Abstract
The objective of the present review was to evaluate the time-course of recovery of biochemical marker levels and physical performance after strenuous military training, and identify which biomarkers are affected. A systematic literature search was conducted using the databases MedLine (Ovid) and Web of Science (WoS) to identify studies until January 2023. Varying relevant search terms were used, related to military training, Special Forces, physical performance, and biomarkers. Records were based on strict inclusion and exclusion criteria. Twelve studies met the inclusion criteria and were selected for this review. A variety of physiological and psychological markers were measured, and military training lasted from 4 to 62 days, with recovery periods varying from 24 h to 6 weeks. Among these studies, full recovery was observed in two studies, while seven studies showed almost full (79–90%) recovery, and in three studies, 44–63% of markers recovered after the measured recovery period. However, in some studies, additional markers could be defined as recovered, depending on the criterion for recovery. In the majority of the studies, most of the measured variables recovered during the follow-up, but often, some variables remained unrecovered, and at times, only modest recovery was seen. It is important to point out that recovery duration depends on the duration and intensity of the military training stressor. Overall, resolution varies between the markers, and sometimes, recovery might not occur, even after prolonged recovery. Therefore, it is important to measure the recovery status of soldiers with both biomarkers and physical performance markers, especially after strenuous training, to maximize operational capability during prolonged missions.
1. Introduction
Soldiers experience several psychophysiological stressors during military training and operations. Operative duties such as load carriage, carrying and handling heavy loads, maneuvering in difficult environments, and casualty evacuation are physically strenuous tasks [1,2,3]. In addition, the psychological demands are also high, including the possibility of death and the possible long duration of operations influencing the ability to maintain vigilance [3].
The goal of military training is to train and prepare soldiers to be resilient to high loads of physical and mental stress that are prevalent in combat situations [4,5]. Therefore, soldiers are frequently exposed to strenuous field training, which simulates the demands of military operations. This training includes high levels of physical activity, often accompanied by sleep deprivation and calorie restriction. As a result, fatigue may accumulate concurrently with physiological impairments, leading to decreased performance. The management of fatigue and recovery can be disturbed by high operational tempo, as optimal recovery might not be achieved between and during operations [3].
Intense and long-lasting training can lead to altered recovery status such as functional (FOR) or non-functional (NFOR) overreaching states [6]. In FOR, the soldier trains hard and makes an effort to accomplish supercompensation, which can lead to performance gains after a recovery period. On the other hand, in NFOR and OTS, the soldier does not have proper recovery period after hard training, and physical performance is negatively affected. FOR recovery can take from days to weeks [6]. NFOR is often seen as a cause of imbalance between the amount and intensity of training together with insufficient recovery that may lead to attenuated physical performance or other maladaptive responses. NFOR recovery typically takes from weeks to months [6]. Ultimately, this imbalance can lead to overtraining syndrome (OTS), which is characterized by decreased performance for a long period of time, and recovery can take several months [7]. In the military, NFOR, FOR, and even OTS can be developed during training and operations [8].
Recovery is a multifaceted restorative process, occurring relative to time. If psychological or physical stressors disturb recovery, fatigue may occur [9]. Fatigue can be compensated with different recovery methods, which means that the organismic balance is returned [10]. There are several methods such as physical performance tests (e.g., jumps, strength tests, and aerobic and anaerobic tests) to measure recovery status in soldiers. Additionally, biochemical markers (e.g., testosterone and cortisol), as well as heart rate variability [11], could identify the recovery level of the actual physiological status [12]. The physiology of common stress biomarkers used in military studies with operational troops was recently documented by Beckner et al. [13]. Recovery is extensively studied in athletes, but this research is not directly applicable to all military populations as athletes optimize their sleep and food intake and quality, and use other recovery methods during their training periods [6]. Within an operational military context, the extreme conditions must be endured until the mission is complete, with limited possibility to individually optimize recovery during the operative stress. If recovery is not optimal between operations, performance may be impaired when starting the next operation [6,8]. Therefore, knowledge about the recovery time-course after exposure to severe military training induced stress is important.
The present systematic review aimed to synthesize data from studies measuring physiological biomarkers and physical performance recovery time among soldiers during and after stress induced by strenuous military training and operations. A better understanding of the physiological recovery state and performance of soldiers during and after operations may improve fatigue management.
2. Methods
2.1. Experimental Approach to the Problem
The present systematic review was conducted during the first quarter of 2023 in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [14]. The following electronic databases were used: Medline (Ovid) and Web of Science (WoS). No limits were placed on the age of the article, although articles were limited to those written in English. Boolean search was used with several military training-related words and word combinations (Figure 1).
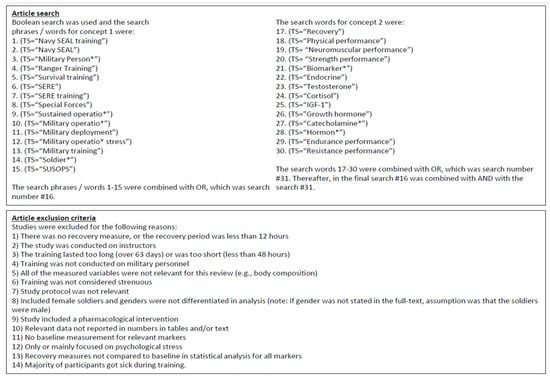
Figure 1.
Boolean search procedure and search phrases/words and article exclusion criteria.
2.2. Procedures
2.2.1. Article Screening/Study Selection
The screening of articles for potential relevance was first determined based on the title of the article, and second, on the abstract. Articles consisting of data from strenuous military training or operations/deployment and measuring metabolic, endocrinological, or physical performance factors before, during, and after strenuous military training or operations were included. Of the abstract-screened and included articles, full texts were obtained and read.
2.2.2. Quality Assessment
The quantitative quality assessment tool “QualSyst” was used when assessing the methodological quality of each selected study. It includes 14 questions, which are scored from 0 (criterion not met) to 1 (criterion met partially) and 2 (criterion met fully). Items not applicable to the study design were marked as “N/A” and were not included in the calculation of the overall score [15]. The outcome score was then divided by the total possible score. A study was considered of high quality if the score was 75% or higher, moderate quality if the score was between 55% and 75%, and weak quality if the score was lower than 55%. This assessment toolkit has also been used in previous systematic reviews in a military context and in a review about overtraining syndrome in soldiers [6]. Slight modifications were made to better suit the military context: Item 3 was shortened to only include “Method of subject selection?”, item 4 was shortened to “Subject characteristics sufficiently described?”, and item 8 was shortened to “Outcome measures well defined and robust to measurement/misclassification bias? Means of assessments reported?”.
3. Results
3.1. Study Selection
The literature search conducted on both databases yielded an overall number of 3681 results. Following abstract screening, 146 records were included for full-text review. Of these, 54 duplicates were removed. Thus, 92 reports were sought for retrieval (for 7 articles, the full text could not be retrieved). Therefore, 85 reports were assessed for eligibility in the full-text screening. Following the full-text screening, 12 studies were included in the review. Reasons for exclusion following the full-text screening are presented in the PRISMA flow diagram (Figure 2).
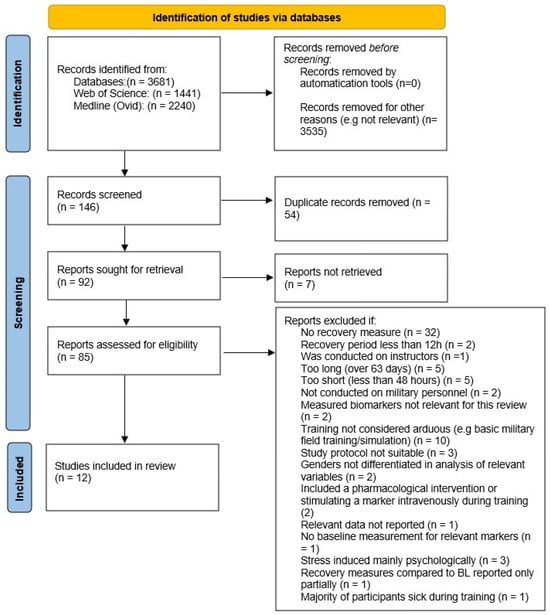
Figure 2.
PRISMA flow diagram.
3.2. Characteristics of Included Studies
All 12 studies included male participants (n = 7–43/study) with ages ranging from 18 to 35 years, with the most common mean age being 22–24 years. Three of these studies described the U.S. Army Ranger course, two described survival, evasion, resistance, and escape courses (SERE), and the rest described strenuous military training or selection courses. The duration of the studies ranged from 4 to 62 days, and all studies were both physically and mentally stressful due to continuous physical exertion, load carriage, sleep deprivation, and energy deficit. Participant details, description of training, and measurement outcomes of the included studies are presented in Table 1 and Table 2.
3.3. Measured Outcomes
The physical performance of the soldiers was measured in 5 studies, and biomarkers in 11 studies. The methodological measures of physical performance and biomarkers varied widely between the studies. Common physical performance assessments included different types of strength tests in five studies. Endurance performance (aerobic or anaerobic) was measured in two studies.
A variety of blood biomarkers consisting of hormones, muscle damage and inflammatory as well as oxidative stress markers (Table 2) were measured. A total of 61 different biomarkers were measured across the studies. The most commonly measured biomarkers were basic stress-related outcome markers, such as testosterone (n = 8) and cortisol (n = 6).
3.4. Recovery Assessments
The recovery status of soldiers was assessed for no longer than 2 weeks in 8 studies, and in 4 studies, recovery was followed for 2–6 weeks. The majority of studies included multiple recovery measurements over different time points. One study assessed recovery for all parameters only after 24 h [16]. For two studies, recovery was assessed at only the time point of 30 [17] or 35 days [18]. In two studies, the post-measurements were not performed directly after the course but at 8 h [19] and 2 weeks [20] after the end of the course. Two other studies measured adverse changes during and after the course [16,21]. The remaining studies performed the post-measurements immediately after the course.

Table 1.
Participant details and description of training.
Table 1.
Participant details and description of training.
| Study | Participants | Type of Training | Description of Training | Energy Expenditure or Deficit/Amount of Food Provided | Sleep |
|---|---|---|---|---|---|
| Mourtakos et al., 2021 [17] | n = 14, age 22.7 ±1.7 yr, male Greek Special Forces volunteers | “Hell Week” of Basic Underwater Demolition(s) (BUD/S) of the Hellenic Navy SEALs | 5-day “Hell Week” of the 32 week “brutal” BUD/s schedule. During “Hell Week”, candidates participate in training course characterized by extreme mental and physical fatigue, e.g., walking 300 km and performing physical training for more than 20 h per day in harsh conditions. | Not reported | No sleep at all during the entire week |
| Conkright et al., 2020 [20] | n = 10, age 24.0 ± 5.0, active-duty male U.S. Army 75th Ranger Regiment soldiers | Ranger course | 62-day length, one of the military’s most challenging courses. Training small unit tactics and leadership under conditions of severe stress created by sleep and caloric restriction, physical exertion, and graded evaluations. Approx. 20 h of training per day, 7 days a week, with 30–40 kg extra weight to carry. | Energy deficit approx. 1200 kcal per day, on average | Less than 4 h per night |
| Vikmoen et al., 2020 [22] | n = 23 men, age 19.3 ± 1.8 yr, Norwegian conscripts who completed a selection exercise | Armed Forces Special Command, Parachute Ranger Platoon selection | Selection exercise; extremely demanding field exercise that lasts ~5 and half days. Designed to test physical and mental resilience in extreme situations in sub-optimal conditions. Consisted of large amounts of physical activity in addition to sleep and food restriction. Main activities: loaded marching and various mentally and physically challenging tasks. Carried load varied between 20 and 40 kg during exercise. | Energy expenditure estimated at 7235 ± 408 kcal/day. Food intake was 575 kcal/day, except for day 3, when it was 3755 kcal. | 1–6 h/day |
| Hamarsland et al., 2018 [19] | n = 15, aged over 18, apprentices applying for Norwegian Naval Special forces | First 6 weeks of Naval Special Forces selection course | First 3 weeks: military camp with heavy physical activity and sleep restriction in a stressful environment; week 4: “hell week”, consisting of sleep and calorie restriction and extreme amounts of physical activity for 20 h per day in a very stressful and difficult environment with about 35 kg of carried load. Weeks 5–6: recovery. | First 3 weeks food intake: ad libitum. Hell week: 10,000 kcal combat ration provided at the start, for the whole week | First 3 weeks: not stated. Hell week: 2–3 h of sleep per night |
| Kyröläinen et al., 2008 [23] | n = 7, Finnish male soldiers, age 24 ± 2 years | Prolonged military field exercise | 20-day field exercise, three phases: First 7 days: Phase 1, very heavy, consisting of walking 20–25 km per day in the forest carrying approx. 50 kg of gear. Six days of phase 2: Easy, walking 5–10 km per day with 20–25 kg of gear. Last week phase 3: heavy, approx. 15 km per day with 30 kg of gear. | Daily energy intake average: 2938 ± 454 kcal/day, with no differences between different phases. Energy deficits were 4000, 450 and 1000 kcal/day in P1 (~7000 kcal EE), P2 (~3200 kcal EE) and P3 (3500 kcal EE). | Average of 6 h sleep per night during the whole field exercise |
| Santos et al., 2018 [21] | n = 43, age 18–23, Brazilian 1st Command Action Battalion male soldiers | Army Corporal Training Course, Combat Simulation exercise | A total of 4 full days of 24 h continuous operations; evaluation of leadership potential in combat. Included 25 kg of added weight + other material to carry. | R2 ration includes 3000–3600 kcal of energy. Day 1: full R2 ration, Day 2: ½ R2 ration, Day 3: 1/3 R2 Ration, Day 4: - | Day 1: 2 h, Day 2: 2 h, Day 3: 1 h, Day 4: - |
| Szivak et al., 2018 [8] | n = 20, age 18–35, active-duty men serving in the U.S. Navy and Marine Corps | Navy SERE course | Highly classified. ~2 weeks of highly realistic SERE training including multiple stressors: environmental extremes, physical demands, food and sleep deprivation, psychological stress. First 4 days was didactic phase, followed by field training phases: Evasion phase: several days of practicing evasion techniques in difficult terrain. Capture phase: several high-stress training scenarios of realistic captivity experience. | Several days of food restriction | Several days of sleep deprivation |
| Henning et al., 2013 [24] | N = 23, age 23.0 ± 2.8 U.S. Army 2/75th Ranger Regiment male soldiers who completed Ranger Training without recycling | Ranger Training Course | 61 days, 30–40 kg load carry, over 200 miles of movement during the course, and food and sleep deprivation. Same course as Conkright et al., 2018. [20] | 2200 kcal of food provided per day | 0–5 h of sleep per night |
| Nindl et al., 1997 [18] | n = 10, U.S. male soldiers from Army Ranger Training Course | Army Ranger Course | Demanding 62-day training program designed to teach and evaluate leadership and small unit tactics under physically and mentally challenging conditions. Multi-stressor environment, 20 h of training each day in forest, forested mountains, coastal swamp, and desert | Estimated energy expenditure: 4200 kcal/day. Caloric intake: 3200 kcal/day. A deficit of 1000 kcal/day | Description indicates maximum of 4 h per night; might be lower |
| Gunga et al., 1996 [25] | n = 29, age 22.2 ± 2.8, male members of Austrian Army special forces training unit | Survival training course | 5-day survival training; 430–570 m above sea level in a wooded area. Incl. 90 km marching, tactical missions with 22.3 kg weight. | 1st day breakfast of 1500 kcal; after that, mean energy intake was 150 kcal/day. Water was limited to 1 L/day (+1 L 1st day morning and 4th day afternoon) | Overall, 20 h of sleep over 5 days (no tent and no sleeping bag) |
| Opstad, 1994 [26] | n = 10, age 22–26, male cadets of the Norwegian Military Academy | Military training course | 5 days continuous physical exercise (infantry activities) around the clock in a forest area at a 500 m altitude | Energy expenditure of 40,000 kj/24 h (9560 kcal); energy intake 5000 kj/24 h (1195 kcal) | No organized sleep, some minutes between activities, total 1–3 h during the whole course. |
| Opstad, 1982 [27] | n = 11, two groups (iso-calorie: n = 5, age 22.9. low-calorie: n = 6, age 22.8). Norwegian Military Academy male cadets | Norwegian Military Ranger training course | 5-day ranger training course with continuous and heavy activities | Energy expenditure of 8000–11,000 kcal/day. Low-calorie group intake was 1500 kcal; deficit 7000–10,000 kcal. Iso-calorie group intake was 6400 kcal/day | Less than 2 h of total sleep during the course |
3.5. Quality Assessment
According to the “QualSyst” checklist, seven studies were rated as “moderate” quality and five as “weak”. Supplementary Table S1 presents the critical appraisal of the studies. The studies scored 10 to 15 “QualSyst” points. Despite the low sample sizes, substantial outcomes were observed for the majority of the main variables. Nevertheless, considerably low sample sizes still accommodate bias, which is especially relevant in hormonal measurements with wide measurement ranges. In addition, variances were rarely reported for the main outcomes, which lowered the overall scores for all except four studies. In addition, mainly due to the nature of field experiments, the study designs tended to lack appropriateness to be rated a full two points.
3.6. The Effects of Training Courses on Physical Performance
Statistically significant decreases in physical performance were observed after the training course (ranging from 6 to 62 days) in four out of five studies [1,18,19,20], except the study by Szivak et al. [16]. In these studies, all the measured physical performance outcomes declined, apart from the study by Conkright et al. [20], in which all other performance measures, except deadlift repetition strength with 225 lbs and bench press repetition strength with 185 lbs, decreased after the course.
3.7. Recovery of Physical Performance
Among studies reporting decrements in physical performance with a recovery follow-up, Nindl et al. [18] was the only one to report the recovery of all outcomes (muscle strength measured as machine simulating power clean, vertical jump height, and explosive power), occurring after 5 weeks. In the other studies, recovery occurred only for some outcomes. Conkright et al. [20] reported that only push-ups and pull-ups returned to baseline after 6 weeks of recovery following a 62-day Ranger course, while other measures (speed/mobility, anaerobic capacity, aerobic fitness) remained non-recovered. Hamarsland et al. [19] reported no clear signs of recovery 72 h after the 6 weeks of the Naval Special Forces selection course. Chest press recovered after 1 week and leg press after 2 weeks, but counter-movement jump (CMJ) remained depressed after 2 weeks. Follow-up from a five-and-a-half-day selection exercise to Special Forces reported that upper body power recovered after 1 week and anaerobic performance measured by an evacuation test recovered after two weeks, but CMJ still remained decreased after two weeks [22]. These results are summarized in Supplementary Table S2.

Table 2.
Measurement times, main outcomes, main findings, and recovery.
Table 2.
Measurement times, main outcomes, main findings, and recovery.
| Study | When Testing Was Conducted | What (Relevant) Markers Were Measured | Main Findings | Recovery of Markers? |
|---|---|---|---|---|
| Mourtakos et al., 2021 [17] | Baseline (BL): 7 days prior to “Hell Week”. During: on each of the 5 days of “Hell Week”. Recovery: 30 days after completion. | Plasma protein concentration, plasma heat capacity profiles, albumin and globulin peak enthalpies and temperatures | The main finding was that the thermal stability of plasma albumin was enhanced and denaturational transition to higher temperatures shifted. The major effect of exercise was a continuous upward shift in the albumin peak by 2–3 Celsius, tending to plateau on the 5th day. Some redistribution of the denaturational enthalpy was also observed during exercise: globulin peak increased relative to albumin peak, especially during first 4 days. Total recovery to the initial signature pattern after 30 days’ recovery. | Yes. |
| Conkright et al., 2020 [20] | Baseline (BL) pre-Ranger School, two-weeks post (P1)-, and six-weeks post (P2)-Ranger School | Physical performance with modified Ranger Athlete Warrior assessment. Speed and mobility: Illinois Agility Test (IAT) test, muscular endurance (push): metronome push-up, muscular strength/endurance (pull): overhand pull-up, core strength: heel clap, anaerobic capacity: 300 yd shuttle run, aerobic fitness 20 m multistage beep test. Strength: 185 lbs bench press and 225 lbs deadlift rep max. | Significant declines across time points in all performance measures except deadlift and bench. BL to P1 declines: push-ups ↓~24%, pull-ups ↓~28%, heel claps ↓~35%, IAT ↓~9%, beep test ↓~20%. The 300 yd run did not decline at P1, only at P2. Push-up and pull-up returned to BL by P2. Other measures related to speed/mobility, anaerobic capacity, and aerobic fitness remained under-recovered at P2 related to BL: IAT ↓~15% and 300-yard run ↓~7% slower, heel clap ↓~27% decline, beep test ↓~23% decline related to BL. | Partial. Push-up and pull-up recovered to BL after 6 weeks; other variables did not. |
| Vikmoen et al., 2020 [22] | Baseline (BL) and 0 h (only physical perf), 24 h (physical perf + blood), 1, 3, 7, and 14 days after field exercise. | Blood: Cortisol (COR), testosterone (T), creatine kinase (CK), insulin-like growth factor 1 (IGF-1). Physical performance: counter-movement jump (CMJ) (n = 17), medicine ball throw (MBT) (n = 18), evacuation test for anaerobic performance (EVAC) (n = 18). | Physical performance: CMJ height decreased after the exercise (↓7.5 cm) and was still ↓6.6 cm reduced after two weeks recovery. CMJ max power followed a similar pattern. EVAC test times were about 50% slower after exercise, with recovery to BL after 2 weeks. MBT: ↓0.5 m; back to BL after 1 week of recovery. Blood: T ↓58% 24 h after the exercise. Still ↓20% at 72 h rec. Increase compared to BL after 1 (↑87%) and 2 weeks of (↑113%) recovery. COR: Increase during exercise (↑26%); back to BL after 72 h of recovery. IGF-1: decrease during exercise; was ↓28% lower at post. After that, IGF-1 increased gradually, and levels were higher than BL after one week of recovery. CK was increased significantly 24 h after exercise (↑353 ± 430%), back to pre-values after 72 h of recovery, and decreased to below pre- values after 1 wk and 2 wk of recovery (↑85%). | Partial. Blood biomarkers recovered after 1 week. CMJ did not recover at 2 weeks, MBT recovered after 1 wk and EVAC after 2 wk. |
| Hamarsland et al., 2018 [19] | Baseline (BL): day 2 of 1st week and Pre: day before hell week (HW). Post: Blood samples immediately after termination of hell week; physical performance 8 h later. Recovery: all measures after 24 h, 72 h, 1 wk, and phys perf 2 wk. | Physical performance: counter-movement jump (CMJ), isometric leg press, isometric chest press. Blood samples: T, COR, T/C ratio, sex hormone-binding globulin (SHBG), CK, C-reactive protein (CRP), thyroid-stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), T3/T4 ratio, IGF-1 and insulin-like growth factor-binding protein 3 (IGFBP-3). Free testosterone (FT) was calculated. | After HW: Physical performance at post: CMJ ↓28%, leg press ↓20%, chest press ↓10%. No clear signs of recovery after 72 h. One week after, chest press returned to pre- levels. Leg press recovered after 2 wk, CMJ still depressed after 2 wk (↓14%). T pre–post ↓70%; after 1 wk, returned to normal. FT ↓39% at post-, ↓60% after 24 h, ↓50% at 72 h, and normal after 1 wk. SHBG pre–post ↑24%, still elevated at 72 h, normalized after 1 wk. COR ↑154% at post-, elevated after 1 wk (↑43%). T/C ratio ↓87% at post-, ↓63% at 24 h, ↓58% at 72 h, back to baseline after 1 wk. IGF-1 and IGFBP-3 both ↓(45/37%) at post-, gradual rec and normalized after 1 wk. T3 and T4 ↓(32%/12%) at post-, gradual recovery to pre- within 1 wk. T3/T4 ratio ↓77% at post-, gradual recovery toward pre- within 1 wk. TSH significantly increased (↑58%) only after 1 wk. CK elevated at post- (700%), decreased to below pre- values after 1 wk. CRP ↑1300% at post-, ↑1500% at 24 h, and below pre- values within 1 wk. | Partial. Some hormones normalized after 1 wk; some did not. Recovery of chest press after 1 wk, leg press after 2 wk. CMJ still depressed after 2 weeks |
| Kyröläinen et al., 2008 [23] | Pre/BL: one day before start, days 5 (P-1mid), 8 (P-2pre), 14 (P-3pre), 16 (P-3mid) and 21 (P-3post) (NOTE = Only first 7 days were considered as the “intervention”. All else were recovery) | Blood: COR, growth hormone (GH), glucose (GLU), CK, urea (U), T, FT, T4, follicle-stimulating hormone (FSH), luteinizing hormone (LH), insulin (INS), Plasma volume (PV) (limited data on plasma volume to assess recovery). | Blood GLU not changed by day 5, ↓13,3% at the end of P-1 (day 7). Back to BL on day 8. At P1-mid (5 days), COR ↑32%, GH ↑616% and INS ↓70%. After these initial rises, COR and GH returned to BL at P-2pre, and INS at the end of P-3post. At P1-mid, T ↓27%, FT ↓26% and LH ↓46%; no change in FSH. All these returned to BL by P-3pre. Serum T4 p1mid ↓9% non-significant, was lower and urea concentration was higher after the whole exercise than BL. No changes in T4 and urea during the first part of the exercise. PV changed slightly during the course. CK increased at P-1mid ↑555% and returned to BL on day 16. | Yes, except T4 was lower and urea concentration was higher after the exercise. |
| Santos et al., 2018 [21] | BL/T0 before beginning of activities (fasted), T1 at 72 h after baseline after 100 km march, and T2 at 63 h after the end of military activity | Blood samples: CK, myoglobin (MB), CRP, alpha 1-acid glycoprotein (AGPA), lactate dehydrogenase (LDH), lactate | CK ↑1035% at T1; returned to baseline at T2. LDH: ↑122% at T1; still ↑37% increased at T2. Lactate ↑127% at T1; returned to baseline at T2. MB: ↑728% at T1; returned to baseline at T2. CRP: ↑182% at T1; returned to baseline at T2. AGPA: ↑14.7% at T1; returned to baseline at T2. Thus, markers increased significantly at T1 and returned to levels close to baseline at T0, except LDH, which did not. | Yes, except one marker (LDH); marker recovery occurred after 63 h |
| Szivak et al., 2018 [16] | (BL)/T1, first day of SERE. Stress assessment (T2), 10 d after T1. Recovery assessment (T3), 24 h after T2. | Blood samples: Epinephrine, norepinephrine, dopamine, COR, T, and neuropeptide-y (NPY) at all testing points. Physical performance: vertical jump, dominant handgrip, nondominant handgrip at test points T1 and T2, and no recovery measure. | Physical performance did not decrease from T1 to T2. Exposure to stress resulted in significant increases in plasma epinephrine ↑70%, plasma norepinephrine ↑191%, plasma dopamine ↑186% and serum COR concentration ↑525%, and a reduction in TES concentrations ↓63%. No significant elevations in plasma NPY. However, NPY decreased significantly at T3 (↓56%). Of the markers that showed increase at T2, only epinephrine recovered at T3; others were still elevated from BL values after 24 h (Norep ↑82%, Dop ↑79% COR ↑172%, Test ↓54%). | No. Of the affected markers, only epinephrine levels recovered after 24 h. |
| Henning et al., 2013 [24] | Before (BL) and immediately after (Post) Army Ranger course. Recovery measures after 2–6 weeks. Note = n = 23 at BL and post-; n = 9 on the recovery measures (no R.D. = no recovery data). | Blood samples: COR (no R.D.), T3, T4 (no R.D.), TSH (no R.D.), dehydroepiandrosterone-sulfate (DHEA-S) (no R.D.), brain-derived neurotrophic factor (BDNF), total and free IGF-1, IGFBP-1 (no R.D. On 2–6), Cytokines (INF-y (no R.D.), IL-1 (no R.D.), IL-4, IL-6, IL-8, IL-10 (no R.D.), TNF-alpha (no R.D.), CRP (no R.D.)), T, SHBG. | T decreased ↓70% at post. Serum SHBG ↑46% at post. COR nonsignificant increase, DHEA-S no change at post. BDNF ↑33% at post. T3 showed a trend to decrease (↓8%) at post. TSH ↑85% at post. No change in T4 at post. Total IGF-1 decreased ↓38.7% and free IGF-1 ↓41% at post. IGFBP-1 ↑534.4%, IGFBP-2 ↑98.3% and IGFBP-3 ↑14.7% at post. IGFBP-6 ↓23.4% at post. Il-4 ↑135.3%, IL-6 ↑217.2%, and IL-8 ↑101,.4%. No changes in INF-y, IL-1B, Il-10, TNF-alpha or CRP. After 2–6 weeks, all markers with recovery data recovered to BL concentrations except T3 (↑17%). | All markers with recovery data recovered to BL after 2–6 weeks, except T3 elevated. |
| Nindl et al., 1997 [18] | Pre: before the start of the course. Post: 62 days after initial testing (at the end of Ranger course). Recovery at 35 days after completion of the course. | Physical performance: Machine simulating power clean (strength) vertical jump (jump height and calculated explosive power). Serum hormones: IGF-1, T3, T4, thyroxine-binding globulin (TBG), TSH, LH, SHBG, T. Metabolic markers: Transferrin, prealbumin, ferritin (not reported), glycerol, nonesterified fatty acids (not reported), HDL (not reported), lactate. | Strength declined ↓21.2%, explosive power ↓22%, vertical jump height ↓18% at post. IGF-1 (↓50%), LH (~↓28%), T3 (↓22%), T4 (↓10%) declined. SHBG (~↑100%), TBG (~↑15%) and TSH (~↑125%) increased. T declined most: ↓86%. Ferritin, HDL and nonesterified fatty acids could not be reported due to dichotomies in text and tables. Prealbumin was significantly lower (↓21%); no differences in transferrin, glycerol, or lactate. Recovery: Physical performance recovered to pre levels at 5 weeks of recovery. Most hormones recovered to pre levels, but T3 and IGF-1 increased compared to pre, and TBG and SHBG only recovered to normal values, not BL. All metabolic markers recovered or were in the normal range except for lactate, which interestingly showed an increase (↑96%) at recovery. | Partial. Phys. Perf recovered. TBG and SHBG only to normal values (not considered recovered), lactate high at rec. T3 and IGF-1 increased. All else recovered. |
| Gunga et al., 1996 [25] | T1: day 1, before course started; T2: after 72 h; T3: after 120 h at the end of the course; T4: after course, 48 h; and T5: 72 h of recovery. | Hemoglobin (Hb), Hematocrit, packed cell volume (PCV), erythropoietin (EPO), iron (Fe), haptoglobin (Hapto), Transferrin, and Ferritin. | EPO decreased during the course but was over control (pre) values during the recovery period. Fe increased during the course and remained above control (pre) concentrations after recovery. Hapto decreased during the course and remained below control concentrations at T4 and T5. Transferrin decreased during training and recovery continuously. Fer increased during the course and returned to control (pre) concentration at T5. Hb increased from T1 to T2, but had decreased below control levels at T5. PCV increased from T1 to T2, but was below control levels at recovery. | Partial. |
| Opstad, 1994 [26] | BL/control the week prior to the course, 1st day of course (day 1–2), last day of the course (day 4–5), and 4–5 days after course (recovery) (REC). | Circadian rhythm blood measures performed seven times during 24 h. Measures: Dopamine (Dop), noradrenaline (Norad), adrenaline (Ad), COR and Plasma Cortisol, progesterone (PS), estradiol (ES), T, dehydroepiandrosterone (DHEA), 17a-hydroxy-progresterone (17a-Hp), DHEA-S, androstenedione (AS), T4, free T4 (FT4), T3, free T3 (FT3), TSH, human growth hormone (HGH), and glucose. | Circadian rhythms: COR: almost extinguished during last 24 h, normalized during REC, and plasma COR rhythm still different. PS: almost extinguished on last day; normal after REC. DHEA-S: No rhythm on last day; no significant alterations during recovery. AS: almost extinguished on last day; not re-established during REC. DHEA: almost abolished on last day; re-established during REC. 17a-Hp: abolished on the last day of course; not re-established during REC. TES: last day no rhythm; not re-established at REC. E: No rhythm shown. Norad: did not show rhythm. Ad, Dop did not show rhythm. HGH: no apparent circadian variations. TSH: alterations in rhythm; re-established at REC. T4: no circadian rhythm shown. FT4: no circadian variations at control and recovery; slight variation in levels during course. T3, FT3: no circadian variation. Glucose: no rhythm was found. | Partial. |
| Opstad, 1982 [27] | Pre, every morning of 5-day combat course and 6 days after. | Prolactin (PRL), T, ES | ES: remained at stable a level during the first two days of activity, decrease from day three, with lowest value on day four (↓50% from precourse values). Recovery to pre- values after 6 days. T decreased after 12 h of activity, decreased about ↓75% from pre-course values on day 3 and remained low. However, it recovered 6 days after. PRL decreased after 12 h and lowered after that point, but recovered after 6 days. No effect on group. | Yes, all hormone levels recovered after 6 days. |
3.8. Effects of Training Course on Biomarkers
In general, large stress-related responses were observed in most biomarkers for all studies, indicating that a stress-induced reaction of a biomarker was evident after the training, affecting homeostasis, which would have to be restored (Supplementary Table S3). Hamarsland et al. [19] reported that all biomarkers except thyroid-stimulating hormone (TSH) were decreased after the first 6 weeks of a Naval Special Forces’ selection course. TSH increased above the baseline at the 1-week recovery point. Szivak et al. [16] documented that all markers but neuropeptide-y (NPY) were decreased at the post-measurement immediately after a 2-week Navy SERE course. However, NPY did decrease at the recovery measurement point, which was 24 h after the “stress assessment”. It is worth noting that the final phase of the course before the post-measurement was mentally very stressful (interrogation), with multiple days of physically demanding SERE training before that. In the study by Henning et al. [24], all others but the cytokines interferon-y, interleukin-1 beta, TNF-alpha, or c-reactive protein (CRP) were decreased during a 61-day Ranger course, and triiodothyronine (T3) only showed a decreasing trend. In addition, no changes in thyroxine (T4) or cortisol were observed. Opstad [26] reported decreases in concentrations or circadian rhythm in all others but glucose, T3, T4, free T3, human growth hormone, adrenaline, noradrenaline, dopamine, and estrogen circadian rhythms following a five-day military training course. Kyröläinen et al. [23] reported changes in all markers except follicle-stimulating hormone (FSH) during/following a 20-day exhaustive field exercise. T4 and urea were not affected until after the first 7 days.
Among the commonly used anabolic biomarkers, testosterone decreased significantly in all available studies: Hamarsland et al. [19]: −70% (with a concomitant increase of +24% in sex hormone-binding globulin (SHBG)); Henning et al. [24]: −70% (with a concomitant increase in SHBG +46%); Nindl et al. [18]: −86%; Opstad and Aakvaag [27]: −75%; Kyröläinen et al. [23]: −27%; Szivak et al. [16]: −63%; and Vikmoen et al. [22]: −58%. Opstad [26] reported that the circadian rhythm of testosterone was extinguished on the last day. An important anabolic hormone, insulin-like growth factor 1 (IGF-1), was measured in four studies, and decreased somewhat similarly (between −28% and −50%) in all studies [6,7,14,22].
Cortisol, often considered the “main” stress hormone of the hypothalamus–pituitary–adrenal (HPA) axis, increased in all studies where it was measured, except for Henning et al. [24]. In the study by Hamarsland et al. [19], cortisol increased +154% and the testosterone/cortisol ratio decreased 87%. Szivak et al. [16] reported that cortisol increased +525%, while in the study by Opstad [26], the cortisol circadian rhythm was “almost extinguished”. In addition, increases of +32% and +26% were reported by Kyröläinen et al. [23] and Vikmoen et al. [22], respectively. The effects on all the most often (at least in two studies) measured biomarkers are provided in detail in Supplementary Table S3.
3.9. Recovery of Biomarkers
The most studied biomarker, testosterone, returned to the baseline during the follow-up in six out of eight studies. In these studies, the length of the recovery period varied from six days to six weeks, while in the two studies that did not show recovery, the recovery period was 24 h or five days. Cortisol recovered to the baseline in three studies and did not recover in two studies [16,19], where a short recovery time (24 h and one week, respectively) does not explain this phenomenon. However, Szivak et al. [16] reported a much greater increase in cortisol compared to studies in which cortisol recovered to the baseline in a relatively short time (3 days) [22,23]. A greater increase in cortisol could mean a longer time to recover. T3, creatine kinase, T4, IGF-1, and CRP recovered to the baseline in all studies in which recovery could be assessed.
Out of the 11 studies, in 3 [17,22,23], all measured biomarkers recovered to the baseline while in 4 [19,21,24,27], 1–2 biomarkers were not recovered. In three studies [18,25,28], only 3–4 biomarkers recovered, and in 1 study [16], 5 biomarkers were not recovered to the baseline. Two studies [17,23] showed a full recovery of the studied biomarkers, seven studies [9,18,21,22,27,28] showed that 79–90% of the measured markers were recovered (or were not affected), and in three studies [16,20,25], recovery (or no effect) was seen for 44–63% of biomarkers. The recovery results of biomarkers are summarized in Supplementary Table S4.
4. Discussion
The present review examined the current evidence for the recovery of physical performance and biomarkers after strenuous military training. “Stress related” biomarkers such as cortisol and testosterone, which were the most studied biomarkers, are mainly affected by the sympathetic–adreno–medullar (SAM) and HPA axes. However, many other biomarkers were also measured in the studies and, therefore, a larger picture of the physiological response to and recovery from extremely strenuous activity can be discussed.
It appears that large physiological decrements occur during and after strenuous military training. Full recovery seems to vary between the reviewed studies (Supplementary Table S5) and there are no clear time periods for recovery that are putatively accepted. However, as we can observe from Table 2, it appears that a minimum of 6 days is likely required, and after 1–2 weeks, the majority of markers (as well as variables of physical performance) have likely fully or nearly fully recovered. Although, Conkright et al. [20] found that 50% of physical performance variables were unrecovered even after 6 weeks because of the duration and intensity of the training. Although a complete recovery of all measured biomarkers occurred only in two studies, almost full recovery (79–90% of markers recovered) occurred in the majority (seven) of the studies. In three studies, recovery occurred only for 44–63% of the studied biomarkers. Among these studies, Conkright et al. [20] reported that more than 6 weeks is required for physical performance to recover after an arduous and long training course such as the Ranger training course. In this particular study, recovery was assessed only after 2 and 6 weeks. Such a long (non-standardized) recovery could also be explained by maladaptation, if the participants did not conduct identical physical training and activity prior to the study [6]. Otherwise, such symptoms can refer to NFOR, or even OTS, as 50% of physical performance variables remained unrecovered after 6 weeks.
The present review included two other studies with a similar study setting, the U.S. Army Ranger course [18,24]. Interestingly, Nindl et al. [18] reported that the measured physical performance values recovered completely after 5 weeks of the Ranger course, while 3/12 biomarkers still had not recovered. Thus, the overall recovery for both physical performance values and biomarker values was 80% of their pre-stress levels after 5 weeks. The notion that all physical performance measures recovered but all biomarkers did not could indicate that even the commonly used recovery outcome, physical performance, might not truly confirm complete physiological recovery status, and that physical performance could recover faster than some biomarkers. However, in the study by Vikmoen et al. [22], which describes a much shorter (5.5 days) extremely demanding field exercise, the opposite result occurred: all biomarkers in this study recovered fully within 1 week, but one physical performance marker (CMJ) remained unrecovered even after 2 weeks. Despite the fact that only four biomarkers were measured in this study, all of them were comparable to other studies and decrements were large; for example, testosterone declined by −58%. In other studies, which measured both biomarkers and physical performance, only physical performance was not affected in one study [16], while some biomarkers and some physical performance markers remained unrecovered in another [19].
The second study on the U.S. Army Ranger training course, Henning et al. [24], reported that 9/10 of markers recovered after 2–6 weeks. Only T3 was not recovered and was elevated at the 2–6-week recovery points. However, T3 was not affected at post-measurement. Therefore, it is difficult to distinguish if it was affected due to the training or for other reasons. In the present review, markers which responded this way were not counted as recovered due to differences in the pre-measurements. However, the results of 90% markers’ recovery also differed from the results of Conkright et al. [20]. In addition, the study setting of Hamarsland et al. [19] compares best to the U.S. Army Ranger courses, as it was similar in length (six weeks). Their study showed that a total of 81% of markers recovered (11/13 biomarkers after 1 week and 2/3 physical performance markers after 2 weeks), even though the decrements were robust. For example, testosterone decreased by −70%, but 1 week of recovery seemed to be effective to return to pre-stress levels. TSH was not affected at post-measurement but only at 1 week of recovery. Therefore, it could be counted as recovered due to not being affected at post-measurement. Therefore, it could be counted as recovered due to not being affected at post-measurement, making cortisol the only biomarker remaining unrecovered in this study [19]. It can be concluded that in these longer-lasting training courses, in all studies except Conkright et al. [20], a great majority of markers recovered in 1 to 6 weeks.
After a two-week highly demanding SERE training course [16], only one (epinephrine) out of six measured biomarkers of the acute SAM axis had recovered, while the three assessed physical performance variables were not affected. Thus, 44% of markers were considered as recovered or not affected within a 24 h follow-up period. Unfortunately, comparison to other studies is not possible as no other training courses lasted for 2 weeks. It is interesting that physical performance (strength) was not affected by the SERE course [16], while all the measured biomarkers were. However, although the participants conducted strenuous SERE training [16] for several days, a particular difference was that the stress induced prior to the actual stress measurement was mainly psychological.
Gunga et al. [25], who reported the least recovery of biomarkers, observed mainly (5/8) different variables than most other studies. Markers were related to blood oxygen transportation, such as erythropoietin and hemoglobin. The follow-up of recovery was only 3 days, which is a probable explanation as to why no full recovery occurred. In addition, the training course lasted only 5 days. The majority of the included courses (n = 7) lasted between 4 and 7 days. In the rest of such studies, the recovery was more complete. For example, in the studies by Opstad and Aakvaag [27] and Mourtakos et al. [17] where full recovery occurred, the training was conducted for 5 days. However, in these two studies, only a few markers were measured. It should also be noted that in the study by Mourtakos et al. [17], the markers (plasma protein denaturation profiles) were different compared the other studies. Full physiological recovery status may be inadequately determined based on the use of these markers as compared to most of the other studies. The recovery periods were 6 days [29] and 30 days [17]. Despite the short duration of training, Opstad [28] observed the second largest decrement in testosterone (−75%) across all studies of this review, but it recovered after only 6 days. Opstad [28] had two groups with energy intakes of 1500 and 6400 kcal per day, with the energy expenditure in both groups being 8000–11,000 kcal/day. Additionally, total sleep was less than 2 h across the 5-day course. Interestingly, there were no group differences in the studied biomarkers and their recovery times. Therefore, it seems that the calorie intake and energy deficit are not the only main driving factors causing stress-related alterations in biomarkers. This also seems to be the case in other stressful situations, such as when looking at burn injuries and sepsis—regardless of nutritional status, whole-body protein catabolism increases, as it seems that despite an abundance of amino acids in circulation, their transportation to muscle is compromised [28]. For the other four studies that lasted between four to seven days [21,22,26,27], 79–86% of the studied biomarkers recovered, although recovery was measured for different time-courses: in the study of Santos et al. [21], 63 h, with 83% recovery; Opstad [26], 4–5 days, with 79% recovery; Kyröläinen et al. [23], 2 weeks, with 82% recovery; and Vikmoen et al. [22], 1 week, showing 86% recovery of markers. Although the duration of training courses was similar (from 4 to 7 days), the recovery times varied between the studies (from 63 h to 2 weeks), while the differences in the percentage of markers recovered were small (from 79% to 86%). However, it should also be kept in mind that different measurements and their time-courses were used. Although the courses were the same in length, the intensity and the amount of food and sleep varied between the studies. According to the inclusion and exclusion criteria, the intensity should be somewhat similar across studies, but quantification is difficult since in the majority of studies, only a general description of training along with the amount of food and sleep was provided. In the future, it is important to try to find a more standardized way to measure physical strain and recovery during military training. This could include validated measurements of physical activity (e.g., 3D accelerometers), physical load (e.g., biomarkers and heart rate variability), and energy expenditure (e.g., doubly labeled water).
The quality of studies in the literature is limiting due to difficulty in reaching high scores on quality assessments for field studies (from 10 to 15 out of 22 in Supplementary Table S1). In addition, due to the nature of the majority of the markers, measurement errors and time-courses may influence results. Furthermore, the number of participants was generally low (from 7 to 43). Also, the intensity could have varied between the reported studies. However, significant changes occurred for the majority of the measured markers, which indicates the stressful nature of the training courses. Finally, the content of recovery periods was not clearly documented, but in the majority of cases, it was likely lighter military-related activity. In the future, it is important to use new technologies, e.g., saliva and sweat measurements, to collect hormonal data from a larger number of military participants time- and cost-effectively. In addition, more controlled recovery periods should be used to follow recovery. Another thing to consider is the fact that even the most strenuous military training courses cannot replicate the major stressors of war. Nevertheless, studies of stress responses to life-threatening situations have been limited. For example, in the study by Trousselard et al. [30], the participants were trained and qualified submariners and participated in underwater escape training. The participants had to escape from a land-based tank simulating a submarine that was close to the surface at a depth of 6 m, and another time from an actual submarine at a depth of 30 m on the sea floor. Compared to the simulated exercise at 6 m, the physiological responses were vastly greater in the experiment on the sea floor (e.g., salivary cortisol was doubled). This suggests that the realism of conditions significantly influences the physiological responses, even though the mission is quite similar.
5. Conclusions and Future Directions
The data and results presented are noteworthy due to the extreme nature of the military courses, and provide information about the capacity to recover from extremely strenuous activity in a relatively short timeframe. To conclude, all studies elicited large effects on the stress system and multiple biomarkers. In most of the studies, almost a full recovery of the studied biomarkers occurred within the appropriate time, although the time-course seemed to vary markedly, and in one study, only 50% of markers were recovered, even after six weeks. Importantly, a full recovery occurred in only two studies. From these data, it can be stated that after 1–2 weeks of strenuous military training, most measured markers have likely recovered, although recovery for all markers does seem to take a longer period. Also, it is important to note that physical performance markers could recover faster than some biomarkers. It seems necessary to measure both physical performance and biomarkers when assessing recovery from military training. It is also worth noting that, if only one or two markers are not recovered, it might not necessarily be related to recovery status but to other factors that also may play a role, as the majority of markers would indicate recovery. It could also be of use to consider the cut-off point for total recovery—is it necessary for 100% of markers to be measured as completely recovered, or is, for example, a 90% recovery rate of measured markers enough to demonstrate a physiologically recovered state? In addition, studies on recovery strategies after strenuous military training are warranted. As a takeaway, it is important to measure recovery with various markers, especially after longer strenuous training courses, to prepare soldiers for real military operations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/physiologia3040046/s1, Table S1: QualSyst; Table S2: Summary of physical performance recovery in studies; Table S3: Results of the most commonly measured single biomarkers (measured in at least two studies), and recovery time course (or no recovery time course) of markers; Table S4: Summary of physical performance and biomarker recovery; Table S5: Summary of biomarker recovery in studies.
Author Contributions
Conceptualization, J.G., H.K. and T.O.; methodology, J.G., H.K. and T.O.; writing—original draft, J.G., H.K., K.P., M.S., B.C.N. and T.O.; writing—review and editing, J.G., H.K., K.P., M.S., B.C.N. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Notes
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The views and opinions presented in this manuscript are solely those of the authors and do not necessarily represent any official policy or position of the represented agencies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Henning, P.C.; Park, B.S.; Kim, J.S. Physiological decrements during sustained military operational stress. Mil. Med. 2011, 176, 991–997. [Google Scholar] [CrossRef]
- Knapik, J.J.; Harman, E.A.; Steelman, R.A.; Graham, B.S. A systematic review of the effects of physical training on load carriage performance. J. Strength Cond. Res. 2012, 26, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Szivak, T.K. Warfighter Adrenal Response to Extreme Military Stress. Doctoral Dissertation, Graduate School of the Ohio State University, Columbus, OH, USA, 2016. [Google Scholar]
- Friedl, K.E.; Knapik, J.J.; Häkkinen, K.; Baumgartner, N.; Groeller, H.; Taylor, N.A.; Duarte, A.F.; Kyröläinen, H.; Jones, B.H.; Kraemer, W.J.; et al. Perspectives on aerobic and strength influences on military physical readiness: Report of an international military physiology roundtable. J. Strength Cond. Res. 2015, 29 (Suppl. S11), S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Nindl, B.C.; Castellani, J.W.; Warr, B.J.; Sharp, M.A.; Henning, P.C.; Spiering, B.A.; Scofield, D.E. Physiological employment standards III: Physiological challenges and consequences encountered during international military deployments. Eur. J. Appl. Physiol. 2013, 113, 2655–2672. [Google Scholar] [CrossRef] [PubMed]
- Vrijkotte, S.; Roelands, B.; Pattyn, N.; Meeusen, R. The overtraining syndrome in soldiers: Insights from the sports domain. Mil. Med. 2019, 184, e192–e200. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Nederhof, E.; Buyse, L.; Roelands, B.; de Schutter, G.; Piacentini, M.F. Diagnosing overtraining in athletes using the two-bout exercise protocol. Br. J. Sports Med. 2010, 44, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Szivak, T.K.; Kraemer, W.J. Physiological readiness and resilience: Pillars of military preparedness. J. Strength Cond. Res. 2015, 29 (Suppl. S11), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44 (Suppl. S2), S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, M. Enhancing Recovery: Preventing Underperformance in Athletes; Human Kinetics: Champaign, IL, USA, 2002. [Google Scholar]
- Taylor, M.K.; Sausen, K.P.; Mujica-Parodi, L.R.; Potterat, E.G.; Yanagi, M.A.; Kim, H. Neurophysiologic methods to measure stress during survival, evasion, resistance, and escape training. Aviat. Space Environ. Med. 2007, 78 (Suppl. S5), B224–B230. [Google Scholar]
- Nédélec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in soccer: Part I—Post-match fatigue and time course of recovery. Sports Med. 2012, 42, 997–1015. [Google Scholar] [CrossRef]
- Beckner, M.E.; Main, L.; Tait, J.L.; Martin, B.J.; Conkright, W.R.; Nindl, B.C. Circulating biomarkers associated with performance and resilience during military operational stress. Eur. J. Sport Sci. 2022, 22, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kmet, L.M.; Lee, C.L.; Cook, L.S. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; HTA Initiative #13; Health Technology Assessment Unit Alberta Heritage Foundation for Medical Research: Edmonton, AB, Canada, 2004. [Google Scholar]
- Szivak, T.K.; Lee, E.C.; Saenz, C.; Flanagan, S.D.; Focht, B.C.; Volek, J.S.; Maresh, C.M.; Kraemer, W.J. Adrenal stress and physical performance during military survival training. Aerosp. Med. Hum. Perform. 2018, 89, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mourtakos, S.; Philippou, A.; Papageorgiou, A.; Lembessis, P.; Zaharinova, S.; Hasanova, Y.; Koynova, R.; Bersimis, F.; Tenchov, B.; Geladas, N.; et al. The effect of prolonged intense physical exercise of special forces volunteers on their plasma protein denaturation profile examined by differential scanning calorimetry. J. Therm. Biol. 2021, 96, 102860. [Google Scholar] [CrossRef] [PubMed]
- Nindl, B.C.; Friedl, K.E.; Frykman, P.N.; Marchitelli, L.J.; Shippee, R.L.; Patton, J.F. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int. J. Sports Med. 1997, 18, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hamarsland, H.; Paulsen, G.; Solberg, P.A.; Slaathaug, O.G.; Raastad, T. Depressed physical performance outlasts hormonal disturbances after military training. Med. Sci. Sports Exerc. 2018, 50, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Conkright, W.R.; Barringer, N.D.; Lescure, P.B.; Feeney, K.A.; Smith, M.A.; Nindl, B.C. Differential recovery rates of fitness following U.S. Army Ranger training. J. Sci. Med. Sport 2020, 23, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.M.S.; Neves, E.B.; Fortes, M.S.R.; Martinez, E.C.; Ferreira, O. The influence of combat simulation exercises on indirect markers of muscle damage in soldiers of the Brazilian army. Biosci. J. 2018, 34, 1051–1061. [Google Scholar] [CrossRef]
- Vikmoen, O.; Teien, H.K.; Raustøl, M.; Aandstad, A.; Tansø, R.; Gulliksrud, K.; Skare, M.; Raastad, T. Sex differences in the physiological response to a demanding military field exercise. Scand. J. Med. Sci. Sports 2020, 30, 1348–1359. [Google Scholar] [CrossRef]
- Kyröläinen, H.; Karinkanta, J.; Santtila, M.; Koski, H.; Mäntysaari, M.; Pullinen, T. Hormonal responses during a prolonged military field exercise with variable exercise intensity. Eur. J. Appl. Physiol. 2008, 102, 539–546. [Google Scholar] [CrossRef]
- Henning, P.C.; Scofield, D.E.; Spiering, B.A.; Staab, J.S.; Matheny, R.W., Jr.; Smith, M.A.; Bhasin, S.; Nindl, B.C. Recovery of endocrine and inflammatory mediators following an extended energy deficit. J. Clin. Endocrinol. Metab. 2014, 99, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Gunga, H.C.; Wittels, P.; Günther, T.; Kanduth, B.; Vormann, J.; Röcker, L.; Kirsch, K. Erythropoietin in 29 men during and after prolonged physical stress combined with food and fluid deprivation. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Opstad, K. Circadian rhythm of hormones is extinguished during prolonged physical stress, sleep and energy deficiency in young men. Eur. J. Endocrinol. 1994, 131, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Opstad, P.K.; Aakvaag, A. Decreased serum levels of oestradiol, testosterone and prolactin during prolonged physical strain and sleep deprivation, and the influence of a high calorie diet. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Opstad, P.K. The hypothalamo-pituitary regulation of androgen secretion in young men after prolonged physical stress combined with energy and sleep deprivation. Acta Endocrinol. 1992, 127, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Gwin, J.A.; Wolfe, R.R.; Pasiakos, S.M.; Ferrando, A.A. Mitigation of muscle loss in stressed physiology: Military relevance. Nutrients 2019, 11, 1703. [Google Scholar] [CrossRef] [PubMed]
- Trousselard, M.; Cian, C.; Barraud, P.-A.; Ferhani, O.; Roux, A.; Claverie, D.; Canini, F.; Baert, P. Physiological and psychological effects of escape from a sunken submarine on shore and at sea. Aviat. Space Environ. Med. 2009, 80, 850–856. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).