Heart Rate Variability Parameters to Evaluate Autonomic Functions in Healthy Young Subjects during Short-Term “Dry” Immersion
Abstract
1. Introduction
2. Results
3. Discussion
3.1. “Heating” Effect Per Se
3.2. “Water Immersion” Effect Per Se
3.3. “Warm Water Immersion” (WWI) Effect
3.4. “Dry Immersion” (DI) Effect
4. Materials and Methods
4.1. Participants
4.2. The DI Session
4.3. Outcome Measures
- Time-domain parameters
- The SDNN and RMSSD inform on the variation of parasympathetically-mediated respiratory sinus arrhythmia.
- The pNN50 is closely correlated with parasympathetic nervous activity.
- The TINN and RMSSD can jointly distinguish between normal heart rhythms and arrhythmias.
- Frequency-domain parameters
- HF power reflects the parasympathetic activity and is related to the respiratory cycle.
- LF power is frequently considered to be related to sympathetic nervous activity. However, the LF power may be produced by both the PNS and SNS, and BP regulation via baroreceptors.
- VLF power correlates with renin–angiotensin and endothelial influences on the heart.
- LF/HF ratio measures “sympatho-vagal balance”, but this model is challenged.
- Nonlinear parameters
- ApEn values indicate the predictability of fluctuations in successive RR intervals.
- DFA describes brief (α1) and long-term (α2) fluctuations. α1 reflects the baroreceptor reflex, while α2 reflects the regulatory mechanisms that limit fluctuation of the RR interval.
- the non-linear metric SD1 of Poincare plot is identical to the RMSSD
- The SD2 measures correlate with LF power and baroreflex sensitivity.
- SD1/SD2 correlates with the LF/HF ratio.
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandiarajan, M.; Hargens, A.R. Ground-Based Analogs for Human Spaceflight. Front. Physiol. 2020, 11, 716. [Google Scholar] [CrossRef]
- Watenpaugh, D.E. Analogs of microgravity: Head-down tilt and water immersion. J. Appl. Physiol. 2016, 120, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry Immersion as a Ground-Based Model of Microgravity Physiological Effects. Front. Physiol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Kermorgant, M.; Nasr, N.; Czosnyka, M.; Arvanitis, D.N.; Hélissen, O.; Senard, J.M.; Pavy-Le Traon, A. Impacts of Microgravity Analogs to Spaceflight on Cerebral Autoregulation. Front. Physiol. 2020, 11, 778. [Google Scholar] [CrossRef]
- Amirova, L.; Navasiolava, N.; Rukavishvikov, I.; Gauquelin-Koch, G.; Gharib, C.; Kozlovskaya, I.; Custaud, M.A.; Tomilovskaya, E. Cardiovascular System Under Simulated Weightlessness: Head-Down Bed Rest vs. Dry Immersion. Front. Physiol. 2020, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Amirova, L.E.; Plehuna, A.; Rukavishnikov, I.V.; Saveko, A.A.; Peipsi, A.; Tomilovskaya, E.S. Sharp Changes in Muscle Tone in Humans Under Simulated Microgravity. Front. Physiol. 2021, 12, 661922. [Google Scholar] [CrossRef] [PubMed]
- Navasiolava, N.M.; Custaud, M.A.; Tomilovskaya, E.S.; Larina, I.M.; Mano, T.; Gauquelin-Koch, G.; Gharib, C.; Kozlovskaya, I.B. Long-term dry immersion: Review and prospects. Eur. J. Appl. Physiol. 2011, 111, 1235–1260. [Google Scholar] [CrossRef] [PubMed]
- Linossier, M.T.; Amirova, L.E.; Thomas, M.; Normand, M.; Bareille, M.P.; Gauquelin-Koch, G.; Beck, A.; Costes-Salon, M.C.; Bonneau, C.; Gharib, C.; et al. Effects of short-term dry immersion on bone remodeling markers, insulin and adipokines. PLoS ONE 2017, 12, e0182970. [Google Scholar] [CrossRef] [PubMed]
- Genin, A.M.; AIu, M.; Shashkov, V.S. Status of human hemodynamics during water immersion in different postures of immersion. Kosm. Biol. I Aviakosmicheskaia Meditsina 1988, 22, 7–10, (In Russian, English summary). [Google Scholar] [PubMed]
- Gerasimova-Meigal, L.; Meigal, A.; Sireneva, N.; Saenko, I. Autonomic Function in Parkinson’s Disease Subjects Across Repeated Short-Term Dry Immersion: Evidence From Linear and Non-linear HRV Parameters. Front. Physiol. 2021, 12, 712365. [Google Scholar] [CrossRef]
- Miroshnichenko, G.G.; Meigal, A.Y.; Saenko, I.V.; Gerasimova-Meigal, L.I.; Chernikova, L.A.; Subbotina, N.S.; Rissanen, S.M.; Karjalainen, P.A. Parameters of Surface Electromyogram Suggest That Dry Immersion Relieves Motor Symptoms in Patients With Parkinsonism. Front. Neurosci. 2018, 12, 667. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Yamamoto, S.; Iwamoto, M.; Inoue, M.; Harada, N. Evaluation of the effect of heat exposure on the autonomic nervous system by heart rate variability and urinary catecholamines. J. Occup. Health. 2007, 49, 199–204. [Google Scholar] [CrossRef]
- Brenner, I.K.; Thomas, S.; Shephard, R.J. Spectral analysis of heart rate variability during heat exposure and repeated exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 145–156. [Google Scholar] [CrossRef]
- Bonde-Petersen, F.; Schultz-Pedersen, L.; Dragsted, N. Peripheral and central blood flow in man during cold, thermoneutral, and hot water immersion. Aviat. Space Environ. Med. 1992, 63, 346–350. [Google Scholar]
- Boussuges, A. Immersion in thermoneutral water: Effects on arterial compliance. Aviat. Space Environ. Med. 2006, 77, 1183–1187. [Google Scholar]
- Sugawara, J.; Tomoto, T. Effects of short-term warm water immersion on cardiac baroreflex sensitivity in healthy men. J. Physiol. Sci. 2020, 70, 34. [Google Scholar] [CrossRef]
- Brunt, V.E.; Howard, M.J.; Francisco, M.A.; Ely, B.R.; Minson, C.T. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J. Physiol. 2016, 594, 5329–5342. [Google Scholar] [CrossRef]
- Parker, R.; Higgins, Z.; Mlombile, Z.N.P.; Mohr, M.J.; Wagner, T.L. The effects of warm water immersion on blood pressure, heart rate and heart rate variability in people with chronic fatigue syndrome. S. Afr. J. Physiother. 2018, 74, 442. [Google Scholar] [CrossRef]
- Shah, P.; Pellicori, P.; Kallvikbacka-Bennett, A.; Zhang, J.; Pan, D.; Clark, A.L. Warm water immersion in patients with chronic heart failure: A pilot study: Shah immerse: HF. Clin. Res. Cardiol. 2019, 108, 468–476. [Google Scholar] [CrossRef]
- Taylor, J.A.; Carr, D.L.; Myers, C.W.; Eckberg, D.L. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 1998, 98, 547–555. [Google Scholar] [CrossRef]
- Tripathi, K.K. Very low frequency oscillations in the power spectra of heart rate variability during dry supine immersion and exposure to non-hypoxic hypobaria. Physiol. Meas. 2011, 32, 717–729. [Google Scholar] [CrossRef]
- Rusanov, V.B.; Pastushkova, L.K.; Larina, I.M.; Chernikova, A.G.; Goncharova, A.G.; Nosovsky, A.M.; Kashirina, D.N.; Brzhozovsky, A.G.; Navasiolava, N.; Kononikhin, A.S.; et al. The Effect of Five-Day Dry Immersion on the Nervous and Metabolic Mechanisms of the Circulatory System. Front. Physiol. 2020, 11, 692. [Google Scholar] [CrossRef]
- Miwa, C.; Sugiyama, Y.; Iwase, S.; Mano, T.; Ohira, Y.; Grigoriev, A.; Kozlovskaya, I.; Egorov, A.; Shenkman, B. Effects of three days of dry immersion on heart rate and blood pressure variabilities during head-up tilting in humans. Environ. Med. 1997, 41, 135–137. [Google Scholar]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef]
- Mäkikallio, T.H.; Tapanainen, J.M.; Tulppo, M.P.; Huikuri, H.V. Clinical applicability of heart rate variability analysis by methods based on nonlinear dynamics. Card. Electrophysiol. Rev. 2002, 6, 250–255. [Google Scholar] [CrossRef]
- Penttilä, J.; Helminen, A.; Jartti, T.; Kuusela, T.; Huikuri, H.V.; Tulppo, M.P.; Scheinin, H. Effect of cardiac vagal outflow on complexity and fractal correlation properties of heart rate dynamics. Auton. Autacoid. Pharmacol. 2003, 23, 173–179. [Google Scholar] [CrossRef]
- Gerasimova-Meigal, L.I.; Sireneva, N.V.; Meigal, A.Y. Estimation of the effect of the course of short-term sessions “dry” immersion on autonomic regulation in patients with parkinsonism. Hum. Physiol. 2021, 47, 51–57. [Google Scholar] [CrossRef]
- Meigal, A.Y.; Tretjakova, O.G.; Gerasimova-Meigal, L.I.; Sayenko, I.V. Program of seven 45-min dry immersion sessions improves choice reaction time in Parkinson’s disease. Front. Physiol. 2021, 11, 621198. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Voss, A.; Schulz, S.; Schroeder, R.; Baumert, M.; Caminal, P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 277–296. [Google Scholar] [CrossRef]
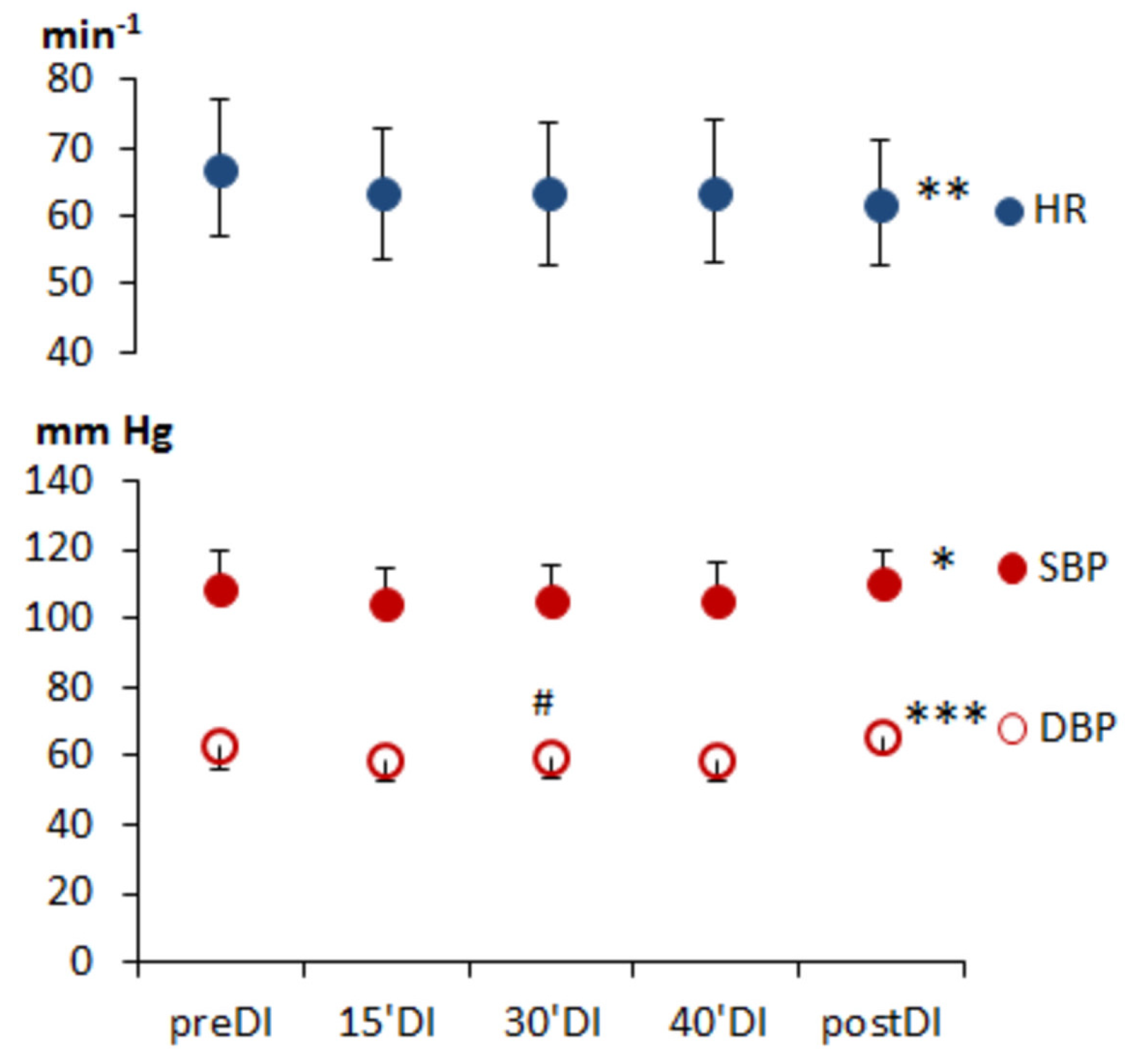
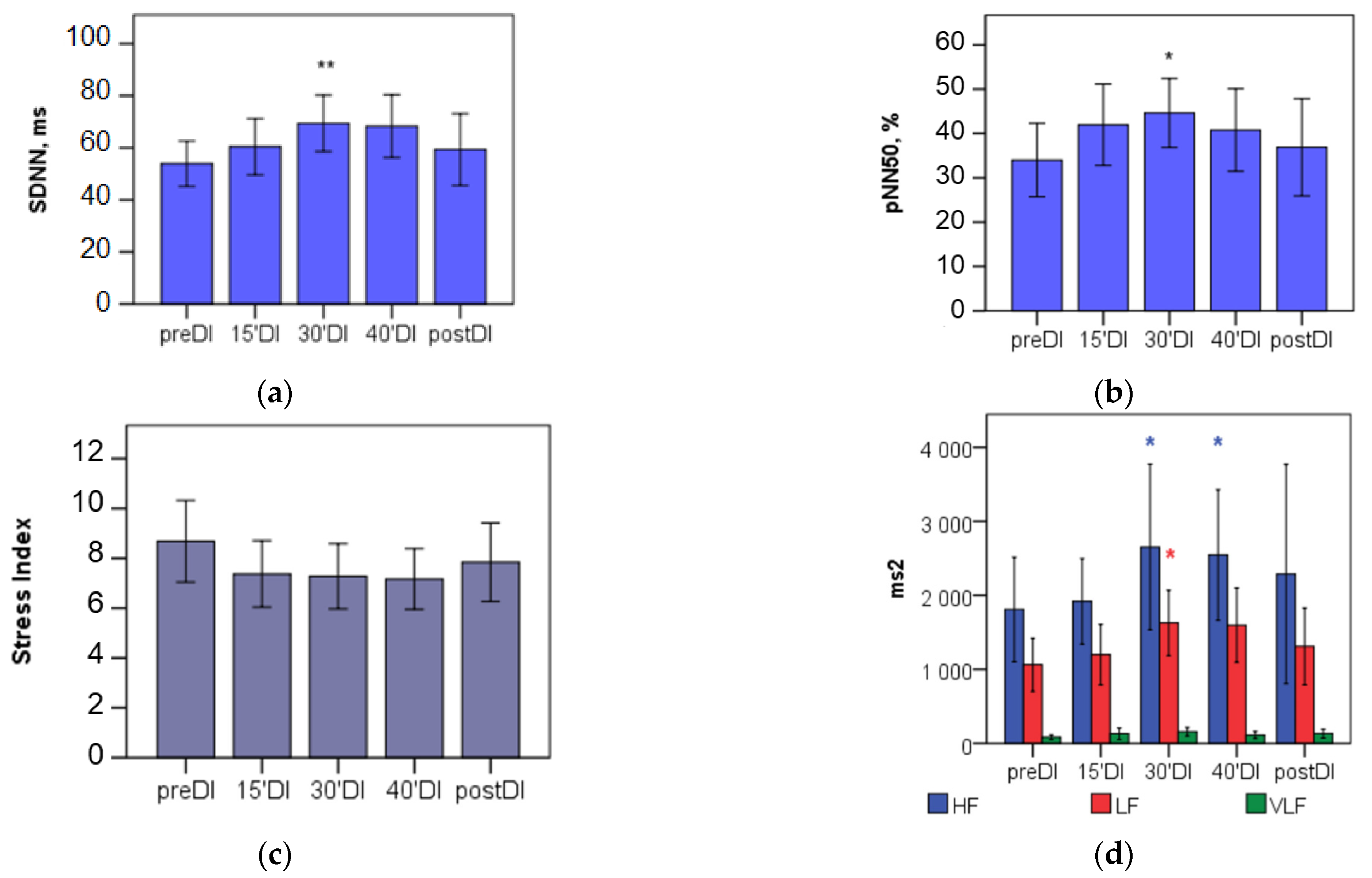
| Parameter | preDI | 15′DI | 30′DI | 40′DI | postDI | Significance |
|---|---|---|---|---|---|---|
| RR, ms | 910 (784; 1007) | 984 (903; 1058) | 1002 (835; 1091) | 998 (853; 1071) | 976 (862; 1073) ** | 0.01 |
| HR, min−1 | 66 (60; 76) | 61 (57; 67) | 60 (55; 72) *** | 60 (56; 71) *** | 61 (56; 67) *** | 0.001 |
| minHR, min−1 | 58 (52; 66) | 54 (49; 61) | 53 (47; 63) * | 53 (47; 65) * | 54 (51; 61) * | 0.01 |
| maxHR, min−1 | 76 (71; 86) | 71 (66; 79) | 76 (69; 87) | 79 (68; 90) | 72 (67; 83) | 0.05 |
| SDNN, ms | 43.9 (34.4; 74.6) | 55.4 (42.4; 77.0) | 68.8 (44.7; 87.2) ** | 63.4 (41.9; 86.5) | 53.6 (41.1; 72.6) | 0.01 |
| RMSSD, ms | 56.4 (34.6; 89.7) | 67.9 (45.6; 92.1) | 78.8 (46.0; 118.6) ** | 74.4 (44.6; 110.6) | 58.9 (41.0; 89.4) | 0.01 |
| pNN50, % | 33.43 (8.42; 57.53) | 43.81 (28.29; 60.41) | 46.91 (24.64; 65.03) ** | 44.33 (19.65; 61.15) | 35.89 (19.63; 53.43) | 0.05 |
| TINN | 300.0 (200.0; 413.0) | 305.0 (239.5; 408.0) | 345.5 (237.8; 419.8) | 366.0 (244.8; 413.5) | 304.0 (207.0; 389.0) | n.s. |
| SI | 8.2 (5.1; 10.4) | 7.0 (5.2; 9.2) | 6.3 (4.7; 9.5) * | 6.2 (5.1; 10.2) | 7.8 (5.4; 9.0) | 0.05 |
| Parameter | preDI | 15′DI | 30′DI | 40′DI | postDI | Significance |
|---|---|---|---|---|---|---|
| TP, ms2 | 1494 (911; 4982) | 2443 (1461; 4481) | 3219 (1740; 6265) ** | 4003 (1512; 6578) * | 2298 (1441; 4779) | 0.01 |
| HF, ms2 | 876 (394; 2752) | 1668 (906; 2442) | 1542 (793; 3764) * | 1772 (694; 3730) * | 1320 (496; 3165) | 0.01 |
| LF, ms2 | 632 (407; 1606) | 933 (426; 1814) | 1284 (596; 2505) * | 1204 (652; 2072) | 1103 (709; 1751) | 0.05 |
| VLF, ms2 | 50 (24; 105) | 61 (38; 108) | 115 (38; 261) | 65 (41; 147) | 100 (55; 168) | n.s. |
| HF, % | 58.80 (41.07; 66.93) | 57.62 (50.49; 74.92) | 54.09 (37.32; 66.43) | 53.63 (37.40; 67.86) | 50.33 (37.42; 68.93) | n.s. |
| LF, % | 37.81 (30.85; 54.00) | 37.61 (23.90; 46.12) | 40.57 (27.93; 56.71) | 45.69 (27.24; 54.29) | 46.58 (29.30; 54.24) | n.s. |
| VLF, % | 2.88 (1.57; 5.18) | 2.97 (1.56; 4.69) | 3.28 (2.35; 5.14) | 2.63 (1.66; 5.35) | 3.08 (2.27; 7.81) | n.s. |
| LF, n.u. | 39.49 (31.77; 56.79) | 39.35 (24.21; 47.32) | 42.84 (28.87; 61.15) | 46.00 (28.65; 58.81) | 48.05 (29.82; 59.76) | n.s. |
| HF, n.u. | 60.47 (43.19; 68.18) | 60.53 (52.65; 75.79) | 57.12 (38.85; 71.02) | 53.99 (41.17; 71.33) | 51.92 (40.23; 70.16) | n.s. |
| LF/HF | 0.637 (0.466; 1.315) | 0.650 (0.320; 0.899) | 0.840 (0.455; 1.831) | 0.852 (0.402; 1.443) | 0.925 (0.425; 1.487) | n.s. |
| Parameter | preDI | 15’DI | 30’DI | 40’DI | postDI | Significance |
|---|---|---|---|---|---|---|
| Poincare Plot | ||||||
| 39.9 (24.5; 63.5) | 48.1 (32.3; 65.2) | 55.9 (32.6; 84.0) | 52.7 (31.6; 78.3) | 41.7 (29.1; 63.3) | n.s. |
| 49.2 (40.6; 82.8) | 64.2 (49.3; 82.4) | 75.2 (52.2; 100.1) ** | 72.9 (50.7; 97.6) | 59.8 (47.4; 82.5) | 0.01 |
| 1.387 (1.186; 1.686) | 1.293 (1.136; 1.591) | 1.379 (1.160; 1.675) | 1.308 (1.222; 1.690) | 1.509 (1.247; 1.760) | n.s. |
| ApEn | 1.127 (1.080; 1.176) | 1.070 (1.012; 1.130) | 1.058 (1.016; 1.162) | 1.082 (1.019; 1.130) ** | 1.069 (1.021; 1.128) ** | 0.001 |
| SampEn | 1.810 (1.589; 1.919) | 1.776 (1.502; 1.892) | 1.754 (1.544; 1.886) | 1.766 (1.570; 1.889) | 1.800 (1.621; 1.948) | n.s. |
| Detrended Fluctutation Analysis (DFA) | ||||||
| 0.871 (0.699; 0.929) | 0.758 (0.617; 0.951) | 0.830 (0.646; 0.969) | 0.847 (0.659; 1.028) | 0.848 (0.649; 1.027) | n.s. |
| 0.238 (0.170; 0.388) | 0.254 (0.184; 0.289) | 0.232 (0.183; 0.296) | 0.270 (0.203; 0.300) | 0.269 (0.170; 0.335) | n.s. |
| Parameter | Men (n = 18) | Women (n = 15) |
|---|---|---|
| Body Mass, kg | 73.9 ± 7.3 | 60.8 ± 7.2 |
| Height, m | 1.84 ± 0.05 | 1.66 ± 0.05 |
| BMI 1 | 21.9 ± 2.0 | 22.0 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova-Meigal, L.; Meigal, A.; Sireneva, N.; Gerasimova, M.; Sklyarova, A. Heart Rate Variability Parameters to Evaluate Autonomic Functions in Healthy Young Subjects during Short-Term “Dry” Immersion. Physiologia 2023, 3, 119-128. https://doi.org/10.3390/physiologia3010010
Gerasimova-Meigal L, Meigal A, Sireneva N, Gerasimova M, Sklyarova A. Heart Rate Variability Parameters to Evaluate Autonomic Functions in Healthy Young Subjects during Short-Term “Dry” Immersion. Physiologia. 2023; 3(1):119-128. https://doi.org/10.3390/physiologia3010010
Chicago/Turabian StyleGerasimova-Meigal, Liudmila, Alexander Meigal, Nadezhda Sireneva, Maria Gerasimova, and Anna Sklyarova. 2023. "Heart Rate Variability Parameters to Evaluate Autonomic Functions in Healthy Young Subjects during Short-Term “Dry” Immersion" Physiologia 3, no. 1: 119-128. https://doi.org/10.3390/physiologia3010010
APA StyleGerasimova-Meigal, L., Meigal, A., Sireneva, N., Gerasimova, M., & Sklyarova, A. (2023). Heart Rate Variability Parameters to Evaluate Autonomic Functions in Healthy Young Subjects during Short-Term “Dry” Immersion. Physiologia, 3(1), 119-128. https://doi.org/10.3390/physiologia3010010









