Physiological Performance of Mimosa pudica L. under Different Light Quality and Photoperiods
Abstract
1. Introduction
2. Material and Methods
2.1. Growth and Light Conditions
2.2. Biochemical Analysis
2.3. Fluorescence Analysis
2.4. Statistics
3. Results
3.1. Plant Morphology and Growth Characteristics
3.2. Biochemical Changes in Light
3.2.1. Effects on SOD Activity
3.2.2. Effects on Catalase Activity
3.2.3. Effects on GPOD Activity
3.2.4. Effects on MDA Content
3.2.5. Effects on Proline Content
3.2.6. Effects of Starch Content
3.2.7. Effects of Chlorophyll Content
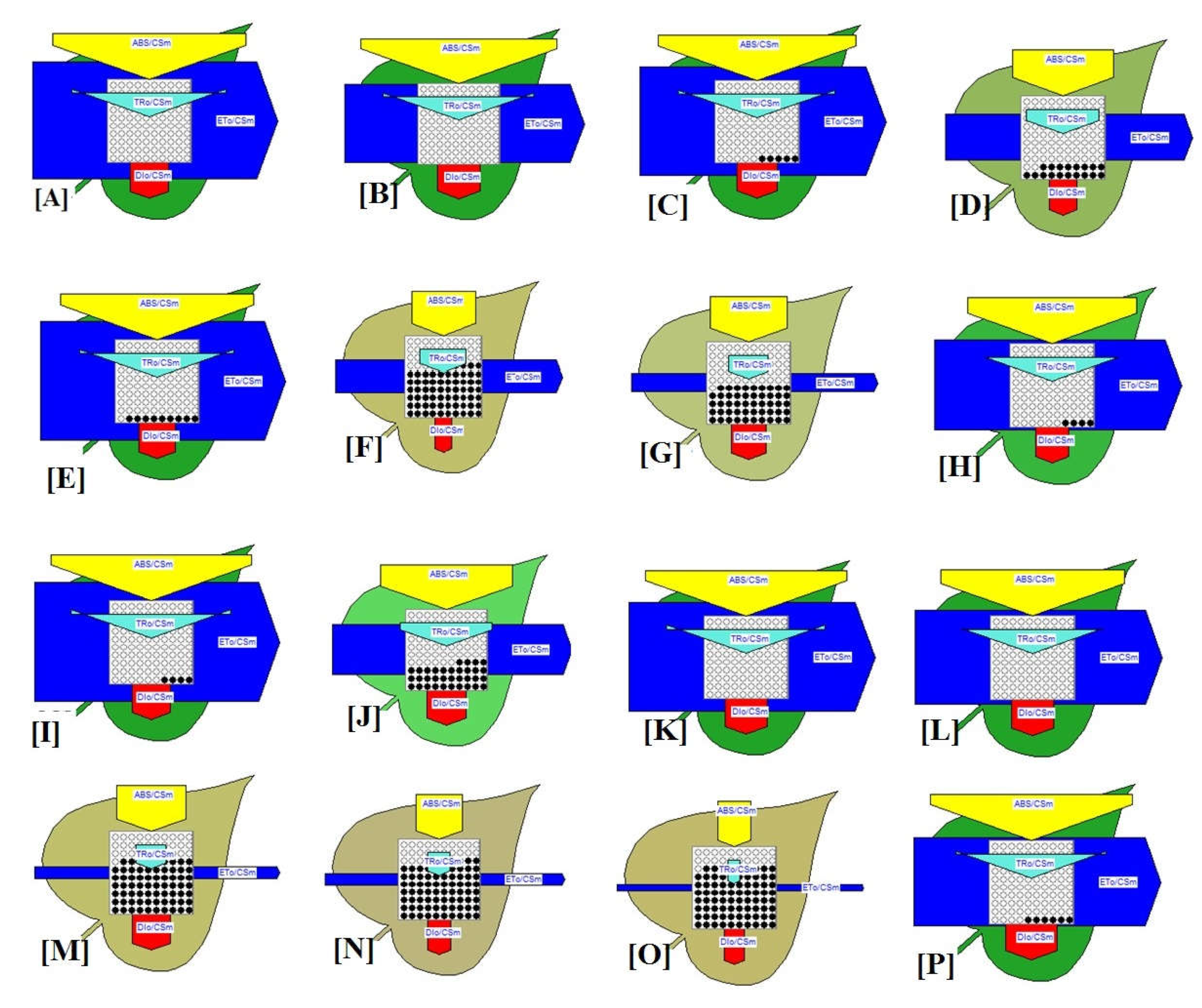
3.3. Biophysical Studies of Light Quality in M. pudica
3.3.1. Chlorophyll a Fluorescence
3.3.2. Phenomenological Energy Fluxes
3.3.3. Density of Active Reaction Centers
3.3.4. Yield and Flux Ratio
3.3.5. Performance Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BL | Blue light |
| GL | Green light |
| RL | Red light |
| WL | White light |
| CL | Continuous light |
| ROS | Reactive oxygen species |
| Chl | Chlorophyll |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPOX | Guaiacol peroxidases |
| PS | Photosystem |
| RC | Reaction center |
| Fm | Maximal fluorescence intensity |
| ABS/CSM | Absorbed photon flux per excited cross-section |
| ET0/CSM | Electron transport flux from QA−to PQ per cross-section |
| DI0/CSM | Dissipated energy flux per cross-section of PSII |
| RC/CSM | Density of active PSII RCs |
| ΦPo | Maximum quantum yield of primary PSII photochemistry (TR0/ABSM or Fv/FM) |
| ΦEo | Quantum yield of electron transport from QA to PQ (ET0/ABS) |
| ΦDo | Quantum yield of energy dissipation in PSII antenna (DI/ABS) |
| PIABS | Performance index on absorbance basis |
| PQ | plastoquinone |
References
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of Light Quality on the Accumulation of Phytochemicals in Vegetables Produced in Controlled Environments: A Review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant Photoreceptors: Multi-Functional Sensory Proteins and Their Signaling Networks. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2019; Volume 92, pp. 114–121. [Google Scholar]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2021, 1–39. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, M.-J.; Guo, Y.-P. Regulation of Photosynthesis by Light Quality and Its Mechanism in Plants. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2008, 19, 1619–1624. [Google Scholar]
- Smith, H. Light Quality, Photoperception, and Plant Strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Xu, D.Q.; Gao, W.; Ruan, J. Effects of Light Quality on Plant Growth and Development. Plant Physiol. J. 2015, 51, 1217–1234. [Google Scholar]
- Wang, X.Y.; Xu, X.M.; Cui, J. The Importance of Blue Light for Leaf Area Expansion, Development of Photosynthetic Apparatus, and Chloroplast Ultrastructure of Cucumis sativus Grown under Weak Light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Weston, E.; Thorogood, K.; Vinti, G.; López-Juez, E. Light Quantity Controls Leaf-Cell and Chloroplast Development in Arabidopsis thaliana Wild Type and Blue-Light-Perception Mutants. Planta 2000, 211, 807–815. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; Van Kooten, O.; Harbinson, J.; Van Ieperen, W. Plasticity of Photosynthesis after the ‘Red Light Syndrome’in Cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Takemiya, A.; Inoue, S.; Doi, M.; Kinoshita, T.; Shimazaki, K. Phototropins Promote Plant Growth in Response to Blue Light in Low Light Environments. Plant Cell 2005, 17, 1120–1127. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves Are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- O’Carrigan, A.; Babla, M.; Wang, F.; Liu, X.; Mak, M.; Thomas, R.; Bellotti, B.; Chen, Z.-H. Analysis of Gas Exchange, Stomatal Behaviour and Micronutrients Uncovers Dynamic Response and Adaptation of Tomato Plants to Monochromatic Light Treatments. Plant Physiol. Biochem. 2014, 82, 105–115. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The Effect of Light Quality on Growth, Photosynthesis, Leaf Anatomy and Volatile Isoprenoids of a Monoterpene-Emitting Herbaceous Species (Solanum Lycopersicum L.) and an Isoprene-Emitting Tree (Platanus Orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Kim, Y.M.; Sung, J.K.; Lee, Y.J.; Lee, D.B.; Yoo, C.H.; Lee, S.B. Varying Effects of Artificial Light on Plant Functional Metabolites. Korean J. Environ. Agric. 2019, 38, 61–67. [Google Scholar] [CrossRef]
- Ali, M.B.; Khandaker, L.; Oba, S. Comparative Study on Functional Components, Antioxidant Activity and Color Parameters of Selected Colored Leafy Vegetables as Affected by Photoperiods. J. Food Agric. Environ. 2009, 7, 392–398. [Google Scholar]
- Torres, A.P.; Lopez, R.G. Photosynthetic Daily Light Integral during Propagation of Tecoma Stans Influences Seedling Rooting and Growth. HortScience 2011, 46, 282–286. [Google Scholar] [CrossRef]
- Weaver, G.; Van Iersel, M.W. Longer Photoperiods with Adaptive Lighting Control Can Improve Growth of Greenhouse-Grown ‘Little Gem’Lettuce (Lactuca Sativa). HortScience 2020, 55, 573–580. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Long-Term Continuous Light Provided by Red and Blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Copper and Mercury Induced Oxidative Stresses and Antioxidant Responses of Spirodela Polyrhiza (L.) Schleid. Biochem. Biophys. Rep. 2020, 23, 100781. [Google Scholar] [CrossRef]
- Bhatt, U.; Singh, H.; Kumar, D.; Strasser, R.J.; Soni, V. Severe Leaf-Vein Infestation Upregulates Antioxidant and Photosynthetic Activities in the Lamina of Ficus religiosa. Acta Physiol. Plant. 2022, 44, 15. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-Induced Reactive Oxygen Species Accumulation by Altering the Enzymatic Antioxidant System and Associated Cytotoxic, Genotoxic, Ultrastructural, and Photosynthetic Changes in Plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef] [PubMed]
- Pandhair, V.; Sekhon, B.S. Reactive Oxygen Species and Antioxidants in Plants: An Overview. J. Plant Biochem. Biotechnol. 2006, 15, 71–78. [Google Scholar] [CrossRef]
- Frąkaszczak, B.; Kula-Maximenko, M. The Preferences of Different Cultivars of Lettuce Seedlings (Lactuca Sativa L.) for the Spectral Composition of Light. Agronomy 2021, 11, 1211. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Performance of Chlorophyll a Fluorescence Parameters in Lemna minor under Heavy Metal Stress Induced by Various Concentration of Copper. Sci. Rep. 2022, 12, 10620. [Google Scholar]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of Light Quality on CO2 Assimilation, Chlorophyll-Fluorescence Quenching, Expression of Calvin Cycle Genes and Carbohydrate Accumulation in Cucumis Sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of Different Light Intensities on Chlorophyll Fluorescence Characteristics and Yield in Lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, H.; Bhatt, U.; Soni, V.; Allakhverdiev, S. Effect of Continuous Light on Antioxidant Activity, Lipid Peroxidation, Proline and Chlorophyll Content in Vigna Radiata L. Funct. Plant Biol. 2021, 49, 145–154. [Google Scholar] [CrossRef]
- Tamilarasi, T.; Ananthi, T. Phytochemical Analysis and Anti Microbial Activity of Mimosa pudica Linn. Res. J. Chem. Sci. 2012, 2231, 606X. [Google Scholar]
- Zhang, J.; Yuan, K.; Zhou, W.; Zhou, J.; Yang, P. Studies on the Active Components and Antioxidant Activities of the Extracts of Mimosa pudica Linn. from Southern China. Pharmacogn. Mag. 2011, 7, 35. [Google Scholar]
- Baghel, A.; Rathore, D.S.; Gupta, V. Evaluation of Diuretic Activity of Different Extracts of Mimosa pudica Linn. Pak. J. Biol. Sci. PJBS 2013, 16, 1223–1225. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro Assay Methods. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Racusen, D.; Foote, M. Protein Synthesis in Dark-Grown Bean Leaves. Can. J. Bot. 1965, 43, 817–824. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- McCready, R.M.; Guggolz, J.; Silviera, V.; Owens, H.S. Determination of Starch and Amylose in Vegetables. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta (BBA) Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Strasser, R.J. Govindjee The Fo and the OJIP Fluorescence Rise in Higher Plants and Algae. In Regulation of Chloroplast Biogenesis; Springer: Boston, MA, USA, 1992; pp. 423–426. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 1, 445–483. [Google Scholar]
- Causin, H.F.; Barneix, A.J. The Role of Oxidative Metabolism in the Regulation of Leaf Senescence by the Light Environment. Int. J. Plant Dev. Biol. 2007, 1, 239–244. [Google Scholar]
- Wang, H.; Jiang, Y.; Shi, K.; Zhou, Y.; Yu, J. Effects of Light Quality on Leaf Senescence and Activities of Antioxidant Enzymes in Cucumber Plants. Sci. Agric. Sin. 2010, 43, 529–534. [Google Scholar]
- Wang, H.B.; Wang, S.; Wang, X.D.; Shi, X.B.; Wang, B.L.; Zheng, X.C.; Wang, Z.Q.; Liu, F.Z. [Effects of Light Quality on Leaf Senescence and Endogenous Hormones Content in Grapevine under Protected Cultivation]. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2017, 28, 3535–3543. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Photosynthetic Characteristics and Chloroplast Ultrastructure of Welsh Onion (Allium Fistulosum L.) Grown under Different LED Wavelengths. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shao, Q.; Xu, M.; Li, S.; Wu, M.; Tan, X.; Su, L. Effects of Light Quality on Morphology, Enzyme Activities, and Bioactive Compound Contents in Anoectochilus roxburghii. Front. Plant Sci. 2017, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Amore, T.; Lazaro, M. Light-Emitting Diodes (LEDs) for Miniature Hydroponic Lettuce. Opt. Photonics J. 2013, 3, 29024. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Growth, Photosynthetic and Antioxidant Parameters of Two Lettuce Cultivars as Affected by Red, Green, and Blue Light-Emitting Diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.; Tripathy, B.C. Photoregulation of the Greening Process of Wheat Seedlings Grown in Red Light. Plant Mol. Biol. 2005, 59, 269–287. [Google Scholar] [CrossRef]
- Moradi, S.; Kafi, M.; Aliniaeifard, S.; Salami, S.A.; Shokrpour, M.; Pedersen, C.; Moosavi-Nezhad, M.; Wróbel, J.; Kalaji, H.M. Blue Light Improves Photosynthetic Performance and Biomass Partitioning toward Harvestable Organs in Saffron (Crocus Sativus L.). Cells 2021, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein Is Needed for Efficient Chlorophyll Triplet Quenching in the Major LHCII Antenna Complex of Higher Plants and Effective Photoprotection in Vivounder Strong Light. BMC Plant Biol. 2006, 6, 1–20. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. In Vivo Assessment of Stress Impact on Plant’s Vitality: Applications in Detecting and Evaluating the Beneficial Role of Mycorrhization on Host Plants. In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2008; pp. 679–703. [Google Scholar]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) Response to Ozone Exposure Assessed with a Chlorophyll a Fluorescence Performance Index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Strasser, B.J. Donor Side Capacity of Photosystem II Probed by Chlorophyll a Fluorescence Transients. Photosynth. Res. 1997, 52, 147–155. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. The Energy Flux Theory 35 Years Later: Formulations and Applications. Photosynth. Res. 2013, 117, 289–320. [Google Scholar] [CrossRef]
- Hamed, S.B.; Lefi, E.; Chaieb, M. Effect of Phosphorus Concentration on the Photochemical Stability of PSII and CO2 Assimilation in Pistacia Vera L. and Pistacia Atlantica Desf. Plant Physiol. Biochem. 2019, 142, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Fang, S.; Zhou, M.; Qin, J. Responses of Morphology, Gas Exchange, Photochemical Activity of Photosystem II, and Antioxidant Balance in Cyclocarya paliurus to Light Spectra. Front. Plant Sci. 2018, 9, 1704. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of Photosystem II. Inactivation, Protein Damage and Turnover. Biochim. Biophys. Acta (BBA) Bioenerg. 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhong, Q.; Chen, C.; Ruan, Q.; Chen, Z.; You, X. Carbon Dioxide Assimilation and Photosynthetic Electron Transport of Tea Leaves under Nitrogen Deficiency. Bot. Stud. 2016, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a Fluorescence Kinetics of Mung Bean (Vigna radiata L.) Grown under Artificial Continuous Light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A Non-Invasive Assay of the Plastoquinone Pool Redox State Based on the OJIP-Transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Xu, Z.; Lv, X.; Wang, Y. Detection of Photosynthetic Performance of Stipa Bungeana Seedlings under Climatic Change Using Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2016, 6, 1254. [Google Scholar] [CrossRef]
- Na, Y.-W.; Jeong, H.J.; Lee, S.-Y.; Choi, H.G.; Kim, S.-H.; Rho, I.R. Chlorophyll Fluorescence as a Diagnostic Tool for Abiotic Stress Tolerance in Wild and Cultivated Strawberry Species. Hortic. Environ. Biotechnol. 2014, 55, 280–286. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Ottosen, C.-O.; Rosenqvist, E. Spectral Effects of LEDs on Chlorophyll Fluorescence and Pigmentation in Phalaenopsis ‘Vivien’ and ‘Purple Star’. Physiol. Plant. 2015, 154, 314–327. [Google Scholar] [CrossRef]
- Janeeshma, E.; Johnson, R.; Amritha, M.S.; Noble, L.; Aswathi, K.P.R.; Telesiński, A.; Kalaji, H.M.; Auriga, A.; Puthur, J.T. Modulations in Chlorophyll a Fluorescence Based on Intensity and Spectral Variations of Light. Int. J. Mol. Sci. 2022, 23, 5599. [Google Scholar] [CrossRef]
- Vass, I.; Kirilovsky, D.; Etienne, A.-L. UV-B Radiation-Induced Donor-and Acceptor-Side Modifications of Photosystem II in the Cyanobacterium Synechocystis Sp. PCC 6803. Biochemistry 1999, 38, 12786–12794. [Google Scholar] [CrossRef]
- Oguchi, R.; Douwstra, P.; Fujita, T.; Chow, W.S.; Terashima, I. Intra-Leaf Gradients of Photoinhibition Induced by Different Color Lights: Implications for the Dual Mechanisms of Photoinhibition and for the Application of Conventional Chlorophyll Fluorometers. New Phytol. 2011, 191, 146–159. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Zhao, J.; Park, Y.G.; Jeong, B.R. Light Quality Affects Growth and Physiology of Carpesium triste Maxim Cultured in Vitro. Agriculture 2020, 10, 258. [Google Scholar] [CrossRef]
- Miyake, C. Molecular Mechanism of Oxidation of P700 and Suppression of ROS Production in Photosystem I in Response to Electron-Sink Limitations in C3 Plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Sarin, N.B. Overexpression of $γ$-Tocopherol Methyl Transferase Gene in Transgenic Brassica juncea Plants Alleviates Abiotic Stress: Physiological and Chlorophyll a Fluorescence Measurements. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Seif, M.; Arab, M.; Zare Mehrjerdi, M.; Li, T.; Lastochkina, O. Growth and Photosynthetic Performance of Calendula officinalis under Monochromatic Red Light. Int. J. Hortic. Sci. Technol. 2018, 5, 123–132. [Google Scholar]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of Growth under Different Light Spectra on the Subsequent High Light Tolerance in Rose Plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; ASGHAR, M.A.; Ali, R.; FAN, Y.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop Photosynthetic Response to Light Quality and Light Intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED Light Enhances Growth, Phytochemical Contents, and Antioxidant Enzyme Activities of Rehmannia glutinosa Cultured in Vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.; Liu, G.; Liu, H. Growth, Photosynthetic Characteristics, Antioxidant Capacity and Biomass Yield and Quality of Wheat (Triticum Aestivum L.) Exposed to LED Light Sources with Different Spectra Combinations. J. Agron. Crop Sci. 2014, 200, 219–230. [Google Scholar] [CrossRef]
- Simlat, M.; Ślękzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The Effect of Light Quality on Seed Germination, Seedling Growth and Selected Biochemical Properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Prochazkova, D.; Wilhelmova, N. Leaf Senescence and Activities of the Antioxidant Enzymes. Biol. Plant. 2007, 51, 401–406. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of Different Spectral Lights with Biomass Accumulation and Production of Antioxidant Secondary Metabolites in Callus Cultures of Medicinally Important Prunella Vulgaris L. J. Photochem. Photobiol. B Biol. 2016, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, Y.; Song, L.; Jacobs, D.F.; Du, X.; Ying, Y.; Shao, Q.; Wu, J. Effect of Differential Light Quality on Morphology, Photosynthesis, and Antioxidant Enzyme Activity in Camptotheca Acuminata Seedlings. J. Plant Growth Regul. 2017, 36, 148–160. [Google Scholar] [CrossRef]
- Saleem, M.H.; Rehman, M.; Zahid, M.; Imran, M.; Xiang, W.; Liu, L. Morphological Changes and Antioxidative Capacity of Jute (Corchorus Capsularis, Malvaceae) under Different Color Light-Emitting Diodes. Braz. J. Bot. 2019, 42, 581–590. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.-C. Chrysanthemum Morphology, Photosynthetic Efficiency and Antioxidant Capacity Are Differentially Modified by Light Quality. J. Plant Physiol. 2017, 213, 66–74. [Google Scholar] [CrossRef]
- Juwei, H.U.; Xin, D.A.I.; Guangyu, S.U.N. Morphological and Physiological Responses of Morus Alba Seedlings under Different Light Qualities. Not. Bot. Horti Agrobot. 2016, 44, 382–392. [Google Scholar]
- Vitale, E.; Izzo, L.G.; Amitrano, C.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light Quality Modulates Photosynthesis and Antioxidant Properties of B. Vulgaris L. Plants from Seeds Irradiated with High-Energy Heavy Ions: Implications for Cultivation in Space. Plants 2022, 11, 1816. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under Continuous Light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Ottosen, C.O. Continuous Light Increases Growth, Daily Carbon Gain, Antioxidants, and Alters Carbohydrate Metabolism in a Cultivated and a Wild Tomato Species. Front. Plant Sci. 2015. [Google Scholar] [CrossRef]
- Xiao-jing, W.; Xiao-li, C.; Wen-zhong, G.U.O.; Hai-ping, L.I.; Ling-zhi, L.I. Effects of LED Green Light on Growth and Quality of Lettuce. Chin. J. Agrometeorol. 2019, 40, 25. [Google Scholar]
- Ajdanian, L.; Babaei, M.; Aroiee, H. Investigation of Photosynthetic Effects, Carbohydrate and Starch Content in Cress (Lepidium Sativum) under the Influence of Blue and Red Spectrum. Heliyon 2020, 6, e05628. [Google Scholar] [CrossRef]
- Azcón-Bieto, J. Inhibition of Photosynthesis by Carbohydrates in Wheat Leaves. Plant Physiol. 1983, 73, 681–686. [Google Scholar] [CrossRef]


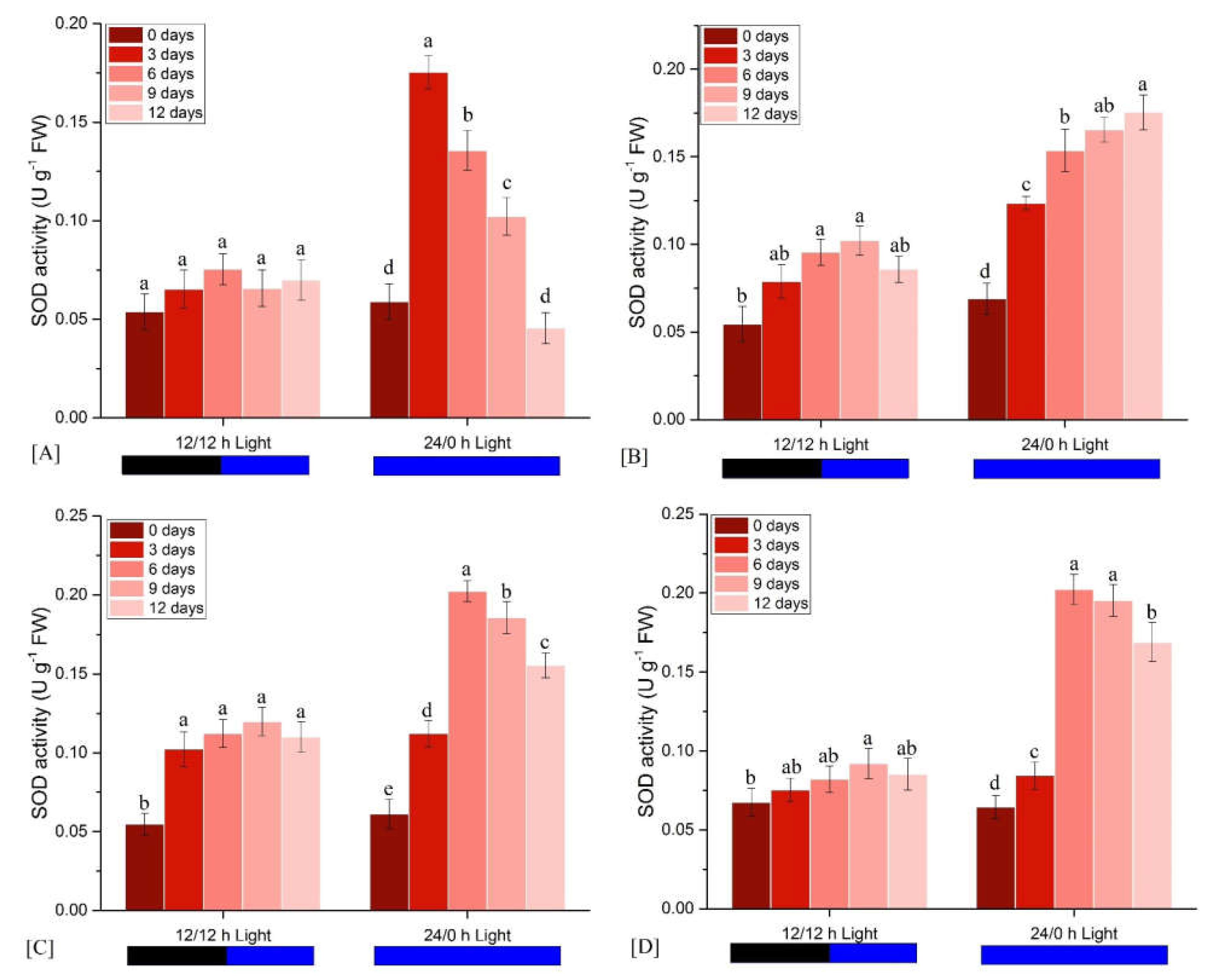
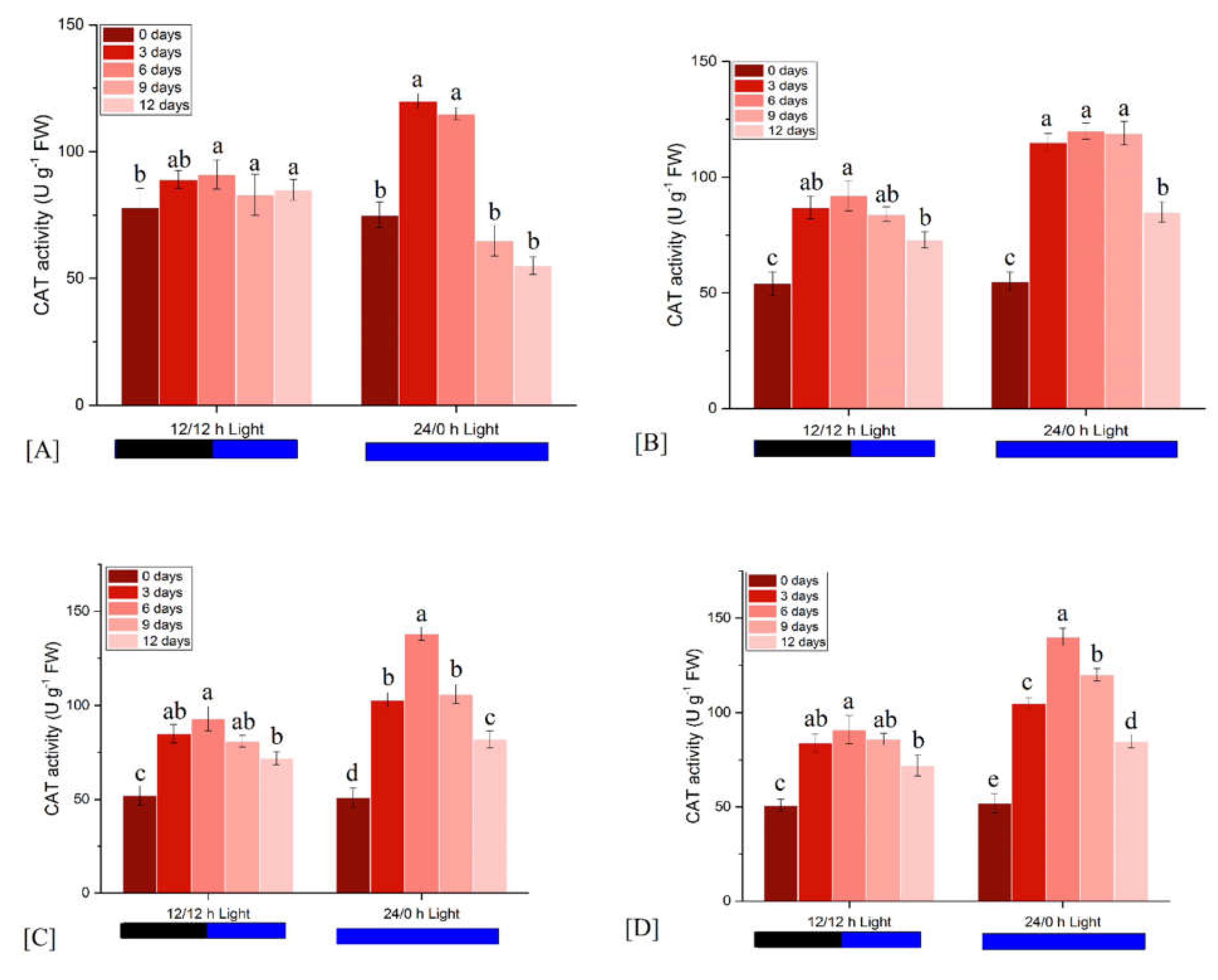

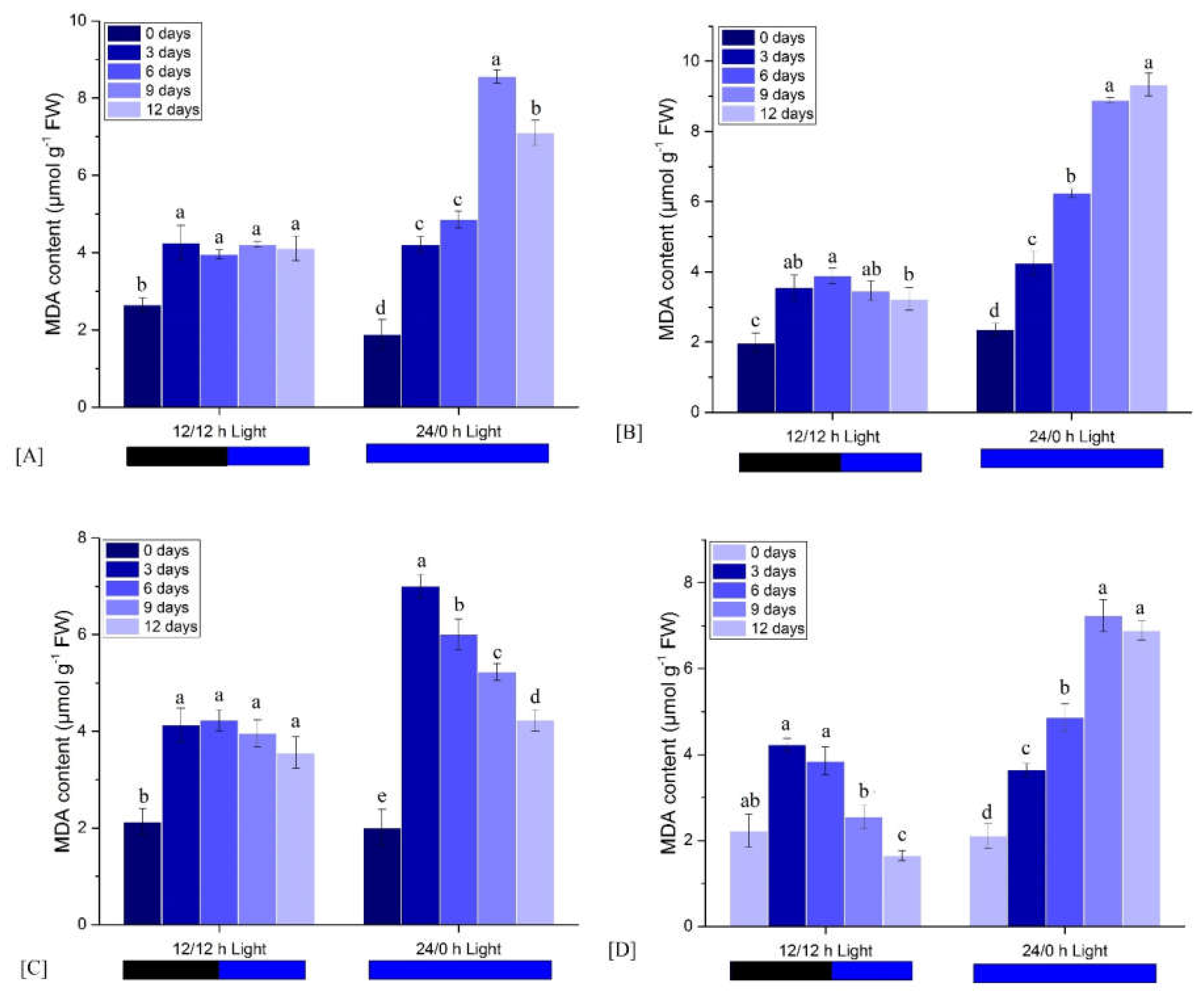
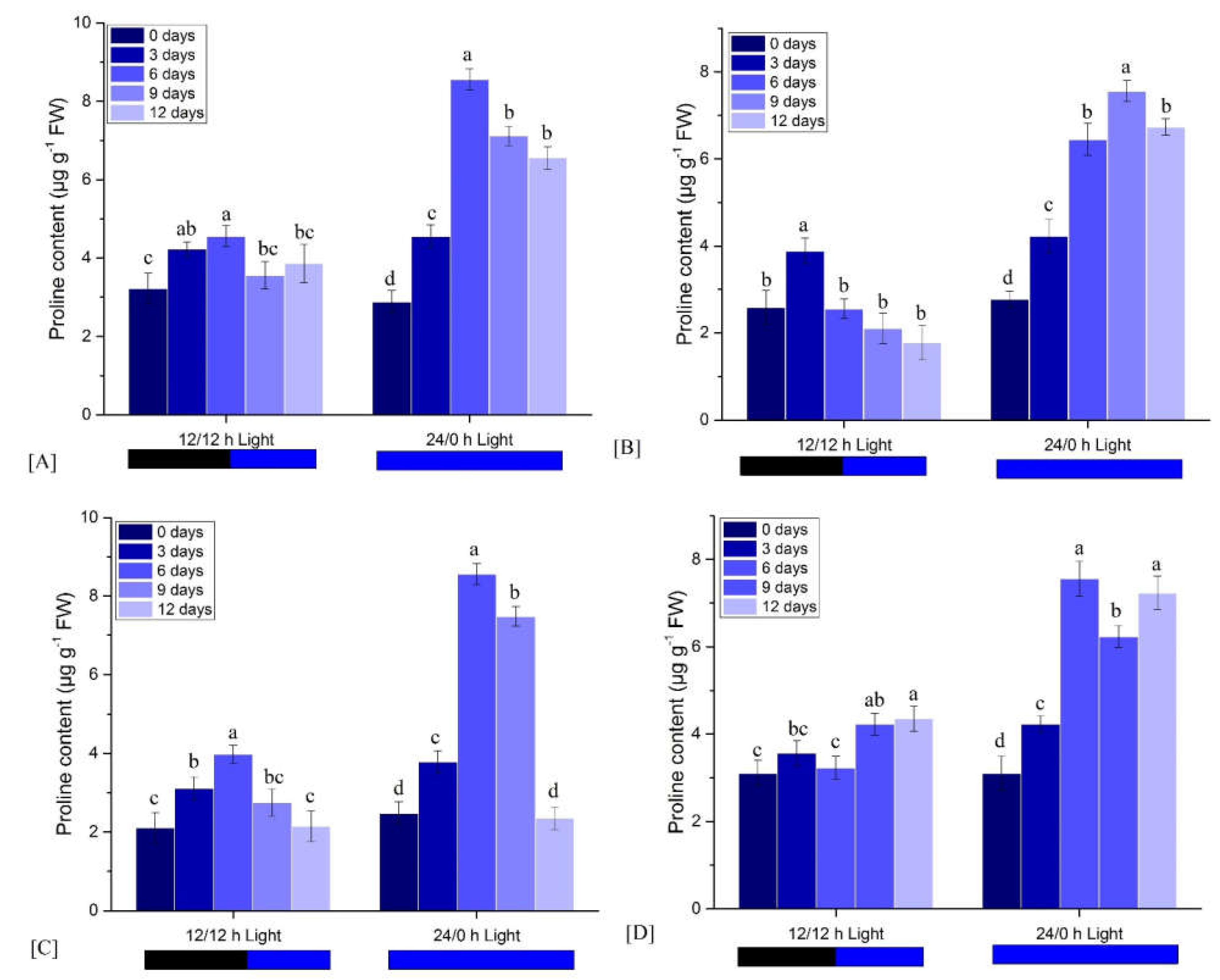



| Light Condition | Observation Time (Days) | FM | ΦPo | ΦEo | ΦDo | PIabs |
|---|---|---|---|---|---|---|
| W12 | 0 | 1317 ± 175.53 ns | 0.8033 ± 0.05 ns | 0.6112 ± 0.01 ns | 0.1967 ± 0.01 ns | 124.5912 ± 7.56 ns |
| W12 | 12 | 1321 ± 196.23 ns | 0.8055 ± 0.03 ns | 0.62 ± 0.03 ns | 0.1945 ± 0.03 ns | 121.9445 ± 10.23 ns |
| W24 | 0 | 1365 ± 151.36 a | 0.8029 ± 0.02 ns | 0.6029 ± 0.09 ns | 0.1971 ± 0.04 a | 108.5457 ± 11.98 a |
| W24 | 12 | 475 ± 85.23 b | 0.4505 ± 0.05 ns | 0.1937 ± 0.07 ns | 0.5495 ± 0.05 b | 1.8076 ± 13.78 b |
| B12 | 0 | 1232 ± 168.96 a | 0.7792 ± 0.10 ns | 0.4074 ± 0.05 a | 0.2208 ± 0.06 ns | 31.6599 ± 5.87 a |
| B12 | 12 | 430 ± 74.25 b | 0.7256 ± 0.04 ns | 0.5116 ± 0.04 b | 0.2744 ± 0.02 ns | 40.5881 ± 8.23 b |
| B24 | 0 | 834 ± 156.36 ns | 0.7014 ± 0.05 ns | 0.3897 ± 0.02 a | 0.2986 ± 0.02 a | 17.3892 ± 12.47 a |
| B24 | 12 | 291 ± 48.65 ns | 0.4983 ± 0.03 ns | 0.2955 ± 0.06 b | 0.5017 ± 0.04 b | 6.2586 ± 13.48 b |
| G12 | 0 | 1346 ± 198.23 a | 0.7964± 0.06 ns | 0.5683 ± 0.08 ns | 0.2036 ± 0.03 a | 78.3994 ± 9.72 a |
| G12 | 12 | 523 ± 79.56 b | 0.5373 ± 0.08 ns | 0.2658 ± 0.03 ns | 0.4627 ± 0.05 b | 5.559 ± 13.56 b |
| G24 | 0 | 1372 ± 145.84 a | 0.7934 ± 0.05 ns | 0.5591 ± 0.05 a | 0.207 ± 0.06 a | 74.6763 ± 10.89 a |
| G24 | 12 | 231 ± 56.87 b | 0.3939 ± 0.07 ns | 0.2121 ± 0.04 b | 0.6061 ± 0.08 b | 2.6136 ± 11.43 b |
| R12 | 0 | 1465 ± 199.23 a | 0.8096 ± 0.08 ns | 0.6089 ± 0.02 a | 0.1904 ± 0.03 a | 121.8065 ± 15.98 a |
| R12 | 12 | 535 ± 98.57.25 b | 0.5869 ± 0.09 ns | 0.2953 ± 0.10 b | 0.4131 ± 0.02 b | 4.9906 ± 18.25 b |
| R24 | 0 | 1434 ± 174.84 a | 0.7964 ± 0.06 a | 0.5886 ± 0.09 a | 0.2036 ± 0.01 a | 93.8863 ± 11.78 a |
| R24 | 12 | 117 ± 23.38 b | 0.1795 ± 0.10 b | 0.0684 ± 0.10 b | 0.8205 ± 0.02 b | 0.1964 ± 9.58 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Singh, H.; Bhatt, U.; Sharma, J.; Sharma, S.; Soni, V. Physiological Performance of Mimosa pudica L. under Different Light Quality and Photoperiods. Physiologia 2022, 2, 132-153. https://doi.org/10.3390/physiologia2040012
Kumar D, Singh H, Bhatt U, Sharma J, Sharma S, Soni V. Physiological Performance of Mimosa pudica L. under Different Light Quality and Photoperiods. Physiologia. 2022; 2(4):132-153. https://doi.org/10.3390/physiologia2040012
Chicago/Turabian StyleKumar, Deepak, Hanwant Singh, Upma Bhatt, Jyotshana Sharma, Shubhangani Sharma, and Vineet Soni. 2022. "Physiological Performance of Mimosa pudica L. under Different Light Quality and Photoperiods" Physiologia 2, no. 4: 132-153. https://doi.org/10.3390/physiologia2040012
APA StyleKumar, D., Singh, H., Bhatt, U., Sharma, J., Sharma, S., & Soni, V. (2022). Physiological Performance of Mimosa pudica L. under Different Light Quality and Photoperiods. Physiologia, 2(4), 132-153. https://doi.org/10.3390/physiologia2040012








