Assessment of Bacterial Presence Among New and “In Use” Resealable Biomaterials Within the Pediatric Dental Clinic
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental Sampling
2.2. Estimated Usage
2.3. Bacterial Culture
2.4. DNA Isolation
2.5. qPCR Screening
2.6. Statistical Analysis
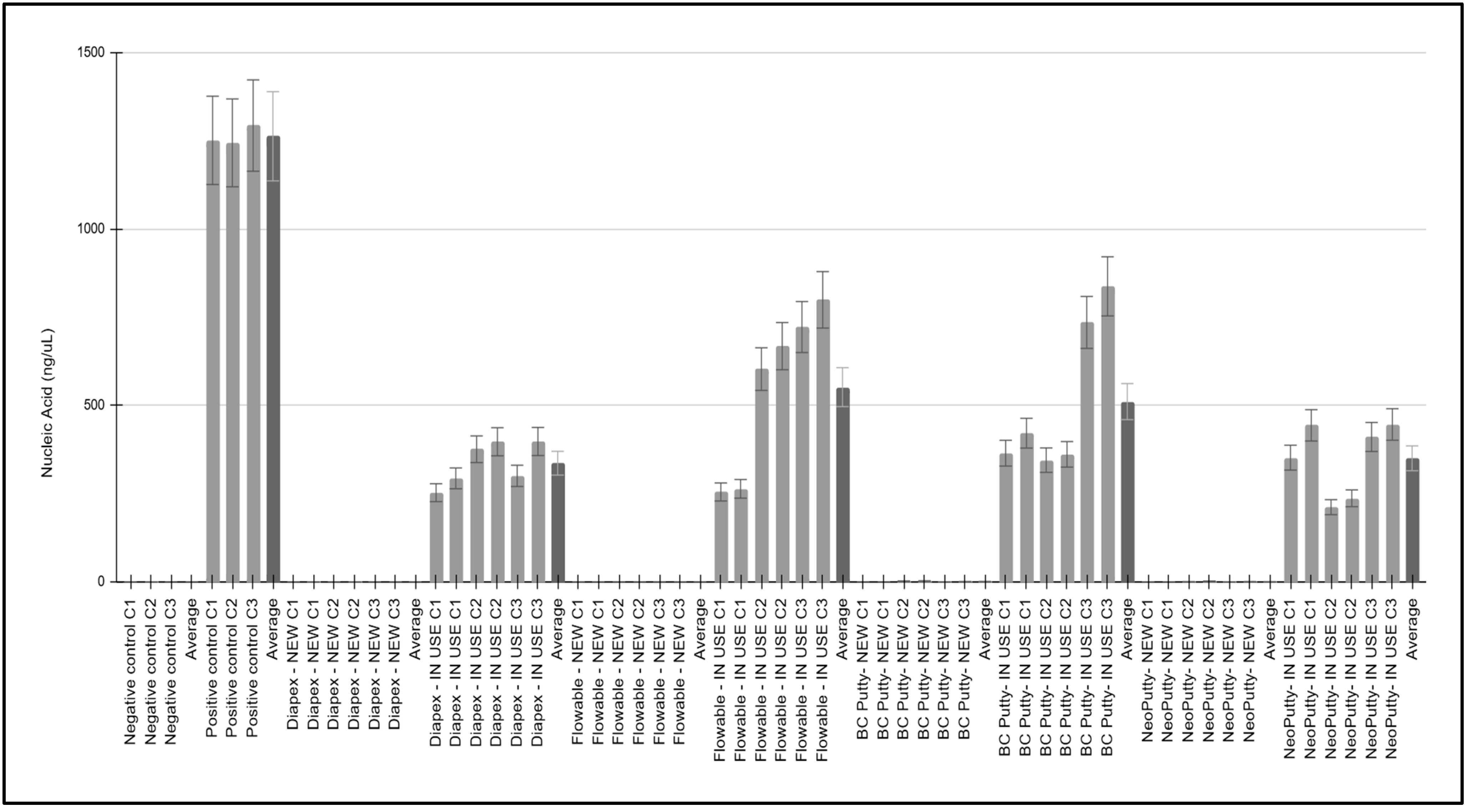
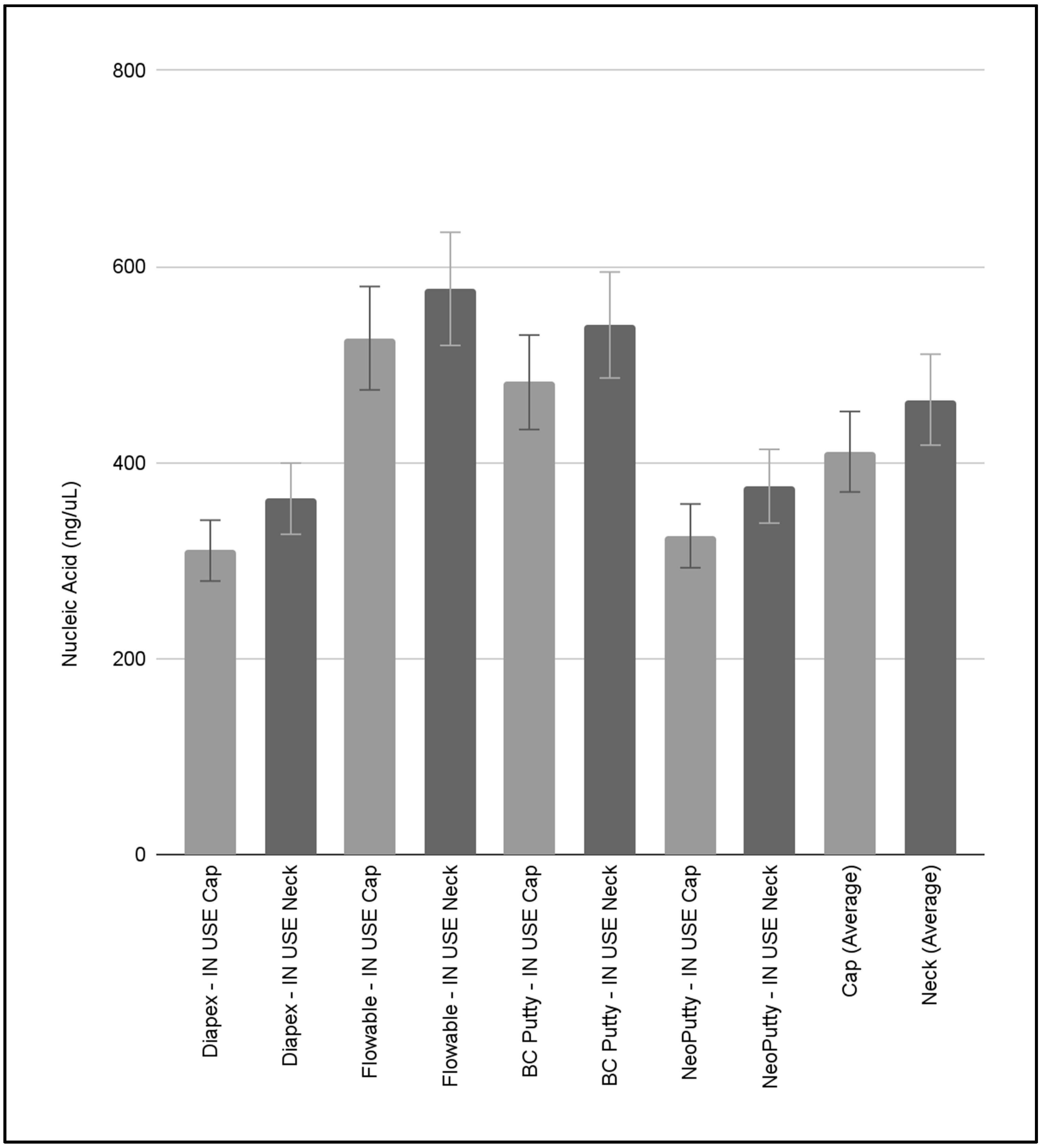
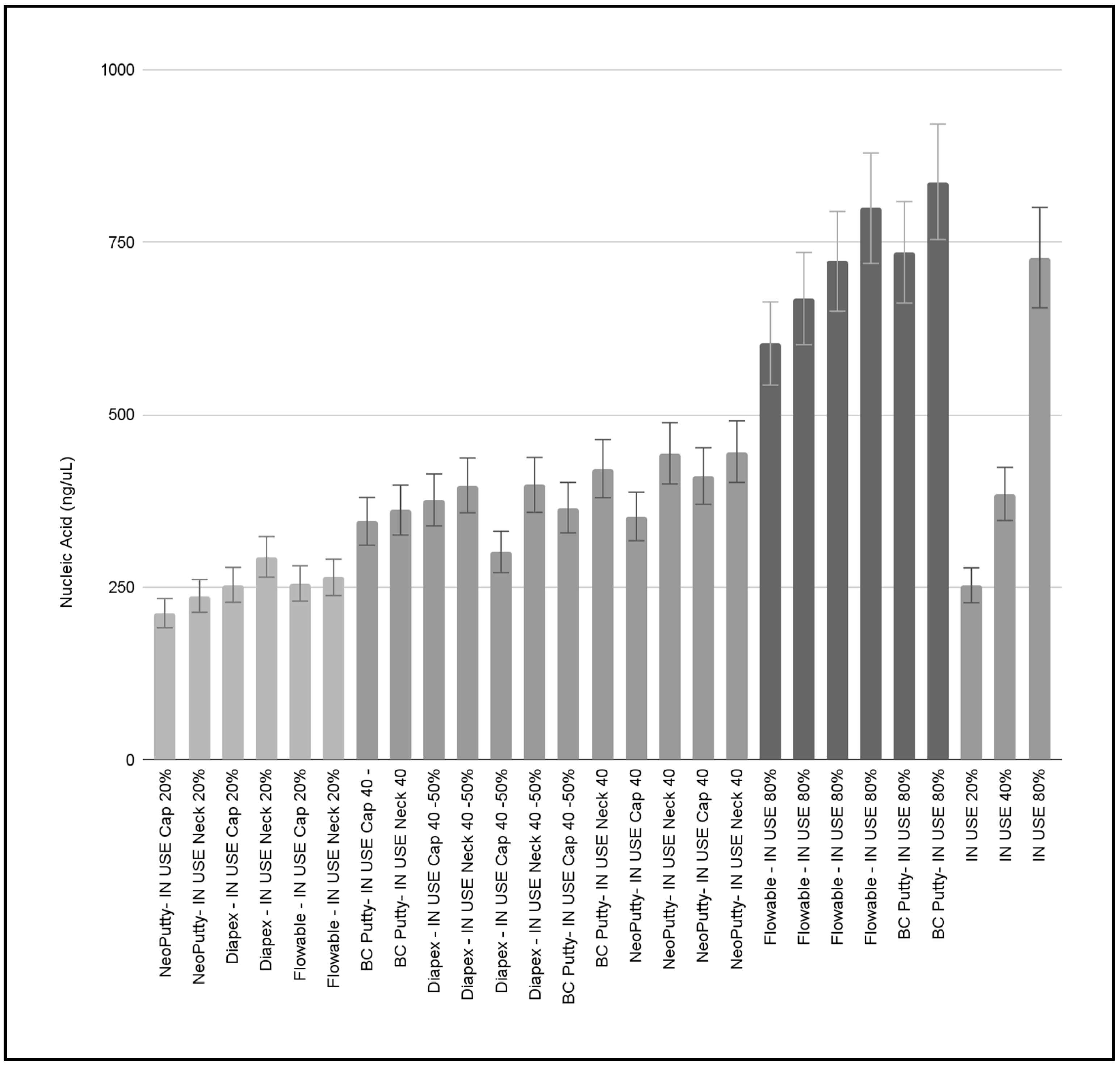
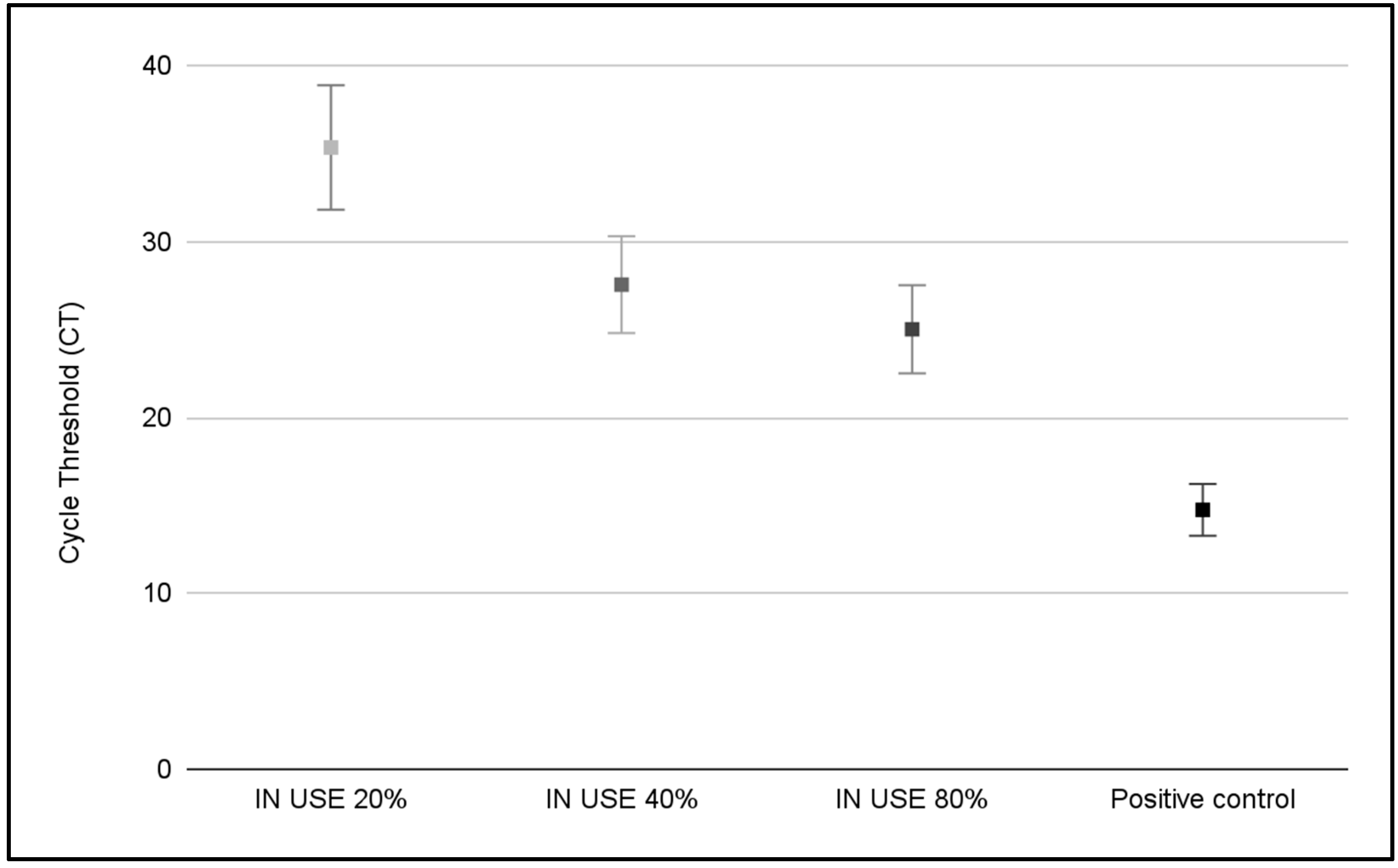
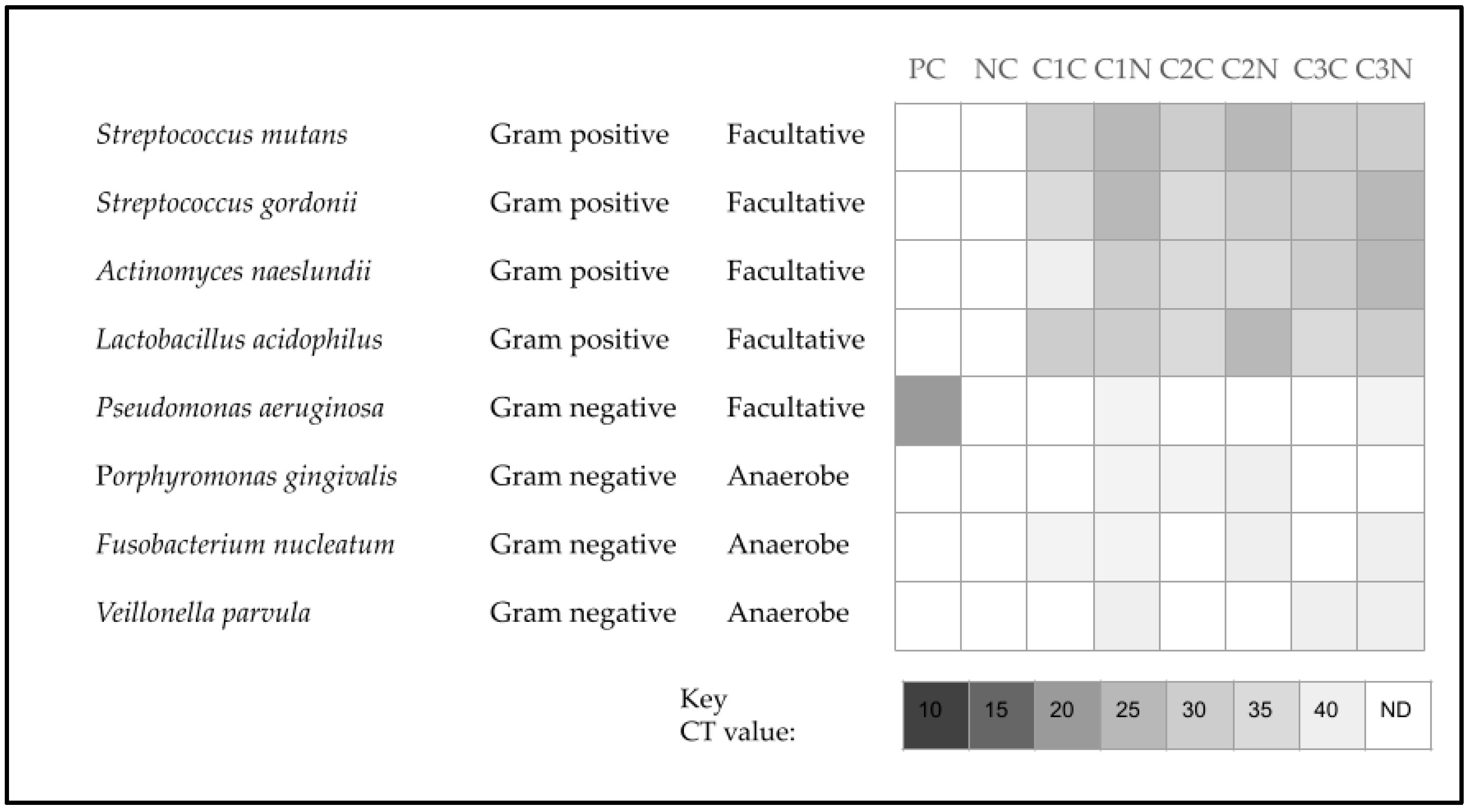
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| qPCR | Quantitative polymerase chain reaction |
| LB | Luria–Bertani |
| ATCC | American Type Culture Collection |
| RT-PCR | Real-Time Polymerase Chain Reaction |
Appendix A
| Sample, Collection | Location | Estimated Use | Nucleic Acid (ng/uL) | A260/A280 Ratio |
|---|---|---|---|---|
| Negative controls (C1, C2, C3) | N/A | Not applicable | 0.568, 0.981, 0.126 | 1.62, 1.54, 1.56 |
| ave = 0.558 ± 0.43 | ave = 1.58 ± 0.04 | |||
| Positive controls (C1, C2, C3) | N/A | Not applicable | 1251.077, 1244.016, 1293.019 | 1.73, 1.53, 1.69 |
| ave = 1262.704 ± 25.49 | ave = 1.65 ± 0.10 | |||
| Diapex—NEW C1 | Cap, Neck | Estimated use: 0% | 0.716, 0.993 | 1.60, 1.67 |
| Diapex—NEW C2 | Cap, Neck | Estimated use: 0% | 0.615, 0.939 | 1.85, 1.65 |
| Diapex—NEW C3 | Cap, Neck | Estimated use: 0% | 0.558, 0.699 | 1.71, 1.61 |
| ave = 0.753 ± 0.18 | ave = 1.68 ± 0.09 | |||
| Diapex—IN USE C1 | Cap, Neck | Estimated use: 20% | 253.36, 293.94 | 1.58, 1.57 |
| Diapex—IN USE C2 | Cap, Neck | Estimated use: 45% | 376.45, 397.56 | 1.66, 1.65 |
| Diapex—IN USE C3 | Cap, Neck | Estimated use: 50% | 300.99, 398.27 | 1.62, 1.62 |
| ave = 336.76 ± 61.85 | ave = 1.62 ± 0.04 | |||
| Flowable—NEW C1 | Cap, Neck | Estimated use: 0% | 0.851, 0.521 | 1.62, 1.64 |
| Flowable—NEW C2 | Cap, Neck | Estimated use: 0% | 0.313, 0.421 | 1.75, 1.76 |
| Flowable—NEW C3 | Cap, Neck | Estimated use: 0% | 0.385, 0.675 | 1.64, 1.65 |
| ave = 0.5276 ± 0.20 | ave = 1.67 ± 0.06 | |||
| Flowable—IN USE C1 | Cap, Neck | Estimated use: 20% | 255.358, 264.22 | 1.94, 1.76 |
| Flowable—IN USE C2 | Cap, Neck | Estimated use: 80% | 603.275, 668.304 | 1.69, 1.59 |
| Flowable—IN USE C3 | Cap, Neck | Estimated use: 80% | 722.407, 799.445 | 1.76, 1.63 |
| ave = 552.168 ± 235.47 | ave = 1.73 ± 0.13 | |||
| BC Putty—NEW C1 | Cap, Neck | Estimated use: 0% | 0.246, 0.431 | 1.47, 1.48 |
| BC Putty—NEW C2 | Cap, Neck | Estimated use: 0% | 3.454, 3.764 | 1.65, 1.49 |
| BC Putty—NEW C3 | Cap, Neck | Estimated use: 0% | 1.029, 1.656 | 1.48, 1.66 |
| ave = 1.763 ± 1.52 | ave = 1.54 ± 0.09 | |||
| BC Putty—IN USE C1 | Cap, Neck | Estimated use: 50% | 365.218, 421.81 | 1.70, 1.56 |
| BC Putty—IN USE C2 | Cap, Neck | Estimated use: 50% | 345.515, 361.863 | 1.64, 1.44 |
| BC Putty—IN USE C3 | Cap, Neck | Estimated use: 80% | 735.484, 837.633 | 1.57, 1.55 |
| ave = 511.253 ± 217.11 | ave = 1.58 ± 0.09 | |||
| NeoPutty—NEW C1 | Cap, Neck | Estimated use: 0% | 0.908, 0.565 | 1.57, 1.54 |
| NeoPutty—NEW C2 | Cap, Neck | Estimated use: 0% | 1.516, 2.968 | 1.50, 1.79 |
| NeoPutty—NEW C3 | Cap, Neck | Estimated use: 0% | 0.921, 1.638 | 1.59, 1.62 |
| ave = 1.419 ± 0.86 | ave = 1.60 ± 0.07 | |||
| NeoPutty—IN USE C1 | Cap, Neck | Estimated use: 40% | 352.523, 444.019 | 1.75, 1.81 |
| NeoPutty—IN USE C2 | Cap, Neck | Estimated use: 20% | 212.296, 237.404 | 1.69, 1.99 |
| NeoPutty—IN USE C3 | Cap, Neck | Estimated use: 50% | 411.098, 446.499 | 1.68, 1.84 |
| ave = 350.639 ± 103.47 | ave = 1.79 ± 0.11 |
References
- Byrne, D.; Saget, S.; Davidson, A.; Haneef, H.; Abdeldaim, T.; Almudahkah, A.; Basquille, N.; Bergin, A.M.; Prida, J.; Lyne, A.; et al. Comparing the environmental impact of reusable and disposable dental examination kits: A life cycle assessment approach. Br. Dent. J. 2022, 233, 317–325. [Google Scholar] [CrossRef]
- Paczkowski, I.; Stingu, C.S.; Hahnel, S.; Rauch, A.; Schierz, O. Cross-Contamination Risk of Dental Tray Adhesives: An In Vitro Study. Materials 2021, 14, 6138. [Google Scholar] [CrossRef]
- Naidu, S.; Dhawan, P.; Raman, S. Improving the Accuracy of Dental Impressions: A Study of Tray Adjustments and Materials. Eur. J. Prosthodont. Restor. Dent. 2025, 33, 49–56. [Google Scholar] [CrossRef]
- Patil, R.V.; Vijayraghavan, V.; Jadhav, M.; Jajoo, S.; Desai, S.; Jagtap, C. Comparison of Tensile Bond Strength of Addition Silicone with Different Custom Tray Materials Using Different Retentive Methods. J. Contemp. Dent. Pract. 2021, 22, 278–283. [Google Scholar]
- Schierz, O.; Müller, H.; Stingu, C.S.; Hahnel, S.; Rauch, A. Dental tray adhesives and their role as potential transmission medium for microorganisms. Clin. Exp. Dent. Res. 2021, 7, 829–832. [Google Scholar] [CrossRef]
- Chukwu, S.; Munn, A.; Wilson, J.C.; Ibrahim, H.; Gosling, D.; Love, R.M.; Bakr, M.M. Efficacy of an impression disinfectant solution after repeated use: An in vitro study. Heliyon 2023, 10, e23792. [Google Scholar] [CrossRef] [PubMed]
- Cruz Hondares, T.; Hao, X.; Zhao, Y.; Lin, Y.; Napierala, D.; Jackson, J.G.; Zhang, P. Antibacterial, biocompatible, and mineralization-inducing properties of calcium silicate-based cements. Int. J. Paediatr. Dent. 2024, 34, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Pelepenko, L.E.; Saavedra, F.; Antunes, T.B.M.; Bombarda, G.F.; Gomes, B.P.F.A.; Zaia, A.A.; Camilleri, J.; Marciano, M.A. Physicochemical, antimicrobial, and biological properties of White-MTAFlow. Clin. Oral Investig. 2021, 25, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, I.M.; Ahmed, S.A. Effect of microwave disinfection on mechanical properties of denture base acrylic resin. J. Mech. Behav. Biomed. Mater. 2010, 3, 480–487. [Google Scholar] [CrossRef]
- AlSaiari, A.K.A.; Alonazi, M.S.; Alotaibi, N.M.; AlQahtani, H.; Alotaibi, W.M.; Yhyha, A.S.S.A.; Aldarorah, A.; Alaklabi, N.M.; Al-Obayli, T.A.S.; Alsaud, O.A.S.; et al. Bioengineering Innovations in Global Dental Infection Control: Applications and Adaptations in Clinical Settings. Int. Dent. J. 2025, 75, 2222–2238. [Google Scholar] [CrossRef]
- Nóbrega, M.T.C.; Bastos, R.T.D.R.M.; Mecenas, P.; de Toledo, I.P.; Richardson-Lozano, R.; Altabtbaei, K.; Flores-Mir, C. Aerosol generated by dental procedures: A scoping review. J. Evid. Based Med. 2021, 14, 303–312. [Google Scholar] [CrossRef]
- Innes, N.; Johnson, I.G.; Al-Yaseen, W.; Harris, R.; Jones, R.; Kc, S.; McGregor, S.; Robertson, M.; Wade, W.G.; Gallagher, J.E. A systematic review of droplet and aerosol generation in dentistry. J. Dent. 2021, 105, 103556. [Google Scholar] [CrossRef]
- Kumar, P.S.; Subramanian, K. Demystifying the mist: Sources of microbial bioload in dental aerosols. J. Periodontol. 2020, 91, 1113–1122. [Google Scholar] [CrossRef]
- Zemouri, C.; de Soet, H.; Crielaard, W.; Laheij, A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS ONE 2017, 12, e0178007. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, A.; Proaño, D.; Kaur, H.; Singhal, S. Aerosol-generating procedures and associated control/mitigation measures: Position paper from the Canadian Dental Hygienists Association and the American Dental Hygienists’ Association. Can. J. Dent. Hyg. 2024, 58, 48–63. [Google Scholar] [PubMed]
- Hung, C.C.U.; Costa, R.C.; Pereira, G.; Abdo, V.L.; Noronha, M.D.S.; Retamal-Valdes, B.; Bertolini, M.; Feres, M.; Shibli, J.A.; Barão, V.A.R.; et al. Oral microbial colonization on titanium and polyetheretherketone dental implant healing abutments: An in vitro and in vivo study. J. Prosthet. Dent. 2023, 133, 1545–1552. [Google Scholar] [CrossRef]
- Frenzel, N.; Maenz, S.; Sanz Beltrán, V.; Völpel, A.; Heyder, M.; Sigusch, B.W.; Lüdecke, C.; Jandt, K.D. Template assisted surface microstructuring of flowable dental composites and its effect on microbial adhesion properties. Dent. Mater. 2016, 32, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.; Barbosa, J.M.; Jardim, L.; Carrilho, E.; Portugal, J. COVID-19 Management in Clinical Dental Care. Part I: Epidemiology, Public Health Implications, and Risk Assessment. Int. Dent. J. 2021, 71, 251–262. [Google Scholar] [CrossRef]
- Dolev, E.; Eli, I.; Mashkit, E.; Grinberg, N.; Emodi-Perlman, A. Fluorescent Marker as a Tool to Improve Strategies to Control Contaminated Surfaces and Decrease Danger of Cross-Contamination in Dental Clinics, during and beyond the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2023, 20, 5229. [Google Scholar] [CrossRef]
- Miguita, L.; Martins-Chaves, R.R.; Geddes, V.E.V.; Mendes, S.D.R.; Costa, S.F.D.S.; Fonseca, P.L.C.; Menezes, D.; de Souza, R.M.; Queiroz, D.C.; Alves, H.J.; et al. Biosafety in Dental Health Care During the COVID-19 Pandemic: A Longitudinal Study. Front. Oral Health 2022, 3, 871107. [Google Scholar] [CrossRef]
- Emett, J.; David, R.; McDaniel, J.; McDaniel, S.; Kingsley, K. Comparison of DNA Extracted from Pediatric Saliva, Gingival Crevicular Fluid and Site-Specific Biofilm Samples. Methods Protoc. 2020, 3, 48. [Google Scholar] [CrossRef]
- Pearson, M.; Stewart, S.; Ma, L.; Kingsley, K.; Sullivan, V. Differential Antimicrobial Effects of Endodontic Irrigant Endocyn on Oral Bacteria. Hygiene 2025, 5, 11. [Google Scholar] [CrossRef]
- Wilson, J.; Swanbeck, S.; Banning, G.; Alhwayek, T.; Sullivan, V.; Howard, K.M.; Kingsley, K. Assessment of Sodium Diamine Fluoride (SDF) with Light Curing Technique: A Pilot Study of Antimicrobial Effects. Methods Protoc. 2022, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Gamble, T.; Wilkerson, C.; Kim, C.; Kingsley, K.; Sullivan, V. Assessment of Trends in Non-Restorative and Preventative Dental Treatment Pre- and Post-COVID-19: A Health Informatics Pilot Study. Children 2025, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Marín, N.; Villa García, L.D.; Aguirre López, E.C.; Medina-Solís, C.E.; Martínez Zumarán, A.; Martínez Rider, R.; Márquez Preciado, R.; Rosales García, P.; Salas Orozco, M.F. Sterilization and Disinfection: Ensuring Infection Control in Dental Practices. Cureus 2025, 17, e79041. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.; Li, H.; Ren, Z.; Zhu, R.; Zhu, Z. Recent advances in sterilization and disinfection technology: A review. Chemosphere 2022, 308 Pt 3, 136404. [Google Scholar] [CrossRef]
- Klumdeth, J.; Jantaratnotai, N.; Thaweboon, S.; Pachimsawat, P. Sterility maintenance of reused disposable paper/plastic sterilization pouches in actual clinical practice. Heliyon 2020, 6, e03672. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Jayanthi; Das, U.M.; Bhatnagar, S. Comparison of the effectiveness of sterilizing endodontic files by 4 different methods: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2010, 28, 2–5. [Google Scholar] [CrossRef]
- Brannan, D.K.; Dille, J.C. Type of closure prevents microbial contamination of cosmetics during consumer use. Appl. Environ. Microbiol. 1990, 56, 1476–1479. [Google Scholar] [CrossRef]
- Coad, C.T.; Osato, M.S.; Wilhelmus, K.R. Bacterial contamination of eyedrop dispensers. Am. J. Ophthalmol. 1984, 98, 548–551. [Google Scholar] [CrossRef]
- Høvding, G.; Sjursen, H. Bacterial contamination of drops and dropper tips of in-use multidose eye drop bottles. Acta Ophthalmol. 1982, 60, 213–222. [Google Scholar] [CrossRef]
- Franz, J.; Scheier, T.C.; Aerni, M.; Gubler, A.; Schreiber, P.W.; Brugger, S.D.; Schmidlin, P.R. Bacterial contamination of air and surfaces during dental procedures-An experimental pilot study using Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 2024, 45, 658–663. [Google Scholar] [CrossRef]
- Wang, B.Y.; Henrichs, L.E.; Arricale, K.; Lien, W.; Savett, D.A.; Vandewalle, K.S. Efficacy of a chairside extraoral suction system in the reduction of aerosol contamination. Gen. Dent. 2023, 71, 16–21. [Google Scholar]
- Kumbargere Nagraj, S.; Eachempati, P.; Paisi, M.; Nasser, M.; Sivaramakrishnan, G.; Verbeek, J.H. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database Syst. Rev. 2020, 10, CD013686. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Di Spirito, F.; D’Ambrosio, F.; De Caro, F.; Pecora, D.; Giorgio, R.; Fortino, L.; Longanella, W.; Franci, G.; Santella, B.; et al. Microbial Air Contamination in a Dental Setting Environment and Ultrasonic Scaling in Periodontally Healthy Subjects: An Observational Study. Int. J. Environ. Res. Public Health 2023, 20, 2710. [Google Scholar] [CrossRef] [PubMed]
- Triggiano, F.; Veschetti, E.; Veneri, F.; Montagna, M.T.; De Giglio, O. Best practices for disinfection in dental settings: Insights from Italian and European regulations. J. Ann. Ig. 2025, 37, 292–301. [Google Scholar] [CrossRef]
- Barreiros, P.; Braga, J.; Faria-Almeida, R.; Coelho, C.; Teughels, W.; Souza, J.C.M. Remnant oral biofilm and microorganisms after autoclaving sterilization of retrieved healing abutments. J. Periodontal Res. 2021, 56, 415–422. [Google Scholar] [CrossRef]
- Sharon, E.; Pietrokovski, Y.; Engel, I.; Assali, R.; Houri-Haddad, Y.; Beyth, N. Biocompatibility, Surface Morphology, and Bacterial Load of Dental Implant Abutments following Decontamination Protocols: An In-Vitro Study. Materials 2023, 16, 4080. [Google Scholar] [CrossRef]
- Gul, M.; Zafar, K.; Ghafoor, R.; Khan, F.R. Frequency of Contamination on Used Healing Abutments after Sterilization: An In Vitro Study. Int. J. Prosthodont. 2024, 37, 109. [Google Scholar] [CrossRef]
- Hardy, A.; Sabatier, V.; Rosello, O.; Salauze, B.; Barbut, F.; Vialle, R. More than just teddy bears: Unconventional transmission agents in the operating room. Arch. Pediatr. 2018, 25, 416–420. [Google Scholar] [CrossRef]
- Held, M.; Mignemi, M.; O’Rear, L.; Wise, M.; Zane, G.; Murphy Zane, M.S.; Schoenecker, J.G. Stuffed Animals in the Operating Room: A Reservoir of Bacteria with a Simple Solution. J. Pediatr. Orthop. 2015, 35, e110–e112. [Google Scholar] [CrossRef]
- Marui, V.C.; Souto, M.L.S.; Rovai, E.S.; Romito, G.A.; Chambrone, L.; Pannuti, C.M. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: A systematic review. J. Am. Dent. Assoc. 2019, 150, 1015–1026.e1. [Google Scholar] [CrossRef] [PubMed]
- Shayegh, M.; Sorenson, C.; Downey, J.; Lin, S.; Jiang, Y.; Sodhi, P.; Sullivan, V.; Howard, K.M.; Kingsley, K. Assessment of SARS-CoV-2 (COVID-19) Clinical Mouthwash Protocol and Prevalence of the Oral Pathogen Scardovia wiggsiae: A Pilot Study of Antibacterial Effects. Methods Protoc. 2023, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, P.; Jiang, Y.; Lin, S.; Downey, J.; Sorenson, C.; Shayegh, M.; Sullivan, V.; Kingsley, K.; Howard, K.M. Administration of Clinical COVID-19 Mouthwashing Protocol and Potential Modulation of Pediatric Oral Bacterial Prevalence of Selenomonas noxia: A Pilot Study. Pediatr. Rep. 2023, 15, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.S.; Binduhayyim, R.I.H.; Mosaddad, S.A.; Heboyan, A. Strategies for preventing aerosol-generated microbial contamination in dental procedures: A systematic review and meta-analysis. Am. J. Infect. Control. 2025, 53, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Engsomboon, N.; Pachimsawat, P.; Thanathornwong, B. Comparative Dissemination of Aerosol and Splatter Using Suction Device during Ultrasonic Scaling: A Pilot Study. Dent. J. 2022, 10, 142. [Google Scholar] [CrossRef]
- Puljich, A.; Jiao, K.; Lee, R.S.B.; Walsh, L.J.; Ivanovski, S.; Han, P. Simulated and clinical aerosol spread in common periodontal aerosol-generating procedures. Clin. Oral Investig. 2022, 26, 5751–5762. [Google Scholar] [CrossRef]
- Melzow, F.; Mertens, S.; Todorov, H.; Groneberg, D.A.; Paris, S.; Gerber, A. Aerosol exposure of staff during dental treatments: A model study. BMC Oral Health 2022, 22, 128. [Google Scholar] [CrossRef]
- Yang, M.; Chaghtai, A.; Melendez, M.; Hasson, H.; Whitaker, E.; Badi, M.; Sperrazza, L.; Godel, J.; Yesilsoy, C.; Tellez, M.; et al. Mitigating saliva aerosol contamination in a dental school clinic. BMC Oral Health 2021, 21, 52. [Google Scholar] [CrossRef]
- Gonçalves Lomardo, P.; Nunes, M.C.; Arriaga, P.; Antunes, L.A.; Machado, A.; Quinelato, V.; Aguiar, T.R.D.S.; Casado, P.L. Concern about the risk of aerosol contamination from ultrasonic scaler: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 417. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J.; Koo, H. Oral polymicrobial communities: Assembly, function, and impact on diseases. Cell Host Microbe 2023, 31, 528–538. [Google Scholar] [CrossRef]
- Lamont, R.J.; Hajishengallis, G.; Koo, H. Social networking at the microbiome-host interface. Infect. Immun. 2023, 91, e0012423. [Google Scholar] [CrossRef]
- Pittet, L.F.; Posfay-Barbe, K.M. Vaccination of immune compromised children-an overview for physicians. Eur. J. Pediatr. 2021, 180, 2035–2047. [Google Scholar] [CrossRef]
- Aranda, C.S.; Gouveia-Pereira, M.P.; da Silva, C.J.M.; Rizzo, M.C.F.V.; Ishizuka, E.; de Oliveira, E.B.; Condino-Neto, A. Severe combined immunodeficiency diagnosis and genetic defects. Immunol. Rev. 2024, 322, 138–147. [Google Scholar] [CrossRef]
- Le, T.T.; Hoang, T.N.; Do, D.H.; Nguyen, X.H.; Huynh, C.; Viet, H.D.; Dat, V.Q.; Zengler, K.; Gilbert, J.A.; Avedissian, S.N.; et al. Current state of microbiota clinical applications in neonatal and pediatric bacterial infections. Gut Microbes 2025, 17, 2529400. [Google Scholar] [CrossRef]
- Westergard, E.J.; Romito, L.M.; Kowolik, M.J.; Palenik, C.J. Controlling bacterial contamination of dental impression guns. J. Am. Dent. Assoc. 2011, 142, 1269–1274. [Google Scholar] [CrossRef]
- Rice, C.D.; Dykstra, M.A.; Gier, R.E. Bacterial contamination in irreversible hydrocolloid impression material and gingival retraction cord. J. Prosthet. Dent. 1991, 65, 496–499. [Google Scholar] [CrossRef] [PubMed]
| Sample, Collection | Location | Estimated Use | Nucleic Acid (ng/uL) | A260/A280 Ratio |
|---|---|---|---|---|
| Negative controls (C1, C2, C3) | N/A | Not applicable | ave = 0.56 ± 0.43 | ave = 1.58 ± 0.04 |
| Positive controls (C1, C2, C3) | N/A | Not applicable | ave = 1262.70 ± 25.49 | ave = 1.65 ± 0.10 |
| Diapex—NEW C1,C2,C3 | Cap, Neck | Estimated use: 0% | ave = 0.75 ± 0.18 | ave = 1.68± 0.09 |
| Diapex—IN USE C1,C2,C3 | Cap, Neck | Estimated use: 20%, 45%, 50% | ave = 336.76 ± 61.85 | ave = 1.62 ± 0.04 |
| Flowable—NEW C1,C2,C3 | Cap, Neck | Estimated use: 0% | ave = 0.53 ± 0.20 | ave = 1.67 ± 0.06 |
| Flowable—IN USE C1,C2,C3 | Cap, Neck | Estimated use: 20%, 80%, 80% | ave = 552.17 ± 235.47 | ave = 1.73 ± 0.13 |
| BC Putty- NEW C1,C2,C3 | Cap, Neck | Estimated use: 0% | ave = 1.76 ± 1.52 | ave = 1.54 ± 0.09 |
| BC Putty- IN USE C1,C2,C3 | Cap, Neck | Estimated use: 50%, 50%, 80% | ave = 511.25 ± 217.11 | ave = 1.58 ± 0.09 |
| NeoPutty- NEW C1,C2,C3 | Cap, Neck | Estimated use: 0% | ave = 1.42 ± 0.86 | ave = 1.60 ± 0.07 |
| NeoPutty- IN USE C1,C2,C3 | Cap, Neck | Estimated use: 40%, 20%, 50% | ave = 350.64 ± 103.47 | ave = 1.79 ± 0.11 |
| Sample | Turbidity | Range | Statistical Analysis |
|---|---|---|---|
| Negative controls | 0.21 ± 0.01 | 0.20 to 0.23 | Two-tailed t-test |
| NEW samples | 0.23 ± 0.01 | 0.21 to 0.23 | p = 0.411 |
| IN USE samples | 0.32 ± 0.05 | 0.26 to 0.37 | p = 0.012 |
| Positive controls | 0.49 ± 0.01 | 0.49 to 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banning, G.; Kim, C.; Wilkerson, C.; Williams, S.J.; Kingsley, K.; Sullivan, V. Assessment of Bacterial Presence Among New and “In Use” Resealable Biomaterials Within the Pediatric Dental Clinic. Hygiene 2025, 5, 42. https://doi.org/10.3390/hygiene5030042
Banning G, Kim C, Wilkerson C, Williams SJ, Kingsley K, Sullivan V. Assessment of Bacterial Presence Among New and “In Use” Resealable Biomaterials Within the Pediatric Dental Clinic. Hygiene. 2025; 5(3):42. https://doi.org/10.3390/hygiene5030042
Chicago/Turabian StyleBanning, Gavin, Cindy Kim, Carter Wilkerson, Shelley J. Williams, Karl Kingsley, and Victoria Sullivan. 2025. "Assessment of Bacterial Presence Among New and “In Use” Resealable Biomaterials Within the Pediatric Dental Clinic" Hygiene 5, no. 3: 42. https://doi.org/10.3390/hygiene5030042
APA StyleBanning, G., Kim, C., Wilkerson, C., Williams, S. J., Kingsley, K., & Sullivan, V. (2025). Assessment of Bacterial Presence Among New and “In Use” Resealable Biomaterials Within the Pediatric Dental Clinic. Hygiene, 5(3), 42. https://doi.org/10.3390/hygiene5030042






