Abstract
Plants are constantly interacting with the diverse microbial community as well as insect pests throughout their life cycle. Due to their sessile nature, plants rely solely on the intracellular signaling and reprogramming of cellular events to resist against pathogens. Insect pests are usually dependent on the nutrient-rich fluid obtained from plants or directly consume plant parts to sustain their life cycle. Plants possess a plethora of microbial communities; these microbiomes constantly influence the physiology, growth, development, and immunity in plants. Hence, the phyllosphere and rhizosphere are believed to play a major role in plant-insect interaction. The phyllosphere, rhizosphere, and endosymbiotic microbiome are currently under extensive scientific investigation. Recently, the advancement of metagenomic next-generation sequencing (mNGS) platforms revolutionized plant-associated microbiome analysis and has unveiled many beneficial microbial communities protecting against diverse pathogenic microorganisms and insect pests. Mycorrhiza is also an important component of the rhizosphere, as it may play a role in soil microbiota, thus indirectly influencing the interaction of insects with plants. In this regard, the present review tries to focus on some major insect pests of plants, the molecular mechanism of plant–insect interaction, and the probable role of phyllosphere and rhizosphere microbiome in this plant–insect encounter. This review is believed to open up a new dimension in developing resistance in plants against insect pests.
1. Introduction
Plants are the primary producers of the ecosystem. They can entrap solar energy into starch molecules through photosynthesis. Heterotrophic organisms primarily depend on plants for food. There are a variety of microorganisms that thrive on the leaves and roots of plants, either benefitting from or harming their hosts. With the massive increase in human population, it is imperative to increase plant production. This is dependent on plant health, and thus understanding the network associated with the plant is essential. The United Nations declared the year 2020 as the International Year of Plant Health (IYPH) [https://www.fao.org/3/cb7056en/cb7056en.pdf, accessed on 29 November 2022]. Because plants do not exist as individual units, they should be considered complex communities associated with a microbial population [1]. Co-existence between plants and insects is not a new phenomenon; it is almost 400 million years old [2]. Around two-thirds of herbivorous insects are mainly leaf-eating beetles or caterpillars. Beetles can exhibit a positive role through nutrient recycling and/or as pollinators [3]. Most insects have been linked to crop plant loss, either directly or indirectly, by facilitating pathogen entry. Chewing insects like the Colorado potato beetle, the European corn borer, and the cotton boll weevil can consume plant tissue, resulting in crop damage. Sap-sucking insects like aphids, whiteflies, and leafhoppers use phloem contents as food when they insert specialized mouthparts (stylets) into the phloem of the host plant. In addition, some insects serve as vectors, transmitting viruses from one host to another. If a virus is introduced into the phloem, it can spread rapidly. The wounds created by insects play a pivotal role in the entry of pathogens like bacteria, fungi, or viruses into the plant [4]. The outcome of the plant–insect bidirectional interaction may be affected by plant-associated microbes like epiphytic, endophytic, pathogenic, and mycorrhizal fungi [5], creating a tri-trophic interaction. Microbes may modulate insect behaviour, such as attracting more insects due to increased pheromone production, feeding behaviour, or perception of odour or taste [6]. Microbes have the ability to alter insect metabolism and behaviour. The primary or secondary metabolism of plants may be modulated by microbes. Microbes can alter plant defence mechanisms against insects, which may be beneficial to either plants or insects [2]. Endophytes associated with the plant may serve as insecticides or fungicides [7]. Feeding of Pseudomonas syringae pv. Tomato DC3000-infected tomato leaves were able to reduce the growth of leaf-chewing caterpillars [8]. Generally, microbial communities in plants are grouped into two major categories based on their locations: rhizospheres (microbial populations present in below-ground tissues) and phyllospheres (microbial communities present in above-ground tissues) [2]. Around 106–107 bacterial cells/cm2 can be observed in the leaf area [9], whereas g−1 of rhizospheric tissue contains 106–109 bacterial cells [10]. Microbes may be epiphytic, living on leaves, or endophytic, living within leaves [9]. Together, these organisms play a critical role in plant health. We need to understand these tripartite interactions between plants, pests, and microorganisms. The current review focuses on major plant insect pests, the microbial population associated with the phyllosphere and rhizosphere, and how said population affects plant–insect interaction.
2. Major Insect Pests to the Crop Plants
Insects are the major cause of global agricultural loss annually. According to FAO, insect pests cause 40% of global yield loss which accounts for about USD 220 billion (https://www.fao.org, accessed on 5th December 2022). It has been reported that crop plants are attacked by more than 10,000 species of insects and 1000 species of nematodes among them, although 10% are considered major insect pests. The situation has been gradually aggravated due to rapid climate change. The massive mixing of pests and the introduction of invasive insects into newer locations have also multiplied due to international travel and exports. Climate change further alters the route of many migratory pests, and may also alter the nature of pests. For example, in the cotton ecosystem, American and spotted bollworms are now secondary pests, whereas caterpillars, pink bollworms, mirids, and mealy bugs are now considered to be the emerging major pests [11]. Additionally, global warming has developed conducive environments for many pests to infect in agricultural and forest ecosystems of cooler Arctic, boreal, temperate, and subtropical regions. Fall armyworms and desert locusts are gradually approaching these colder regions and impose massive crop loss [12].
The insects infect the plants mainly for food, nutrition, and shelter for reproduction. The majority of invertebrate Arthropods are characterized by joint legs, external skeletons, and one pair of antennae. The majority of insects have four distinct developmental stages called complete metamorphosis, e.g., egg, larva, pupa, and adult. In some sap-sucking and chewing insects, the developmental stages are restricted to three distinct phases e.g., egg, nymph, and adult [13,14]. In general, insects can thus be classified into the following groups, (i) consume foliage for food, (ii) suck sap from leaves and stems for nutrition, (iii) consume or tunnel roots and stems for shelter, laying eggs or food, and (iv) feed on flowers and/or seeds [15]. Despite direct devastating roles, many insect pests also harbour diverse secondary infections e.g., bacterial or viral infections inhabiting particularly mouth parts and salivary glands of the infecting primary insects. Some insects rely on internal tissues of different plant parts for their food, e.g., western flower thrips, Frankliniella occidentalis (Pergande), is known to be an invasive pest for different food, fiber, and ornamental crops globally. These insects also act as a vector for the tospoviruses, which acts as secondary infector [16]. Recently the draft genome of Frankliniella occidentalis has been reported as being used to decode infection biology, control strategy, and vector biology associated with thrips [17]. The other major group of plant insects belong to the sap-sucking category (e.g., scales, leafhoppers, aphids). Scale insects are the Hemipteran group of insects belonging to the Coccoidea, and have many unusual characteristics like sexual dimorphism and sometimes sexual dichronism [18]. The mealybug (e.g., Planococcus ficus, Planococcus citri, etc.), soft scale insect (Saissetia oleae), and armoured scale (Parlatoria ziziphi) are some examples of devastating pests in this category for plants [19]. The mealy bugs for cotton plants include, Phenacoccus solenopsis, Ferrisa virgata, Maconellicoccus hirsutus, etc., among which P. solenopsis is the dominant species for cotton plants in the Indian subcontinent [20]. It has been reported that the mango tree is also infected with the two types of mealy bugs, Planococcus citri and Icerya seychellarum [21]. Leaf-hoppers are also members of the Hemipteran group but belong to Cicadellidae which also causes massive crop damage in the Indian subcontinent [22]. The leaf-hoppers generally attack woody plants and damage plants in four major ways: destruction of leaf chlorophyll, sucking off nutrients from plants, laying eggs on plants (particularly leaf lower surface), and acting as a vector for many plant viruses [23]. Tea production is dramatically curtailed by green tea leaf-hoppers [Empoasca (Matsumurasca) onukii Matsuda] in the tea-growing hilly regions [24]. On the other hand, the brown leaf-hopper (BPH) Nilaparvata lugens is considered the major insect pest for rice (Oryza sativa). Next-generation sequencing-based analysis has revealed that the internal genetic map, oxidative properties, and starvation stress response are the major determinative factors in BPH and rice interactions [25]. The green leaf-hoppers (Nephotettix cincticeps (Uhler)) can also infect rice plants. Recently some resistant varieties of Oryza longistaminata (A. Chev. & Roehrich) against the green leaf-hoppers are reported [26]. Aphids are another group of sap-sucking insects causing huge damage to crop plants. They are very small, pear-shaped, and have extraordinary reproductive ability. These insects readily infect the soft tissue organs of plants, such as leaves and flowers. Excessive numbers together weaken the plants by leaching excessive nutrients and the plants exhibit stunted growth, yellowing, and curling of leaves, etc. [27,28]. Aphids (Aphis craccivora) are known to damage chickpea plants across the Indian subcontinent (http://dalhangyanmanch.res.in). Many works have been carried out to resist aphids and to understand the mechanism of aphid interaction with plants. Unlike other insects, aphids also harbour different deleterious plant viruses. Recently many novel viruses have been identified in aphids which could be potential plant viruses e.g., chickpea chlorotic stunt virus (CpCSV), bean leafroll virus (BLRV), beet western yellows virus (BWYV), soybean dwarf virus (SbDV), cotton leafroll dwarf virus (CLRDV), cucurbit aphid-borne yellows virus (CABYV), and phaseolus bean mild yellows virus (PhBMYV). Similarly, the Geminiviridae family includes chickpea chlorotic dwarf virus (CpCDV), chickpea chlorosis virus (CpCV), chickpea red leaf virus (CpRLV), chickpea yellows virus (CpYV), mastrevirus. Additionally, faba bean necrotic yellows virus (FBNYV) (Nanoviridae), cucumber mosaic virus (CMV), and alfalfa mosaic virus (AMV) (Bromoviridae) were also reported [29]. In rice, aphids are more likely to infect the roots than shoot. Rhopalosiphum rufiabdominale (Sasaki) and Tetraneura nigriabdominalis (Sasaki) were the two potent root-invading aphids for rice [30]. In the soil, the root infecting aphid Rhopalosiphum rufiabdominale (Sasaki) shares other hosts. Roots of grasses and sedges show a common association, but Cannabis sativa L has also been reported to be the host for this aphid recently [31].
3. Molecular Mechanism of Plant–Insect Interplay
The primary requirement for any host-insect interaction is the successful establishment and recognition of the insect on the respective plant host. Plants possess myriads of receptors for the recognition of pathogens and pests. Insect recognition differs from microbial pathogen recognition by plants. The insect pest resides on the plant surface and causes mechanical damage simultaneously. The recognition of insects has been carried out by chemicals, especially the small polypeptides and fatty acid conjugates (FACs) predominantly present in the saliva and ovipositional fluid secreted by the insects [32]. Inceptins, caeliferins, systemins are also recognition molecules of insects [33,34,35]. Systemin receptors were first identified in tomato plants. Systemin is derived from the larger precursor peptide and activated by the leucine-rich repeat receptor kinase (LRR-RK), SR 160, which in turn homologous to Brassinosteroid Insensitive 1 (BRI1). The plants bear two distinct systemin receptors SYR1 and SYR2 for herbivory [36]. In cowpea (Vigna unguiculata) an inceptin receptor (INR), also in the class of leucine-rich repeats, was identified against armyworms (Spodoptera exigua) [37]. There are some small precursor proteins (PROPEPs) that act as an elicitor in insect herbivory. In Arabidopsis it has been shown that PEP receptors (PEPR1, PEPR2, and PROPEP3) are strongly activated during insect herbivory [38]. Lectin-like receptor kinase (LecRK) is also involved in insect herbivory. It was reported that oviposition fluid or insect eggs of Pieris brassicae were recognised by LecRK 1.8 of Arabidopsis thaliana [39]. These receptors then transduce the signal in the intracellular milieu through different signalling intermediates. Insect-derived elicitors e.g., FACs, β glucanase, and glucose oxidase (GOX) are responsible for separate downstream signalling [40]. Insects usually induce jasmonic acid (JA) mediated pathways; in some other cases salicylic acid-mediated pathways were also documented [32]. The JA-dependent insect resistance was observed in Arabidopsis thaliana against Egyptian cotton worms (Spodoptera littoralis Boisd.). The coi1 mutant, with a deficiency in the JA synthesis, was insect-susceptible; contrarily, npr1 mutant with deficiency of SA production showed greater insect resistance [41]. On the other hand, SA-mediated induction of TGA transcription factor and NPR1-dependent pathogenesis-related protein (PR) formation in some cases restricts insect infection [32]. Precisely, insect eggs induced systemic response by SA and showed cross-tolerance to several microbial as well as fungal pathogens [42]. Interestingly, it has been found recently that Dorsal switch protein 1 (DSP1), an insect homologous protein of human high mobility group box 1 (HMGB1), is responsible for immune response. SA interacts with this DSP1 and hampers immune response in insects [43]. The induction of wound-induced protein kinase (WIPK), SA-induced protein kinase (SIPK), and further activation of mitogen-activated protein kinase (MAPK) cascade are operated to induce resistance response against insect pests [44]. In a separate pathway, WRKY transcription factors (TF) are activated. WRKY TFs are also involved in post-transcriptional and epigenetic regulation of insect herbivory resistance response [45]. Reactive oxygen species (ROS) also play crucial roles in herbivory signal transduction. It was observed that herbivore-associated elicitors (HAEs) can directly induce NADPH oxidase-mediated ROS induction, which in turn activates calcium channels and Ca+2 accumulation in the cytosol [35]. A chlorophyll fluorescence imaging-based study confirmed increased levels of ROS and photosystem II (PSII) activity in potatoes in response to sap-sucking insect Halyomorpha halys [46]. Such ROS production is intricately associated with calcium-dependent signalling in response to insect pests [47]. This Ca+2 influx induces calcium-dependent protein kinase (CDPKs), calmodulin proteins, and calcineurin to regulate defence response against herbivory [48]. Inversely, calcium sensor proteins are also known to negatively regulate stress response by modulating hormonal crosstalk [49] (Figure 1).
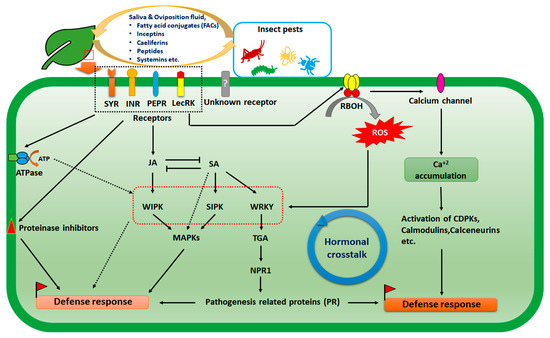
Figure 1.
Schematic diagram highlighting molecular mechanism of plant–insect interaction.
4. Plant-Associated Microbiome
4.1. Microbiome of Phyllosphere
The aerial part of the plant, mainly the leaf surface where microbes are present, is known as the phyllosphere [50]. In 1955, the term “phyllosphere” was introduced by plant pathologist F.T. Last [51]. Microbiologist J. Ruinen described the phyllosphere as “the external surface of the leaf as an environment for microorganisms” [52]. Morris stated that “the phyllosphere is the microenvironment extended from the leaf surface outward to the outer edge of the boundary layer surrounding the leaf and inward into the leaf tissues” [53]. According to Doan and Leveau [54], the phyllosphere consists of two interconnected but distinct environments: “phylloplane” (leaf surface landscape) and “phyllotelma” (leaf surface waterscape). The phyllosphere indicates the above-ground aerial part of the plant composed of stem and leaf, which is the habitat for various microbes [55]. The phyllosphere could cover one billion square kilometres [56]. Various microbes, including bacteria, filamentous fungi, yeast, and viruses have been found in the zone of the phyllosphere [57] (Table 1).
The average number of bacteria may be 106–107 cells/cm2 of leaf (http://eagri.org/eagri50/AMBE101/pdf/lec19.pdf accessed on 2 March 2023). Bacteria may have a positive, negative, or neutral impact on the host plant. Proteobacteria (Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria), Actinobacteria, Firmicutes, and Bacteroidetes are among them. Bacillus and Pseudomonas may be dominant genera [55]; in addition to other genera of nitrogen-fixing bacteria, Phytomonas, Erwinia, Sarcina, Xantomonas, and Gluconobacter have been observed on the surface of the plant [55,58]. Plant pathogens can enter the inner leaf through stomata or wounds [4]. Bacteria in the phyllosphere have a wide range of functions. Plant cell walls contain the methyl ester of pectin. Pectin methylesterase naturally produces methanol from the hydrolysis of pectin methyl ester. Methanol is produced by stomata and used as a carbon source by microbes in the phyllosphere. The concentration of methanol in the phyllosphere varies during the light-dark cycle. The concentration of methanol was lower in the light period and higher in the dark period. Plant leaf surfaces may be dominantly colonised by methanol-utilizing bacteria known as pink-pigmented facultative methanotrophs (PPFMs). Some of them have the ability to enhance plant growth as well as elevate crop yield [59]. Methylobacterium, a methanol-using bacterium, may be present in the phyllosphere [60]. The phyllosphere is also a habitat for methane-producing bacteria [61]. Isoprene-degrading bacteria were discovered in the phyllosphere of the willow, Salix fragilis, a high-isoprene emitting plant in the northern hemisphere, according to a DNA stable isotope probing (SIP) experiment. The relative abundance of the bacterial taxa was obtained from 13C-labelled willow leaf samples after a DNA-SIP experiment. They were Acidovorax, Mycolicibacterium, Variovorax, Mycobacterium, Polaromonas, Hydrogenophaga, Ramlibacter, Rhodoferax, Streptomyces, Bradyrhizobium, Pseudomonas, Methylobacterium, Mesorhizobium, Diaphorobacter, Comamonas, etc. [62]. Isoprene-degrading bacteria like Rhodococcus and Variovorax were isolated from the phyllosphere of a temperate, isoprene-emitting poplar tree [63]. According to the report, Acinetobacter genera dominated the phyllosphere of the oil palm tree, followed by Clostridium and Enterobacter [64]. Hence, microbes in the phyllosphere have a role in reducing methanol as well as degrading isoprene. The phyllosphere bacteria may be associated with the nitrogen cycle. A good number of chemolithoautotrophic ammonia oxidizers (CAO) and chemolithoautotrophic nitrite oxidizers (CNO) were observed in the phyllosphere of spruce trees in a forest ecosystem receiving high atmospheric nitrogen. They were most likely present in the stomatal cavity [65]. When plants from Costa Rica’s lowland tropical rainforest were studied, nitrogen fixation was found to occur primarily in the leaves by microbes associated with epiphytes. Fixation took place primarily on the surface of the leaves rather than the interior. It was likely mediated by cyanobacteria associated with epiphytes. Along with cyanobacteria, diazotrophic proteobacteria may be involved in nitrogen fixation [66]. Microbes in the phyllosphere can also use vitamins by consuming pantothenic acid, -alanine, and other host plant precursors, Pantothenic auxotrophic Methylobacterium sp. OR01 can grow in leaves [59]. Yeast is another member of the phyllosphere, and can colonize the surface as well as internal tissues of the phyllosphere [57]. The study showed that the densities of the yeast, filamentous fungi, and bacteria of the phyllosphere of sugar beet were 1010, 107, and 1011 CFU per gram of dry leaf, respectively. Senescing leaves contain more yeast than mature and immature leaves [67], and the abundance of yeast may vary with seasonal fluctuation in mature and immature leaves. Fluctuating temperature, secretion of specific nutrients, and leakage of the plant during various seasons and developmental stages contribute to the seasonal dynamics of yeast. Competition for space, nutrients, degradation of mycotoxins and fungicides, and interaction with host plants and microbes like bacteria and fungi may influence the diversity and abundance of yeast [57]. Leaf epiphytic bacteria have the ability to cross the cuticle and superficial tissue layer, and endophytic bacteria can migrate from root form epiphytic, surface living bacteria [68]. Various factors are responsible for the microbial community of the phyllosphere. Microbes of the phyllosphere face various stress conditions like limited resources, heat stress, osmotic stress, exposure to UV light, and fluctuating water availability [69]. Global warming also has an impact on the microbial community; extreme temperatures may be responsible for lowering the relative humidity. As many microbes cannot survive in a dry environment, plant surface microbes are influenced by extreme temperatures and low humidity [70]. In the warming effect, Alphaproteobacteria and Bacteroidetes were relatively less abundant, whereas Gammaproteobacteria, Actinobacteria, and Firmicutes were most abundant. In addition, warming also caused a reduction in the abundance of beneficial bacteria and enhanced pathogenic bacteria in leaf samples [71]. Air pollution contributes to the number of living microbes in the phyllosphere. The number and species composition of yeast and bacteria were observed to be altered in the microflora of the phyllosphere of Tiladansia plants in a tropical urban environment compared with an unpolluted plant. Nitrogen-fixing bacteria and fungi were found to be less affected [72]. Leaf physiology also plays a pivotal role in the abundance and diversity of the microbial population. Bacteria may develop larger-sized colonial associations on leaf surfaces [73].

Table 1.
Major phyllosphere microbes.
Table 1.
Major phyllosphere microbes.
| Phyllosphere Microbes | Examples | Reference |
|---|---|---|
| Bacteria (considered to be the most abundant microbes in phyllosphere) | Proteobacteria (Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria), Actinobacteria, Firmicutes, and Bacteroidetes are common in phyllosphere | Thapa et al. [55] |
| Bacillus and Pseudomonas may be prevalent genera | Thapa et al. [55] | |
| Methylotrophic bacteria are prevalent in phyllosphere | Sivakumar et al. [73] | |
| Methane-producing bacteria are also present | Iguchi et al. [61] | |
| Bacteria involved in nitrogen cycle may be present in phyllosphere including diazotrophic bacteria | Papen et al. [65], Furnkranz et al. [66] | |
| Bacterial pathogen may be present like Pseudomonas syringae | Sivakumar et al. [73] | |
| Biosurfactant producing bacteria may be present | Sivakumar et al. [73] | |
| Actinomycetes is also present in phyllosphere | Sivakumar et al. [73] | |
| Fungi | Epiphytic and endophytic fungi may be involved in decomposition of litter, recycling of carbon and nitrogen. Endophytic fungi may be involved in resistance against biotic and abiotic stress | Sivakumar et al. [73] |
| Yeast like fungi and filamentous fungi are present | Sivakumar et al. [73] | |
| Genus of Aspergillus, Penicillium, Alternaria, Trichoderma, Fusarium are observed | Sivakumar et al. [73] |
4.2. Microbiome of Rhizosphere
The rhizosphere is the narrow, dynamic region of the soil where microbial interaction is prevalent under the influence of plant roots. [74,75]. The term “rhizosphere” is derived from the Greek words “rhiza” and “sphere,” meaning root and field of influence, respectively [76]. German scientist Hiltner defined the rhizosphere as “the zone of soil immediately adjacent to legume roots that support the high bacterial activity.” [77]. The rhizosphere is divided into three regions: endo rhizosphere, consisting of root tissue including the endodermis and cortical layers; rhizoplane, consisting of the root–soil interface where soil particles and microbes get adhered to the surface of the roots; and ectorhizosphere, consisting of soil immediately adjacent to the roots. Various organic compounds are released from the roots by secretion, exudation, and deposition, which makes the rhizosphere a nutrient-enriched region of the soil. Root cap cells, root tissue, sloughed root hairs, epidermal tissues, simple carbohydrates, amino acids, vitamins, plant hormones, organic acids, sugar phosphate ester, phenolics, carbon-containing secondary metabolites, proteins, enzymes, and polysaccharides are key ingredients of exudate by rhizodeposition. In addition, this enrichment obviously promotes the growth of various microbial populations. Hence, the rhizosphere becomes a unique region in the soil and a separate region from the bulk of the soil [76]. In addition to bacteria and fungi, the rhizosphere contains oomycetes, nematodes, protozoa, algae, viruses, and archaea. The rhizosphere microbiome becomes part of the complex food web. Some microbes from the rhizosphere exert beneficial effects on plants. They are mainly plant growth-promoting rhizobacteria (PGPR), nitrogen-fixing bacteria, mycorrhizal fungi, and protozoa (Table 2). In contrast to pathogenic bacteria, oomycetes, and nematodes, pathogenic fungi can exhibit a negative effect on plant growth. Rhizosphere microbes are responsible for the uptake of nutrients like trace elements (iron). Low concentrations of phenolic compounds in exudates may promote the conidial germination of pathogenic fungi [78]. Bacteria are more prevalent microbes in this rhizosphere region [76]. The root tip zone, which has the highest sucrose or tryptophan exudation, has a higher number of bacteria than the mature root zone, which has a lower number of bacteria [74]. The more common genera found in the rhizosphere are Pseudomonas, Bacillus, Azotobacter, Arthobacter, Rhizobium, Agrobacterium, Flavobacter, and Micrococcus. Gram-negative, rod-shaped, non-sporulating bacteria belonging to Proteobacteria and Actinobacteria are more commonly found in the rhizosphere, due to the utilisation of root exudates. Aerobic bacteria become less abundant in the rhizosphere as oxygen levels fall. Various strains of Bacillus are predominant among the Gram-positive bacteria in the rhizosphere. It may be the result of the Bacillus’ ability to form endospores and the inhibition of competitors by its production of antimicrobial substances [76]. Plant growth-promoting rhizobacteria (PGPR) are bacteria that promote plant growth, also known as plant health-promoting rhizobacteria (PHPR) and yield-increasing bacteria (YIB). Genera of Azospirillium, Arthobacter, Acinetobacter, Bradyrhizobium, Pseudomonas, Bacillus, Erwinia, Flavobacterium, and Rhizobium have been associated with the rhizosphere and exhibit a positive effect on plants. They carry out various functions for improving plant health, like nitrogen fixation, phosphate solubilization, and the production of phytohormones. They also serve as biocontrol agents through competition, inducing resistance, cell wall lysis, and antibacterial mechanisms [79]. Rhizosphere bacteria can synthesise volatile organic compounds, which have an inhibitory effect on pathogens [80]. The bacterial population in the rhizosphere is determined by the plant itself due to exudate secretion, age, and developmental stage. These factors, in addition to soil pH, salinity, nutrient concentration, organic matter, cropping, and fertiliser and pesticide application, all contribute to the composition of the bacterial population in the rhizosphere [76]. In addition to PGPR, plant growth-promoting fungi (PGPF) are also present in the rhizosphere [81]. PGPFs are non-pathogenic, soil-dwelling microbes; they can colonise the root and are involved in improving plant growth. Many PGPFs may be involved in inducing resistance against pathogens. Important PGPFs in the rhizosphere are Penicillum, Trichoderma, Fusarium, and Phoma [82]. PGPFs may help in root development, promote flowering, stimulate photosynthetic ability, and increase crop yield [83]. Some rhizosphere fungi are responsible for producing antimicrobial compounds (ex., Tricoderma sp.) [78]. Arbuscular mycorrhizae fungi (AMF), an endotrophic mycorrhiza mostly associated with terrestrial plants, play an important role in the rhizosphere region [84]. AMF can obtain carbohydrates from plants and provide minerals like phosphorus and nitrogen to plants [85]. Exudates from mycorrhizal roots also contribute to the proliferation of soil bacteria [83].

Table 2.
Main rhizosphere microbes.
4.3. The Effect of Phyllosphere and Rhizosphere Microbiome on Plant–Insect Interaction
Plants are also habitats for various microbes. Soil bacteria are more diverse than phyllosphere bacteria, and the highest richness is observed in soil bacteria. A mutualistic relationship exists between plants and the bacterial population. Although some bacteria may be pathogenic [86]. Root exudate and host genome-dependent factors are key components for the composition of the bacterial population in the rhizosphere. In contrast, the phyllosphere microbial population is characterised by the immediate leaf surface. Plants can also interact with insects. Interactions between insects and plants have been accounted for since more than 400 million years ago. Microbes can either alter the primary or secondary metabolism of plants, and the defence system of plants, or directly or indirectly affect insects [2]. Instead of bidirectional interactions made by microbes with the host plant or microbes with insects, three-way interactions among microbes, plants, and insects have been observed. Induction of phytohormones like jasmonic acid (JA), salicylic acid (SA), ethylene (ET), plant defensive proteins, and the biosynthesis of secondary metabolites are main players in providing defence against leaf-chewing and phloem-feeding insects [81].
4.3.1. Phyllosphere Interaction
Beetles are important plant pathogen vectors. Phyllosphere microorganisms also play a significant role in the interaction of beetles with plants. The presence of the rust fungus Melamposora allii-fragilis in the willow hybrid Salix x Cuspidata influenced the feeding and oviposition behaviour of the willow leaf beetle Plagiodera versicolora. Rust-infected leaves were free of adult willow beetles because adult willow leaves were kept away from feeding and ovipositing on rust-infected leaves [87].
Leaf fungi can also affect the performance of the insect. The effect of birch rust fungi Melampsoridium betilunum on autumnal moth larvae on mountain birch trees was investigated to know the performance of autumnal moth larvae. The number of rust fungi on the leaves of birch trees was a key determinant of the performance of moth larvae. One tree had no visible fungal infection, while another had low infection and still another had high infection. The highest and lowest pupal weights were found in an asymptomatic tree and a high-infection tree, respectively [5]. It was reported that Ostrinia nubilalis, the European corn borer, was prevented from ovipositing on the maize leaf surface if Sporobolomyces roseus, a leaf-colonising epiphytic yeast, was sprayed over the leaf surface [88]. Infection of Fusarium verticillioides, a maize pathogen, is accelerated by the European corn borer [89].
4.3.2. Effect of Plant Growth Promoting Rhizobacteria (PGPR)/Rhizosphere Bacteria on Plant–Insect Interaction
The effect of rhizobacteria on insects may differ, depending on the specific insect. Different outcomes may be obtained when the same rhizobacteria treatment takes place on different insects. The insects may be negatively affected, positively affected, or not affected at all. Plant–insect interaction may be affected by inducing systemic resistance (ISR) triggered by PGPR. PGPR, Pseudomonas fluorescens could induce systemic resistance against chewing insects in Arabidopsis thaliana. While Pseudomonas fluorescens exerted a positive effect on the performance including weight gain of generalist aphid Myzus persicae, no effect was observed on the crucifer specialist aphid Brevicoryne brassicae in Arabidopsis model. This data clearly shows that rhizobacteria could induce not only resistance but also systemic susceptibility to insects [90].
PGPR mediates resistance against insect herbivores through (ISR). Priming is a key event for ISR [91]. Rhizobacterium, Pseudomonas fluorescens strain SS101 was observed to exhibit resistance against the leaf-chewing insect pest Spodptera exigua in Arabidopsis thaliana. This resistance was mediated through indolic glucosinolate [92]. The presence of PGPR Pseudomonas putida and Rothia sp. reduced the negative impact of an inoculated Spodoptera litura tomato plant. Plant biomass was increased by 60% and yield was enhanced by 40% in insect-inoculated plants in the presence of PGPR. In PGPR-treated plants, increased proline production, antioxidant enzyme activation, phenolic content, and chlorophyll content were observed [93]. Higher weight, a significantly lower mortality rate, and a high pupation rate of beet armyworm, Spodptera exigua larvae, were observed in untreated cotton plants more frequently than in PGPR-treated plants. Plants treated with PGPR also produced more JA and expressed more JA-responsive genes. An enhanced level of the insecticidal compound gossypol and higher expression of genes involved in its biosynthesis was also observed in plants by rhizobacteria [94]. Application of both PGPR, Bacillus sp. strain 6 and Pseudomonas sp. strain 6K, was able to generate good productivity in wheat with the lowest aphid population, the highest plant height, and the highest grain yield [95]. Whitefly (Bemicia tabaci) is an important pest for many plants and can infect around 600 plant species. It can form chlorosis in infested leaves [96]. The report also showed that the development of whitefly (Bemicia tabaci) was retarded in tomato plants in the presence of PGPR Bacillus subtilis [97]. In contrast to this result, pre-inoculated PGPR, Pseudomonas fluorescens WCS417r promoted the performance of whitefly (Bemicia tabaci), a phloem-feeding insect, in the tomato plant. It might be due to the lower ability of bacteria to activate plant defence mechanisms [96]. A significant decrease in the population of the striped cucumber beetle Acalyma vittatum and the spotted cucumber beetle Diabrotica undecimpunctata howrdi was observed when systemic resistance was promoted by PGPR strains such as P. putida strain 89B-27, S. marcescens strain 90–166, Flavomonas oryzihabitans strain INR-5, and Bacillus pumilus strain INR-5 in cucumbers. Moreover, the efficacy of S. marcescens strain 90-166 was better than the others and more effective than the insecticide esfenvalerate [98]. Another report showed that the rhizobacterium Pseudomonas simiae WCS417r elevated ISR against the leaf-chewing insect, Mamestra brassicae, in Arabidopsis thaliana. The JA and ET pathways were activated to promote resistance. In addition, camalexin and aliphatic glucosinolates (GLS) were synthesised by rhizobacteria, and they had the ability to inhibit the herbivore-mediated level of indole GLS. After rhizobacteria colonised Arabidopsis thaliana roots, the signalling pathway was altered by prioritising expression of genes in the JA/ET-dependent ORA59 branch over the JA-dependent MYC2 branch. Hence, the interaction between plant and insect was modified due to the colonisation of rhizobacterium at the level of transcription and the performance of the insect and plant chemistry. Hence, it is clear that the bidirectional interaction of plants and rhizosphere bacteria determines the outcome of the plant–insect interaction, leading to the conversion of bidirectional interaction to tripartite interaction [99]. The weight of Mamestra brassicae caterpillar larvae was reduced after rhizobacteria were inoculated into Arabidopsis roots. Treatment with rhizobacteria exerted a negative effect on Mamestra brassicae in soil and sand mixtures, whereas the result was variable only in soil. It is proposed that rhizobacteria–plant–insect interaction is also dependent on soil composition [100]. A significant reduction in tomato production has been associated with Spodoptera litura. Tomato rhizosphere bacteria exhibited resistance against the tobacco cutworm, Spodoptera litura, by inducing a JA-dependent pathway in tomato plants. Four defence-related genes, allene oxide cyclase (AOC), allene oxide synthase (AOS), lipoxygenase D (LOXD), and proteinase inhibitor II (PI-II), were found to be induced by bacterial isolates after six hours of inoculation with insects. The expression levels of the JA biosynthesis genes AOC, AOS, and LOXD were 2.2, 1.7, and 2.7-fold, respectively [101]. AMF are members of the Phylum Glomeromycota and can interact symbiotically with more than 80% of terrestrial plants. Inoculation of AMF (Glomus intraradices) enhanced tolerance against Spodoptera litura in black gram [102]. AMF exerted resistance against gypsy moth larvae by reducing larval growth and survival. The body weight, length, and head capsule width of the 4th and 5th instar gypsy moth larvae were significantly reduced by AMF-inoculated poplar plants. In this way, AMF protected poplar seedlings. Metabolites with insecticidal activity like coumarin, stachydrine, vanillic acid, and abietic acid were found to be increased in AMF-infested poplar plants [103]. Inoculation of AMF into plants may affect the interaction between aboveground plants and pollinators. The report showed that visitation of all pollinators did not depend on AMF treatment, but there was a taxon-specific response. The behaviour of bumblebees, honeybees, and Lepidoptera varied with AMF treatment [104].
In summary, microbes associated with the phyllosphere and rhizosphere have positive and negative effects on insects. This may lead to the development of resistance to insects in plants. Several mechanisms are regulated by microbes for resistance (Figure 2).
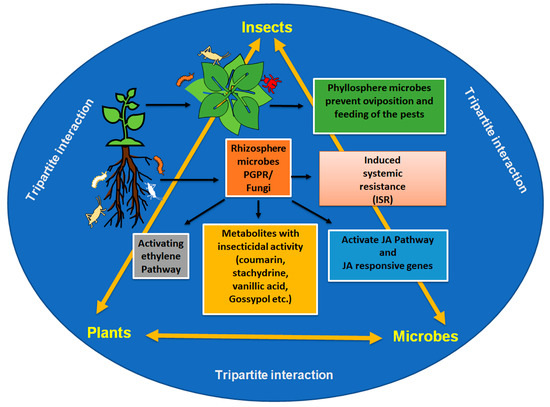
Figure 2.
The influence of phyllosphere and rhizosphere microbiome on plant–insect interplay.
5. Plant–Microbe Interaction Shaping the Ecological Dynamics
In plant immunity, adaptive reactions are paramount due to the large absence of humoral reactions. In the ecological niche distinction between pathogenic and non-pathogenic microbes by plants is very interesting. The microbes possess structural components specific to the entire group (e.g., chitin for the fungi) called pathogen-associated molecular patterns (PAMP) or microbe-associated molecular patterns (MAMP). These PAMPs/MAMPs are recognized by cognate receptors found on the host cell surface of plants, called pattern recognition receptors (PRRs). The PAMP–PRR interaction determines successful plant–microbe interaction. Now, pathogenic microbes possess effector molecules that lead to toxicity due to particular diseases [105]. The arms race between plants and microbes either beneficial or harmful instigates several metabolite syntheses in plants. The induction of secondary metabolites due to plant–microbe interaction is highly evident in different studies [106]. The functional characterization of bacteriomes and associated metabolites have been studied in relation to plant–insect interaction [107]. The secreted metabolites from the root have changed the rhizosphere microbiota [108]. These findings would bridge a huge knowledge gap in communication between plants and microbes. This will not only provide novel information on plant–microbe interplay but have immense application in human welfare and animal sciences too in the future.
6. Conclusions and Future Remarks
There are two types of tri-trophic interactions between plants, insects, and microbes. Microbe-mediated plant–insect interactions and insect-mediated plant–microbe interactions. In this review, we discussed the effects of plant microbes on plant–insect interactions. Plant-associated microbes have positive and negative effects on insect–plant interactions. The development of susceptibility to insects may occur as a result of the positive effect. In contrast, plants show resistance to insects under the influence of microbes. This result has another implication for the agricultural field. Microbes from the phyllosphere and rhizosphere have been studied for their ability to control insects in plants. They can be used as bioprotectants for plants against insects. Since insects are plant pests, they cause major crop losses worldwide. This eco-friendly, natural system based on microbes offers several advantages over conventional insecticides. The development of insecticide resistance is eliminated. The environment is not polluted by the use of microbes. Therefore, knowledge of the interaction between plants, insects, and microbes is crucial for maintaining plant health and developing bioinsecticides using microbes associated with plants. Furthermore, because specific pollinators may visit AMF-treated plants on a regular basis, they receive much more attention in the field of pollination. This field can be explored to enrich plant pollination chemistry. Hence, tri-trophic interaction among plants, insects, and microbes is essential for maintaining plant health as well as pollination chemistry.
Author Contributions
Conceptualization, A.B. (Anirban Bhar), writing—original draft preparation, T.D., A.B. (Anindya Bhattacharyya) and A.B. (Anirban Bhar); writing—review and editing, T.D., A.B. (Anindya Bhattacharyya) and A.B. (Anirban Bhar); visualization, A.B. (Anirban Bhar); supervision, A.B. (Anirban Bhar). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are highly thankful to the Editor-in-chief, guest editor, and all other editorial members of the journal to grant full waiver to publish this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saikkonen, K.; Neuvonen, S. European pine sawfly and microbial interactions mediated by the host plant. In Sawfly Life History Adaptations to Woody Plants; Wagner, M., Raffa, K., Eds.; Academic Press, Inc.: New York, NY, USA, 1993; pp. 431–450. [Google Scholar]
- Sugio, A.; Dubreuil, G.; Giron, D.; Simon, J.C. Plant–insect interactions under bacterial influence: Ecological implications and underlying mechanisms. J. Exp. Bot. 2015, 66, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Wielkopolan, B.; Obrępalska-Stęplowska, A. Three-way interaction among plants, bacteria, and coleopteran insects. Planta 2016, 244, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Coupland, G.; Dolan, L.; Harberd, N.; Jones, J.; Martin, C.; Sablowski, R.; Amey, A. Plant Biology; Garland Science, Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Lappalainen, J.; Helander, M.L.; Palokangas, P. The performance of the autumnal moth is lower on trees infected by birch rust. Myco. Res. 1995, 99, 994–996. [Google Scholar] [CrossRef]
- Franco, F.P.; Túler, A.C.; Gallan, D.Z.; Gonçalves, F.G. Fungal phytopathogen modulates plant and insect responses to promote its dissemination. ISME J. 2021, 15, 3522–3533. [Google Scholar] [CrossRef] [PubMed]
- Sturz, A.V.; Christie, B.R.; Matheson, B.G.; Arsenault, W.J.; Buchanan, N.A. Endophytic bacterial communities in the periderm of potato tubers and their potential to improve resistance to soilborne plant pathogens. Plant Pathol. 1999, 48, 360–369. [Google Scholar] [CrossRef]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P.H.J. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef]
- Humphrey, P.T.; Nguyen, T.T.; Villalobos, M.M.; Whiteman, N.K. Diversity and abundance of phyllosphere bacteria are linked to insect herbivory. Mol. Ecol. 2014, 23, 1497–1515. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant. Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Jindal, V.; Dhawan, A.K. Insect pest problems and crop losses: Changing trends. Indian J. Ecol. 2010, 37, 1–7. [Google Scholar]
- IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests—A Global Challenge to Prevent and Mitigate Plant Pest Risks in Agriculture, Forestry and Ecosystems; FAO on behalf of the IPPC Secretariat: Rome, Italy, 2021. [Google Scholar]
- Bishop, C.D.; Erezyilmaz, D.F.; Flatt, T.; Georgiou, C.D.; Hadfield, M.G.; Heyland, A.; Hodin, J.; Jacobs, M.W.; Maslakova, S.A.; Pires, A.; et al. What is metamorphosis? Integr. Comp. Biol. 2006, 46, 655–661. [Google Scholar] [CrossRef]
- Truman, J.W. The evolution of insect metamorphosis. Curr. Biol. 2019, 29, R1252–R1268. [Google Scholar] [CrossRef]
- App, B.A.; Manglitz, G.R. Insects and related pests. Alfalfa Sci. Technol. 1972, 15, 527–554. [Google Scholar]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef]
- Rotenberg, D.; Baumann, A.A.; Ben-Mahmoud, S.; Christiaens, O.; Dermauw, W.; Ioannidis, P.; Jacobs, C.G.; Vargas Jentzsch, I.M.; Oliver, J.E.; Poelchau, M.F.; et al. Genome-enabled insights into the biology of thrips as crop pests. BMC Biol. 2020, 18, 169. [Google Scholar] [CrossRef]
- Gullan, P.J.; Kosztarab, M. Adaptations in scale insects. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef]
- Mansour, R.; Grissa-Lebdi, K.; Suma, P.; Mazzeo, G.; Russo, A. Key scale insects (Hemiptera: Coccoidea) of high economic importance in a Mediterranean area: Host plants, bio-ecological characteristics, natural enemies and pest management strategies—A review. Plant Prot. Sci. 2017, 53, 1–14. [Google Scholar] [CrossRef]
- Joshi, M.D.; Butani, P.G.; Patel, V.N.; Jeyakumar, P. Cotton mealy bug, Phenacoccus solenopsis Tinsley-a review. Agric. Rev. 2010, 31, 113–119. [Google Scholar]
- Mokhtar, A.H. Biochemical and anatomical characteristics of some mango tree cultivars infested by two mealybugs, planococcus citri (risso) and icerya seychellarum (westood) in Egypt. Egypt. J. Agric. Res. 2022, 100, 570–580. [Google Scholar] [CrossRef]
- Prabhakar, M.; Prasad, Y.G.; Thirupathi, M.; Sreedevi, G.; Dharajothi, B.; Venkateswarlu, B. Use of ground based hyperspectral remote sensing for detection of stress in cotton caused by leafhopper (Hemiptera: Cicadellidae). Comput. Electron. Agric. 2011, 79, 189–198. [Google Scholar] [CrossRef]
- Shorthouse, J.D. INSECTS AND OTHER ANIMALS|Overview of Insects. Encyclopedia of Rose Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 415–425. [Google Scholar]
- Zhao, X.; Chen, S.; Wang, S.; Shan, W.; Wang, X.; Lin, Y.; Su, F.; Yang, Z.; Yu, X. Defensive responses of tea plants (Camellia sinensis) against tea green leafhopper attack: A multi-omics study. Front. Plant Sci. 2020, 10, 1705. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, W.; Huang, C.; Hu, Y.; Chen, Y.; Guo, J.; Zhou, C.; Chen, R.; Du, B.; Zhu, L.; et al. Combining next-generation sequencing and single-molecule sequencing to explore brown plant hopper responses to contrasting genotypes of japonica rice. BMC Genom. 2019, 20, 682. [Google Scholar] [CrossRef] [PubMed]
- Thein, H.W.; Yamagata, Y.; Van Mai, T.; Yasui, H. Four resistance alleles derived from Oryza longistaminata (A. Chev. & Roehrich) against green rice leafhopper, Nephotettix cincticeps (Uhler) identified using novel introgression lines. Breed Sci. 2019, 69, 573–584. [Google Scholar] [PubMed]
- Will, T.; van Bel, A.J. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006, 57, 729–737. [Google Scholar] [CrossRef]
- Giordanengo, P.; Brunissen, L.; Rusterucci, C.; Vincent, C.; van Bel, A.; Dinant, S.; Girousse, C.; Faucher, M.; Bonnemain, J.L. Compatible plant-aphid interactions: How aphids manipulate plant responses. Comptes Rendus Biol. 2010, 333, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Cun, Z. Identification of New Chickpea Virus and Control of Chickpea Virus Disease. Evid-Based Complment. Altern. Med 2022, 2022, 6465505. [Google Scholar] [CrossRef]
- Hidayat, P.; Harleni, H.; Maharani, Y.; Triwidodo, H. Biology and demography statistic aphids Rhopalosiphum rufiabdominale (Sasaki) and Tetraneura nigriabdominalis (Sasaki) (Hemiptera: Aphididae) in rice roots. Indones. J. Entomol. 2019, 16, 180–186. [Google Scholar] [CrossRef]
- Cranshaw, W.; Wainwright-Evans, S. Cannabis sativa as a host of rice root aphid (Hemiptera: Aphididae) in North America. J. Integr. Pest Manag. 2020, 11, 15. [Google Scholar] [CrossRef]
- War, A.R.; Taggar, G.K.; Hussain, B.; Taggar, M.S.; Nair, R.M.; Sharma, H.C. Plant defence against herbivory and insect adaptations. AoB Plants 2018, 10, ply037. [Google Scholar]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981. [Google Scholar] [CrossRef]
- Gust, A.A.; Nürnberger, T. A plant surface receptor for sensing insect herbivory. Proc. Natl. Acad. Sci. USA 2020, 117, 32839–32841. [Google Scholar] [CrossRef]
- Malook, S.U.; Maqbool, S.; Hafeez, M.; Karunarathna, S.C.; Suwannarach, N. Molecular and Biochemical Mechanisms of Elicitors in Pest Resistance. Life 2022, 12, 844. [Google Scholar] [CrossRef]
- Coppola, M.; Di Lelio, I.; Romanelli, A.; Gualtieri, L.; Molisso, D.; Ruocco, M.; Avitabile, C.; Natale, R.; Cascone, P.; Guerrieri, E.; et al. Tomato plants treated with systemin peptide show enhanced levels of direct and indirect defense associated with increased expression of defense-related genes. Plants 2019, 8, 395. [Google Scholar] [CrossRef]
- Steinbrenner, A.D.; Muñoz-Amatriaín, M.; Venegas, J.M.A.; Lo, S.; Shi, D.; Holton, N.; Zipfel, C.; Abagyan, R.; Huffaker, A.; Close, T.J.; et al. A receptor for herbivore-associated molecular patterns mediates plant immunity. Biorxiv 2019, 679803. [Google Scholar]
- Klauser, D.; Desurmont, G.A.; Glauser, G.; Vallat, A.; Flury, P.; Boller, T.; Turlings, T.C.; Bartels, S. The Arabidopsis Pep-PEPR system is induced by herbivore feeding and contributes to JA-mediated plant defence against herbivory. J. Exp. Bot. 2015, 66, 5327–5336. [Google Scholar] [CrossRef]
- Gouhier-Darimont, C.; Stahl, E.; Glauser, G.; Reymond, P. The Arabidopsis lectin receptor kinase LecRK-I 8 is involved in insect egg perception. Front. Plant Sci. 2019, 10, 623. [Google Scholar] [CrossRef]
- Chen, C.Y.; Mao, Y.B. Research advances in plant–insect molecular interaction. F1000Res 2020, 9, 198. [Google Scholar] [CrossRef]
- Stotz, H.U.; Koch, T.; Biedermann, A.; Weniger, K.; Boland, W.; Mitchell-Olds, T. Evidence for regulation of resistance in Arabidopsis to Egyptian cotton worm by salicylic and jasmonic acid signaling pathways. Planta 2002, 214, 648–652. [Google Scholar] [CrossRef]
- Alfonso, E.; Stahl, E.; Glauser, G.; Bellani, E.; Raaymakers, T.M.; Van den Ackerveken, G.; Zeier, J.; Reymond, P. Insect eggs trigger systemic acquired resistance against a fungal and an oomycete pathogen. New Phytol. 2021, 232, 2491–2505. [Google Scholar] [CrossRef]
- Mollah, M.M.I.; Choi, H.W.; Yeam, I.; Lee, J.M.; Kim, Y. Salicylic acid, a plant hormone, suppresses phytophagous insect immune response by interrupting HMG-Like DSP1. Front. Physiol. 2021, 12, 744272. [Google Scholar] [CrossRef]
- Wu, J.; Hettenhausen, C.; Meldau, S.; Baldwin, I.T. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 2007, 19, 1096–1122. [Google Scholar] [CrossRef]
- Kundu, P.; Vadassery, J. Role of WRKY transcription factors in plant defense against lepidopteran insect herbivores: An overview. J. Plant Biochem. Biotechnol. 2021, 30, 698–707. [Google Scholar] [CrossRef]
- Sperdouli, I.; Andreadis, S.S.; Adamakis, I.D.S.; Moustaka, J.; Koutsogeorgiou, E.I.; Moustakas, M. Reactive Oxygen Species Initiate Defence Responses of Potato Photosystem II to Sap-Sucking Insect Feeding. Insects 2022, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to Herbivory, Wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef] [PubMed]
- Parmagnani, A.S.; Maffei, M.E. Calcium Signaling in Plant-Insect Interactions. Plants 2022, 11, 2689. [Google Scholar] [CrossRef]
- Heyer, M.; Scholz, S.S.; Reichelt, M.; Kunert, G.; Oelmüller, R.; Mithöfer, A. The Ca2+ sensor proteins CML37 and CML42 antagonistically regulate plant stress responses by altering phytohormone signals. Plant Mol. Biol. 2022, 109, 611–625. [Google Scholar] [CrossRef]
- Barrera, S.E.; Sarango-Flóres, S.W.; Montenegro-Gómez, S.P. The phyllosphere microbiome and its potential application in horticultural crops. A review. Rev. Colomb. Cienc. Hortícolas 2019, 13, 384–396. [Google Scholar] [CrossRef]
- Vacher, C.; Hampe, A.; Porté, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The Phyllosphere: Microbial Jungle at the Plant–Climate Interface. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Ruinen, J. The phyllosphere: I. An ecologically neglected milieu. Plant Soil 1961, 15, 81–109. [Google Scholar] [CrossRef]
- Morris, C.E. Phyllosphere. eLS 2002, 1–8. [Google Scholar]
- Doan, H.K.; Leveau, J.H. Artificial surfaces in phyllosphere microbiology. Phytopathology 2015, 105, 1036–1042. [Google Scholar] [CrossRef]
- Thapa, S.; Prasanna, R. Prospecting the characteristics and significance of the phyllosphere microbiome. Ann. Microbiol. 2018, 68, 229–245. [Google Scholar] [CrossRef]
- Morris, C.; Kinkel, L. Phyllosphere microbiology. In Fifty Years of Phyllosphere Microbiology: Significant Contributions to Research in Related Fields; Lindow, S.E., Hecht-Poinar, E.I., Elliott, V.J., Eds.; APS Press: St. Paul, MO, USA, 2002; pp. 365–375. [Google Scholar]
- Gouka, L.; Raaijmakers, J.M.; Cordovez, V. Ecology and functional potential of phyllosphere yeasts. Trends Plant Sci. 2022, 27, 1109–1123. [Google Scholar] [CrossRef]
- Rao, N.S.S. Soil Microbiology. Oxford & IBH Publishing Co. Pvt Ltd.: New Delhi, India, 1999. [Google Scholar]
- Yurimoto, H.; Shiraishi, K.; Saka, Y. Physiology of Methylotrophs Living in the Phyllosphere. Microorganisms 2021, 9, 809. [Google Scholar] [CrossRef]
- Abanda-Nkpwatt, D.; Musch, M.; Tschiersch, J.; Boettner, M.; Schwab, W. Molecular interaction between Methylobacterium extorquens and seedlings: Growth promotion, methanol consumption, and localization of the methanol emission site. J. Exp. Bot. 2006, 57, 4025–4032. [Google Scholar] [CrossRef]
- Iguchi, H.; Yurimoto, H.; Sakai, Y. Interactions of methylotrophs with plants and other heterotrophic bacteria. Microorganisms 2015, 3, 137–151. [Google Scholar] [CrossRef]
- Gibson, L.; Crombie, A.T.; McNamara, N.P.; Murrell, J.C. Isoprene-degrading bacteria associated with the phyllosphere of Salix fragilis, a high isoprene-emitting willow of the Northern Hemisphere. Environ. Microbiol 2021, 16, 17. [Google Scholar] [CrossRef]
- Crombie, A.T.; Larke-Mejia, N.L.; Emery, H.; Dawson, R.; Pratscher, J.; Murphy, G.P.; McGenity, T.J.; Murrell, J.C. Poplar phyllosphere harbors disparate isoprene-degrading bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 13081–130816. [Google Scholar] [CrossRef]
- Carrión, O.; Gibson, L.; Elias, D.M.O.; McNamara, N.P.; van Alen, T.A.; Huub, J.M.; Op den Camp, H.J.; Supramaniam, C.V.; McGenity, T.J.; Murrell, J.C. Diversity of isoprene-degrading bacteria in phyllosphere and soil communities from a high isoprene-emitting environment: A Malaysian oil palm plantation. Microbiome 2020, 8, 81. [Google Scholar] [CrossRef]
- Papen, H.; Gessler, A.; Zumbusch, E.; Rennenberg, H. Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Curr. Microbiol. 2002, 44, 56–60. [Google Scholar] [CrossRef]
- Fu¨rnkranz, M.; Wane, W.; Richter, A.; Abell, G.; Rasche, F.; Sessitsch, A. Nitrogen fixation by phyllosphere bacteria associated with higher plants and their colonizing epiphytes of a tropical lowland rainforest of Costa Rica. ISME J. 2008, 2, 561–570. [Google Scholar] [CrossRef]
- Thompson, I.P.; Bailey, M.J.; Fenlon, J.S.; Fermor, T.R.; Lilley, A.K.; Lynch, J.M.; McCormack, P.J.; McQuilken, M.P.; Purdy, K.J.; Rainey, P.B.; et al. Quantitative and qualitative seasonal changes in the microbial community from the phyllosphere of sugar beet (Beta vulgaris). Plant Soil 1993, 150, 177–191. [Google Scholar] [CrossRef]
- Bringel, F.; Couée, I. Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front. Microbiol. 2015, 6, 486. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.L.; Sundin, G.W. Effect of Solar UV-B Radiation on a Phyllosphere Bacterial Community. Appl. Environ. Microbiol. 2001, 67, 5488–5496. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y. Effect of climate change on phyllosphere microflora: Plant-microorganism interactions. Climate Change: Global Risks, Challenges and Decisions. In IOP Conference Series. Earth and Environmental Science; IOP Publishing: Bristol, UK, 2009; p. 6. [Google Scholar]
- Aydogan, E.L.; Moser, G.; Müller, C.; Kämpfer, P.; Glaeser, S.P. Long-Term Warming Shifts the Composition of Bacterial Communities in the Phyllosphere of Galium album in a Permanent Grassland Field-Experiment. Front Microbiol. 2018, 9, 144. [Google Scholar] [CrossRef]
- Brighigna, L.; Gori, A.; Gonneli, S.; Favilli, F. The influence of air pollution on the phyllosphere microflora composition of Tillandsia leaves (Bromeliaceae). Rev. Bio. Trop. 2000, 48, 511–517. [Google Scholar] [CrossRef]
- Sivakumar, N.; Sathishkumar, R.; Selvakumar, G.; Shyamkumar, R.; Arjunekumar, K. Phyllospheric Microbiomes: Diversity, Ecological Significance, and Biotechnological Applications. In Plant Microbiomes for Sustainable Agriculture; Yadav, A.N., Singh, J., Rastegari, A.A., Yadav, N., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 25, pp. 113–172. [Google Scholar]
- Lagos, M.L.; Maruyama, F.; Nannipieri, P.; Mora, M.L.; Ogram, A.; Jorquera, M.A. Current overview on the study of bacteria in the rhizosphere by modern molecular techniques: A mini-review. J. Soil Sci. Plant Nutr. 2015, 15, 504–523. [Google Scholar]
- Wang, Y.; Zhang, M.; Li, S.; Li, P.; Lang, Z. Effects of Insect-Resistant Maize HGK60 on Community Diversity of Bacteria and Fungi in Rhizosphere Soil. Plants 2022, 11, 2824. [Google Scholar] [CrossRef]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Rhizosphere: Its structure, bacterial diversity and significance. Rev. Environ. Sci. Biotechnol. 2014, 13, 63–77. [Google Scholar] [CrossRef]
- Hiltner, L. About new experiences and problems in the field of Bodenbakteriologie. Works Ger. Agric. Soc. 1904, 98, 59–78. [Google Scholar]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Sayyed, R.Z.; Reddy, M.S. Plant Growth-Promoting Rhizobacteria: An Eco-friendly Approach for Sustainable Agroecosystem. In Plant, Soil and Microbes; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; Volume 1: Implication in Crop Science, pp. 181–201. [Google Scholar]
- Wang, E.; Liu, X.; Si, Z.; Li, X.; Bi, J.; Dong, W.; Chen, M.; Wang, S.; Zhang, J.; Song, A.; et al. Volatile Organic Compounds from Rice Rhizosphere Bacteria Inhibit Growth of the Pathogen Rhizoctonia solani. Agriculture 2021, 11, 368. [Google Scholar] [CrossRef]
- Rashid, M.H.O.; Chung, Y.R. Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar] [CrossRef]
- El-Maraghy, S.S.; Tohamy, A.T.; Hussein, K.A. Plant protection properties of the Plant Growth-Promoting Fungi (PGPF): Mechanisms and potentiality. Curr. Res. Environ. Appl. Mycol. 2021, 11, 391–415. [Google Scholar] [CrossRef]
- Akinola, S.A.; Babalola, O.O. The fungal and archaeal community within plant rhizosphere: A review on their contribution to crop safety. J. Plant Nutri. 2021, 44, 600–618. [Google Scholar] [CrossRef]
- Cui, J.; Bai, L.; Liua, X.; Jie, W.; Cai, B. Arbuscular mycorrhizal fungal communities in the rhizosphere of a continuous cropping soybean system at the seedling stage. Braz. J. Microbiol. 2018, 49, 240–247. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Simon, M.; Hilker, M. Does Rust Infection of Willow Affect Feeding and Oviposition Behavior of Willow Leaf Beetles? J. Insect Behav. 2005, 18, 115–129. [Google Scholar] [CrossRef]
- Martin, D.S.; Jones, C.P.; Yao, K.F.; Lee, L.E. An epiphytic yeast (Sporobolomyces roseus) influencing in oviposition preference of the European corn borer (Ostrinia nubilalis) on maize. Acta Oecol. 1993, 14, 563–574. [Google Scholar]
- Blandino, M.; Scarpino, V.; Vanara, F.; Sulyok, M.; Krska, R.; Reyneri, A. Role of the European corn borer (Ostrinia nubilalis) on contamination of maize with 13 Fusarium mycotoxins. Food addit. Contam. Chem. Annal. 2015, 32, 533–543. [Google Scholar] [CrossRef]
- Pineda, A.; Zheng, S.J.; van Loon, J.J.A.; Dicke, M. Rhizobacteria modify plant–aphid interactions: A case of induced systemic susceptibility. Plant Biol. 2012, 14 (Suppl. S1), 83–90. [Google Scholar] [CrossRef] [PubMed]
- Disi, J.; Simmons, J.; Zebelo, S. Plant Growth-Promoting Rhizobacteria-Induced Defense Against Insect Herbivores. In Field Crops: Sustainable Management by PGPR; Maheshwari, D.K., Dheeman, S., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 385–410. [Google Scholar]
- Van de Mortel, J.E.; de Vos, R.C.; Dekkers, E.; Pineda, A.; Guillod, L.; Bouwmeester, K.; van Loon, J.J.; Dicke, M.; Raaijmakers, J.M. Metabolic and transcriptomic changes induced in Arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol. 2012, 160, 2173–2188. [Google Scholar] [CrossRef]
- Bano, A.; Muqarab, R. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.). Plant Biol. 2017, 19, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria activates (+)-δ-cadinene synthase genes and induces systemic resistance in cotton against beet armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Aslam, Z.; Khaliq, A.; Ahmed, J.N.; Nawaz, A.; Hussain, M. Plant growth promoting rhizobacteria reduce aphid population and enhance the productivity of bread wheat. Braz. J. Microbiol. 2018, 49 (Suppl. S1), 9–14. [Google Scholar] [CrossRef]
- Shavit, R.; Ofek-Lalzar, M.; Burdman, S.; Morin, S. Inoculation of tomato plants with rhizobacteria enhances the performance of the phloem-feeding insect Bemisia tabaci . Front. Plant Sci. 2013, 4, 306. [Google Scholar] [CrossRef]
- Valenzuela-Soto, J.H.; Estrada-Hernández, M.G.; Ibarra-Laclette, E.; Délano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 2010, 231, 397–410. [Google Scholar] [CrossRef]
- Zehnder, G.; Kloepper, J.; Yao, C.; Wei, G. Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth promoting rhizobacteria. J. Econ. Entomol. 1997, 90, 391–396. [Google Scholar] [CrossRef]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.; Dicke, M.; Pineda, A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar]
- Pangesti, N.; Pineda, A.; Dicke, M.; Van Loon, J.J.A. Variation in plant-mediated interactions between rhizobacteria and caterpillars: Potential role of soil composition. Plant Biol. 2015, 17, 474–483. [Google Scholar] [CrossRef]
- Ling, S.; Zhao, Y.; Sun, S.; Zheng, D.; Sun, X.; Zeng, R.; Chen, D.; Song, Y. Enhanced anti-herbivore defense of tomato plants against Spodoptera litura by their rhizosphere bacteria. BMC Plant Biol. 2022, 22, 254. [Google Scholar] [CrossRef]
- Selvaraj, A.; Thangavel, K.; Uthandi, S. Arbuscular mycorrhizal fungi (Glomus intraradices) and diazotrophic bacterium (Rhizobium BMBS) primed defense in blackgram against herbivorous insect (Spodoptera litura) infestation. Microbiol. Res. 2020, 231, 126355. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, M.; Wu, S.; Zheng, L.; Wang, Q.; Wang, G.; Yan, S. Defense responses of arbuscular mycorrhizal fungus-colonized poplar seedlings against gypsy moth larvae: A multiomics study. Hortic. Res. 2021, 8, 245. [Google Scholar] [CrossRef]
- Barber, N.A.; Kiers, E.T.; Hazzard, R.V.; Adler, L.S. Context-dependency of arbuscular mycorrhizal fungi on plant–insect interactions in an agroecosystem. Front. Plant. Sci. 2013, 4, 338. [Google Scholar] [CrossRef]
- Bhar, A.; Chakraborty, A.; Roy, A. Plant responses to biotic stress: Old memories matter. Plants 2022, 11, 84. [Google Scholar] [CrossRef]
- Rangel, L.I.; Bolton, M.D. The unsung roles of microbial secondary metabolite effectors in the plant disease cacophony. Curr. Opin. Plant Biol. 2022, 68, 102233. [Google Scholar] [CrossRef]
- Mayoral-Peña, Z.; Lázaro-Vidal, V.; Fornoni, J.; Álvarez-Martínez, R.; Garrido, E. Studying Plant–Insect Interactions through the Analyses of the Diversity, Composition, and Functional Inference of Their Bacteriomes. Microorganisms 2022, 11, 40. [Google Scholar] [CrossRef]
- Koprivova, A.; Kopriva, S. Plant secondary metabolites altering root microbiome composition and function. Curr. Opin. Plant Biol. 2022, 67, 102227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).