Abstract
Background: In sporting and combat settings, optimal fluid replacement is rarely achieved, exacerbating physiological strain. It is unknown if prescribed fluid replacement following exercise in heat impacts heart rate variability (HRV). Purpose: Compare prescribed drinking (PD) and ad libitum (AL) fluid replacement on HRV following exercise in heat. Methods: Twelve participants (26 ± 5 years, VO2max: 58.44 ± 7.05 mL·kg−1·min−1) completed three trials in heat (36 °C, 36% humidity) on separate days, and were placed into groups, PD or AL. Recovery was assessed ~24 h later (hydration and HRV). HRV time and frequency was measured using a 3-lead electrocardiogram. Two-way repeated measures analysis of variance measured changes in HRV pre-trial, post-trial, and follow-up between groups. Data reported: p-value, mean difference (MD). Results: Fluid consumption was greater in PD during recovery (p = 0.012, MD = 1245 mL). Both groups were euhydrated at follow-up. HRV time (p < 0.001, MD = 24.23) and frequency (p < 0.001, MD = −1.98 ms2) decreased post-trial and increased by follow-up (time, p < 0.001, MD = −32.12; frequency, p < 0.001, MD = 2.38 ms2). HRV was similar between groups (p > 0.05). Conclusions: Replacing ≥60% fluid sufficiently rehydrates and restores HRV 24 h post-exercise in heat and mild dehydration (BML ≤ 3%). Prescribed fluid consumption during recovery was ~30% greater. Additional measures of recovery sensitive to heat strain may provide a more holistic understanding of specific mechanisms of recovery.
1. Introduction
During exercise in the heat, great demand is placed on both the cardiovascular and thermoregulatory systems [1,2,3,4,5]. Core body temperature and heart rate increase, resulting in simultaneous competition for the available blood volume by the skin, exercising muscles, and cardiovascular system [1,2,3,4,5]. Thermoregulatory strain while exercising in the heat leads to increases in internal body temperature as the body works to dissipate heat via mechanisms such as cutaneous vasodilation and sweating [6,7]. While sweating is beneficial to remove excess heat from the body during exercise, the loss of fluid from the body results in dehydration, which places additional stress on the cardiovascular system [3]. Accumulated cardiovascular and thermoregulatory strain without proper recovery during successive bouts of work or exercise in the heat diminishes performance and increases the risk for cardiac and heat-related events [8,9]. Furthermore, rehydration, as commonly done via ad libitum or prescribed drinking strategies, is essential to allow for proper cardiovascular and thermoregulatory functioning. This is of particular importance in warfighter, laborer, and athletic populations where successive days of exertion in the heat along with dehydration are a common and frequent experience [9,10,11]. Essential to repeated days of exertion in the heat is proper recovery from each exposure to allow for response and adaptation from the physical exertion and heat stress [9]. Both dehydration and exercise in the heat can place immense stress on the cardiovascular system, specifically the autonomic nervous system (ANS). Recently, heart rate variability (HRV) has been used to monitor ANS function in such populations during and following exercise in the heat [4,10,11,12,13,14,15,16,17,18].
Previous research has shown that following exercise in the heat, and potentially for days thereafter, ANS measures remain at levels indicative of sustained sympathetic dominance (an adverse effect), revealing a lag in the body’s ability to restore vagal tone [10,11,13,19,20,21]. This is critical because inadequate recovery increases susceptibility to cardiac or heat-related conditions in a subsequent session [7,9,20,22].
The influence of fluid replacement on cardiac autonomic modulation during prolonged exercise in the heat to offset dehydration, and its effects on cardiac autonomic modulation, have demonstrated that cardiac function was impaired to a higher degree with heat stress and the absence of fluid replacement [3,4]. Specifically, associations were examined between dehydration and ANS recovery during and acutely following exercise, revealing that cardiac function was impaired to a higher degree with heat stress and the absence of fluid replacement [3,4]. This exemplifies the pertinence in appropriately replacing fluid when exercising in the heat to decrease the strain placed on the ANS and thermoregulatory systems, to optimize safety [5,7,23,24].
Improper recovery from previous heat exposures and inadequate hydration can increase susceptibility to catastrophic conditions [25,26]. In many sporting and laborer settings, optimal fluid replacement during physical exertion is extremely challenging and rarely achieved [2,5,24,27,28]. For this reason, it may be beneficial to consider fluid replacement strategies following exercise in the heat. It is not currently known if a prescribed fluid replacement strategy following exertional heat stress impacts HRV recovery the following day. Therefore, the purpose of this study is to investigate and compare the impact of a prescribed drinking versus ad libitum drinking fluid replacement strategy on HRV recovery following prolonged exercise in the heat. We hypothesized that HRV recovery would be greater when prescribed drinking fluid replacement strategies are utilized following prolonged exercise in the heat.
2. Materials and Methods
Procedures in this study were approved by the University of Connecticut Institutional Review Board and the U.S. Army Office of Human Research Oversight, Ft. Detrick, Maryland. This data is part of a larger study that investigated the impact of intermittent cooling between repeated bouts of exercise in the heat [29]. However, the current study tested a different hypothesis regarding fluid replacement and HRV recovery. From all three exercise trials, only data regarding urine, HRV, rectal temperature, and fluid replacement (consumption) were utilized for further analysis.
2.1. Participants
Twelve physically-active and healthy males between 18–35 years of age with a VO2max ≥ 45 mL·kg−1·min−1 were included in this research study. Participants completed a total of seven laboratory visits including the baseline trial, three exercise trials, and three follow-up visits. Participants were randomly placed into one of two groups; Ad Libitum (AL) or Prescribed Drinking (PD), and matched for age and aerobic fitness level. Participants were excluded if they had any chronic health problems that affect thermoregulatory ability (disorders affecting the liver, kidneys or the ability to sweat normally), fever or current illness at the time of testing, history of cardiovascular, metabolic, or respiratory disease, current musculoskeletal injury that limits physical activity, and/or current use of a medication that is known to influence body temperature (i.e., amphetamines, antihypertensives, anticholinergics, acetaminophen, diuretics, NSAIDs, aspirin). All participants provided verbal and written consent prior to participating in study-related procedures.
2.2. Experimental Design
2.2.1. Participants
Participants completed three exercise trial visits on three separate days in a randomized order. Exercise trials were separated by at least three days [13]. HRV was measured before exercise (HRVpre), approximately 10 min following the final rest block after exercise (HRVpost), and 20–24 h following the exercise trial (HRVfollow-up). Participants were also assigned to one of two groups, AL or PD, to determine which fluid replacement strategy they would be using following all trials for the duration of the study. Participants utilized the same fluid replacement strategy following each of the three exercise trials. Fluid replacement groups were matched.
Participants in the AL group (n = 6) were an average age of 27 ± 5 years, with an aerobic fitness level of 58.93 ± 7.61 mL·kg−1·min−1. Participants in the PD group (n = 6) were an average age of 25 ± 5 years, with an aerobic fitness level of 57.95 ± 7.14 mL·kg−1·min−1. Participant characteristics are presented in Table 1.

Table 1.
Participant demographics and anthropometrics.
2.2.2. Baseline and Familiarization (Visit 1)
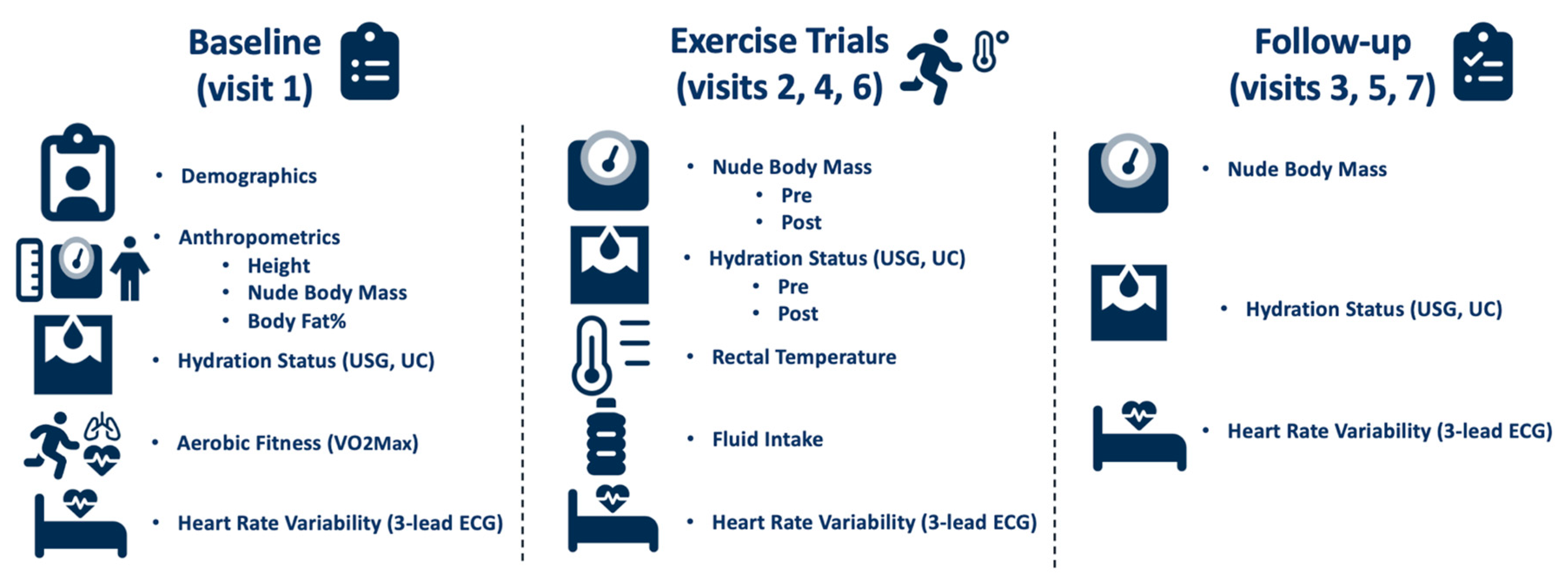
Upon arrival to the lab on the baseline day, urine specific gravity (USG) using a handheld refractometer (Model TS400; Reichert Inc., Depew, NY, USA), and urine color (UC) were assessed to confirm euhydration (USG ≤ 1.020). Height was measured with a tape measure during this baseline visit. Nude body mass (NBM) was measured using a platform scale (Defender® 7000XtremeW; OHASUS Corp., Parsippany, NJ, USA). Following these measurements, HRV was recorded using a 3-lead electrocardiogram with a recording frequency of 1000 Hz (ECG100C, BIOPAC Systems Inc., Goleta, CA, USA) and Polar H10 heart rate monitor (H10®, Polar Electro™, Kempele, Finland) with the participants lying in a supine position in a dark, thermoneutral room for 10 min. Next, participants completed a maximal oxygen consumption (VO2max) test on a motorized treadmill (T150; COSMED, Traunstein, Germany) in a thermoneutral environment to determine aerobic fitness. The VO2max test consisted of participants running on a treadmill at a 2% grade for 3-min stages, until volitional exhaustion. Parvo Medics software (TrueOne 2400; Parvo Medics, Salt Lake City, UT, USA) was used to determine VO2max. Finally, body fat percentage was calculated using a 7-site evaluation of skin fold thickness (chest, iliac, tricep, calf, shoulder, abdomen, and thigh) with skin fold calipers (Baseline®, Medical Skinfold Caliper, Fabrication Enterprises, NY, USA). All measurements are listed in Figure 1.
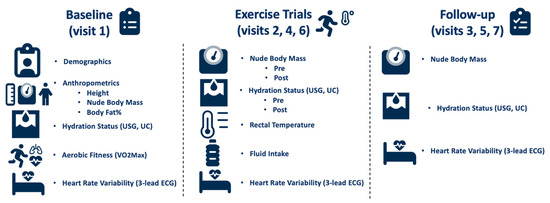
Figure 1.
Study timeline and measurements.
2.2.3. Exercise Trials (Visits 2, 4, and 6)
Before each trial, participants abstained from alcohol for 24 h and caffeine for 12 h. Nude body mass (NBM) and hydration status were recorded before and after each trial. Participants inserted a rectal thermistor (YSI reusable temperature probe, Zoll Medical corporation, Chelmsford, MA, USA) approximately 10–15 cm beyond the anal sphincter [30]. Internal body temperature was measured using Biopac software (MP160; BIOPAC Systems Inc., Goleta, CA, USA). Participants wore the heart rate monitor strap during trials and a battle dress uniform during all exercise and rest blocks.
2.2.4. Exercise Protocol
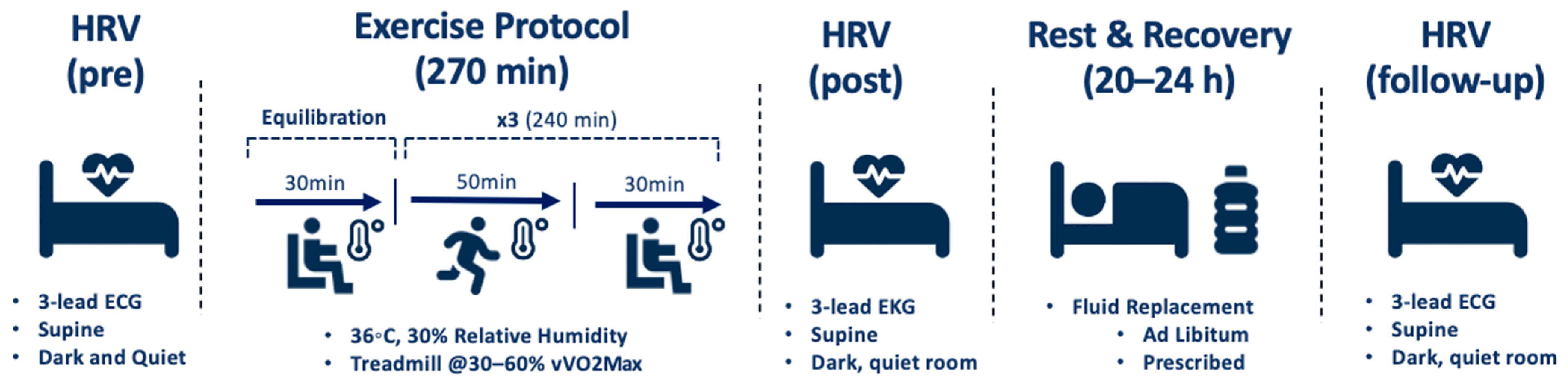
Participants equilibrated for 30 min in an environmental chamber set to 36 °C and 30% relative humidity. Heart rate (HR) and rectal temperature (Trec) were recorded at baseline and every 10 min during this time. HR and Trec were measured continuously before, during, and after exercise and cooling to monitor participants. Exercise trials consisted of three 50-min bouts of exercise, each followed by a 30-min rest and cooling block, respectively, in the environmental chamber. The exercise protocol consisted of 10-min intervals of walking or running at low, moderate, and high intensities. Running velocity ranged from 30–60% of the participant’s velocity measured at VO2max (vVO2max). The exercise protocol was used in support of previous research to represent that of warfighters, laborers, and athletes in regards to modality, duration, intensity, and rest [4,9,10,11,13]. HRV was assessed before, during, and 10 min after the final rest block of each exercise trial using a 3-lead ECG and a Polar H10 heart rate monitor [31,32]. Participants were permitted to drink ad libitum throughout the exercise trial. Figure 2 displays a detailed schematic of the exercise and recovery protocol.
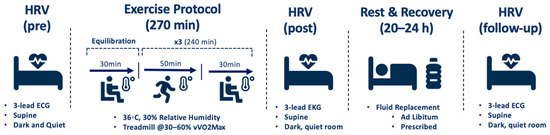
Figure 2.
Schematic of exercise and recovery protocol.
2.2.5. Fluid Replacement Strategies
Ad libitum fluid consumption during exercise trials was recorded and NBM was measured after each trial to calculate body mass loss (BML) from sweat and urine. This was used to determine a fluid replacement strategy for participants in the PD group. Following each exercise trial, participants in the PD group were prescribed and consumed a fluid replacement equivalent to that of their fluid loss during the trial plus an additional 1.5 L (the amount typically excreted in urine in one day) [24]. Participants were permitted to add zero-calorie flavoring to this amount of water if they desired to make it more palatable. Participants in the AL group were instructed to drink as they normally would. Participants tracked their diet and fluid intake, including any supplements added to fluid, for the entire day of their exercise trial until their follow-up visit the next morning, using MyFitnessPal (MyFitnessPal LLC, Version 21.4.6.34430, San Francisco, CA, USA). Upon departure from the laboratory following exercise trials, all participants were provided a urine cup to collect their first morning urine sample if they needed to urinate before arrival to the laboratory for the follow-up visit the next morning. Participants were also provided with a water bottle to help measure the amount of water they drank throughout the remainder of the day following the exercise trial and before their follow-up visit the next morning.
2.2.6. Follow-Up Visits (Visits 3, 5, and 7)
Participants returned to the lab the morning following each exercise trial (between 5:00 a.m. and 8:30 a.m.) for a total of three follow-up visits. Follow-up visits took approximately 30 min. During each follow-up visit, NBM, hydration status (USG and urine color), and HRV were measured and recorded using the exact same methods as those conducted before and after exercise trials. Diet and fluid intake records from the remainder of the previous day up until arrival at the laboratory the following morning, were also collected during follow-up visits.
2.2.7. Measurements
Cardiac frequency (R-R interval) data from the ECG (3-channel at a sampling rate of 1000 Hz) recordings were extracted from the Biopac AcqKnowledge software [33]. Heart rate and HRV indices were then derived for analysis (10 min windowed analysis with 10 s time step) for time and frequency domains. HRVAnalysis software HRVanalysis 1.0 was used for further analysis of HRV data [34]. Five different HRV variables were measured in total: two time domains and three frequency domains. Time domain indices included HR, the natural logarithm of root-mean-square of successive differences (lnRMSSD) of normal-to-normal intervals, and the proportion of RR intervals greater than 50 ms (pNN50). Frequency domain indices included low (LF, 0.04–0.15 Hz) and high (HF, 0.15–0.4 Hz) frequency components, and LF:HF ratio [20,35]. Directionality for variables is as follows: higher values for lnRMSSD, pNN50, HF and LF:HF ratio are favorable, whereas lower values for LF are favorable. The respective data from all exercise trials were combined regardless of cooling intervention as no impact of cooling was found and therefore, we did not include this as part of our analyses.
3. Statistical Analyses
Based on data from a study related to HRV, fluid strategies, and exercise in the heat, a minimum of six participants was needed to give a power of 0.88 with an effect size of 1.72 [4]. Power calculations were computed using G*Power (G*Power 3.1.9.6 for macOS) [36]. Each participant (12 total, 6 in each group) participated in three trials, and measures were averaged from the three trials to represent one value for pre-trial, one value for post-trial, and one value for follow-up, for each participant. Data is an average of the data from a single trial. All data were tested for normality using the Shapiro-Wilk test and homogeneity of variances using Levene’s test prior to further analyses. Two-way mixed repeated measures analysis of variance (ANOVAs) for each of the HRV indices (RMSSD, pNN50, LF power, HF power, LF:HF ratio) analyzed differences in HRVpre, HRVpost, and HRV24h within and between groups (PD and AL). Post-hoc independent and dependent t-tests examined where differences occurred. Independent T-tests examined differences in peak HR during exercise trials, peak Trec during exercise trials, fluid consumption during exercise trials, percent body mass loss (BML) during exercise trials, and hydration status pre- and post-trials and at follow-up visits using USG and UC. Statistical significance was set at p < 0.05, a priori. Cohen’s d was used to determine the magnitude of differences [28,29,30,31]. Effect size was determined as small (0.2), moderate (0.5), large (0.8), and very large (>1.0) [28,29,30,31]. Data are reported as p-value, t-value, F-value, mean difference (MD), standard error (SE), and effect size (ES). All statistical analyses were performed using Jamovi (The jamovi project [2020], jamovi [Version 1.2] [Computer Software]. Retrieved from https://www.jamovi.org accessed on 22 January 2021) [37].
4. Results
4.1. Fluid Replacement Protocol
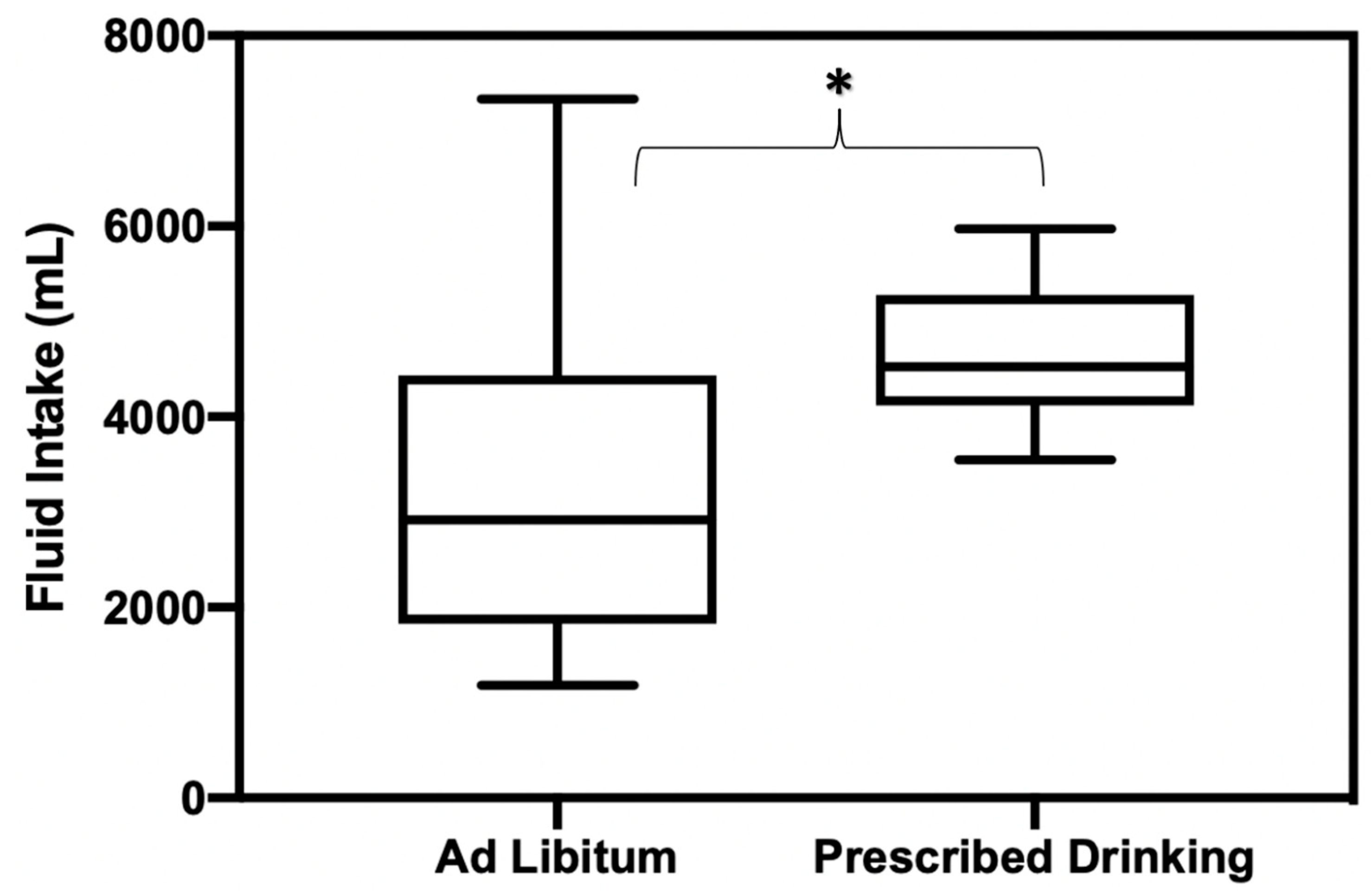
Fluid consumption during the recovery period (the 20–24 h following exercise trials) was significantly different between PD and AL groups, with PD groups drinking significantly more than AL (p = 0.012, mean difference [MD] = −1245 mL, SE = 470, ES = 0.88) (Figure 3). Participants in the AL group only replaced 60% of fluid loss in comparison to the amount of fluid the PD group replaced.
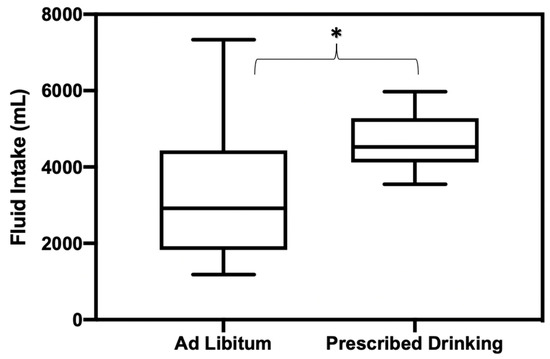
Figure 3.
Fluid replacement amounts consumed during 20–24 h recovery period following exercise trials for AL and PD groups. * Indicates statistically significant differences in fluid consumption amounts between groups.
There were no significant differences in fluid consumption between PD and AL groups at baseline or during any exercise trials (p = 0.14) (Table 2a,b).

Table 2.
a. Physiological and behavioral variables for prescribed drinking (PD) and ad libitum (AL) groups during exercise trials. b. Physiological and behavioral variables for prescribed drinking (PD) and ad libitum (AL) groups at follow-up visits.
4.2. Body Mass Loss and Hydration Status
There was no significant difference in percent BML or BML between PD or AL groups during any of the exercise trials (%BML: p = 0.64, BML: p = 0.97). There were no significant differences between PD and AL groups in USG or UC prior to any trials (USG: p = 0.77, UC: p = 0.82), after any trials (USG: p = 0.19, UC: p = 0.18), or during any of the follow-up visits (USG: p = 0.14, UC: p = 0.12) (Table 2a).
4.3. Physiological and Behavioral Variables
There were no significant differences in average Trec or peak Trec between PD and AL groups during exercise trials (avg Trec: p = 0.59, peak Trec: p = 0.32). There were no significant differences in average HR or peak HR between PD and AL groups during any trials (avg HR: p = 0.97, peak HR: p = 0.40). Table 2a presents average values of physiological variables (Trec, HR, and hydration status) and Table 2b presents behavioral variables (fluid consumption) for each group among exercise trials and follow-up visits.
4.4. HRV Time Domains
4.4.1. RMSSD
Time domain analyses for the natural logarithm of the root mean square of successive differences between normal heartbeats (LnRMSSD) revealed no significant differences (p = 0.15, F = 2.42, ES = 0.13). No differences were found between groups (p = 0.54, F = 0.43, ES = 0.02) (Table 3).

Table 3.
Heart rate variability time and frequency measures pre-trial, post-trial, and at follow-up.
4.4.2. pNN50
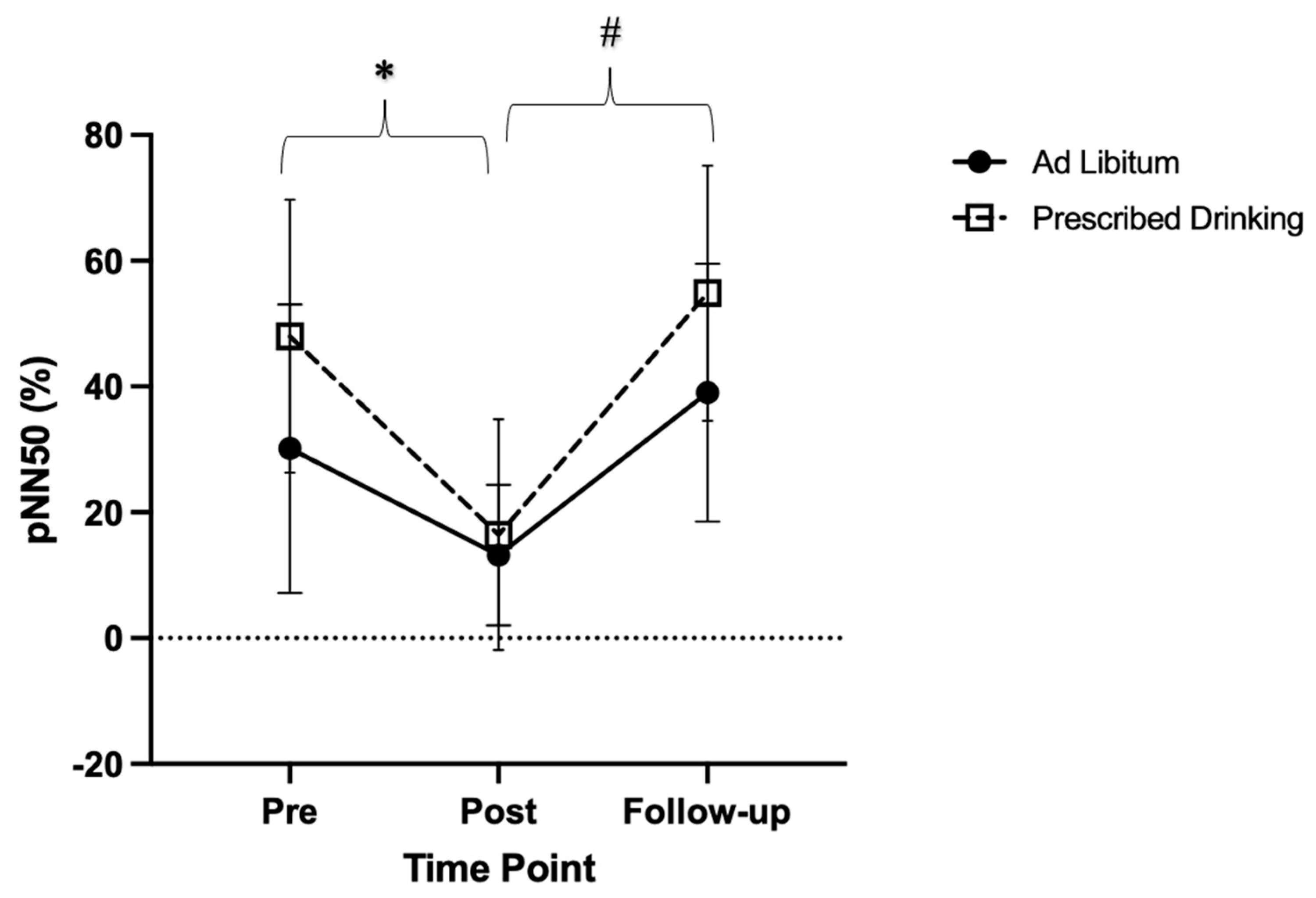
There were significant main effects in percentage of adjacent NN intervals (pNN50) over time (p < 0.001, F = 24.38, ES = 0.36). (Figure 4). Specifically, post-trial pNN50 measures were lower compared to pre-trial measures (p = 0.005, t = 4.15, MD = 24.23, SE = 5.84), and follow-up measures were significantly higher than post-trial measures (p < 0.001, t = −7.07, MD = −32.12, SE = 4.54). Follow-up pNN50 measures were similar to pre-trial measures (p = 0.141, t = −2.09, MD = −7.88, SE = 3.77).
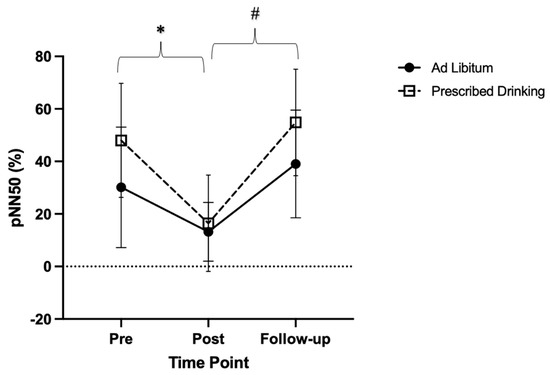
Figure 4.
Change in the proportion of the number of successive NN intervals that differ >50 milliseconds in relation to the total number of NN intervals (pNN50), for each drinking group (ad libitum and prescribed drinking) at pre-, post-, and follow-up for each exercise trial. Clear squares and dashed lines represent the prescribed drinking group, and black circles and solid lines represent the ad libitum group. Data are presented as averages and standard deviations. * Indicates statistically significant differences from pre-trial to post-trial. # Indicates statistically significant differences from post-trial to follow-up within groups. Measures were calculated as the change in values from baseline.
4.5. HRV Frequency Domains
4.5.1. Low Frequency
No significant differences were found in LF components of HRV for either group at pre-trial, post-trial, or at follow-up (p = 0.33, F = 1.06, ES = 0.06). No differences were found between groups (p = 0.30, F = 1.19, ES = 0.07) (Table 3).
4.5.2. High Frequency
No significant differences were found in HF components of HRV for either group at pre-trial, post-trial, or at follow-up (p = 0.31, F = 1.15, ES = 0.06). No differences were found between groups (p = 0.35, F = 0.99, ES = 0.05) (Table 3).
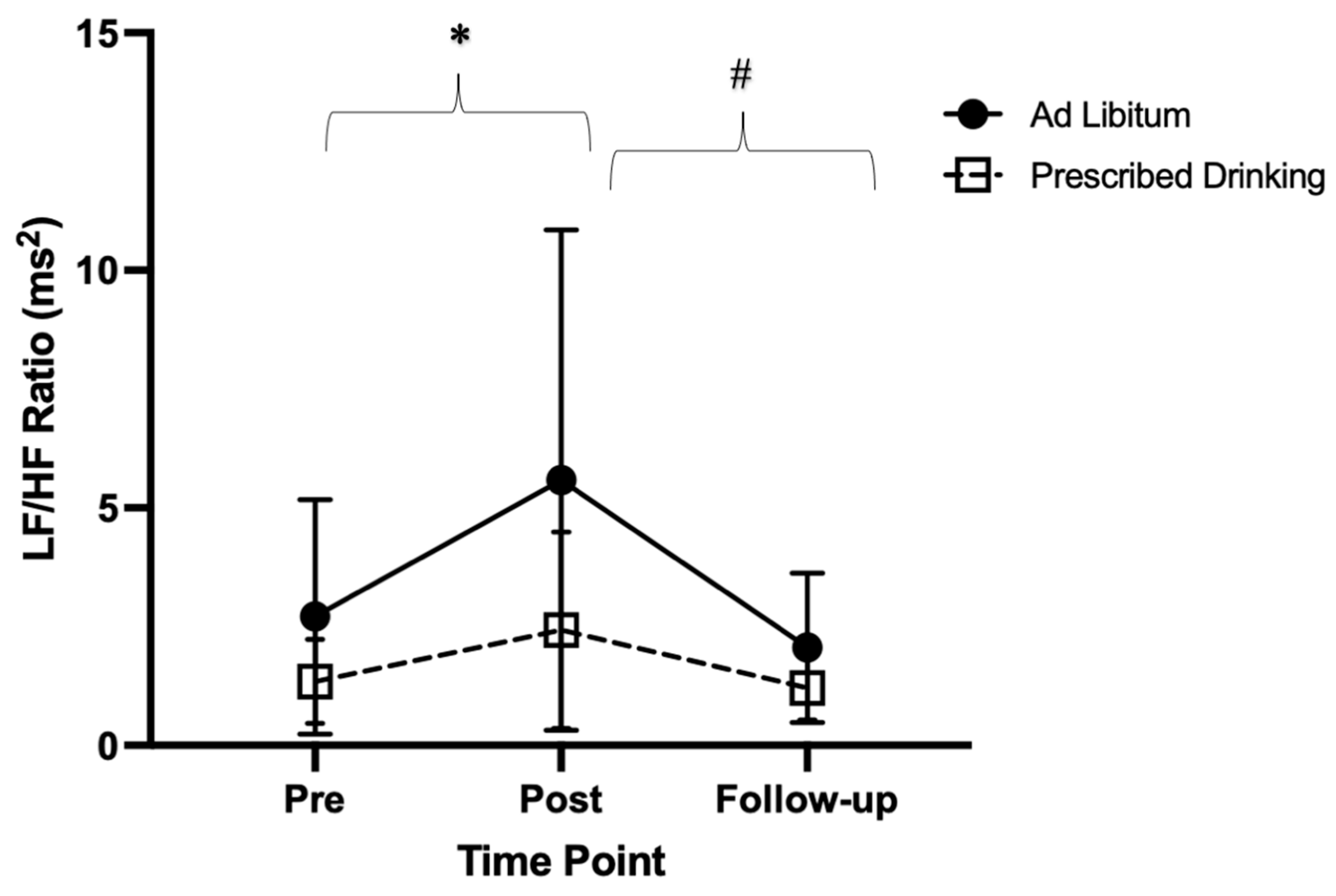
4.5.3. Low Frequency: High Frequency Ratio
Significant differences in the ratio of LF:HF components were found within groups over time (p < 0.001, F = 10.64, ES = 0.17). Specifically, the LF:HF ratio was lower post-trial compared to pre-trial (p = 0.023, t = −3.22, MD = −1.98, SE = 0.61). Additionally, LF:HF ratio was greater at follow-up compared to post-trial (p = 0.013, t = 3.59, MD = 2.38, SE = 0.66). Follow-up LF:HF ratio was similar to pre-trial (p = 0.441, t = 1.27, MD = 0.40, SE = 0.31). (Figure 5).
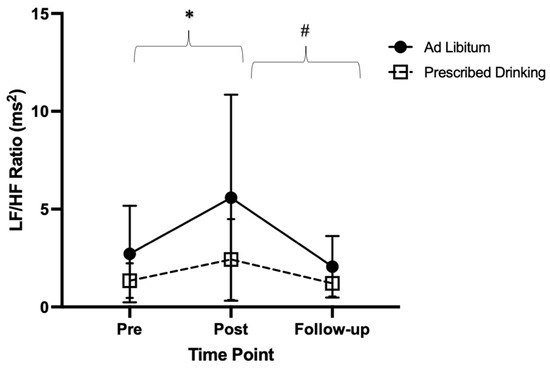
Figure 5.
Low frequency/high frequency ratio (LF:HF ratio) pre-, post-, and follow-up measures for ad libitum and prescribed drinking groups. Clear squares and dashed lines represent the prescribed drinking group, and black circles and solid lines represent the ad libitum group. Data are presented as averages and standard deviations. * Indicates statistically significant differences from pre-trial to post-trial within groups. # Indicates statistically significant differences from post-trial to follow-up within groups. Measures were calculated as the change in values from baseline.
5. Discussion
The purpose of this study was to investigate and compare the impact of a PD versus AL drinking fluid replacement strategy on ANS recovery following prolonged exercise in the heat. Contrary to our hypothesis, we found no difference in HRV when a PD versus AL drinking fluid replacement strategy was utilized following prolonged exercise in the heat (p > 0.05). Time and frequency domain measurements of HRV revealed significant disturbances immediately post-trial compared to pre-trial (p < 0.05), independent of groups. Disturbances in HRV post-trial were alleviated by the follow-up visit, with follow-up HRV measures significantly higher than post-trial measures (p < 0.05), and not significantly different from pre-trial measures (p > 0.05). These findings suggest that in general, when fluid is replaced following prolonged exercise in the heat, regardless of PD or AL strategies, ANS function is restored in aerobically fit individuals. Furthermore, ANS function may have been restored despite the environmental stress, due to the moderate physical stress (30–60% of speed at VO2max) performed.
Prior findings show that fluid replacement in comparison with no fluid replacement decreases the strain that is placed on the ANS [4,15,37,38,39,40]. Additional research has investigated the effects of prescribed fluid replacement during exercise in the heat on various physiological markers including core temperature and ANS strain [40]. Our findings expand this literature by utilizing a prescribed fluid replacement amount in comparison to ad libitum drinking specifically during recovery to alleviate ANS strain. Our study was novel in that we utilized a prescribed fluid replacement strategy during the recovery post-trial period, while allowing fluid intake ad libitum during trials. We chose to utilize a fluid replacement strategy specifically during recovery due to the fact that ad libitum drinking, as well as planned fluid replacement during exercise, is sometimes not possible in certain circumstances (in military and laborer contexts where fluid is not accessible or rehydration is not feasible, or in sporting contexts where minimal breaks are provided and consuming enough fluid is challenging), and often falls short in replacing fluid loss [41,42]. Additionally, it has been noted that ad libitum drinking during exercise in the heat does not reduce the magnitude of cardiovascular strain [4,38]. Therefore, we focused on fluid replacement strategies during recovery.
The fluid replacement strategy prescribed to participants in our study was equivalent to that of BML during trials in addition to the amount of typical daily water loss that occurs through urination [41,42]. Interestingly, participants in the AL group only replaced 60% of fluid loss in comparison to the amount of fluid the PD group replaced. However, both groups drank enough fluid to replace BML from trials and return to a state of euhydration by the time of follow-up (measured by USG and UC) (Figure 3). This may be due to the fact that both groups only experienced dehydration of approximately 3% BML during exercise trials, which is readily achievable to replace ad libitum, compared to dehydration of 4% or greater BML [43]. Further, these findings revealed interesting aspects of drinking behavior and support existing fluid replacement positions stands [41,42]. Specifically, when given an extended time to rest and recover (>12 h), ad libitum drinking behaviors are sufficient to adequately rehydrate [40]. Our findings add to this existing knowledge by revealing that when given approximately 20–24 h to recover following moderate exercise in the heat, ad libitum drinking (and potentially foods with increased water content) that amounts to 60% of fluid loss, is adequate in rehydration as well as restoring ANS function following 150 min of moderate exercise and 90 min of rest in the heat. When at least 60% of fluid was replaced following prolonged exercise in the heat, ANS strain was relieved almost completely, and HRV measured in time and frequency domains was restored close to pre-trial levels.
Impairments in HRV time domain measurements have been reported with exercise in the heat, most commonly LnRMSSD, which indicates differences in time between normal heartbeats. LnRMSSD and pNN50 (consecutive intervals that differ more than 50 ms) measures largely coincide with the duration of time the ANS is under a state of stress. In our study, impairments in time domain measures such as pNN50 (Figure 4) revealed less variability post-trial compared to pre-trial. This decrease in short-term HRV measures post-trial identifies significant ANS strain following prolonged exercise in the heat and is synonymous with previous research [12,15]. In relation to fluid replacement during exercise, this exemplifies that ad libitum drinking during prolonged exercise in the heat did not reduce ANS strain caused by the duration and intensity of the exercise performed.
Frequency domains are also commonly utilized to measure disturbances in HRV as these components differentiate the sympathetic (low frequency) and parasympathetic (high frequency) influences of R-R intervals [20]. In support of previous findings, our study revealed sympathetic dominance and a diminished vagal tone post-trial with LF:HF increasing post-trial in our study [44,45,46,47,48]. Additionally, LF:HF ratios revealed the restoration of vagal tone at follow-up close to pre-trial levels (Figure 5). This reveals the large contribution of the parasympathetic division in cardiac autonomic restoration [12]. Macarteney et al. (2020) found a decrease LF:HF immediately following exercise [4]. The findings in our study may be attributed to the fact that participants in our study were able to drink ad libitum during exercise trials, whereas in the aforementioned they were not.
In relation to fluid replacement, our findings reveal that ad libitum drinking during exercise did not reduce ANS strain. In our study, during exercise in the heat, the sympathetic division of the ANS was dominant and parasympathetic tone diminished, even when ad libitum drinking was utilized. Several factors could have contributed to a reduction in parasympathetic tone, including that the amount of fluid loss during trials was greater than that being restored through ad libitum drinking, and the exercise itself (both intensity and duration). This process of dehydration decreases blood volume, causing the heart to work harder to pump a sufficient amount of blood to working muscles, ultimately straining the ANS [49,50]. Additionally, the duration of exercise trials in our study was very long (approximately 5–6 total hours in the heat), which required the ANS to handle this strain for an extended period of time, likely exacerbating the disturbances found in post-exercise HRV measures. Practically, this supports current recommendations of maximum time limits for practices and games in athletic settings in the heat, as well as utilization of fluid replacement beyond that of ad libitum during exercise [26,41,50,51,52]. This also supports that a period of rest between prolonged exercise in the heat is essential in allowing individuals to rehydrate even if ANS function is not restored in such a short time [50,51,52]. In military and occupational settings, this stresses the importance of ensuring that there is a way for warfighters and laborers to fully replace fluid loss and have adequate recovery time. Combined, the aforementioned support of recommendations and findings of this study help advance knowledge of strategies to enhance safety during exertion in the heat.
A limitation of this study was that we investigated only a prescribed and ad libitum fluid replacement group and only had an exercising group. Future research should investigate the effects of no fluid replacement during the recovery period (fluid restriction) in comparison to prescribed and ad libitum strategies, as well as including a non-exercise group. This would be beneficial as many populations may not have the opportunity to replace fluids between events and do not have opportunities to rest. This would also allow researchers to further understand the independent effects of fluid replacement, or lack thereof, on ANS recovery. A minor limitation to this study is the possibility that the Hawthorne effect may have influenced fluid consumption in the AL fluid replacement group. However, no indications were given to participants of monitoring drinking behavior, and the participants were instructed to eat and drink as they normally would. Lastly, a limitation of this study was the limited time-points in which HRV was measured. Repeated sequential measurement of HRV post-trial may elucidate the precise moment or window following exercise in the heat that HRV is restored to pre-trial values. This would be beneficial for populations who have multiple competitive, training, or occupational events within the same day, to understand how much time is required to restore ANS function before successive events. Understanding the impact of practical strategies such as fluid replacement would also be helpful to develop practical plans to accelerate recovery if recovery is needed in a shorter time window, such as in certain athletic and military settings.
To our knowledge, no studies have explored the use of fluid replacement strategies specifically during the post-trial recovery period on ANS recovery following prolonged exercise in the heat. Therefore, this study was novel in its approach of fluid replacement during recovery and supplements the work of researchers who have found beneficial effects of fluid replacement on ANS function during exercise in the heat. Moreover, our study suggests that fluid replacement during recovery equating to at least 60% of fluid loss, whether prescribed amount or ad libitum, aids in restoring HRV to pre-trial levels, as no difference was found in HRV recovery between the two strategies with this protocol. Ad libitum drinking during exercise may be insufficient in maintaining a state of euhydration and reducing ANS strain. However, our findings suggest that during recovery, when BML does not exceed 3% and extended time (~24 h) is given following moderate exercise in the heat, replacing at least 60% of fluid loss is sufficient to restore a state of euhydration as well as ANS function. This is important to understand that rehydration impacts autonomic nervous system recovery moderately but may not be the primary factor or mechanism of autonomic nervous system recovery. This reveals the need to assess autonomic nervous system recovery using a more holistic approach encompassing various factors sensitive to heat strain, and additional variables reflective of autonomic nervous system recovery, to better understand the mechanisms of recovery of the autonomic nervous system following heat strain.
Author Contributions
Conceptualization, C.N.M., M.C.M., R.L.S., R.A.H., R.M.C., Y.S., S.P.L., J.R. and D.J.C.; Methodology, C.N.M., M.C.M., R.L.S., R.A.H., R.M.C., Y.S., S.L. (Srinivas Laxminarayan), J.R. and D.J.C.; Formal analysis, C.N.M., M.C.M., S.L. (Sean P. Langan), R.L.S., R.A.H., R.M.C. and Y.S.; Investigation, C.N.M., M.C.M., S.L. (Sean P. Langan) and S.L. (Srinivas Laxminarayan); Data curation, C.N.M., M.C.M., S.L. (Sean P. Langan), R.L.S., R.A.H., R.M.C. and Y.S.; Writing—original draft, C.N.M.; Writing—review and editing, C.N.M., M.C.M., S.L. (Sean P. Langan), R.L.S., R.A.H., R.M.C., Y.S., S.L. (Srinivas Laxminarayan), J.R. and D.J.C.; Supervision, R.L.S., R.A.H. and D.J.C.; Project administration, C.N.M., M.C.M., S.L. (Sean P. Langan), R.L.S., R.A.H., R.M.C., S.L. (Srinivas Laxminarayan), J.R. and D.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Military Operational Medicine Research Program Area Directorate of the U.S. Army Medical Research and Development Command, Fort Detrick, MD, and the Defense Medical Research and Development Program. In addition, the HJF was supported under Contract Numbers W81XWH-17-2-0024 and W81XWH-20-C-0031.
Institutional Review Board Statement
This study was approved the University of Connecticut Institutional Review Board (Protocol: H20-0010).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data reported can be found in this manuscript as well as in an additional manuscript at: https://link.springer.com/article/10.1007/s00421-023-05139-x accessed on 24 January 2023.
Conflicts of Interest
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the United States (U.S.) Army, the U.S. Department of Defense, or The Henry M. Jackson Foundation (HJF) for the Advancement of Military Medicine, Inc. This paper has been approved for public release with unlimited distribution.
References
- Kenny, G.P.; Journeay, W.S. Human thermoregulation: Separating thermal and nonthermal effects on heat loss. Front. Biosci. 2010, 15, 259–290. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Schlader, Z.J. Human behavioral thermoregulation during exercise in the heat. Scand. J. Med. Sci. Sport. 2015, 25, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.; Cheuvront, S.N.; Wray, D.W.; Kolka, M.A.; Stephenson, L.A.; Sawka, M.N. The influence of hydration status on heart rate variability after exercise heat stress. J. Therm. Biol. 2005, 30, 495–502. [Google Scholar] [CrossRef]
- Macartney, M.J.; Meade, R.D.; Notley, S.R.; Herry, C.L.; Seely, A.J.E.; Kenny, G.P. Fluid Loss during Exercise-Heat Stress Reduces Cardiac Vagal Autonomic Modulation. Med. Sci. Sport. Exerc. 2020, 52, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Armstrong, L.E.; Edward, C.F.; Mack, G.W.; Sawaka, M.N.; Senay, L.C.; Scherman, M. Exercise and Fluid Replacement. Medicine & Science in Sports & Exercise. Available online: https://www.researchgate.net/profile/Michael_Sawka/publication/13917600_Exercise_and_fluid_replacement_Position_stand_American_College_of_Sports_Medicine/links/558a8ad808aee1fc9174f2f1.pdf (accessed on 20 August 2020).
- Casa, D.J.; Csillan, D.; Armstrong, L.E.; Baker, L.B.; Bergeron, M.F.; Buchanan, V.M.; Carroll, M.J.; Cleary, M.A.; Eichner, E.R.; Ferrara, M.S.; et al. Preseason Heat-Acclimatization Guidelines for Secondary School Athletics. J. Athl. Train. 2009, 44, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J. Sport and Physical Activity in the Heat: Maximizing Performance and Safety; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Brenner, I.K.M.; Thomas, S.; Shephard, R.J. Spectral analysis of heart rate variability during heat exposure and repeated exercise. Eur. J. Appl. Physiol. 1997, 76, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Pryor, R.; Pryor, J.L.; Vandermark, L.W.; Adams, E.L.; Brodeur, R.M.; Armstrong, L.E.; Lee, E.C.; Maresh, C.M.; Anderson, J.M.; Casa, D.J. Exacerbated heat strain during consecutive days of repeated exercise sessions in heat. J. Sci. Med. Sport 2019, 22, 1084–1089. [Google Scholar] [CrossRef]
- Macartney, M.J.; Notley, S.R.; Meade, R.D.; Herry, C.L.; Kenny, G.P. Heart rate variability in older men on the day following prolonged work in the heat. J. Occup. Environ. Hyg. 2020, 17, 383–389. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Colburn, D.; Hostler, D. Heat Strain Is Exacerbated on the Second of Consecutive Days of Fire Suppression. Med. Sci. Sport. Exerc. 2017, 49, 999–1005. [Google Scholar] [CrossRef]
- Michael, S.; Graham, K.S.; Davis, G.M.O. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals—A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Abellán-Aynés, O.; López-Plaza, D.; Alacid, F.; Naranjo-Orellana, J.; Manonelles, P. Recovery of Heart Rate Variability After Exercise Under Hot Conditions: The Effect of Relative Humidity. Wilderness Environ. Med. 2019, 30, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kaltsatou, A.; Flouris, A.D.; Herry, C.L.; Notley, S.R.; Macartney, M.J.; Seely, A.J.E.; Kenny, G.P.; E Seely, A.J. Heart rate variability in older workers during work under the Threshold Limit Values for heat exposure. Am. J. Ind. Med. 2020, 63, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.G.; Ahmad, S.; Seely, A.J.; Kenny, G.P. Heart rate variability and baroreceptor sensitivity following exercise-induced hyperthermia in endurance trained men. Eur. J. Appl. Physiol. 2012, 112, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Papelier, Y.; Laursen, P.B.; Ahmaidi, S. Noninvasive assessment of cardiac parasympathetic function: Postexercise heart rate recovery or heart rate variability? Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H8–H10. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Friesen, B.J.; Herry, C.L.; Seely, A.J.E.; Notley, S.R.; Kenny, G.P. Heart rate variability dynamics during treatment for exertional heat strain when immediate response is not possible. Exp. Physiol. 2019, 104, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Stacey, M.M.J.; Delves, S.K.; Woods, D.R.; Britland, S.E.; Macconnachie, L.; Allsopp, A.J.; Brett, S.J.; Fallowfield, J.L.; Boos, C.J. Heart rate variability and plasma nephrines in the evaluation of heat acclimatisation status. Eur. J. Appl. Physiol. 2018, 118, 165–174. [Google Scholar] [CrossRef]
- García Iglesias, D.; Roqueñi Gutiérrez, N.; De Cos, F.J.; Calvo, D. Analysis of the High-Frequency Content in Human QRS Complexes by the Continuous Wavelet Transform: An Automatized Analysis for the Prediction of Sudden Cardiac Death. Sensors 2018, 18, 560. [Google Scholar] [CrossRef]
- Ebrahimzadeh, E.; Pooyan, M.; Bijar, A. A Novel Approach to Predict Sudden Cardiac Death (SCD) Using Nonlinear and Time-Frequency Analyses from HRV Signals. PLoS ONE 2014, 9, e81896. [Google Scholar] [CrossRef]
- Laurent, C.M.; Green, J.M.; A Bishop, P.; Sjökvist, J.; E Schumacker, R.; Richardson, M.T.; Curtner-Smith, M. A Practical Approach to Monitoring Recovery: Development of a Perceived Recovery Status Scale. J. Strength Cond. Res. 2011, 25, 620–628. [Google Scholar] [CrossRef]
- Harmon Kimberly, G.; Asif Irfan, M.; Maleszewski Joseph, J.; Owens, D.; Prutkin, J.; Salerno, J.; Zigman, M.; Ellenbogen, R.; Rao, A.; Ackerman, M.; et al. Incidence, Cause, and Comparative Frequency of Sudden Cardiac Death in National Collegiate Athletic Association Athletes. Circulation 2015, 132, 10–19. [Google Scholar] [CrossRef]
- Adams, W.M.; Hosokawa, Y.; Casa, D.J. Body-Cooling Paradigm in Sport: Maximizing Safety and Performance During Competition. J. Sport Rehabil. 2016, 25, 382–394. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.P.; Anderson, S.A.; Armstrong, L.E.; Casa, D.J.; Cheuvront, S.N.; Cooper, L.; Kenney, W.L.; O’Connor, F.G.; Roberts, W.O. National Athletic Trainers’ Association Position Statement: Fluid Replacement for the Physically Active. J Athl. Train. 2017, 52, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; Guskiewicz, K.M.; Anderson, S.A.; Courson, R.W.; Heck, J.F.; Jimenez, C.C.; McDermott, B.P.; Miller, M.G.; Stearns, R.L.; Swartz, E.E.; et al. National Athletic Trainers’ Association Position Statement: Preventing Sudden Death in Sports. J. Athl. Train. 2012, 47, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; Curtis, R.M.; Stearns, R.L. Heart rate variability offers additional applications in heat-stressed. Exp. Physiol. 2019, 104, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; Almquist, J.; Anderson, S.A.; Baker, L.; Bergeron, M.F.; Biagioli, B.; Boden, B.; Brenner, J.S.; Carroll, M.; Colgate, B.; et al. The Inter-Association Task Force for Preventing Sudden Death in Secondary School Athletics Programs: Best-Practices Recommendations. J. Athl. Train. 2013, 48, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Hostler, D.; Bednez, J.C.; Kerin, S.; Reis, S.; Kong, P.W.; Morley, J.; Gallagher, M., Jr.; Suyama, J. Comparison of Rehydration Regimens for Rehabilitation of Firefighters Performing Heavy Exercise in Thermal Protective Clothing: A Report from the Fireground Rehab Evaluation (FIRE) Trial. Prehospital Emerg. Care 2010, 14, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.P.; Manning, C.N.; Morrissey, M.C.; Gulati, T.; Laxminarayan, S.; Reifman, J.; Casa, D.J. Efficacy of two intermittent cooling strategies during prolonged work-rest intervals in the heat with personal protective gear compared with a control condition. Eur. J. Appl. Physiol. 2023, 1–10. [Google Scholar] [CrossRef]
- Miller, K.C.; Hughes, L.E.; Long, B.C.; Adams, W.M.; Casa, D.J. Validity of Core Temperature Measurements at 3 Rectal Depths During Rest, Exercise, Cold-Water Immersion, and Recovery. J. Athl. Train. 2017, 52, 332–338. [Google Scholar] [CrossRef]
- Hernando, D.; Garatachea, N.; Almeida, R.; Casajús, J.A.; Bailón, R. Validation of Heart Rate Monitor Polar RS800 for Heart Rate Variability Analysis During Exercise. J. Strength Cond. Res. 2018, 32, 716–725. [Google Scholar] [CrossRef]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can Wearable Devices Accurately Measure Heart Rate Variability? A Systematic Review. Folia Med. 2018, 60, 7–20. [Google Scholar] [CrossRef]
- Manual | BIOPAC. BIOPAC Systems, Inc. Available online: https://www.biopac.com/manual/ (accessed on 22 January 2021).
- Frontiers | HRVanalysis: A Free Software for Analyzing Cardiac Autonomic Activity | Physiology. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2016.00557/full (accessed on 5 March 2021).
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- About—Jamovi. Available online: https://www.jamovi.org/about.html (accessed on 16 January 2021).
- Early, K.S.; Earnest, C.P.; Theall, B.; Lemoine, N.P.; Harrell, B.; Johannsen, N.M. Free-living, continuous hypo-hydration, and cardiovascular response to exercise in a heated environment. Physiol. Rep. 2018, 6, e13672. [Google Scholar] [CrossRef] [PubMed]
- Nolte, H.W.; Noakes, T.D.; Nolte, K. Ad Libitum vs. Restricted Fluid Replacement on Hydration and Performance of Military Tasks. Aviat. Space Environ. Med. 2013, 84, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Casa, D.J.; Jensen, K.A.; Stearns, R.L.; DeMartini, J.K.; Pagnotta, K.D.; Roti, M.W.; Armstrong, L.E.; Maresh, C.M. Comparison of Two Fluid Replacement Protocols During a 20-km Trail Running Race in the Heat. J. Strength Cond. Res. 2016, 30, 2609–2616. [Google Scholar] [CrossRef]
- Lopez, R.M.; Casa, D.J.; A Jensen, K.; DeMartini, J.K.; Pagnotta, K.D.; Ruiz, R.C.; Roti, M.W.; Stearns, R.L.; E Armstrong, L.; Maresh, C.M. Examining the Influence of Hydration Status on Physiological Responses and Running Speed During Trail Running in the Heat With Controlled Exercise Intensity. J. Strength Cond. Res. 2011, 25, 2944–2954. [Google Scholar] [CrossRef]
- Casa, D.J.; Armstrong, L.E.; Hillman, S.K.; Montain, S.J.; Reiff, R.V.; Rich, B.S.E.; Roberts, W.O.; Stone, J.A. National Athletic Trainers’ Association Position Statement: Fluid Replacement for Athletes. J. Athl. Train. 2000, 35, 212–224. [Google Scholar]
- Convertino, V.A.; Armstrong, L.E.; Coyle, E.F.; Mack, G.W.; Sawka, M.N.; Senay, L.C.; Sherman, W.M. ACSM Position Stand: Exercise and Fluid Replacement. Med. Sci. Sports Exerc. 1996, 28, 1–9. [Google Scholar] [CrossRef]
- Adams, W.M. Rehydration on Subsequent Performance and Recovery Following Exercise-Induced Dehydration: Ad Libitum Versus Prescribed Fluid Replacement. Ph.D. Thesis, University of Connecticut, Stamford, CT, USA, 2016; p. 109. [Google Scholar]
- Yamamoto, Y.; Hughson, R.L.; Peterson, J.C. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J. Appl. Physiol. 1991, 71, 1136–1142. [Google Scholar] [CrossRef]
- Bernardi, L.; Passino, C.; Robergs, R.; Appenzeller, O. Acute and persistent effects of a 46-kilometre wilderness trail run at altitude: Cardiovascular autonomic modulation and baroreflexes. Cardiovasc. Res. 1997, 34, 273–280. [Google Scholar] [CrossRef]
- Evidence for an Intrinsic Mechanism Regulating Heart Rate Variability in the Transplanted and the Intact Heart During Submaximal Dynamic Exercise? | Cardiovascular Research | Oxford Academic. Available online: https://academic.oup.com/cardiovascres/article/24/12/969/493866?login=true (accessed on 4 March 2021).
- Perini, R.; Veicsteinas, A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur. J. Appl. Physiol. 2003, 90, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, O.; Furlan, R.; Pagani, M.R.; Piazza, S.; Guazzi, M.; Pagani, M.; Malliani, A. Analysis of Neural Mechanisms Accompanying Different Intensities of Dynamic Exercise. Chest 1992, 101 (Suppl. 5), 226S–230S. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, J. Separate and Combined Influences of Dehydration and Hyperthermia on Cardiovascular Responses to Exercise. Int. J. Sports Med. 1998, 19 (Suppl. 2), S111–S114. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N.; Halliwill, J.R.; Morgan, B.J.; Eisenach, J.H.; Joyner, M.J. Influences of hydration on post-exercise cardiovascular control in humans. J. Physiol. 2003, 552, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Binkley, H.M.; Beckett, J.; Casa, D.J.; Kleiner, D.M.; Plummer, P.E. National Athletic Trainers’ Association Position Statement: Exertional Heat Illnesses. J. Athl. Train. 2002, 37, 329–343. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).