1. Introduction
Breath-hold diving has been traditionally practiced by humans exposing their bodies to physiological limits for professional and recreational reasons (e.g., harvesting natural profitable products from the seabed, fishing, freediving for leisure), as well as for participation in underwater sports (e.g., synchronized swimming, underwater hockey, and rugby). More recently freediving has emerged as a competitive sport. Among the most known modalities of freediving is static apnea, where the goal is to voluntarily hold the breath for as long as possible while motionlessly floating face down on the surface of the water, without compromising consciousness. Observable signs of hypoxic loss of muscle control or syncope constitute reasons for breath-hold termination and disqualification from apnea competition [
1]. A series of safety maneuvers are followed during competitions to prevent the divers from becoming severely hypoxic, thus limiting overambitious attempts. The ongoing pursuit for new records has led to the achievement of impressively long breath-hold durations in today’s elite apneists, who are capable of withstanding impressive physiological and psychological challenges. The apnea performance holding the men’s world record in static apnea was achieved in 2009 and lasted for 11:35 min (
https://www.aidainternational.org/ (accessed on 1 September 2023)).
A review of several studies on static apnea suggests that the factors determining the limits of apneic duration are: gas storage capacity in lungs, blood and tissues, metabolic rate, and tolerance to asphyxia [
2]. It has been demonstrated that apnea with face immersion induces more intense physiological responses than apnea without face immersion [
3]. Moreover, exercise concurrent to apnea with water immersion modifies the diving response compared to rest, as the diving reflex is affected by the muscular and oxygen (O
2) demands [
4,
5,
6].
The diving response in humans, which consists mainly of bradycardia and peripheral vasoconstriction to conserve oxygen for the vital organs, is characterized by great individual variability and is affected by lung volumes, chemosensitivity, the ability for mental relaxation, and diving experience, among other factors [
2,
7]. Elite apneists are able to suppress the urge to breathe, sustain greater arterial O
2 desaturation, and develop an earlier and more pronounced bradycardia, thus accomplishing impressively long breath-hold times.
High lung volumes (or forced vital capacity, FVC) are a feature prolonging static apnea duration and distinguishing apnea divers. “Lung packing” or “glossopharyngeal breathing” maneuvers, during which the oral cavity and tongue are used as a pump to add a small volume of air into the lungs, are frequently used by apneists to increase lung volume just before diving [
8]. Another major factor determining blood O
2 storage capacity, but also increasing carbon dioxide (CO
2)-buffering capacity, and thus influencing apneic duration, are the circulating hemoglobin levels [
2,
9].
In static apnea, when no working muscles compete for blood flow, centralization of blood flow is facilitated in order to conserve oxygen for the vital organs. This occurs by decreasing heart rate (HR), thereby reducing the O
2 demand of the cardiac muscle, and by redistributing blood from the periphery to central circulation, resulting in reduced oxidative metabolism and increased reliance on anaerobic metabolism in underperfused areas [
2]. An increased anaerobic metabolism and progressive acidosis due to the accumulation of CO
2 and lactate could also be considered as factors limiting the duration of apnea. Metabolism and apnea performance can be influenced by psychological stamina, thermal factors, dietary status, and the ability to reach a relaxed state before apnea [
2,
10].
Several studies aiming to investigate and understand apnea and its physiological responses have carried out experiments in the laboratory with face immersion (e.g., using water-filled containers) providing great knowledge to the scientific community [
11,
12,
13,
14], but there are scarce data on physiological responses of well-trained apneists during an official apnea competition or under conditions simulating an actual competition [
15,
16,
17,
18,
19].
This study was conducted to explore physiological responses during static apnea performed under conditions simulating an actual competition and their association with apnea duration, in breath-hold divers who have acquired high physiological adaptations resulting from long-term apnea training.
2. Materials and Methods
The methods and procedures employed in the study were in accordance with the Declaration of Helsinki and were approved by the local ethics committee (no. 1452/11-01-2023).
2.1. Participants
In the present study, 9 healthy and well-trained male apneists were selected from a pool of 30 breath-hold divers. The athletes were selected according to the following criteria: (a) non-smoking, (b) a diving experience of at least 1 year, and (c) capability of exerting maximal effort and achieving long apnea times by significantly overcoming inhibiting factors such as physical discomfort, lack of concentration, and psychological fatigue. Those athletes who did not meet the above criteria were excluded from the study. Their weekly training routine consisted of 2–4 sessions per week, each lasting 1–1.5 h of aerobic and anaerobic-based swimming exercise interspersed with dynamic and static apnea efforts. No intense training was performed for 3–5 days preceding the apneic test. On the experimental day, participants were asked to abstain from coffee or caffeine-containing beverages and to consume their last meal (low in fat) at least 6 h prior to reporting to the swimming pool for the breath-hold testing. All the procedures and possible risks were thoroughly explained to the participants, and their written consent was obtained.
2.2. Procedures
2.2.1. Preliminary Visit
One day prior to the experiment, the participants were given all the necessary information about the experimental procedure, including the schedule sheet, and signed a written consent for their participation. On the same day, their body mass and height were measured, and their chronological age as well as the period they followed a systematic apnea training program were recorded.
2.2.2. Apnea Test
Setting
The experiment was carried out in an indoor swimming pool (water temperature 26–27 °C) in the morning/afternoon hours (09:30–15:00). The whole procedure simulated an actual “static apnea competition” under field conditions, according to the competition rules and regulations set out by the International Association for the Development of Apnea (AIDA) [
1], in the presence of an experienced judge and medical supervision. To conduct the experiment, one lane, 25 m long and 2.5 m wide, was split into 2 zones; zone 1 was used as the warm-up area and zone 2 was used as the test area. The participant entered the swimming pool wearing a whole-body neoprene wetsuit with a thickness ranging from 3.5 to 5 mm, goggles, and a special clip for sealing the nose. The participant could start the warm-up in zone 1 no earlier than 45 min before his personal “time 0”. The warm-up consisted of up to 4 static apneas with an interval of at least 10 min between efforts. In each zone, only one participant and the safety attendant of the corresponding zone could be present. Participants entered zone 2 every 20 min.
Before “Time 0”
Prior to starting the warm-up, hemoglobin concentration ([Hb]), blood lactate concentration ([La]), and FVC were measured for each participant. The measurement of FVC was carried out with the trunk vertically submerged in the water and the hands resting on the sidewall of the pool. Moreover, the transmitter of a telemetric HR monitor was placed inside the participant’s wetsuit. Two participants did not perform any static apnea during their warm-up (personal tactic). During relaxation the participants breathed freely but were advised to avoid hyperventilation. While in zone 2 and 5 min before the start of the test, the participants assumed a prone position on the surface of the pool with the left hand and part of the left arm in a relaxed position out of the water resting on the perimeter tiles of the pool or on floating foam boards, if needed, in order to ensure proper connection and smooth operation of the pulse oximeter.
Maximum Static Apnea
Upon completion of the warm-up, participants performed a maximal static apnea, the termination of which was determined by the participants themselves. In zone 2, the participant was informed of the time remaining until “time 0” at regular intervals followed by a 10-s countdown, allowing the participant to coordinate his last maximal inspiration with the start of the test. At “time 0”, the participant, having completed his last maximal inspiration, laid his head prone and relaxed on the surface of the water, so that the forehead and the airways were completely submerged in the water. With the entry of the face into the water, the digital timers used for recording the duration of the apneic effort and coordinating the collection of HR and oxygen saturation (SaO2) data were also activated. The participant exerted his best apneic effort, which he voluntarily terminated when he could not sustain the discomfort any longer without compromising consciousness.
Safety Routine
During the apneic effort the participant was obliged to respond to external stimuli (“checks”) of a specific type which were given to him by the safety attendant at specific time intervals [
1]. The participant’s response had to be identical to those external stimuli. For example, moving the same finger in the same manner (e.g., tapping once, twice, or three times) in each check. The participant could terminate his apneic effort at any moment he felt he had reached his personal limits. If the participant did not appropriately respond to a “check”, the safety attendant would end the test, and pull him out of the water. None of the participants included in this study faced such an event. With the voluntary exit of the face from the water the digital timer stopped recording the apnea duration. The time was considered valid as long as the participant did not show any symptoms of loss of motor control, orientation and senses [
1]. Compliance with the above conditions was confirmed by the physician (a cardiologist with extensive experience in sports apnea competitions) who was present, as well as an official match judge (a rescuer certified as an ‘advanced O
2 advantage instructor’ by DAN, an international Divers Alert Network) who was also present during the experiment.
2.3. Measurements
Body mass and height were measured using a medical scale (Bilance Salus, Milano, Italy) with no shoes and prior to wearing the whole-body neoprene wetsuit. Body mass index (BMI) was then calculated as body mass adjusted for height. FVC was measured before the start of the warm-up with a portable spirometer (Micro Medical, CareFusion, Kent, UK). [Hb] was determined shortly before the start of the warm-up with a portable analyzer using 10 µL samples of capillary blood drawn from a fingertip (Dr. Lange miniphotometer LP2, Oststeinbek, Germany). SaO2 and HR were measured for 1 min before “time 0” (resting HR), during the apneic effort, and for 1 min of the recovery period, recording values every 5 s. SaO2 was measured with a pulse oximeter attached to the index finger (Nellcor Symphony N-3000-I10, Minneapolis, MN, USA). The lowest value of SaO2 observed post-apnea was recorded as the minimum SaO2 value (minSaO2). HR was measured with a telemetric HR monitor (Polar S640, Kempele, Finland). The lowest value of HR observed during 10-s intervals (i.e., for two consecutive 5-s intervals) throughout apnea was recorded as the minimum HR value (minHR) and its difference from resting HR as ΔHR. Blood [La] was measured at rest (i.e., before the start of the warm-up) as well as at the 3rd and 5th minute of recovery using fingertip blood sampling and a portable analyzer (BM-Lactate Accusport Analyzer, Roche, Indianapolis, IN, USA). The highest of the two recovery [La] values was used for calculating the post- to pre-apnea difference in [La] (Δ[La]). A video camera (Sony, DCR-TRV 140, Tokyo, Japan) recorded the effort of each participant from the time he entered the test zone (zone 2) until his exit from it. Each participant’s total duration of apnea was measured with a digital stopwatch (Casio, HS-70W-1DF, Tokyo, Japan) in seconds. From this maximal static apnea, the data of the present research were collected. Upon completion of the test, all data were transferred to a PC.
2.4. Statistical Analysis
Statistical analyses were performed using Statistica 10 (StatSoft, Tulsa, OK, USA). All data are reported as mean ± SD. Possible correlations between measured variables were explored via calculating the Pearson product-moment correlation coefficient (r) for nondirectional tests (two-tailed). A regression analysis was conducted using the duration of apnea as the dependent variable and FVC together with minSaO2 as the independent variable. From regression analysis, R squared and the level of significance were calculated. The rate of decline in SaO2 for every participant was calculated from beta values using a linear equation. The level of significance for all statistical analyses was set at p < 0.05. With the sample size set at n = 9 and the level of significance at p = 0.05, the calculated statistical power values for the reported correlation and regression analyses were ≥0.70.
3. Results
Participants’ characteristics are presented in
Table 1. Chronological age ranged from 24 to 35 years, diving experience from 1 to 4 years, body mass from 67 to 85 kg, height from 170 to 191 cm, body mass index (BMI) from 20 to 27, FVC from 4.44 to 8.48 L, and [Hb] from 14.6 to 17.3 g/dL.
The total duration of apnea, average and minimum values of SaO
2% and HR, as well as post- to pre-apnea lactate concentration differences (Δ[La]) are presented in
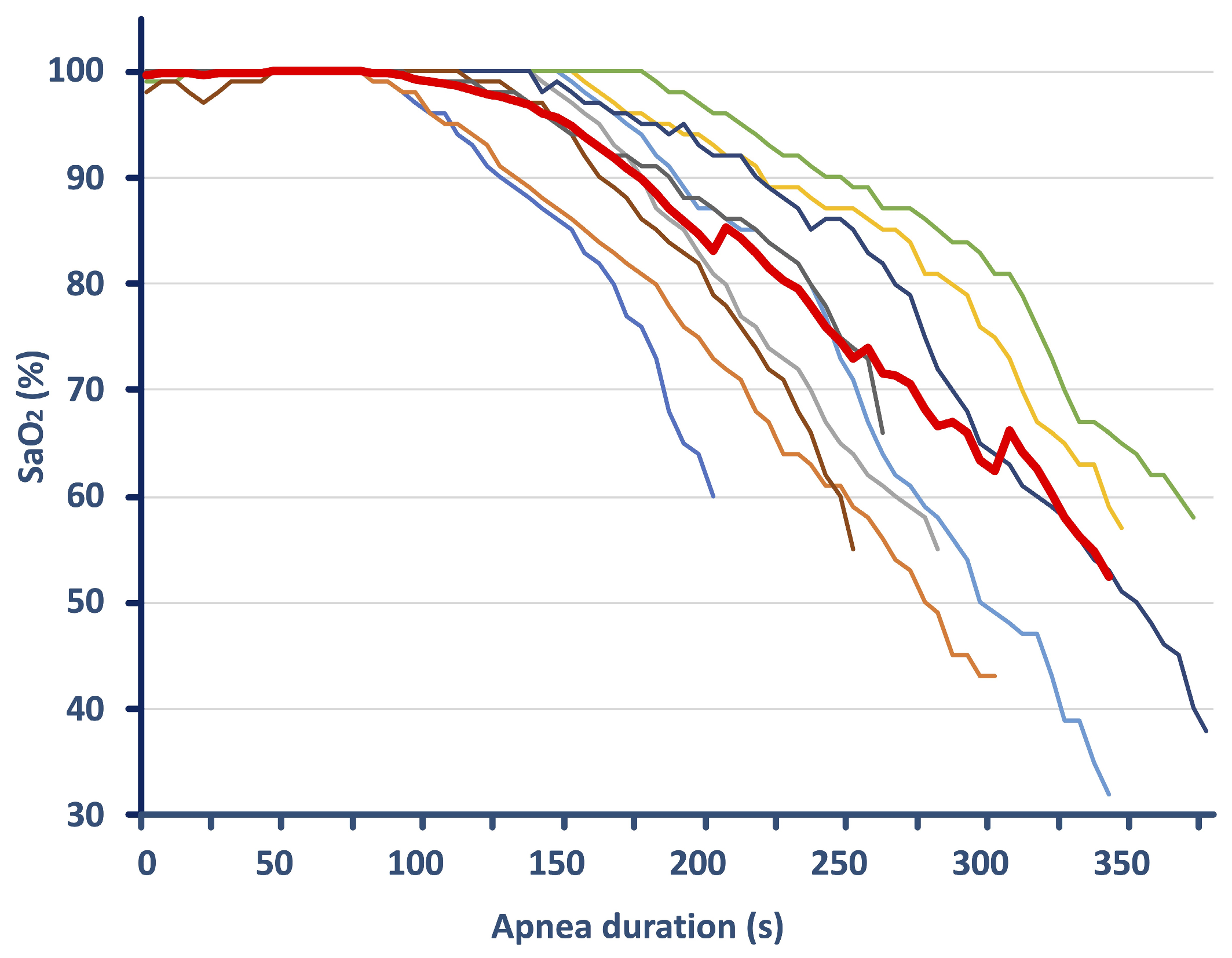
Table 2. The apneic effort lasted on average ~5 ± 1 min, with individual values ranging from 200 to 375 s.
During apneas, approximately 2 min (116 ± 35 s) elapsed without O
2 desaturation, after which SaO
2 dropped, with its average values reduced to 85 ± 3%. MinSaO
2 reached 47 ± 12%, ranging from 29–65%, and was recorded in the first 16 ± 8 s following the termination of apnea. The rate of decline in SaO
2 was similar among subjects (mean beta values of −1.4 ± 0.3,
Figure 1). Over the course of the apneic effort, bradycardia was observed, with average HR being reduced to 69 ± 12 bpm (a reduction of 31%). The HR drop from resting values was 52 ± 15 bpm, reaching minimum HR values of 49 ± 8 bpm with a range of 41–67 bpm. [La] increased by 0.81 ± 0.69 mmol/L from rest to post-apnea.
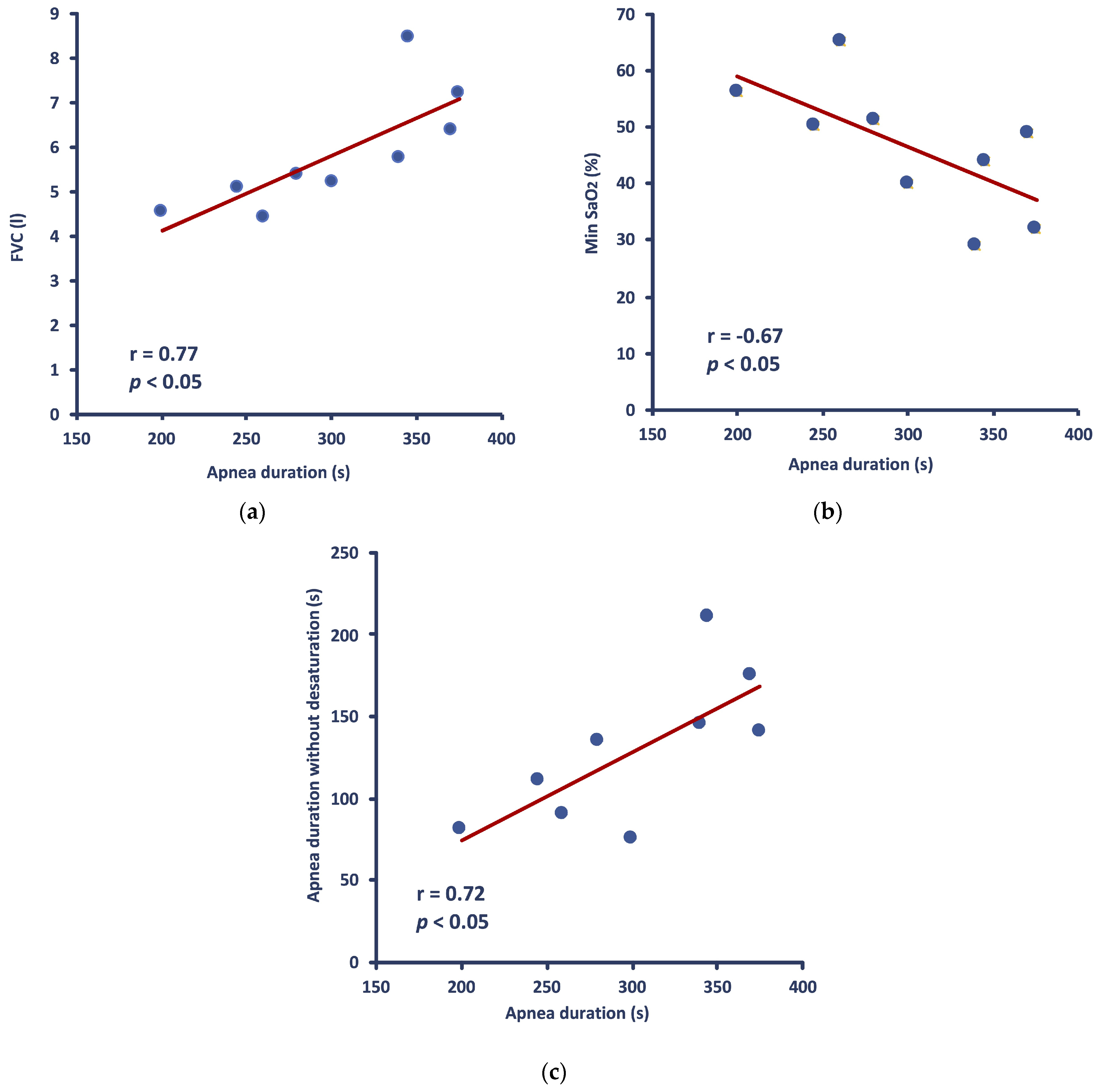
Apnea duration was positively correlated with FVC (r = 0.771,
p = 0.015) (
Figure 2a) and negatively correlated with minSaO
2 (r = −0.672,
p = 0.047) (
Figure 2b). There was also a positive correlation between the total apnea duration and the duration of the apneic effort phase during which no drop in SaO
2 occurred (r = 0.736,
p = 0.024) (
Figure 2c), whereas no correlation was observed between apnea duration and the average SaO
2 throughout apnea. Similarly, no significant correlation was found between apnea duration and either min HR or ΔHR or average HR throughout apnea. Lastly, neither [Hb] nor Δ[La] showed any significant correlation with apnea duration.
HR (either average, min, or Δ values) was not correlated with SaO2 (either average or min values). However, average HR during apnea was significantly correlated with the slope of SaO2 drop (r = 0.717, p = 0.030). Moreover, minHR was significantly correlated (r = 0.685, p = 0.042), and average HR tended to correlate (r = 0.645, p = 0.061) with [Hb]. Also, there was a significant correlation between FVC and the duration of the apneic effort phase during which no drop in SaO2 occurred (r = 0.773, p = 0.015).
When FVC and minSaO2 are combined in a regression model [F(2,6) = 6.84, p = 0.028], they can explain 70% of the variance in apnea duration.
4. Discussion
The present study examined physiological responses in well-trained divers who underwent static apnea testing in a simulated competition setting. They achieved remarkably long breath-hold times and reached severe hypoxemic levels, exhibiting varying levels of O2 desaturation and diverse bradycardic responses. Apnea duration was related to high FVC and delayed onset of O2 desaturation. The minimum level of O2 saturation attained during the apneic effort, which seems to play a role in diving safety, was also associated with maximal breath-hold duration.
The major strength of this piece of research is that all participants were well-trained in apnea, experienced in competitions, and were studied during a protocol simulating an actual static apnea competition, thus increasing the applicability of the study.
Schagatay et al. [
20] have also reported a close association between vital capacity (VC) and apnea performance in elite apneists. However, in that study, apnea performance was judged as accumulated competition scores from several types of dives (not only static apnea) and VC was measured on a different day in the lab with the divers in the standing position, outside the water without lung packing. In our study, we showed a significant correlation between static apnea duration and FVC measured while divers were preparing for performing maximal apnea under conditions simulating actual competition (approximately 1 h before), with the trunk vertically submerged in the water and without lung packing. This difference constitutes the novelty in our findings.
4.1. O2 Desaturation
During breath-holding, the O
2 stores in the lungs and blood are progressively diminished until the partial pressure of O
2 in the brain becomes extremely low, increasing the risk for loss of consciousness in divers [
16,
17]. The minimum O
2 saturation attained in our study (46 ± 11%) is in agreement with the notion made by Bain et al. [
10] that it is common for motivated elite apneists to terminate their maximal apneic effort slightly before reaching an SaO
2 of ~50%, a value approximating the theoretical limit for consciousness in humans [
16,
21]. It is thus suggested that P50 (i.e., the O
2 tension when hemoglobin is 50% saturated with O
2) exerts a significant hypoxic stress for the termination of apnea. There were, however, two of the best performers with apnea durations of about 6 min, who managed to reduce their SaO
2 saturation to much lower values (close to 30%). Overall, in the group of the nine well-trained participants, it is notable that the minSaO
2 showed a significant negative correlation with total apnea duration.
MinSaO
2 was recorded in the first ~20 s of recovery, after breathing was resumed. This delay could be attributed to a slow perfusion rate due to apnea-induced peripheral vasoconstriction. The nadir SaO
2 corresponds to the SaO
2 in the blood leaving the lungs at the end of apnea, but the detection of this minimum value by the finger probe of the pulse oximeter is delayed due to the circulation time from the lungs to the finger [
22,
23]. In order to assure that hypoxic syncope will not occur or will have no major complications [
18], it is important to monitor a diver for at least 20–40 s after the termination of the apneic effort [
22], as was followed in the present study.
During the first ~2 min of the apnea test, no drop in SaO2 was observed, most probably due to the pre-apneic decrease in respiratory rate, resulting from the relaxation techniques performed before the test and the attenuation of metabolism. During this phase, SaO2 remained unchanged for as long as the O2 levels in the lung remained relatively high. This is also evident from the significant correlation found between FVC and the duration of the first phase of apnea, during which no desaturation occurred.
4.2. O2 Storage Capacity
In elite and motivated apneists, breath-hold duration depends on bodily O
2 storage capacity (high lung volumes and [Hb] levels) and the ability to conserve O
2 (redistribution of blood flow to central organs and attenuated oxidative metabolic rate) [
2,
10]. The role of O
2 storage appears to be important for the apneic performance in the specific sample of trained individuals tested in the present study, as judged by the strong correlation between FVC and total apnea duration. Lung volume was “reinforced” due to the lung packing preceding the apneic effort in most (seven out of nine) of the participants in the present study. Even when the two participants who did not perform lung packing were excluded from the analysis, the correlation coefficient still appeared to be significant (r = 0.793,
p = 0.033). The ability to “pack” more air in the lungs attenuates the rate of blood O
2 desaturation during a breath-hold and dilutes CO
2 in the lungs [
2]. Accordingly, trained apneists have larger lung volumes, which could in part be attributed to practiced glossopharyngeal insufflation and strengthened respiratory muscles, as it is not known whether this is an inherent characteristic [
24,
25]. On the other hand, it should be mentioned that the increased intrathoracic pressure associated with lung packing likely impedes venous return, thus increasing risks for substantial decreases in stroke volume, cardiac output, blood pressure, and cerebral blood flow, with detrimental consequences to the diver (varying from dizziness to even fainting) at the beginning of the apnea [
26,
27], while possible lung damage has also been reported [
28].
O
2 storage could be judged also from [Hb] levels, but there was no correlation found between baseline [Hb] and apnea duration in this study. Repeated apneas have been shown to increase [Hb] via splenic contraction [
29] with dynamic apneas being more effective at increasing [Hb] in elite breath-hold divers [
30], but whether the participants of the present study experienced such a response during their single static apneic effort is purely speculative, since we have no post-apneic measurements of [Hb]. However, there was a significant correlation between [Hb] and minHR; the higher the [Hb] the less the bradycardia. Those who had the advantage of higher O
2 storage in their erythrocytes had probably less need to lower their HR during apnea. Nevertheless, this finding could not be a direct cause and effect relationship, and more research is needed to confirm it. Moreover, a slower rate of O
2 desaturation has been observed in divers with a more pronounced bradycardic and hypertensive response to breath-holding [
31] who can also endure apnea for longer durations [
32]. In agreement with this, in our study, average HR during apnea was significantly correlated with the slope of SaO
2 drop. Blood pressure was not measured in the present study to evaluate its influence on HR response and apnea duration, but during prolonged static apnea, cardiac and vascular adjustments may be separately controlled [
33,
34]. Additionally, even though not evaluated in the present study, involuntary breathing movements, more probable to occur during static apnea, as well as cardiac arrhythmias, detected in the later phases of static apnea [
19,
35], may also contribute to the HR response.
4.3. Bradycardia
HR reduction is often monitored to give an indication of the magnitude of the diving response and is related to apnea competition results in elite apnea divers—the lower the heart rate, the better their score in a world championship [
2,
36]. Prior to and during apnea, the divers of this study tried to relax as completely as possible, and this relaxation would facilitate their performance by means of stronger bradycardia and reduced O
2 consumption. In contrast, Lindholm et al. [
17] reported that during actual static apnea competition, HR values were higher than in an apnea training session, concluding that in a competitive situation the mental stress caused by the anticipation of maximally performing on a single dive tends to increase HR prior to apnea, and during the initial stages thereof. In the same study it was also found that the HR declined with apnea time. In the present study, however, the magnitude of bradycardia was not correlated with static apnea duration, probably because our divers were performing under a simulative and not an actual competitive condition, with a lesser stress-induced tachycardia prior to the apneic effort.
4.4. Anaerobic Metabolism
Lactate accumulation has been looked at as an O
2 conserving mechanism in freedivers [
37], as an increase in blood lactate indicates that the O
2 supply to the non-essential organs is effectively cut off. Underperfused muscles do not receive sufficient O
2 and shift rapidly to anaerobic metabolism, with lactate accumulation being more profound in dynamic apnea than in apnea without exercise [
10]. All participants in our study showed an increased blood [La] compared to pre-apneic blood [La], but this increase was not significantly correlated with apnea duration, neither with O
2 desaturation. Interestingly, however, the participant who had the highest pre- to post-apnea [La] difference (2.18 mmol/L) also exhibited the lowest value in average SaO
2 throughout apnea (81%), possibly indicating the effect of acidosis on the reduction of O
2 affinity with hemoglobin (Bohr effect). Such an association might have been clearly observed with a larger group of participants.
4.5. Limitations
A main limitation of the present study was the small number of participants, who, however, exhibited considerable variability in their responses. Moreover, the warm-up routine was not standardized, and hemodynamic measures were not carried out. Future research should be conducted on a larger sample of well-trained apneists with high physiological adaptations acquired from long-term apnea training, and should incorporate measures of blood pressure and HR variability to elucidate the role of the sympathetic and parasympathetic systems on the physiological responses of their apneic performance.