Investigating the Effectiveness of Plant-Mediated Cerium Oxide Nanoparticles as Larvicidal Agents against the Dengue Vector Aedes aegypti
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of B. cylindrica Plant Extract
2.2. Qualitative Phytochemical Analysis of BL Aqueous Extract
2.2.1. Alkaloids
2.2.2. Phenolic Compounds
2.2.3. Flavonoids
2.2.4. Saponins
2.2.5. Steroids
2.2.6. Terpenoids
2.2.7. Tannins
2.2.8. Reducing Sugars
2.2.9. Proteins
2.3. Production of Cerium Oxide Nanoparticles (CeO2 NPs)
2.4. Characterization
2.5. Rearing of Mosquito and Larvicidal Activity
2.6. Enzymatic Studies
2.6.1. Whole-Body Homogenates for Enzyme Source Preparation
2.6.2. AChE Activity
2.6.3. GST Activity
2.7. Statistical Data Analysis
3. Results
3.1. Qualitative Phytochemical Analysis of BL Aqueous Extract
3.2. Characterization of CeO2 NPs
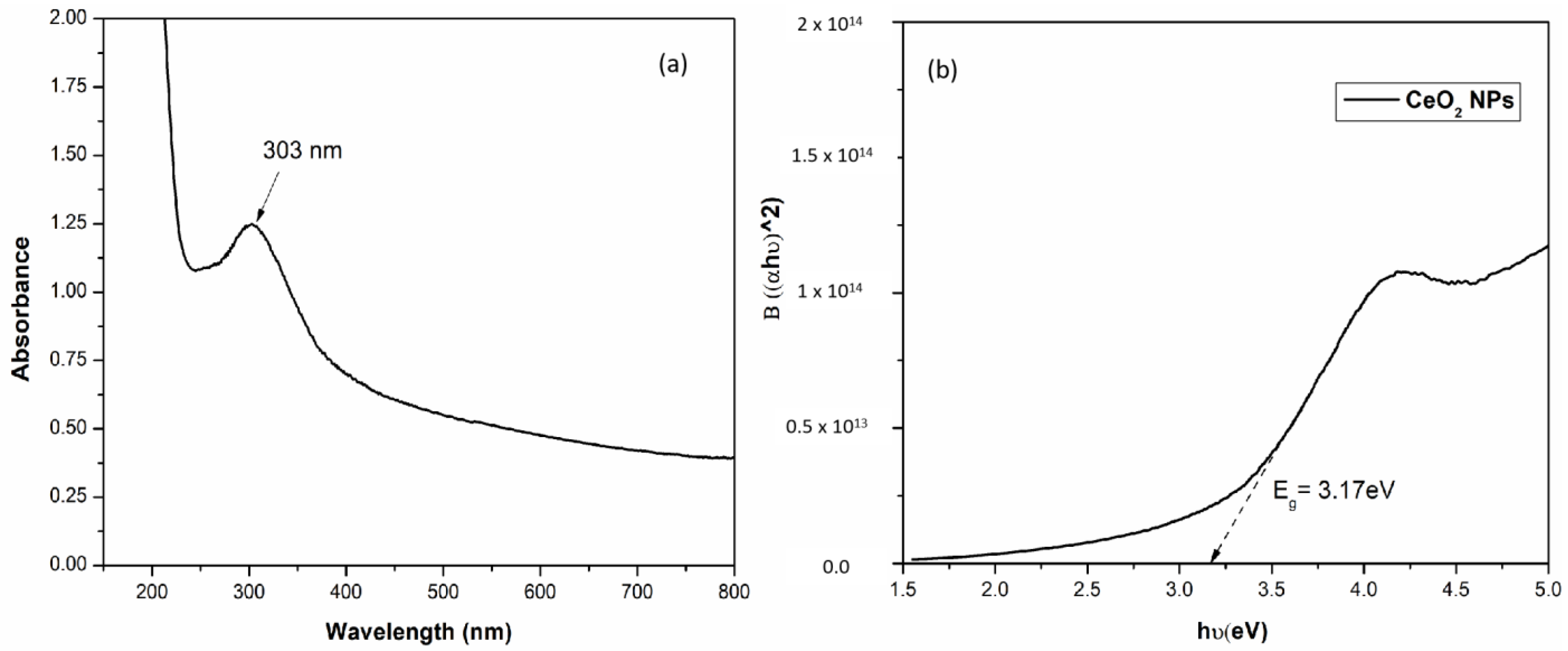
3.2.1. UV–Visible Spectroscopy
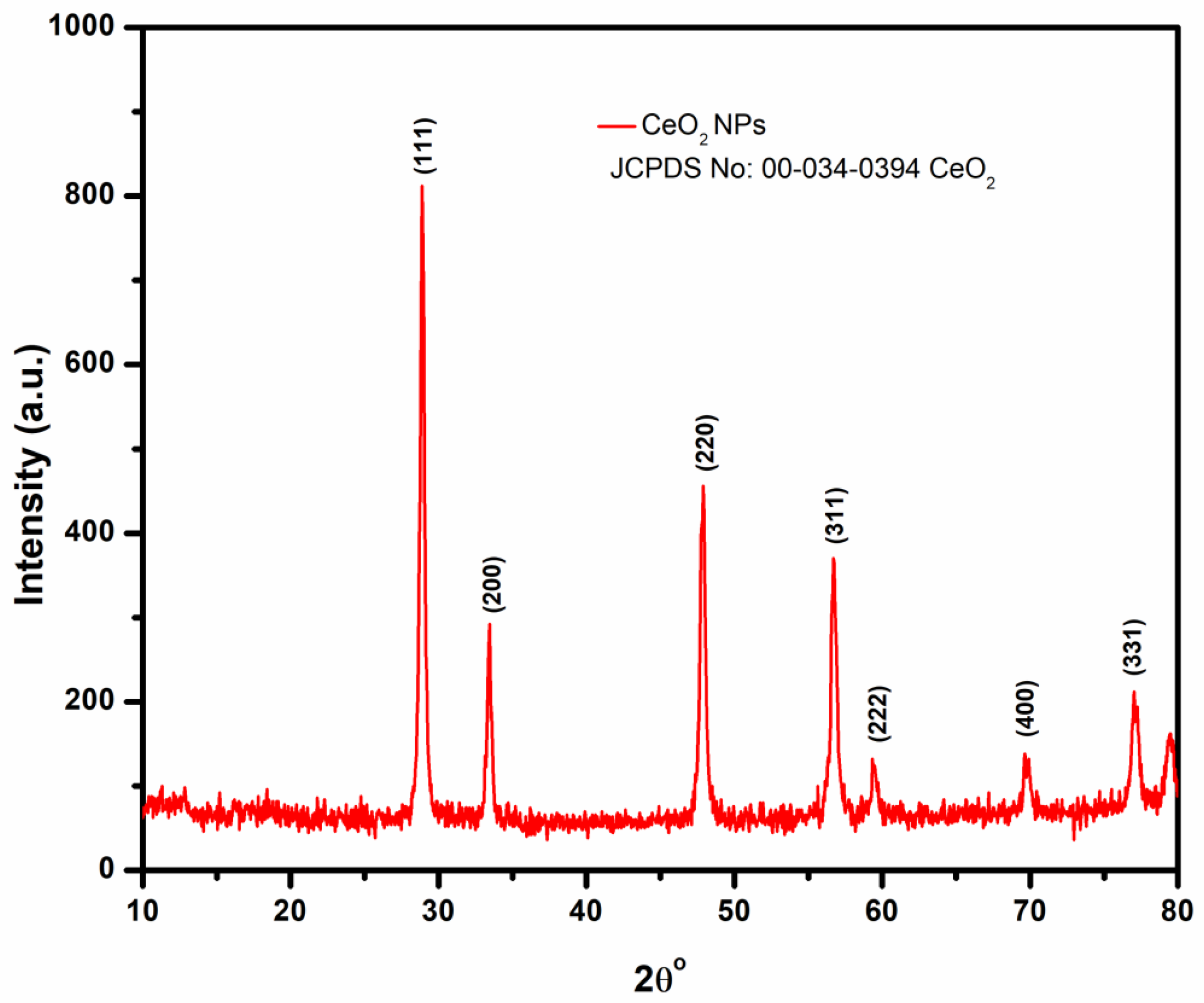
3.2.2. XRD Analysis
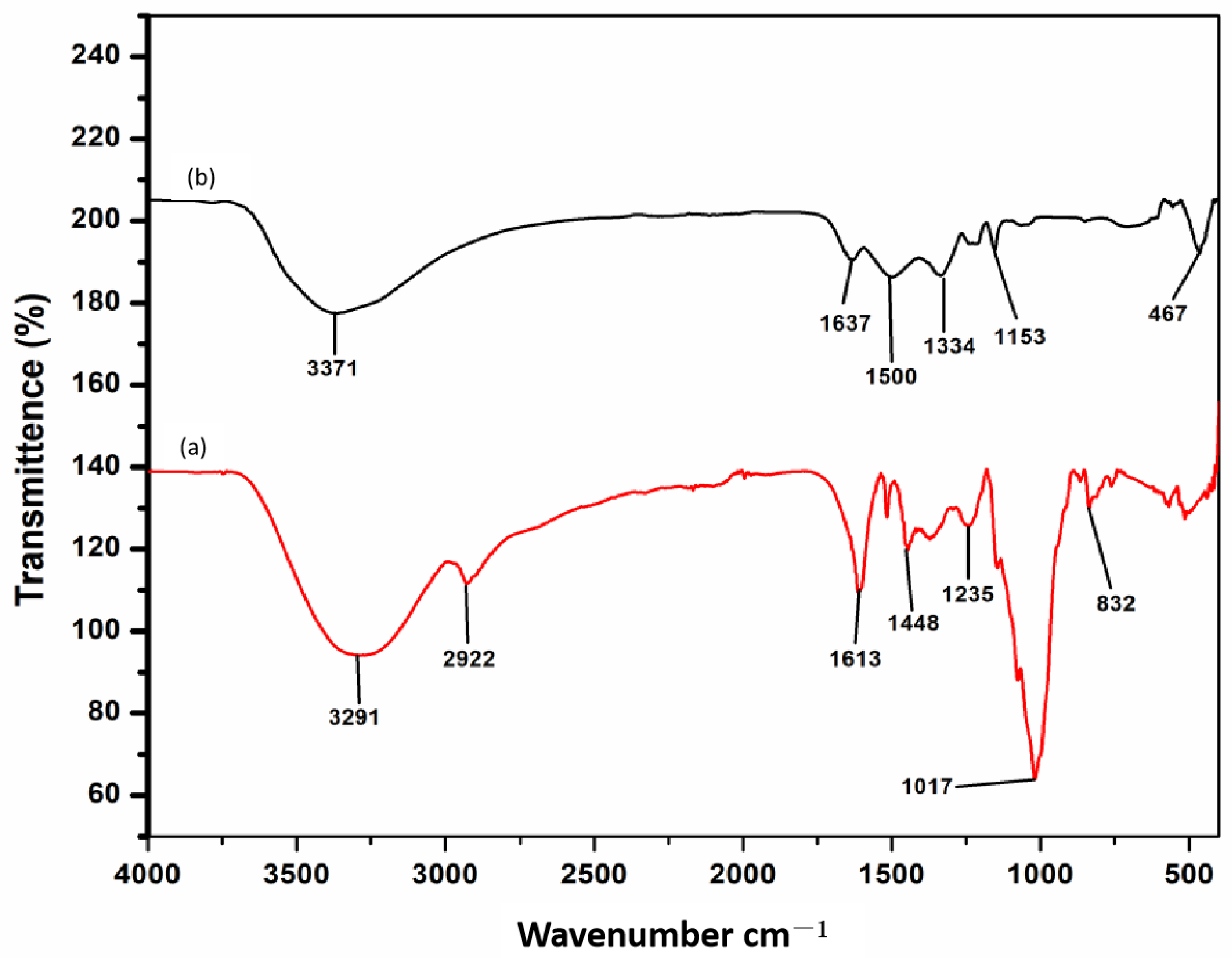
3.2.3. FT-IR Analysis
3.2.4. TEM Analysis
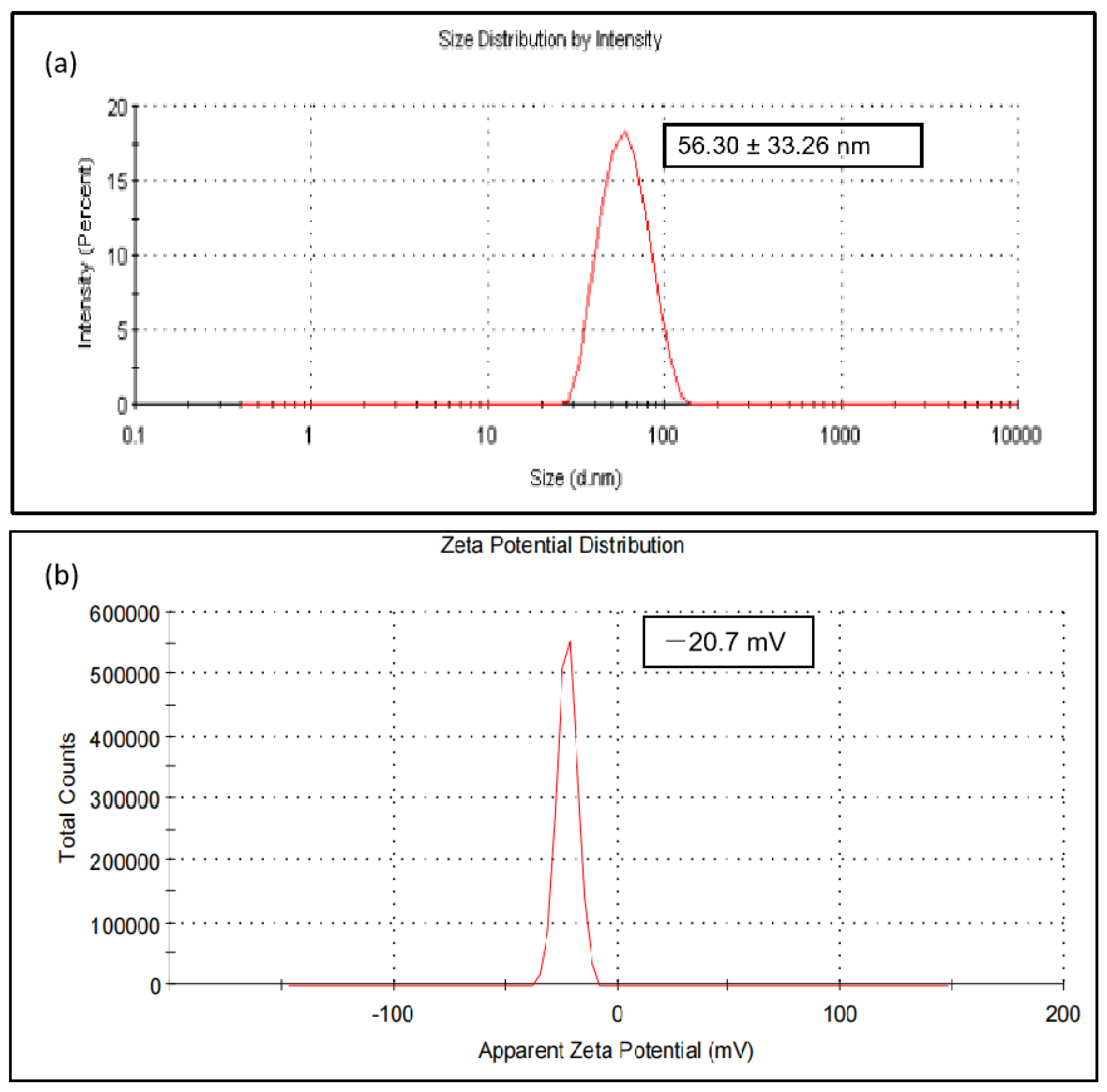
3.2.5. Dynamic Light Scattering (DLS) and Zeta Potential
3.2.6. Energy Dispersive X-ray Spectra (EDS) of CeO2 NPs
3.3. Mosquito Larvicidal Activity
3.4. Enzyme Activity
3.4.1. Acetylcholinesterase (AChE) Activity
3.4.2. Glutathione S-Transferase (GST) Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taubes, G. Malaria Parasite Outwits the Immune System. Science 2000, 290, 435. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Murugan, P.; Noortheen, A. Larvicidal and repellent potential of Albizzia amara Boivin and Ocimum basilicum Linn against dengue vector, Aedes aegypti (Insecta: Diptera: Culicidae). Bioresour. Technol. 2007, 98, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, K.; Malarmagal, R.; Charulatha, H.; Saraswatula, V.L.; Prabakaran, K. Larvicidal effects of crude extracts of dried ripened fruits of Piper nigrum against Culex quinquefasciatus larval instars. J. Vector Borne Dis. 2009, 46, 153. [Google Scholar]
- Kamaraj, C.; Bagavan, A.; Rahuman, A.A.; Zahir, A.A.; Elango, G.; Pandiyan, G. Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol. Res. 2009, 104, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Goncalvez, A.P.; Engle, R.E.; St. Claire, M.; Purcell, R.H.; Lai, C.J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 2007, 104, 9422–9427. [Google Scholar] [CrossRef]
- Wiratsudakul, A.; Suparit, P.; Modchang, C. Dynamics of Zika virus outbreaks: An overview of mathematical modeling approaches. PeerJ 2018, 6, e4526. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; WHO Library: Geneva, Switzerland, 2009; pp. 10–12.
- World Health Organization. Available online: https://www.who.int/srilanka/news/detail/08-07-2019-preventive-action-is-vital-to-curtail-dengue-outbreaks-in-sri-lanka (accessed on 1 February 2023).
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.; Vasquez, C.; Carvajal, A.; Ramirez, M.; Cardenas, G.; Petrie, W.D.; Beier, J.C. Effectiveness of adulticide and larvicide in controlling high densities of Aedes aegypti in urban environments. PLoS ONE 2021, 16, e0246046. [Google Scholar] [CrossRef]
- Subahar, R.; Aulia, A.P.; Yulhasri, Y.; Felim, R.R.; Susanto, L.; Winita, R.; El Bayani, G.F.; Adugna, T. Assessment of susceptible Culex quinquefasciatus larvae in Indonesia to different insecticides through metabolic enzymes and the histopathological midgut. Heliyon 2022, 8, e12234. [Google Scholar] [CrossRef]
- De Castro, B.M.; De Jaeger, X.; Martins-Silva, C.; Lima, R.D.F.; Amaral, E.; Menezes, C.; Lima, P.; Neves, C.M.L.; Pires, R.G.; Gould, T.W.; et al. The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol. Cell. Biol. 2009, 29, 5238–5250. [Google Scholar] [CrossRef]
- Melo-Santos, M.; Varjal-Melo, J.; Araújo, A.; Gomes, T.; Paiva, M.; Regis, L.; Furtado, A.; Magalhaes, T.; Macoris, M.; Andrighetti, M.; et al. Resistance to the organophosphate temephos: Mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop. 2010, 113, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Azizullah, A.; Rehman, Z.U.; Ali, I.; Murad, W.; Muhammad, N.; Ullah, W.; Häder, D.P. Chlorophyll derivatives can be an efficient weapon in the fight against dengue. Parasitol. Res. 2014, 113, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Saddhe, A.A.; Jamdade, R.A.; Kumar, K. Evaluation of multilocus marker efficacy for delineating mangrove species of West Coast India. PLoS ONE 2017, 12, e0183245. [Google Scholar] [CrossRef]
- Revathi, P.; Jeyaseelansenthinath, T.; Thirumalaikolundhusubramaian, P. Preliminary Phytochemical Screening and GCMS Analysis of Ethanolic Extract of Mangrove Plant—Bruguiera cylindrica (Rhizho) L. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 729–740. [Google Scholar]
- Nithyamol Kalappurakkal, V.; Bhattacharya, D.; Chakravarty, S.; Venkata Uppuluri, M. Isolation, synthesis and AC hE inhibitory potential of some novel cinnamyl esters of taraxerol, the major metabolite of the Mangrove Bruguiera cylindrica. Chem. Biodivers. 2018, 15, e1800008. [Google Scholar] [CrossRef]
- Bobbarala, V.; Vadlapudi, V.R.; Naidu, C.K. Antimicrobial potentialities of mangrove plant Avicennia marina. J. Pharm. Res. 2009, 2, 1019–1021. [Google Scholar]
- Dahibhate, N.L.; Saddhe, A.A.; Kumar, K. Mangrove plants as a source of bioactive compounds: A review. Nat. Prod. J. 2019, 9, 86–97. [Google Scholar] [CrossRef]
- Dahibhate, N.L.; Roy, U.; Kumar, K. Phytochemical screening, antimicrobial and antioxidant activities of selected mangrove species. Curr. Bioact. Compd. 2020, 16, 152–163. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Sasikumar, J.M.; Shamna, R.; Pandiarajan, C.; Sofia, P.; Nagarajan, B. Antioxidant activities of bark extract from mangroves, Bruguiera cylindrica (L.) Blume and Ceriops decandra Perr. Indian J. Pharmacol. 2011, 43, 557. [Google Scholar]
- Premanathan, M.; Kathiresan, K.; Nakashima, H. Mangrove halophytes: A source of antiviral substances. South Pac. Study 1999, 19, 49–57. [Google Scholar]
- Murugan, K.; Dinesh, D.; Paulpandi, M.; Althbyani, A.D.M.; Subramaniam, J.; Madhiyazhagan, P.; Wang, L.; Suresh, U.; Kumar, P.M.; Mohan, J.; et al. Nanoparticles in the fight against mosquito-borne diseases: Bioactivity of Bruguiera cylindrica—Synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4349–4361. [Google Scholar] [CrossRef]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitology research 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Mishra, A.K. Immunomodulation, Toxicity, and Therapeutic Potential of Nanoparticles. BioTech 2022, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.J.; Wang, H.; Li, Y.R.; Zhu, J.M.; Zhu, J.J. Ultrasonic-induced synthesis of CeO2 nanotubes. J. Cryst. Growth 2005, 281, 525–529. [Google Scholar] [CrossRef]
- Thakur, S.; Patil, P. Rapid synthesis of cerium oxide nanoparticles with superior humidity-sensing performance. Sens. Actuators B Chem. 2014, 194, 260–268. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Jamal, A. Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci. Total Environ. 2011, 409, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Y.; Zhang, Z.; He, X.; Zhang, J.; Guo, Z.; Tai, R.; Zhao, Y.; Chai, Z. Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano 2012, 6, 9943–9950. [Google Scholar] [CrossRef]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.M. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Alizadeh, S.; Mousavi-Kamazani, M.; Salavati-Niasari, M. Synthesis and characterization of CeO2 nanoparticles via hydrothermal route. J. Ind. Eng. Chem. 2015, 21, 1301–1305. [Google Scholar] [CrossRef]
- Arumugam, A.; Karthikeyan, C.; Hameed, A.S.H.; Gopinath, K.; Gowri, S.; Karthika, V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater. Sci. Eng. C 2015, 49, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Aboyewa, J.A.; Sibuyi, N.R.; Meyer, M.; Oguntibeju, O.O. Green synthesis of metallic nanoparticles using some selected medicinal plants from southern africa and their biological applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed]
- Gawali, P.; Jadhav, B.L. Antioxidant activity and antioxidant phytochemical analysis of mangrove species Sonneratia alba and Bruguiera cylindrica. Asian J. Microbiol. Biotechnol. Environ. Sci. 2011, 13, 257–261. [Google Scholar]
- Ranjana, B.L.; Jadhav, B.; Dhavan, P.; Patel, P. In vitro antidiabetic activity and phytochemical analysis of Lumnitzera racemosa leaves. Int. Res. J. Pharm. 2010, 10, 220–227. [Google Scholar]
- Kumar, S.; Warikoo, R.; Wahab, N. Larvicidal potential of ethanolic extracts of dried fruits of three species of peppercorns against different instars of an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 2010, 107, 901–907. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Rückert, C.; Chotiwan, N.; Nguyen, C.; Luna, S.M.G.; Fauver, J.R.; Foy, B.D.; Perera, R.; Black, W.C.; Kading, R.C.; et al. Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0005101. [Google Scholar] [CrossRef]
- Finlayson, C.; Saingamsook, J.; Somboon, P. A simple and affordable membrane-feeding method for Aedes aegpyti and Anopheles minimus (Diptera: Culicidae). Acta Trop. 2015, 152, 245–251. [Google Scholar] [CrossRef]
- World Health Organization. Safety of Pyrethroids for Public Health Use; No. WHO/CDS/WHOPES/GCDPP/2005.10; World Health Organization: Geneva, Switzerland, 2005.
- Napoleão, T.H.; Pontual, E.V.; Lima, T.D.A.; Santos, N.D.D.L.; Sá, R.A.; Coelho, L.C.B.B.; Navarro, D.M.D.A.F.; Paiva, P.M.G. Effect of Myracrodruon urundeuva leaf lectin on survival and digestive enzymes of Aedes aegypti larvae. Parasitol. Res. 2012, 110, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Ikezawa, H.; Taguchi, R. [84] Phosphatidylinositol-specific phospholipase C from Bacillus cereus and Bacillus thurinǵiensis. Methods Enzymol. 1981, 71, 731–741. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Boyd, M. Japanese consumers’ acceptance of genetically modified (GM) Food: An ordered probit analysis. J. Food Prod. Mark. 2006, 12, 45–57. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium oxide nanoparticles and their efficient antibacterial application in vitro against gram-positive and gram-negative pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef]
- Gu, H.; Soucek, M.D. Preparation and characterization of monodisperse cerium oxide nanoparticles in hydrocarbon solvents. Chem. Mater. 2007, 19, 1103–1110. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Saraswathi, H.; Sangeetha, E.; Soundarya, S.; Kumar, N.M. Green synthesis, characterization and biological activities of nanoceria. Ceram. Int. 2019, 45, 12382–12386. [Google Scholar] [CrossRef]
- Elaziouti, A.; Laouedj, N.; Bekka, A.; Vannier, R.N. Preparation and characterization of p–n heterojunction CuBi2O4/CeO2 and its photocatalytic activities under UVA light irradiation. J. King Saud Univ. Sci. 2015, 27, 120–135. [Google Scholar] [CrossRef]
- Miri, A.; Sarani, M. Biosynthesis, characterization and cytotoxic activity of CeO2 nanoparticles. Ceram. Int. 2018, 44, 12642–12647. [Google Scholar] [CrossRef]
- Arul, N.S.; Mangalaraj, D.; Han, J.I. Facile hydrothermal synthesis of CeO2 nanopebbles. Bull. Mater. Sci. 2015, 38, 1135–1139. [Google Scholar] [CrossRef]
- Baseri, M.K.; Baker, S. Identification of cellular components of medicinal plants using FTIR. Rom. J. Biophys. 2011, 21, 277–284. [Google Scholar]
- Silva, J.P.; Méndez, G.L.; Lombana, J.; Marrugo, D.G.; Correa-Turizo, R. Physicochemical Characterization of Spent Coffee Ground (Coffea arabica L.) and its Antioxidant Evaluation. Adv. J. Food Sci. Technol. 2018, 16, 220–225. [Google Scholar] [CrossRef]
- Al, R.N.; Al-Haidari, K.S. Environmental friendly synthesis of silver nanoparticles using leaf extract of Mureira Tree (Azadirachta indica) cultivated in Iraq and efficacy the antimicrobial activity. J. Nat. Sci. Res. 2016, 6, 2224–3186. [Google Scholar]
- Bakkiyaraj, R.; Balakrishnan, M.; Subramanian, R. Synthesis, structural characterisation, optical studies of CeO2 nanoparticles and its cytotoxic activity. Mater. Res. Innov. 2017, 21, 351–357. [Google Scholar] [CrossRef]
- Hasanzadeh, L.; Oskuee, R.K.; Sadri, K.; Nourmohammadi, E.; Mohajeri, M.; Mardani, Z.; Hashemzadeh, A.; Darroudi, M. Green synthesis of labeled CeO2 nanoparticles with 99mTc and its biodistribution evaluation in mice. Life Sci. 2018, 212, 233–240. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pal, R.; Siddiqi, N.J.; Sharma, B. Acetylcholinesterase from human erythrocytes as a surrogate biomarker of lead induced neurotoxicity. Enzym. Res. 2015, 370705, 1–7. [Google Scholar] [CrossRef]
- Ali, M.S.; Ravikumar, S.; Beula, J.M. Bioactivity of seagrass against the dengue fever mosquito Aedes aegypti larvae. Asian Pac. J. Trop. Biomed. 2012, 2, 570–573. [Google Scholar] [CrossRef]
- Dhavan, P.P.; Ranjana; Jadhav, B.L. Mosquito larvicidal potency of selected halophyte species and their modulation on acetylcholinesterase and glutathione s-transferase against dengue vector: Aedes aegypti. Plant Cell Biotechnol. Mol. Biol. 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Putri, G.E.; Rilda, Y.; Syukri, S.; Labanni, A.; Arief, S. Highly antimicrobial activity of cerium oxide nanoparticles synthesized using Moringa oleifera leaf extract by a rapid green precipitation method. J. Mater. Res. Technol. 2021, 15, 2355–2364. [Google Scholar] [CrossRef]
- Sabouri, Z.; Sabouri, M.; Amiri, M.S.; Khatami, M.; Darroudi, M. Plant-based synthesis of cerium oxide nanoparticles using Rheum turkestanicum extract and evaluation of their cytotoxicity and photocatalytic properties. Mater. Technol. 2022, 37, 555–568. [Google Scholar] [CrossRef]
- Rezvani, E.; Hatamie, A.; Berahman, M.; Simchi, M.; Angizi, S.; Rahmati, R.; Kennedy, J.; Simchi, A. Synthesis, first-principle simulation, and application of three-dimensional ceria nanoparticles/graphene nanocomposite for non-enzymatic hydrogen peroxide detection. J. Electrochem. Soc. 2019, 166, H3167. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M. Green synthesis of silver nanoparticles by Mulberry Leaves Extract. Nanosci. Nanotechnol. 2012, 2, 125–128. [Google Scholar] [CrossRef]
- Dutta, D.; Mukherjee, R.; Patra, M.; Banik, M.; Dasgupta, R.; Mukherjee, M.; Basu, T. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids Surf. B Biointerfaces 2016, 147, 45–53. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Jin, H.; Park, Y. Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C 2019, 101, 204–216. [Google Scholar] [CrossRef]
- Gopinath, K.; Karthika, V.; Sundaravadivelan, C.; Gowri, S.; Arumugam, A. Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. J. Nanostruct. Chem. 2015, 5, 295–303. [Google Scholar] [CrossRef]
- Sundaravadivelan, C.; Nalini Padmanabhan, M.; Sivaprasath, P.; Kishmu, L. Biosynthesized silver nanoparticles from Pedilanthus tithymaloides leaf extract with anti-developmental activity against larval instars of Aedes aegypti L. (Diptera; Culicidae). Parasitol. Res. 2013, 112, 303–311. [Google Scholar] [CrossRef]
- Fournier, D. Mutations of acetylcholinesterase which confer insecticide resistance in insect populations. Chem.-Biol. Interact. 2005, 157, 257–261. [Google Scholar] [CrossRef]
- Suganthy, N.; Pandian, S.K.; Devi, K.P. Cholinesterase inhibitory effects of Rhizophora lamarckii, Avicennia officinalis, Sesuvium portulacastrum and Suaeda monica: Mangroves inhabiting an Indian coastal area (Vellar Estuary). J. Enzym. Inhib. Med. Chem. 2009, 24, 702–707. [Google Scholar] [CrossRef]
- Lu, F.C. Basic Toxicology: Fundamentals, Target Organs, and Risk Assessment; Taylor and Francis: Washington, DC, USA, 1996. [Google Scholar]
- Trang, A.; Khandhar, P.B. Physiology, acetylcholinesterase. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Vanhaelen, N.; Haubruge, E.; Lognay, G.; Francis, F. Hoverfly glutathione S-transferases and effect of Brassicaceae secondary metabolites. Pestic. Biochem. Physiol. 2001, 71, 170–177. [Google Scholar] [CrossRef]
- Parkes, T.L.; Hilliker, A.J.; Phillips, J.P. Genetic and biochemical analysis of glutathione-S-transferase in the oxygen defense system of Drosophila melanogaster. Genome 1993, 36, 1007–1014. [Google Scholar] [CrossRef]
- Giordano, G.; Afsharinejad, Z.; Guizzetti, M.; Vitalone, A.; Kavanagh, T.J.; Costa, L.G. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol. Appl. Pharmacol. 2007, 219, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.G.; Small, G.J.; Hemingway, J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 2001, 357, 65–72. [Google Scholar] [CrossRef]
- Dhavan, P.P.; Jadhav, B.L. Eco-friendly approach to control dengue vector Aedes aegypti larvae with their enzyme modulation by Lumnitzera racemosa fabricated zinc oxide nanorods. SN Appl. Sci. 2020, 2, 843. [Google Scholar] [CrossRef]
- Vorbrodt, A. The role of phosphate in intracellular metabolism. Postepy. Hig. Med. Dosw. 1959, 13, 200–206. [Google Scholar]
- Pratt, H.D. Mosquitoes of Public Health Importance and Their Control (No. 772); US Department of Health, Education, and Welfare, Public Health Service, Communicable Disease Center: Atlanta, GA, USA, 1963.




| Phytochemical Constituents | B. cylindrica Leaf aqueous Extract |
|---|---|
| Alkaloids | + |
| Flavonoids | + |
| Terpenoids | + |
| Steroids | − |
| Tannins | + |
| Saponins | + |
| Phenolic compounds | + |
| Reducing sugars | + |
| Proteins | + |
| Treatment | Concentration (μg/mL) | 24 h Mortality (%) ± SE | LC50 (μg/mL) | LCL–UCL | LC90 (μg/mL) | LCL–UCL | χ2 (d.f.) |
|---|---|---|---|---|---|---|---|
| B. cylindrica aqueous extract | 250 | 4.58 ± 0.96 | 1258.04 | 583.87–1574.89 | 2250.03 | 1787.97–5310.37 | 16.73 * (4) (p = 0.002) |
| 500 | 13.75 ± 1.25 | ||||||
| 750 | 22.91 ± 1.29 | ||||||
| 1000 | 38.33 ± 2.16 | ||||||
| 1500 | 56.25 ± 2.39 | ||||||
| 2000 | 84.58 ± 2.25 | ||||||
| 2500 | 100 ± 0.00 | ||||||
| CeO2 NPs | 10 | 6.66 ± 0.71 | 46.28 | 17.38–53.20 | 64.62 | 56.16–179.54 | 17.06 * (4) (p = 0.002) |
| 20 | 16.25 ± 1.25 | ||||||
| 30 | 23.75 ± 1.08 | ||||||
| 40 | 41.25 ± 1.95 | ||||||
| 50 | 57.50 ± 1.44 | ||||||
| 60 | 83.75 ± 1.64 | ||||||
| 70 | 100 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhavan, P.P.; Sonawane, V.R.; Mishra, A.K. Investigating the Effectiveness of Plant-Mediated Cerium Oxide Nanoparticles as Larvicidal Agents against the Dengue Vector Aedes aegypti. Physiologia 2023, 3, 329-346. https://doi.org/10.3390/physiologia3020023
Dhavan PP, Sonawane VR, Mishra AK. Investigating the Effectiveness of Plant-Mediated Cerium Oxide Nanoparticles as Larvicidal Agents against the Dengue Vector Aedes aegypti. Physiologia. 2023; 3(2):329-346. https://doi.org/10.3390/physiologia3020023
Chicago/Turabian StyleDhavan, Pratik P., Vivek R. Sonawane, and Abhinava K. Mishra. 2023. "Investigating the Effectiveness of Plant-Mediated Cerium Oxide Nanoparticles as Larvicidal Agents against the Dengue Vector Aedes aegypti" Physiologia 3, no. 2: 329-346. https://doi.org/10.3390/physiologia3020023
APA StyleDhavan, P. P., Sonawane, V. R., & Mishra, A. K. (2023). Investigating the Effectiveness of Plant-Mediated Cerium Oxide Nanoparticles as Larvicidal Agents against the Dengue Vector Aedes aegypti. Physiologia, 3(2), 329-346. https://doi.org/10.3390/physiologia3020023






