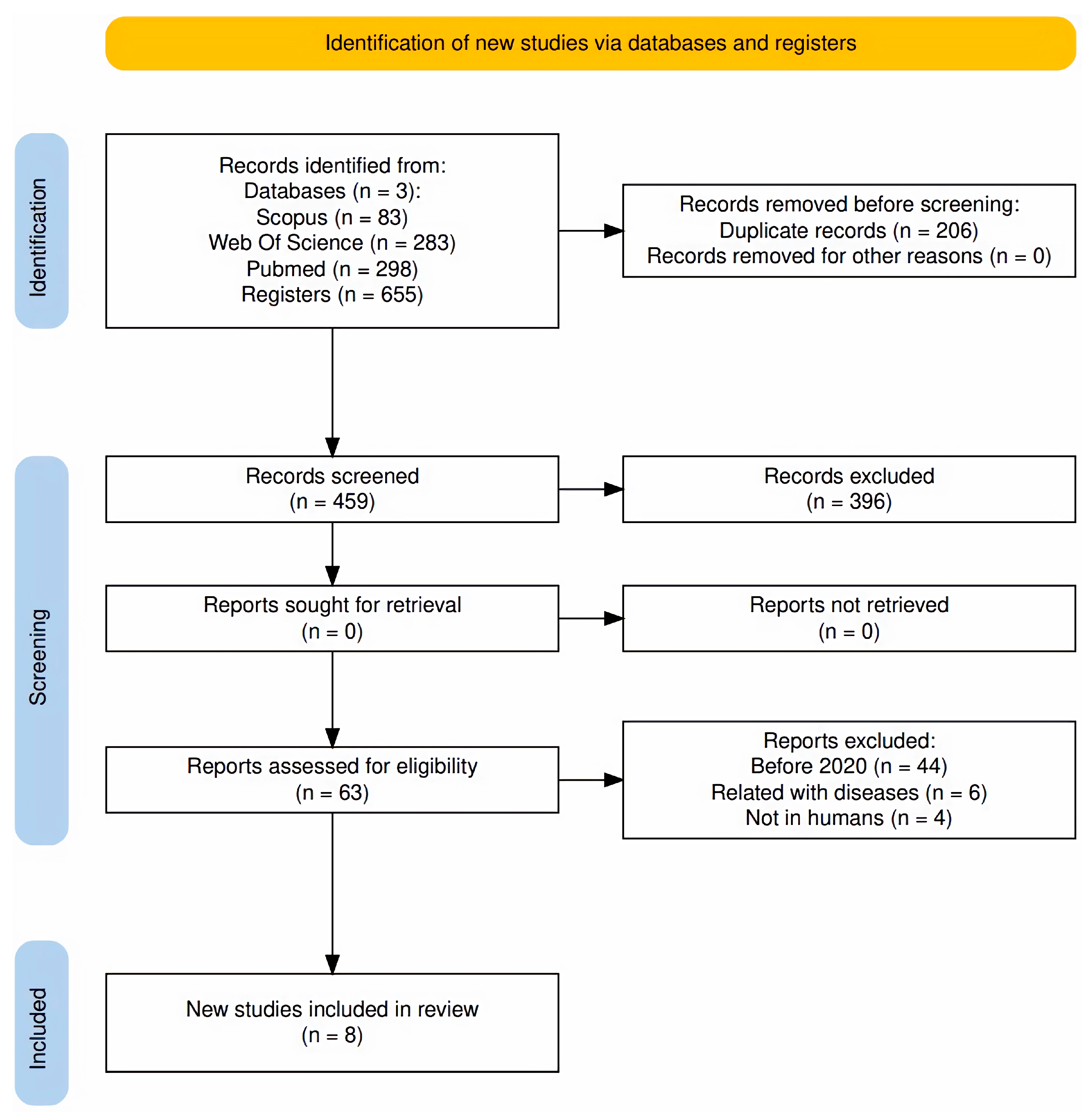
2. Study Determination
3. Results
3.1. Introduction to Analysis of Studies
3.2. Comparative Analysis and Key Results
3.3. Lactate and Physiological Stress
4. Discussion
4.1. Exercise, BDNF, and Cognitive Development
4.2. Influence of Cognitive Development on High-Performance Sports
4.3. The Systemic Effects of BDNF Beyond the Nervous System
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, C.B.; Sallis, J.F.; Needle, R. The relation of physical activity and exercise to mental health. Public Health Rep. 1985, 100, 195. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1424736/ (accessed on 30 October 2024).
- Sara, V.R.; Stuart, M.C.; Rutherford, R.; Moore, S.; Lazarus, L. Brain growth-promoting activity in human serum: Relationship to growth hormone and somatomedin. J. Clin. Endocrinol. Metab. 1978, 47, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Resumen de Plasticidad Sináptica, BDNF y Ejercicio Físico—Dialnet. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5456613&info=resumen&idioma=SPA (accessed on 30 October 2024).
- Walsh, J.J.; Tschakovsky, M.E. Exercise and circulating BDNF: Mechanisms of release and implications for the design of exercise interventions. Appl. Physiol. Nutr. Metab. 2018, 43, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Hoekstra, S.P.; Kamijo, Y.-I.; Goosey-Tolfrey, V.L.; Walsh, J.J.; Tajima F., F.; Leicht, C.A. Serum and plasma brain-derived neurotrophic factor concentration are elevated by systemic but not local passive heating. PLoS ONE 2021, 16, e0260775. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Parada-Sánchez, S.G.; Macias-Cervantes, M.H.; Pérezvázquez, V.; Vargas-Ortiz, K. The Effects of Different Types of Exercise on Circulating Irisin Levels in Healthy Individuals and in People With Overweight, Metabolic Syndrome and Type 2 Diabetes. Physiol. Res. 2022, 71, 457–475. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Bosch, B.M.; Bringard, A.; Logrieco, M.G.; Lauer, E.; Imobersteg, N.; Thomas, A.; Ferretti, G.; Schwartz, S.; Igloi, K. A single session of moderate intensity exercise influences memory, endocannabinoids and brain derived neurotrophic factor levels in men. Sci. Rep. 2021, 11, 14371. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Z.; Wang, T.; Tian, L.; Wang, M.; Liu, R.; Zuo, C.; Jihua, W. Acute effects of two different work-to-rest ratio of high-intensity interval training on brain-derived neurotrophic factor in untrained young men. Front. Physiol. 2022, 13, 988773. [Google Scholar] [CrossRef] [PubMed]
- Rentería, I.; García-Suárez, P.C.; Martínez-Corona, D.O.; Moncada-Jiménez, J.; Plaisance, E.P.; JiméNez-Maldonado, A. Short-term high-Intensity interval training increases systemic brain-derived neurotrophic factor (BDNF) in healthy women. Eur. J. Sport Sci. 2020, 20, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Reycraft, J.T.; Islam, H.; Townsend, L.K.; Hayward, G.C.; Hazell, T.O.M.J.; MacPherson, R.E.K. Exercise Intensity and Recovery on Circulating Brain-derived Neurotrophic Factor. Med. Sci. Sports Exerc. 2020, 52, 1210–1217. [Google Scholar] [CrossRef]
- García-Suárez, P.C.; Rentería, I.; Moncada-Jiménez, J.; Fry, A.C.; Jiménez-Maldonado, A. Acute Systemic Response Of BDNF, Lactate and Cortisol to Strenuous Exercise Modalities in Healthy Untrained Women. Dose Response 2020, 18, 1559325820970818. [Google Scholar] [CrossRef]
- Lodo, L.; Moreira, A.; Bacurau, R.F.P.; Capitani, C.D.; Barbosa, W.P.; Massa, M.; Schoenfeld, B.J.; Aoki, M.S. Resistance Exercise Intensity Does Not Influence Neurotrophic Factors Response in Equated Volume Schemes. J. Hum. Kinet. 2020, 74, 227–236. [Google Scholar] [CrossRef]
- Ospina, B.M.; Cadavid-Ruiz, N. The effect of aerobic exercise on serum brain-derived neurotrophic factor (BDNF) and executive function in college students. Ment. Health Phys. Act. 2024, 26, 100578. [Google Scholar] [CrossRef]
- Banerjee, M.; Shenoy, R.R. Emphasizing roles of BDNF promoters and inducers in Alzheimer’s disease for improving impaired cognition and memory. J. Basic Clin. Physiol. Pharmacol. 2021, 34, 125–136. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and memory processing. Neuropharmacology 2014, 76 Pt C, 677–683. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Cohen, L.G. The Olympic brain. Does corticospinal plasticity play a role in acquisition of skills required for high-performance sports? J. Physiol. 2008, 586, 65–70. [Google Scholar] [CrossRef]
- Duru, A.D.; Balcioglu, T.H. Functional and Structural Plasticity of Brain in Elite Karate Athletes. J. Healthcare Eng. 2018, 2018, 8310975. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça Filho, E.J.; Barth, B.; Bandeira, D.R.; de Lima, R.M.S.; Arcego, D.M.; Dalmaz, C.; Pokhvisneva, I.; Sassi, R.B.; Hall, G.B.; Meaney, M.J.; et al. Cognitive Development and Brain Gray Matter Susceptibility to Prenatal Adversities: Moderation by the Prefrontal Cortex Brain-Derived Neurotrophic Factor Gene Co-expression Network. Front. Neurosci. 2021, 15, 744743. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, Y.; Huang, R.; Li, L.; Xia, F.; Zou, L.; Yu, Q.; Lin, J.; Herold, F.; Perrey, S.; et al. Structural and functional brain signatures of endurance runners. Brain Struct. Funct. 2021, 226, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, Z.; Jiang, X.; Chen, M.B.; Dong, H.; Liu, J.; Südhof, T.C.; Quake, S.R. Spatial transcriptomics reveal neuron-astrocyte synergy in long-term memory. Nature 2024, 627, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Lappi, O. Egocentric Chunking in the Predictive Brain: A Cognitive Basis of Expert Performance in High-Speed Sports. Front. Hum. Neurosci. 2022, 16, 822887. [Google Scholar] [CrossRef]
- Matthews, V.B.; Åström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef]
- Delezie, J.; Weihrauch, M.; Maier, G.; Tejero, R.; Ham, D.J.; Gill, J.F.; Karrer-Cardel, B.; Rüegg, M.A.; Tabares, L.; Handschin, C. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 16111–16120. [Google Scholar] [CrossRef]
- Ahuja, P.; Ng, C.F.; Pang, B.P.S.; Chan, W.S.; Tse, M.C.L.; Bi, X.; Kwan, H.-L.R.; Brobst, D.; Herlea-Pana, O.; Yang, X.; et al. Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy 2022, 18, 1367–1384. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Trombetta, I.C.; Demoura, J.R.; Alves, C.R.; Carbonari-Brito, R.; Cepeda, F.X.; Lemos, J.R. Serum Levels of BDNF in Cardiovascular Protection and in Response to Exercise. Arq. Bras. Cardiol. 2020, 115, 263–269. [Google Scholar] [CrossRef]
| Inclusion and Exclusion Criteria | |
|---|---|
| Inclusion criteria | Exclusion criteria |
|
|
| Description of the Included Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Study Design | Population Gender | Population Status | No. Population | Population Age | Type of Exercise | Duration | Exercise Intensity | BDNF Increase Compared to Control |
| [12] | Cross-over | Male | Regular exercise | 20 | = 23.03 | Cycling | 30 min/15 min | Moderate/acute | Yes |
| [13] | Cross-over | Male | Untrained students | 12 | = 23.7 | Cycling/HIIT | 35 min per test | Acute | Yes |
| [14] | Parallel | Female | Young sedentary | 17 | N/A | HIIT/GTX | 3 sessions × 4 weeks 17.5 min (3 sessions) 22 min (3 sessions) 26.5 min (6 sessions) | Acute | Yes |
| [15] | Cross-over | Male | Active | 8 | = 23.1 | Running (MICT, VICT, and SIT) | 3 h per session | Moderate/acute | Yes |
| [16] | Cross-over | Male and female | 3 months resistance training | 30 (15 and 15) | = 22 | Bench press/squat | 4 × 10 (35% 1RM) 4 × 5 (70% 1RM) | Moderate/acute | Yes |
| [17] | Cross-over | Female | Athletes | 17 | = 20.0 | GXT/HIIT | 12 min per session | Acute | No |
| [18] | Parallel | Male and female | Athletes Regular fitness Sedentary | 20 (11 and 9) 19 (9 and 10) 23 (10 and 13) | = 20.2 | N/A | 30 min | Acute/moderate | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Infantes, F.; Díaz-Quesada, G.; Radesca-Fabiano, K.; Muñoz-Andradas, G.; Domínguez-Balmaseda, D. Impact of High-Intensity Exercise on BDNF Levels and Its Implications in High-Performance Sport: A Systematic Review. Physiologia 2024, 4, 414-423. https://doi.org/10.3390/physiologia4040027
Moreno-Infantes F, Díaz-Quesada G, Radesca-Fabiano K, Muñoz-Andradas G, Domínguez-Balmaseda D. Impact of High-Intensity Exercise on BDNF Levels and Its Implications in High-Performance Sport: A Systematic Review. Physiologia. 2024; 4(4):414-423. https://doi.org/10.3390/physiologia4040027
Chicago/Turabian StyleMoreno-Infantes, Fernando, Gema Díaz-Quesada, Krizia Radesca-Fabiano, Guilermo Muñoz-Andradas, and Diego Domínguez-Balmaseda. 2024. "Impact of High-Intensity Exercise on BDNF Levels and Its Implications in High-Performance Sport: A Systematic Review" Physiologia 4, no. 4: 414-423. https://doi.org/10.3390/physiologia4040027
APA StyleMoreno-Infantes, F., Díaz-Quesada, G., Radesca-Fabiano, K., Muñoz-Andradas, G., & Domínguez-Balmaseda, D. (2024). Impact of High-Intensity Exercise on BDNF Levels and Its Implications in High-Performance Sport: A Systematic Review. Physiologia, 4(4), 414-423. https://doi.org/10.3390/physiologia4040027








