The Involvement of Lipid Mediators in the Mechanisms of Exercise-Induced Muscle Damage
Abstract
1. Introduction
2. Search Methodology
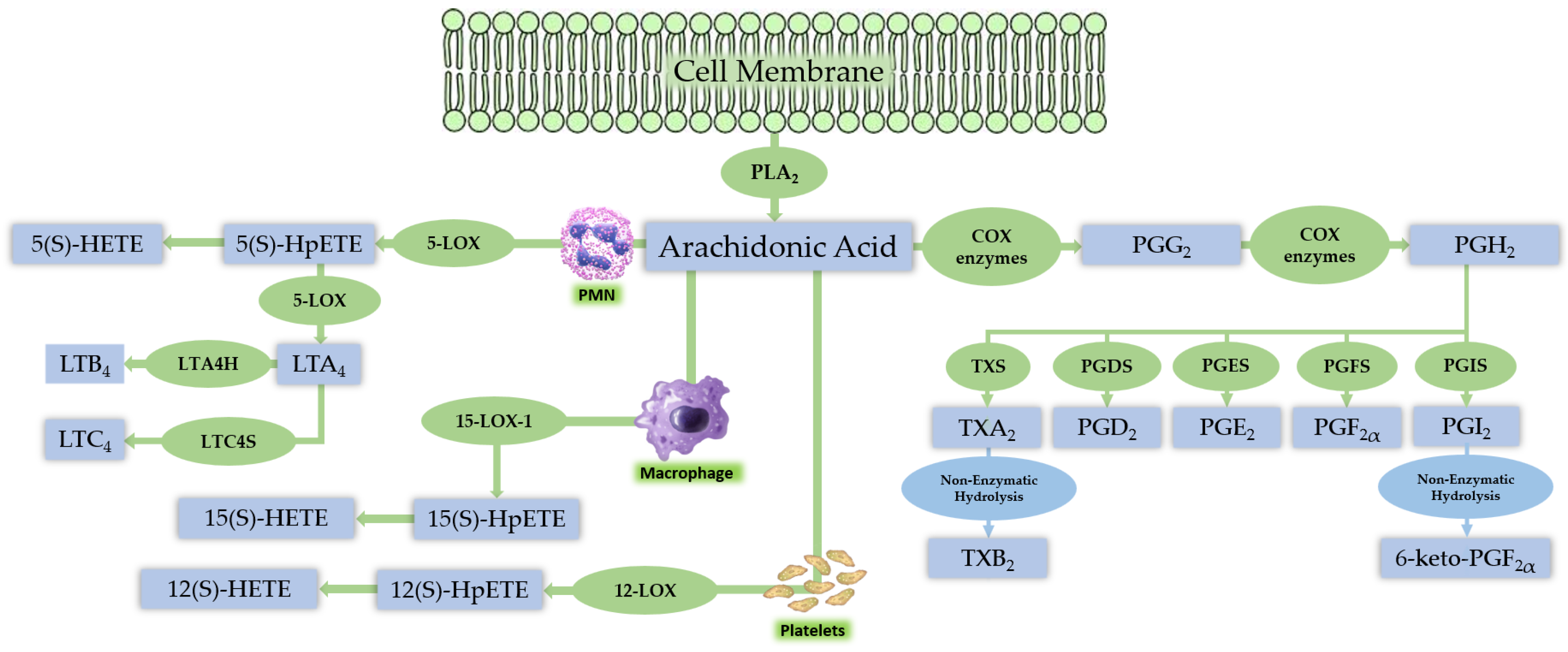
3. Lipid Mediator Pathways
3.1. Polyunsaturated Fatty Acid-Derived Lipid Mediator Pathways
Pro-Resolving Lipid Mediators
3.2. Platelet Activating Factor
3.3. Sphingolipids
Sphingolipid Metabolism in Skeletal Muscle
3.4. The Endocannabinoid System
3.5. Exercise-Induced Muscle Damage
3.5.1. PUFA-Derived Lipid Mediators in EIMD Interventions in Humans
| Subjects | Design/Intervention | Biological Samples/Time Points | Variables | Effect on Lipid Mediators and EIMD Markers | Reference |
|---|---|---|---|---|---|
| 10 ♂, healthy, moderately active (22 ± 0.4 years old) | Intervention Exercise: 8 × 5 maximal EC knee extensor and flexor at 60°/s angular velocity on both legs (eccentric and concentric trials) | Plasma/Serum: pre-ex, 10 min, 30 min, 24 h, 48 h post-exercise | PT (eccentric and concentric), DOMS Plasma elastase, myeloperoxidase, Serum CK, Mb, Plasma PGE2 | ↔ PGE2 ↑ CK post-ex for both concentric and eccentric. Peak CK at 48 h. ↑ CK in eccentric vs. concentric. ↑ DOMS in | Croisier et al., 1996 [64] |
| 10 (9 ♂, 1 ♀) healthy, non-obese (25.7 years old) | Exercise: One side: 2 × 50 concentric/eccentric contractions of the calf muscles 24 h before the start of microdialysis (exercised leg) Other side: Calf (untreated, control) Biceps (normal saline) Microdialysis 24 h after exercise for 80 min (legs and arms) 2 Pain stimulations during dialysis: Legs (2 × 10 dorsal and plantar flexions of the foot, 10 min between sets). Arms/biceps (normal saline and hypertonic saline injection, 5 × 200 μL 5.8 % NaCl, 2 min interval into the biceps muscle). | BC: pre-ex, 0 h, 24 h post-ex Microdialysis: 20 min dialysate sample during pain stimulation on legs | Calf circumference, DOMS (legs and arms) Serum: CK, Lactate Microdialysis: PGE2, Nitric oxide, Substance P, Glutamate | No difference in PGE2 between exercised and control leg without pain stimulation ↑ PGE2 in exercised leg following second pain stimulation ↑ muscle soreness in the exercised leg pre-pains stimulation ↑ muscle soreness in exercised leg during pain stimulation | Tegeder et al., 2002 [58] |
| 10 ♂, healthy, untrained (20.4 ± 2.0 years old) | Intervention Exercise: 2 bouts EC of the elbow flexors on the non-dominant arm separated by 4 weeks | Plasma: pre-ex, 6 h, 24 h, 48 h, 72 h, 96 h post-ex | IL-1β, IL-1ra, IL-4, IL-6, IL-8, IL-10, IL-12p40, TNF-α, G-CSF, MPO, HSP60 HSP70, DOMS, Upper arm circumference (UAC), MIS (90°), ROM (FANG, RANG), CK, Mb, PGE2 | ↔ PGE2 after both bouts of exercise ↑ CK and Mb after first bout up to 4 days ↔ CK and Mb after second bout ↑ DOMS and UAC after first bout vs. second bout ↓ MIS and ROM after first bout vs. second bout | Hirose et al., 2004 [66] |
| 12 ♂, healthy, recreationally active (28 ± 1.5 years old) | Intervention Exercise: Rebounds at 70% of maximal height until exhaustion | Serum: pre-ex, 0 h, 2 h, 2 d, 8 d post-ex | MIF (plantar flexor muscle), Leukocytes, CRP, IL-6, Substance P, CK PGE2 | ↑ PGE2 at 2 h post-ex. Complete recovery at 8-d ↑ CK post-ex (Peak at 2 d) ↓ MIF at 0 h and 2 d post-ex | Dousset et al., 2007 [60] |
| 16 recreationally active (8 ♂: 26 ± 1 yr; 8 ♀: 24 ± 1 yr) | Exercise: 10 × 10 EC with each leg at 120% of their concentric 1 RM | Muscle biopsy: Pre-ex, 4 h, 24 h post-ex | Real-Time RT-PCR: COX-2 COX1b variants (COX-1b1, -1b2, and -1b3) COX-1 (v1 and v2) | ↑ COX-2 at 4 h and 24 h ↔ COX-1 and COX-1b variants | Weinheimer et al., 2007 [67] |
| 40 ♂, army soldiers (19.1 ± 1.8 years old) | Exercise: bench press at 50% 1-RM, 4 x ~20 (n = 8) 75% 1-RM, 5 x ~ 11 (n = 7) 90% 1-RM, 10 x ~4 (n = 7) 110% 1-RM, 8 x ~4 (n = 7) control (no exercise, n = 6) | Plasma/Serum: pre-ex, 24 h, 48 h, 72 h post-ex | DOMS IL-1β, IL-6, TNF-α, CK PGE2 | ↑ PGE2 for all exercise groups. ↑ PGE2 for the 110% group at 24 and 48 h vs. other groups. ↑ CK for all groups post-ex, peak at 24–48 h. Correlation between peak DOMS and peak PGE2. Correlation between peak CK and peak PGE2. | Uchida et al., 2009 [61] |
|
17 ♀, healthy young (YW) (23.89 ± 2.03 years old) Post-menopausal (PMW) (51.13 ± 5.08 years old) | 5 × 6 maximal EC of the elbow flexors | Serum: pre-ex, 0 h, 24 h, 48 h, 72 h post-ex | Isometric Torque, DOMS, upper-arm circumference, ROM, IL-6, 10 IL-10, TNF-α, CK, PGE2 | ↑ PGE2 at 48 h and 72 h post-exercise in PWM vs. YW Intra-groups PGE2 unaltered for both PWM and YW ↓ Isometric Torque and DOMS in 24 h, 48 h, 72 h post-ex in both PMW and YW ↔ Isometric Torque and DOMS in PMW vs. YW ↑ CK in 72 h post-ex in YW ↔ CK in PMW vs. YW Positive correlation between age and PGE2 Negative correlations between age and DOMS at 48 h and 72 h post-ex | Conceição et al., 2012 [72] |
| 8 ♂, young, resistance trained for 2 years, unaccustomed to bench press exercise | 4 × 8 bench press EC at 70% of 1 RM. 2 bouts (2-week interval) | Serum: pre-ex, 24 h, 48 h, 72 h, 96 h post-ex | 1 RM strength measurement, 1 RM eccentric strength measurement (1 Rmecc), DOMS, CK, PGE2 | No significant interaction effect for PGE2 ↑ peak plasma PGE2 at 48 and 72 h after first bout vs. second bout ↓ 1 RM at 24 h after first bout vs. second bout ↑ peak DOMS at 48 h after first bout vs. second bout ↑ CK at 72 h after first bout vs. second bout | Meneghel, Adilson J. 2014 [73] |
| 12 ♂, untrained, recreationally active (18–25 years old) | Acute bout of maximal ECC knee extensions, unilateral | Muscle biopsy: pre-ex, 2 h, 4 h, 24 h post-ex | TXB2, 12(S) HHTrE, PGE2, PGF2α, 15-Deoxy-Δ12,14-prostaglandin J3, 5-hydroperoxy-eicosatetranoic acid (5-HpETE), 12-Oxo-LTB4, 20-COOH-LTB4, 12-hydroxy-eicosatetraenoic acid (12-HETE), Tetranor 12-HETE, 12-hydroxy-eicosapentaenoic acid (12-HEPE), 15-hydroxy-eicosatetranoic acid (15-HETE), 15-hydroxy-eicosapentaenoic acid (15-HEPE), 4-hydroxy-docosahexanoic acid (4-HdoHE), 7-hydroxy-docosahexanoic acid (7-HdoHE), 14-hydroperxy-docosahexanoic acid (14-HpDoHE), 17-hydroxy-docosahexanoic acid (17-HdoHE), lipoxins (LXA4, LXB4, LXA5), E-series resolvins (RvE1 & RvE3) D-series resolvins (RvD1, RvD2, RvD5, RvD6), protectins (PD1 & 10S,17S-DiHDoHE), maresins (MaR1), epoxyeicosatrienoic acid (EpETrE), dihydroxyeicosatrienoic acids (DiHETrEs), CYP epoxidase enzyme products (9(10) -EpOME and 12(13) -EpOME, 9(10)-DiHOME and 12(13)-DiHOME) | TXB2 ↑ to 3.73 ng/g at 2 h from 1.12 ng/g at baseline. 12(S)-HHTrE ↑ to 13.50 ng/g at 2 h from 7.68 ng/g at baseline. PGE2 ↑ to 2.84 ng/g at 2 h from 1.13 ng/g at baseline. PGF2α ↑ to 1.20 ng/g at 2 h from 0.68 ng/g at baseline. 15-Deoxy-Δ12,14-prostaglandin J3 ↑ to 4.45 ng/g at 2 h from 3.09 ng/g at baseline. 5-HETE ↑ to 8.99 ng/g at 2 h from 3.38 ng/g at baseline. 12-Oxo-LTB4 and 20-COOH-LTB4 detected in resting muscle and ↑ at 2 h to 2.29 ng/g and 5.33 ng/g. 12-HETE to 63.81 ng/g ↑ at 2 h from 22.51 ng/g at baseline. Tetranor 12-HETE ↑ to 3.97 ng/g at 2 h from 0.62 ng/g at baseline. 12 HEPE ↑ at 2 h after exercise. 7-HdoHE ↑ to 1.54 ng/g at 2 h from 0.98 ng/g at baseline. 14-HdoHE ↑ to 1.60 ng/g at 2 h from 0.68 ng/g at baseline. 5,6-EpETrE ↑ threefold to 14.12 ng/g at 2 h. 11,12-DiHETrE and 14,15-DiHETrE ↑ significantly at 2 h. 9(10)-and 12(13) -DiHOME were ↑ significantly at 2 h. 7-HdoHE ↑ to 1.54 ng/g at 2 h from 0.98 ng/g. 14-HdoHE ↑ to 1.60 ng/g at 2 h from 0.68 ng/g. 5,6-EpETrE ↑ 3-fold to 14.12 ng/g at 2 h. 11,12-DiHETrE and 14,15-DiHETrE ↑ at 2 h. 9(10)-and 12(13)-DiHOME ↑ at 2 h. | Vella et al., 2019 [10] |
| Subjects | Design/Intervention | Biological Samples/Time Points | Variables | Effect on Lipid Mediators and EIMD Markers | Reference |
|---|---|---|---|---|---|
| 20 ♂, college aged | Randomized placebo-controlled trial Aspirin (ASP, n = 10) (3 g/day) or PLA (n = 10) for 4 days, 1-day prior exercise. Exercise: 3 × 10 reps squat (70% of 1 RM). | Plasma/Serum: pre-ex, 24 h, 48 h, 72 h post-ex | Muscle Soreness, Salicylate, CK, PGF2α, PGE2 | ↑ PGF2α and PGE2 at 24 h returning to baseline at 72 h for PLA ↔ PGF2α and PGE2 at ASP ↑ CK post-ex in ASP and PLA ↔ CK between ASP and PLA No effect on muscle soreness | Boatwright et al., 1991 [56] |
| 10 ♂, healthy, moderately active (22 ± 0.4 years old) | Crossover placebo-controlled with piroxam (20 mg piroxicams/day, 3 days pre-ex) Exercise: 8 × 5 maximal EC knee extensor and flexor at 60°/s angular velocity on both legs | Plasma/Serum: pre-ex, 10 min, 30 min, 24 h and 48 h after exercise | DOMS, PT, Serum CK, Mb, Plasma PGE2 | ↓ PGE2 at rest and after exercise in piroxam group ↓ PGE2 in piroxam vs. PLA ↔ PGE2 over time for both groups No effect of piroxicam in DOMS, PT, CK and Mb | Croisier et al., 1996 [65] |
| 24 ♂, recreationally active | Double blind, placebo-controlled trial Placebo (PLA, n = 8) Ibuprofen (IBU 1200 mg, n = 8) Acetaminophen (ACET, n = 8) Exercise: Unilateral high intensity eccentric exercise 2 days after and ~24 h before muscle biopsies | Muscle biopsies: 2 d pre-ex, 24 h post-ex | PGE2, PGF2α | ↑ PGF2α in PLA (+77%) vs. IBU (−1%) and ACET (−14%) ↑ PGE2 in PLA (+64%) vs. ACET (−16%) | Trappe et al., 2001 [57] |
| 34 ♂, athletes (25.5 ± 3.2 years old) | Double-blind trial CR (4 × 5 g creatine monohydrate + 60 g maltodextrine, n = 18) PLA (carbohydrate, n = 16) 5 days. Exercise: 30 km run | Plasma: 15 min pre-ex, 24 h post-ex | TNF-α, CK, LDH, Creatinine, PGE2 | ↑ PGE2 in PLA and CR post-ex ↑ PGE2 in PLA vs. CR post-ex ↑ CK and TNF-α in PLA and CR post-ex ↑ LDH in PLA post-ex ↔ LDH in CR post-ex | Santos et al., 2004 [59] |
| 29 ♂, recreationally active (22.55 ± 4.42 years old) | Randomized, double-blinded, and placebo-controlled (cellulose) or proteolytic supplement (fungal proteases, bromelain, and papain, SUP). Exercise: 3 × 5 Isokinetic extension/flexion of the quadriceps Downhill running for 45 min at 60% VO2max 21 d after supplementation | Serum: baseline, pre-ex, 0 h, 3 h, 24 h, 48 h post-ex | PT, DOMS CK, IgG, IgA, IgM, IL-6, IL-8, IL-10, IL-12, TNF-α, IL-1β, SOD, Circulating Leucocytes, PGE2, 8-isoprostane (8-iso), COX-2 | ↑ COX2 at PLA vs. SUP ↑ COX2 at PLA vs. SUP group × time interaction for COX2 ↔ PGE2 between SUP and PLA ↔ PT and DOMS between SUP and PLA ↔ CK between SUP and PLA | Buford et al., 2009 [70] |
| 16 ♂, recreationally active (23 ± 1 years old) | Randomized placebo-controlled COX-2 inhibitor (celecoxib 600 mg/d, n = 8) Placebo (n = 8) Exercise: 10 × 10 repetitions of high-intensity eccentric knee extensions with each leg at 120% of their concentric 1 RM | [2H5]phenylalanine infusion at arrival Muscle biopsy: 2 h and 5 h BC: 2.5 h, 3.5 h, 4.5 h | Fractional synthesis rate, mRNA levels of COX-1 (1v1, 1v2) and COX-2, protein levels of COX-1 (1v1, 1v2) and COX-2 | ↑ COX-2 mRNA (3.0 ± 0.9-fold) at COX-2 inhibitor group vs. PLA ↔ COX-1 mRNA and COX-1 protein levels in both groups | Burd et al., 2010 [68] |
| 33 ♂, ♀, healthy, physically active (18–33 years old) | Randomized double-blind, placebo-controlled with celecoxib (400 mg/d, CEL), 3-week wash-out period Exercise: 14 × 5 eccentric unilateral contractions of the elbow flexors Microdialysis in exercised arm and non-exercised arm (control) after the first bout. | Muscle biopsy: 1 h, 2 h, 4 h and 7 d after bout 1 and 1 h and 2 d after bout 2 (n = 24). Serum: 1 h pre-ex, 1 h, 8 h, 20 h, 1 d, 2 d, 3 d, 4 d, 7 d, 9 d post-ex Microdialysis: 2–6 h post-ex and either 24 h (n = 5) or 48 h (n = 5) post-ex. | PT (isometric and concentric), RANG, DOMS Leucocyte number (CD68+), Number of satellite cells (CD56+), CK (serum) PGE2 (microdialysis) | ↔ PGE2 after first bout in both CEL and PLA ↑ CK after first bout but not second bout. ↔ CK between CEL and PLA ↓ DOMS on CEL vs. PLA after both bouts ↓ PT (isometric and concentric) after both bouts in both CEL and PLA. ↔ PT (isometric and concentric) between CEL and PLA | Paulsen et al., 2010 [71] |
| 45 ♂, healthy, untrained (29.7 ± 6.6 years old) | Randomized, double-blinded, repeated measures trial Groups: experimental (Omega-3 fish oil + 100 IU of d-a-tocopherol/dl-a-tocopherol acetate, EXP, n = 15) PLA (soybean/corn oil mixture + 100 IU of d-a-tocopherol/dl-a-tocopherol acetate, n = 15) Control (100 IU of d-a-tocopherol/dl-a-tocopherol acetate, CTL, n = 15) Exercise: Bench stepping exercises with eccentric patterns for 40 min (5 min stepping, 1 rest). | Plasma: baseline, pre-ex, 0 h, 24 h, 48 h post-ex | IL-6, TNF-a, CK, LDH, Mb, PGE2 | ↑ PGE2, CK, LDH, Mb for all groups after exercise ↓ PGE2, CK, LDH, Mb in EXP vs. PLA or control ↓ PGE2 levels post-ex, 24 h and 48 h for EXP vs. PLA and control ↓ PGE2 levels pre-ex vs. baseline in EXP ↓ elevation of PGE2 post-ex, 24 h, 48 h for EXP | Tartibian et al., 2011 [62] |
| 8 ♂, healthy (23 ± 3 years old | Indomethacin (NSAID) microdialysis infusion on vastus lateralis of one leg. Placebo microdialysis infusion on vastus lateralis of the other leg. Exercise: 200 eccentric contractions in each leg (100 at 30°/s and 100 at 120°/s). | Muscle biopsy: Pre-ex, 5 h, 24 h, 28 h and 8 d post-ex RNA extraction Real-time PCR | Growth factor genes, extracellular matrix-related genes, PGC1α, PPARγ, MCP1 Gene expression of COX-1 and COX-2 | ↑ COX-2 expression (6-fold) at 5 h post-ex in NSAID vs. PLA ↔ COX-1 expression in both groups over time ↔ COX-1 expression NSAID vs. PLA | Mikkelsen et al., 2011 [69] |
| 14 ♂, physically active (22.4 ± 1.7) | Randomized, double-blinded, crossover, placebo-controlled with American ginseng supplement 30 day-supplementation with 1600 mg/d American ginseng extract (AG) or 1600 mg/d hydroxymethylcellulose (PLA) Exercise: Downhill running at 60% VO2peak | Plasma: pre-supplementation, pre-ex, 0 h, 2 h, 24 h, 48 h and 72 h post-ex. | DOMS, TNF-α, IL-1β, IL-4, IL-10, CK, 8-iso-PGF2α | ↑ 8-iso- PGF2α at 0 h post-ex vs. pre-ex in AG and PLA ↓ 8-iso- PGF2α at 0 h, 2 h and 24 h post-ex in AG vs. PLA ↑ CK at 0 h, 2 h, 24 h, 48 h, 72 h post-ex in PLA. Peak at 24 h ↑ CK at 0 h, 2 h, 24 h post-ex in AG. Peak at 24 h. ↑ DOMS at 0 h, 2 h, 24 h, 48 h, 72 h post-ex. ↔ DOMS between AG and PLA. | Lin et al., 2021 [63] |
3.5.2. PUFA-Derived Lipid Mediators and EIMD in Animal and In-Vitro Studies
| Animal Model | Protocol | Sample | Variables | Effect on Lipid Mediators and EIMD Markers | Reference |
|---|---|---|---|---|---|
| 36 ♂ Wistar rats (9-weeks old) | Exercise: 100 repeated EC on plantar flexor muscles of left leg. Right leg as control. Precon group (n = 18): 10 repeated EC, 2 days pre-ex. Non-Precon group (n = 18): no EC pre-ex | Plantar flexor muscle at 0 d, 2 d, 4 d post-ex | Histochemical analysis mRNA levels: HGF, Pax7, MyoD, myogenin, BKB2, COX-2 | ↑ COX-2 mRNA at 2 d post-ex for both Precon and Non-Precon ↑ COX-2 mRNA at 4 d in non-Precon ↑ COX-2 mRNA in non-Precon vs. Precon | Nagahisa et al., 2018 [74] |
| Avian myoblasts | Mechanical stimulation: 5 × 20% substratum stretches and relaxations/20 s. 10 s rest. 3 times. (5 h, 24 h, 48 h) | - | Protein synthesis and degradation rate, Proteinase, CK, PGE2, PGF2α, 6-keto-PGF2α | ↑ PGE2 and PGF2α efflux by 97 and 41%, respectively within 4–5 h of mechanical stimulation. ↔ 6-keto-PGF1α | Vandenburgh et al., 1990 [75] |
3.5.3. PAF and EIMD Interventions in Humans
3.5.4. Human Studies with Sphingolipid-Derived Lipid Mediators
3.5.5. Animal Studies with Sphingolipid-Derived Lipid Mediators
| Animal Model | Stimuli | Sample | Assay | Effect on Lipid Mediators and EIMD Markers | Reference |
|---|---|---|---|---|---|
| 30 ♂ Wistar rats | 3 groups: 1-Control (resting). 2-Exercise until exhaustion. 3-gastrocnemius muscle contraction through sciatic nerve stimulation Electrical stimulation of sciatic nerve | Muscle samples: Soleus. Red (slow-twitch oxidative fibers) and white (fast twitch glycolytic fibers) section of the gastrocnemius muscle | Sphinganine, sphingosine | ↑ sphinganine in soleus vs. red gastrocnemiusat rest. ↑ sphinganine in red gastrocnemiusvs. white gastrocnemiusat rest. ↑ sphinganine (~6-fold) in each muscle after prolonged exercise. ↑ sphingosine in soleus and red gastrocnemius s white gastrocnemius. ↑ sphingosine (3-fold) in the soleus and red and white gastrocnemius (~2-fold) after prolonged exercise. | Dobrzyń et al., 2002 [78] |
| Mice ♂ (7-week-old) | Treadmill running at 5 m/min for 5 min, increasing to 10 m/min for 5 min, 15 m/min for 5 min, 20 m/min for 10 min | Serum and gastrocnemius muscle (white and red portion) at rest, 0 h and 24 h post-ex | IL-6, caspase-3, Protein levels of Serine palmitoyltransferase-1 (palmitoyltransferase-1 (SPT-1), acidic sphingomyelinase (A-Smase), neutral sphingomuelinase (N-Smase), Serum CK | ↑ levels of A-Smase at 0–24 h ↔ N-Smase levels ↔ SPT-1 levels ↑ CK at 24 h post-ex | Lee et al., 2019 [79] |
4. Synopsis
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Headland, S.E.; Norling, L.V. The Resolution of Inflammation: Principles and Challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; Kantharidis, P.; Cooper, M.E.; Godson, C. Pro-Resolving Lipid Mediators: Regulators of Inflammation, Metabolism and Kidney Function. Nat. Rev. Nephrol. 2021, 17, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Maddipati, K.R.; Cameron-Smith, D. Emerging Roles of Pro-Resolving Lipid Mediators in Immunological and Adaptive Responses to Exercise-Induced Muscle Injury. Exerc. Immunol. Rev. 2016, 22, 110–134. [Google Scholar]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory Monocytes Recruited after Skeletal Muscle Injury Switch into Antiinflammatory Macrophages to Support Myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and Inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Bernard, C.; Zavoriti, A.; Pucelle, Q.; Chazaud, B.; Gondin, J. Role of Macrophages during Skeletal Muscle Regeneration and Hypertrophy-Implications for Immunomodulatory Strategies. Physiol. Rep. 2022, 10, e15480. [Google Scholar] [CrossRef]
- Deslandes, A.; Moraes, H.; Ferreira, C.; Veiga, H.; Silveira, H.; Mouta, R.; Pompeu, F.A.; Coutinho, E.S.; Laks, J. Exercise and Mental Health: Many Reasons to Move. Neuropsychobiology 2009, 59, 191–198. [Google Scholar] [CrossRef]
- Silveira, L.S.; Antunes, B.M.; Minari, A.L.A.; dos Santos, R.V.T.; Neto, J.C.R.; Lira, F.S. Macrophage Polarization: Implications on Metabolic Diseases and the Role of Exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 115–132. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.F.; Farnfield, M.M.; Maddipati, K.R.; Russell, A.P.; Cameron-Smith, D. Intramuscular Inflammatory and Resolving Lipid Profile Responses to an Acute Bout of Resistance Exercise in Men. Physiol. Rep. 2019, 7, e14108. [Google Scholar] [CrossRef]
- Methenitis, S.; Stergiou, I.; Antonopoulou, S.; Nomikos, T. Can Exercise-Induced Muscle Damage Be a Good Model for the Investigation of the Anti-Inflammatory Properties of Diet in Humans? Biomedicines 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Sinzinger, H.; Virgolini, I. Effects of Exercise on Parameters of Blood Coagulation, Platelet Function and the Prostaglandin System. Sport. Med. 1988, 6, 238–245. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Sun, Y.; Woods, J.A. Chapter Fourteen—Exercise and the Regulation of Inflammatory Responses. In Progress in Molecular Biology and Translational Science; Bouchard, C., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 135, pp. 337–354. ISBN 1877-1173. [Google Scholar]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot Essent Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Capra, V.; Rovati, G.E.; Mangano, P.; Buccellati, C.; Murphy, R.C.; Sala, A. Transcellular Biosynthesis of Eicosanoid Lipid Mediators. Biochim. Biophys. Acta 2015, 1851, 377–382. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of Polyunsaturated Fatty Acids to Produce Lipid Mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating Inflammation and Infection in the 21st Century: New Hints from Decoding Resolution Mediators and Mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Serhan, C.N. Discovery of Specialized Pro-Resolving Mediators Marks the Dawn of Resolution Physiology and Pharmacology. Mol. Asp. Med. 2017, 58, 1–11. [Google Scholar] [CrossRef]
- Vidar Hansen, T.; Serhan, C.N. Protectins: Their Biosynthesis, Metabolism and Structure-Functions. Biochem. Pharmacol. 2022, 206, 115330. [Google Scholar] [CrossRef] [PubMed]
- Gireddy, H.B.; Rajaram, H.; Koduganti, R.R.; Ambati, M.R.A.; Harika, T.S.L. Maresins: The Mainstay in Periodontal Resolution. Cureus 2022, 14, e21742. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Hao, H.; Tu, W.S.; Guo, N.; Zhou, X.Y. Maresins: Anti-Inflammatory Pro-Resolving Mediators with Therapeutic Potential. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7442–7453. [Google Scholar] [CrossRef] [PubMed]
- Demopoulos, C.A.; Pinckard, R.N.; Hanahan, D.J. Platelet-Activating Factor. Evidence for 1-O-Alkyl-2-Acetyl-Sn-Glyceryl-3-Phosphorylcholine as the Active Component (a New Class of Lipid Chemical Mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar] [CrossRef] [PubMed]
- Kono, N.; Arai, H. Platelet-Activating Factor Acetylhydrolases: An Overview and Update. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 922–931. [Google Scholar] [CrossRef]
- Stafforini, D.M.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Platelet-Activating Factor, a Pleiotrophic Mediator of Physiological and Pathological Processes. Crit. Rev. Clin. Lab. Sci. 2003, 40, 643–672. [Google Scholar] [CrossRef]
- Prescott, S.M.; Zimmerman, G.A.; Stafforini, D.M.; McIntyre, T.M. Platelet-Activating Factor and Related Lipid Mediators. Annu. Rev. Biochem. 2000, 69, 419–445. [Google Scholar] [CrossRef]
- Snyder, F.; Fitzgerald, V.; Blank, M.L. Biosynthesis of Platelet-Activating Factor and Enzyme Inhibitors. Adv. Exp. Med. Biol. 1996, 416, 5–10. [Google Scholar] [CrossRef]
- Thudichum, J.L.W. A Treatise on the Chemical Constitution of the Brain: Based Throughout upon Original Researches. Glasg. Med. J. 1884, 22, 363–364. [Google Scholar]
- Merrill, A.H.; Sweeley, C.C. Chapter 12—Sphingolipids: Metabolism and Cell Signalling. In New Comprehensive Biochemistry; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 31, pp. 309–339. ISBN 0167-7306. [Google Scholar]
- Tan-Chen, S.; Guitton, J.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism and Signaling in Skeletal Muscle: From Physiology to Physiopathology. Front. Endocrinol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Cordeiro, A.V.; Silva, V.R.R.; Pauli, J.R.; da Silva, A.S.R.; Cintra, D.E.; Moura, L.P.; Ropelle, E.R. The Role of Sphingosine-1-Phosphate in Skeletal Muscle: Physiology, Mechanisms, and Clinical Perspectives. J. Cell. Physiol. 2019, 234, 10047–10059. [Google Scholar] [CrossRef] [PubMed]
- Ohanian, J.; Ohanian, V. Sphingolipids in Mammalian Cell Signalling. Cell. Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Hodun, K.; Chabowski, A.; Baranowski, M. Sphingosine-1-Phosphate in Acute Exercise and Training. Scand. J. Med. Sci. Sport. 2021, 31, 945–955. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of Ceramide-Mediated Programmed Cell Death by Sphingosine-1-Phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Danieli-Betto, D.; Peron, S.; Germinario, E.; Zanin, M.; Sorci, G.; Franzoso, S.; Sandonà, D.; Betto, R. Sphingosine 1-Phosphate Signaling Is Involved in Skeletal Muscle Regeneration. Am. J. Physiol. Cell Physiol. 2010, 298, C550–C558. [Google Scholar] [CrossRef]
- Maceyka, M.; Milstien, S.; Spiegel, S. Shooting the Messenger: Oxidative Stress Regulates Sphingosine-1-Phosphate. Circ. Res. 2007, 100, 7–9. [Google Scholar] [CrossRef]
- Straczkowski, M.; Kowalska, I.; Baranowski, M.; Nikolajuk, A.; Otziomek, E.; Zabielski, P.; Adamska, A.; Blachnio, A.; Gorski, J.; Gorska, M. Increased Skeletal Muscle Ceramide Level in Men at Risk of Developing Type 2 Diabetes. Diabetologia 2007, 50, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum Sphingolipids: Relationships to Insulin Sensitivity and Changes with Exercise in Humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef]
- Nagata, Y.; Partridge, T.A.; Matsuda, R.; Zammit, P.S. Entry of Muscle Satellite Cells into the Cell Cycle Requires Sphingolipid Signaling. J. Cell Biol. 2006, 174, 245–253. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef]
- Duan, J.; Merrill, A.H. 1-Deoxysphingolipids Encountered Exogenously and Made de Novo: Dangerous Mysteries inside an Enigma. J. Biol. Chem. 2015, 290, 15380–15389. [Google Scholar] [CrossRef] [PubMed]
- Zuellig, R.A.; Hornemann, T.; Othman, A.; Hehl, A.B.; Bode, H.; Güntert, T.; Ogunshola, O.O.; Saponara, E.; Grabliauskaite, K.; Jang, J.H.; et al. Deoxysphingolipids, Novel Biomarkers for Type 2 Diabetes, Are Cytotoxic for Insulin-Producing Cells. Diabetes 2014, 63, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Kitatani, K.; Chalfant, C.E.; Taha, T.A.; Kawamori, T.; Bielawski, J.; Obeid, L.M.; Hannun, Y.A. The Coordination of Prostaglandin E2 Production by Sphingosine-1-Phosphate and Ceramide-1-Phosphate. Mol. Pharmacol. 2005, 68, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Muñoz, A.; Presa, N.; Gomez-Larrauri, A.; Rivera, I.G.; Trueba, M.; Ordoñez, M. Control of Inflammatory Responses by Ceramide, Sphingosine 1-Phosphate and Ceramide 1-Phosphate. Prog. Lipid Res. 2016, 61, 51–62. [Google Scholar] [CrossRef]
- Göggel, R.; Winoto-Morbach, S.; Vielhaber, G.; Imai, Y.; Lindner, K.; Brade, L.; Brade, H.; Ehlers, S.; Slutsky, A.S.; Schütze, S.; et al. PAF-Mediated Pulmonary Edema: A New Role for Acid Sphingomyelinase and Ceramide. Nat. Med. 2004, 10, 155–160. [Google Scholar] [CrossRef]
- Fayyaz, S.; Japtok, L.; Kleuser, B. Divergent Role of Sphingosine 1-Phosphate on Insulin Resistance. Cell. Physiol. Biochem. 2014, 34, 134–147. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.X.; Aucouturier, J.; Di Marzo, V. The Role of the Endocannabinoid System in Skeletal Muscle and Metabolic Adaptations to Exercise: Potential Implications for the Treatment of Obesity. Obes. Rev. 2012, 13, 1110–1124. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? Int. J. Mol. Sci. 2020, 21, 4211. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Zywno, H.; Berk, K.; Bzdega, W.; Kolakowski, A.; Chabowski, A.; Konstantynowicz-Nowicka, K. The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3083. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Stožer, A.; Vodopivc, P.; Križančić Bombek, L. Pathophysiology of Exercise-Induced Muscle Damage and Its Structural, Functional, Metabolic, and Clinical Consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef]
- Boatwright, D.; Byrd, R.; Mangum, M. Relationship between Serum Prostaglandin Formation, Creatine Kinase Activity, and Ratings of Perceived Soreness. Sport. Med. Train. Rehabil. 1991, 2, 85–88. [Google Scholar] [CrossRef]
- Trappe, T.A.; Fluckey, J.D.; White, F.; Lambert, C.P.; Evans, W.J. Skeletal Muscle PGF(2)(Alpha) and PGE(2) in Response to Eccentric Resistance Exercise: Influence of Ibuprofen Acetaminophen. J. Clin. Endocrinol. Metab. 2001, 86, 5067–5070. [Google Scholar] [CrossRef]
- Tegeder, L.; Zimmermann, J.; Meller, S.T.; Geisslinger, G. Release of Algesic Substances in Human Experimental Muscle Pain. Inflamm. Res. 2002, 51, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.V.; Bassit, R.A.; Caperuto, E.C.; Costa Rosa, L.F. The Effect of Creatine Supplementation upon Inflammatory and Muscle Soreness Markers after a 30 km Race. Life Sci. 2004, 75, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Dousset, E.; Avela, J.; Ishikawa, M.; Kallio, J.; Kuitunen, S.; Kyröláinen, H.; Linnamo, V.; Komi, P.V. Bimodal Recovery Pattern in Human Skeletal Muscle Induced by Exhaustive Stretch-Shortening Cycle Exercise. Med. Sci. Sport. Exerc. 2007, 39, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.C.; Nosaka, K.; Ugrinowitsch, C.; Yamashita, A.; Martins, E.; Moriscot, A.S.; Aoki, M.S. Effect of Bench Press Exercise Intensity on Muscle Soreness and Inflammatory Mediators. J. Sport. Sci. 2009, 27, 499–507. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 Fatty Acids Supplementation Attenuates Inflammatory Markers after Eccentric Exercise in Untrained Men. Clin. J. Sport Med. 2011, 21, 131–137. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, Y.A.; Chen, S.L.; Hsu, M.C.; Hsu, C.C. American Ginseng Attenuates Eccentric Exercise-Induced Muscle Damage via the Modulation of Lipid Peroxidation and Inflammatory Adaptation in Males. Nutrients 2021, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Croisier, J.L.; Camus, G.; Deby-Dupont, G.; Bertrand, F.; Lhermerout, C.; Crielaard, J.M.; Juchmès-Ferir, A.; Deby, C.; Albert, A.; Lamy, M. Myocellular Enzyme Leakage, Polymorphonuclear Neutrophil Activation and Delayed Onset Muscle Soreness Induced by Isokinetic Eccentric Exercise. Arch Physiol. Biochem. 1996, 104, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Croisier, J.L.; Camus, G.; Monfils, T.; Deby-Dupon, G.; Fafchamps, M.; Venneman, I.; Crielaard, J.M.; Juchmès-Ferir, A.; Lhermerout, C.; Lamy, M.; et al. Piroxicam Fails to Reduce Myocellular Enzyme Leakage and Delayed Onset Muscle Soreness Induced by Isokinetic Eccentric Exercise. Mediat. Inflamm. 1996, 5, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Hirose, L.; Nosaka, K.; Newton, M.; Laveder, A.; Kano, M.; Peake, J.; Suzuki, K. Changes in Inflammatory Mediators Following Eccentric Exercise of the Elbow Flexors. Exerc. Immunol. Rev. 2004, 10, 75–90. [Google Scholar] [PubMed]
- Weinheimer, E.M.; Jemiolo, B.; Carroll, C.C.; Harber, M.P.; Haus, J.M.; Burd, N.A.; LeMoine, J.K.; Trappe, S.W.; Trappe, T.A. Resistance Exercise and Cyclooxygenase (COX) Expression in Human Skeletal Muscle: Implications for COX-Inhibiting Drugs and Protein Synthesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2241-8. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Dickinson, J.M.; Lemoine, J.K.; Carroll, C.C.; Sullivan, B.E.; Haus, J.M.; Jemiolo, B.; Trappe, S.W.; Hughes, G.M.; Sanders, C.E.; et al. Effect of a Cyclooxygenase-2 Inhibitor on Postexercise Muscle Protein Synthesis in Humans. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E354–E361. [Google Scholar] [CrossRef]
- Mikkelsen, U.R.; Schjerling, P.; Helmark, I.C.; Reitelseder, S.; Holm, L.; Skovgaard, D.; Langberg, H.; Kjaer, M.; Heinemeier, K.M. Local NSAID Infusion Does Not Affect Protein Synthesis and Gene Expression in Human Muscle after Eccentric Exercise. Scand. J. Med. Sci. Sport. 2011, 21, 630–644. [Google Scholar] [CrossRef]
- Buford, T.W.; Cooke, M.B.; Redd, L.L.; Hudson, G.M.; Shelmadine, B.D.; Willoughby, D.S. Protease Supplementation Improves Muscle Function after Eccentric Exercise. Med. Sci. Sport. Exerc. 2009, 41, 1908–1914. [Google Scholar] [CrossRef]
- Paulsen, G.; Egner, I.M.; Drange, M.; Langberg, H.; Benestad, H.B.; Fjeld, J.G.; Hallén, J.; Raastad, T. A COX-2 Inhibitor Reduces Muscle Soreness, but Does Not Influence Recovery and Adaptation after Eccentric Exercise. Scand. J. Med. Sci. Sport. 2010, 20, e195–e207. [Google Scholar] [CrossRef]
- Conceição, M.S.; Libardi, C.A.; Nogueira, F.R.; Bonganha, V.; Gáspari, A.F.; Chacon-Mikahil, M.P.; Cavaglieri, C.R.; Madruga, V.A. Effects of Eccentric Exercise on Systemic Concentrations of Pro- and Anti-Inflammatory Cytokines and Prostaglandin (E2): Comparison between Young and Postmenopausal Women. Eur. J. Appl. Physiol. 2012, 112, 3205–3213. [Google Scholar] [CrossRef]
- Meneghel, A.J.; Verlengia, R.; Crisp, A.H.; Aoki, M.S.; Nosaka, K.; da Mota, G.R.; Lopes, C.R. Muscle Damage of Resistance-Trained Men after Two Bouts of Eccentric Bench Press Exercise. J. Strength Cond. Res. 2014, 28, 2961–2966. [Google Scholar] [CrossRef]
- Nagahisa, H.; Ikezaki, K.; Yamada, R.; Yamada, T.; Miyata, H. Preconditioning Contractions Suppress Muscle Pain Markers after Damaging Eccentric Contractions. Pain. Res. Manag. 2018, 2018, 3080715. [Google Scholar] [CrossRef] [PubMed]
- Vandenburgh, H.H.; Hatfaludy, S.; Sohar, I.; Shansky, J. Stretch-Induced Prostaglandins and Protein Turnover in Cultured Skeletal Muscle. Am. J. Physiol. 1990, 259, C232–C240. [Google Scholar] [CrossRef] [PubMed]
- Milias, G.A.; Nomikos, T.; Fragopoulou, E.; Athanasopoulos, S.; Antonopoulou, S. Effects of Eccentric Exercise-Induced Muscle Injury on Blood Levels of Platelet Activating Factor (PAF) and Other Inflammatory Markers. Eur. J. Appl. Physiol. 2005, 95, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, M.; Górski, J.; Klapcinska, B.; Waskiewicz, Z.; Sadowska-Krepa, E. Ultramarathon Run Markedly Reduces Plasma Sphingosine-1-Phosphate Concentration. Int. J. Sport. Nutr. Exerc. Metab. 2014, 24, 148–156. [Google Scholar] [CrossRef]
- Dobrzyń, A.; Górski, J. Effect of Acute Exercise on the Content of Free Sphinganine and Sphingosine in Different Skeletal Muscle Types of the Rat. Horm. Metab. Res. 2002, 34, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Leem, Y.H. Acid Sphingomyelinase Inhibition Alleviates Muscle Damage in Gastrocnemius after Acute Strenuous Exercise. J. Exerc. Nutr. Biochem. 2019, 23, 1–6. [Google Scholar] [CrossRef]
- Murase, S.; Terazawa, E.; Hirate, K.; Yamanaka, H.; Kanda, H.; Noguchi, K.; Ota, H.; Queme, F.; Taguchi, T.; Mizumura, K. Upregulated Glial Cell Line-Derived Neurotrophic Factor through Cyclooxygenase-2 Activation in the Muscle Is Required for Mechanical Hyperalgesia after Exercise in Rats. J. Physiol. 2013, 591, 3035–3048. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaffer, A.; Portanova, J.; Seibert, K.; Isakson, P.C. Inhibition of Cyclooxygenase-2 Rapidly Reverses Inflammatory Hyperalgesia and Prostaglandin E2 Production. J. Pharmacol. Exp. Ther. 1997, 283, 1069–1075. [Google Scholar]
- Rodemann, H.P.; Goldberg, A.L. Arachidonic Acid, Prostaglandin E2 and F2 Alpha Influence Rates of Protein Turnover in Skeletal and Cardiac Muscle. J. Biol. Chem. 1982, 257, 1632–1638. [Google Scholar] [CrossRef]
- Markworth, J.F.; Cameron-Smith, D. Prostaglandin F2α Stimulates PI3K/ERK/MTOR Signaling and Skeletal Myotube Hypertrophy. Am. J. Physiol. Cell Physiol. 2011, 300, C671–C682. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human Inflammatory and Resolving Lipid Mediator Responses to Resistance Exercise and Ibuprofen Treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1281–R1296. [Google Scholar] [CrossRef] [PubMed]
- Karlstad, M.D.; Buripakdi, D.; Carroll, R.C. Platelet-activating factor (PAF)-induced decreases in whole-body and skeletal muscle protein synthesis. Shock 2000, 14, 490–498. [Google Scholar] [CrossRef]
- Sabbadini, R.A.; Betto, R.; Teresi, A.; Fachechi-Cassano, G.; Salviati, G. The Effects of Sphingosine on Sarcoplasmic Reticulum Membrane Calcium Release. J. Biol. Chem. 1992, 267, 15475–15484. [Google Scholar] [CrossRef] [PubMed]
- Danieli-Betto, D.; Germinario, E.; Esposito, A.; Megighian, A.; Midrio, M.; Ravara, B.; Damiani, E.; Libera, L.D.; Sabbadini, R.A.; Betto, R. Sphingosine 1-Phosphate Protects Mouse Extensor Digitorum Longus Skeletal Muscle during Fatigue. Am. J. Physiol. Cell Physiol. 2005, 288, C1367–C1373. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Jt. Surg. 2020, 102, 1197–1204. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; García Lopez, D.A.; Borrego, J.C.; Murgu, M.; Valdez, J.; López, J.A.; Monárrez-Espino, J. Untargeted Analysis in Post-COVID-19 Patients Reveals Dysregulated Lipid Pathways Two Years after Recovery. Front. Mol. Biosci. 2023, 10, 1100486. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Petsini, F.; Detopoulou, M.; Theoharides, T.C.; Demopoulos, C.A. Is There an Interplay between the SARS-CoV-2 Spike Protein and Platelet-Activating Factor? Biofactors 2022, 48, 1271–1283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gakis, A.G.; Nomikos, T.; Philippou, A.; Antonopoulou, S. The Involvement of Lipid Mediators in the Mechanisms of Exercise-Induced Muscle Damage. Physiologia 2023, 3, 305-328. https://doi.org/10.3390/physiologia3020022
Gakis AG, Nomikos T, Philippou A, Antonopoulou S. The Involvement of Lipid Mediators in the Mechanisms of Exercise-Induced Muscle Damage. Physiologia. 2023; 3(2):305-328. https://doi.org/10.3390/physiologia3020022
Chicago/Turabian StyleGakis, Athanasios G., Tzortzis Nomikos, Anastassios Philippou, and Smaragdi Antonopoulou. 2023. "The Involvement of Lipid Mediators in the Mechanisms of Exercise-Induced Muscle Damage" Physiologia 3, no. 2: 305-328. https://doi.org/10.3390/physiologia3020022
APA StyleGakis, A. G., Nomikos, T., Philippou, A., & Antonopoulou, S. (2023). The Involvement of Lipid Mediators in the Mechanisms of Exercise-Induced Muscle Damage. Physiologia, 3(2), 305-328. https://doi.org/10.3390/physiologia3020022








