Abstract
Breast milk is vital for infant survival, protecting against infections and strengthening the immune system. In addition to nutrients, breast milk contains beneficial microorganisms, antimicrobial peptides and proteins (APPs), including lactoferrin and lysozyme, and peptides such as defensins and cathelicidins that destroy harmful bacteria and regulate the neonatal immune response. Breast milk also promotes the growth of beneficial gut bacteria (Bacteroidaceae and Bifidobacteriaceae) while reducing harmful pathogens, fostering a healthy gut microbiome, and supporting long-term infant health. Traditionally, research on antimicrobial proteins and milk microbiota has been conducted in isolation. However, at the molecular level, these components do not function independently; they interact synergistically, influencing immunomodulation, inflammation, and the composition of the gut microbiome. Therefore, this review aims to provide an overview of the discovery and identification of APPs in breast milk, the dynamic relationship between the breast milk microbiota, and the potentiation of artificial feeding with supplemented formulas when breastfeeding is impossible, benefits on newborn immune systems, and even the benefits to breast tissue.
1. Introduction
Breast milk is crucial for infant survival because it protects against infections and even reduces the risk of developing acute leukemia in infancy by 23%. Breastfeeding reduces neonatal mortality, particularly in low-income countries [1,2,3]. Even in high-income countries, it reduces the risk of respiratory disease and diarrhea, while supporting immune development [4,5,6]. It provides essential nutrients, cells, biomolecules and beneficial microorganisms through the vertical transmission of bacteria from mother to baby, including Bifidobacterium and Lactobacillus. It establishes the infant’s gut microbiome [7,8]. Approximately 22.89% of the bacteria in breast milk originate from the maternal gut, contributing to 25.64% of the infant’s gut microbiota [9,10]. The milk microbiome includes bacterial extracellular vehicles (EVs), dominated by Bacteroides, Acinetobacter, and Lactobacillaceae, which may mediate bacterial communication and modulate infant immunity [11].
The microbiome is also regulated by human glycosylated immunoprotective proteins (HMGPs), including lactoferrin and secretory immunoglobulin A (SIgA), which act as soluble receptor mimetics, preventing pathogen adhesion and fostering the growth of beneficial bacteria, and generate anti-inflammatory metabolites, such as short-chain fatty acids [12,13,14,15]. As prebiotics, human milk oligosaccharides (HMOs) and HMGPs promote the growth of Bifidobacterium species, conferring anti-infective and immunomodulatory properties that have driven the supplementation of infant formula with HMOs [16]. Certain HMOs, such as 3’-sialyl lactose (3’SL), exert epigenetic effects by inhibiting histone deacetylases (HDACs), enhancing the transcription of anti-inflammatory genes, and modulating host–microbe interactions, contributing to protection against chronic diseases like atherosclerosis [17]. These molecules in breast milk have a selective regulatory function on microbes. SIgA exhibits selective specificity, neutralizing pathogens while preserving commensal bacteria, with heterogeneity in reactivity towards the infant gut microbiota to maintain microbiota balance [18,19]. Additionally, the complement system in breast milk selectively eliminates Gram-positive bacteria, like Staphylococcus lentus, through the membrane attack complex (MAC), further contributing to microbiota homeostasis [20].
Moreover, breast milk provides antioxidant protection through molecules such as glutathione, melatonin, superoxide dismutase, catalase, and vitamins A, C, and E. These antioxidants neutralize reactive oxygen species, preserving cellular integrity during rapid growth, particularly in preterm and low-birth-weight infants [17]. Human milk also contains metabolic hormones, including leptin, insulin, ghrelin, adiponectin, and resistin, which regulate energy balance, appetite, adipogenesis, and modulate immune responses and inflammation, linking early nutrition to long-term metabolic health. Notably, leptin has been associated with a reduced risk of obesity in breastfed infants [21]. In addition to these factors, human milk contains microRNAs (miRNAs) encapsulated in extracellular vesicles, which remain stable and bioavailable in the infant gut. These miRNAs can be internalized by epithelial and immune cells, regulating gene expression related to immune maturation, gut barrier function, and metabolic programming, adding an important epigenetic layer of maternal–infant communication with long-term health implications [22].
This review explores the relationship between other important immune molecules, the antimicrobial peptides and proteins (APPs), and the infant microbiome, emphasizing the balance between APPs and beneficial microbiota and the modulation of your immune system. Antimicrobial peptides and proteins (APPs) are small molecules (12–50 amino acids) or polypeptides (>50 amino acids) with a net positive charge and variable sequences, structure, diversity, and specificity. Structure enables integration into anionic pathogen membranes, forming lytic pores and developing functions such as immunomodulation [23,24,25].
Among the multiple bioactive components of breast milk, antimicrobial proteins such as lactoferrin show remarkable functional and individual variability. Lactoferrin stands out among the antimicrobial proteins in breast milk, whose concentration and activity can vary individually depending on maternal factors such as health status, genetics, and lactation stage. It exhibits broad antimicrobial and immunomodulatory functions [26,27,28], with the potential to prevent and treat perinatal complications such as neonatal sepsis and necrotizing enterocolitis. Lactoferrin is effective against pathogens like Streptococcus agalactiae, Escherichia coli, and Acinetobacter baumannii. It reduces group B Streptococcus (GBS) biofilm formation and adhesion, highlighting its role in preventing GBS perinatal infections [29,30]. Given the complexity of neonatal outcomes, it is essential to consider that sample size critically influences the validity of findings; larger cohorts strengthen the reliability of associations between breast milk components and neonatal health by minimizing bias and improving generalizability [31]. Emerging evidence indicates that maternal lifestyle factors—such as body mass index, diet quality, physical activity, and smoking—significantly influence breast milk composition, potentially impacting infant immunity, neurodevelopment, and long-term health outcomes [32].
Breast milk has antimicrobial peptides made either by breaking down proteins or synthesized directly [33], like defensins, cathelicidin, and peptides derived from human and bovine milk proteins. Studies have identified 6645 peptides, some inhibiting Staphylococcus aureus and Escherichia coli growth, while others support Bifidobacterium infantis proliferation [34]. Therefore, this review aims to provide an overview of the discovery and identification of APPs in breast milk, the dynamic relationship between the breast milk microbiota and the potentiation of artificial feeding with supplemented formulas when breastfeeding is impossible, benefits on newborn immune systems, and even the benefits to breast tissue.
2. Antimicrobial Proteins in Human Breast Milk
Breast milk provides essential nutrients and contains a complex array of antimicrobial proteins and peptides that protect the infant from infection [35]. These components are illustrated in Figure 1. Lactoferrin (LF) was the first antimicrobial protein identified in breast milk. This iron-affinity protein limits the availability of iron to pathogens. As a glycoprotein, LF can release and re-bind iron and undergoes glycosylation, particularly fucosylation, to optimize its protective function against pathogens [36,37,38]. LF possesses an antimicrobial domain at the N-terminus that is distinct from its iron-binding sites. This domain consists of an 18 amino acid loop stabilized by a disulfide bond between cysteine residues 20 and 37, demonstrating excellent efficacy against both Gram-negative and -positive bacteria by disrupting bacterial membrane permeability [39]. In human milk, lactoferrin levels significantly decrease during lactation, from 6.7 ± 0.7 g/L in colostrum (1–3 days postpartum) to 3.7 ± 0.1 g/L in transitional milk (4–7 days), and further to 2.6 ± 0.4 g/L in mature milk (20–60 days), which confirms the benefits of early breastfeeding [40].
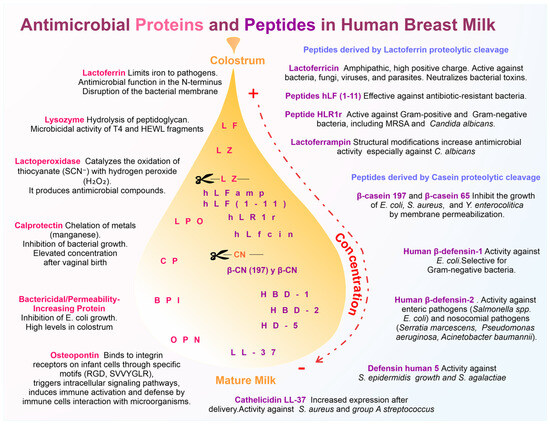
Figure 1.
Schematic representation of the antimicrobial proteins and peptides in breast milk and their role in protection. On the left are shown the major antimicrobial proteins, including lactoferrin (LF), lysozyme (LZ), lactoperoxidase (LPO), calprotectin (CP), bactericidal/permeability-increasing protein (BPI), and osteopontin (OPN), and along with their mechanisms of action. On the right, proteolytic cleavage-derived antimicrobial peptides, such as lactoferricin (Lfcin), peptides Hlf (1-11), HLR1r, and human lactoferrampin (LFampH), peptide derivates from casein (CN) proteolytic cleavage as well as non-cleavage-derived antimicrobial peptides, such as cathelicidin LL-37 and human defensins (HBD-1 and HBD-2), human defensin 5 (HD-5), and its antimicrobial specificity are highlighted. The transition from colostrum to mature milk is represented by a color gradient (dotted arrow indicating higher to lower concentration), indicating the progressive decrease in these molecules.
LF works synergistically with another antimicrobial protein, lysozyme (LZ), to eliminate Streptococcus pneumoniae; while LF disrupts bacterial membrane permeability, LZ hydrolyzes peptidoglycans more effectively [41]. LZ exerts its antimicrobial functions through amphipathic regions. Peptides in C region terminal areas, the T4 and HEWL fragments, exhibit non-enzymatic microbicidal activity and interact with microbial membranes, disrupting and damaging bacterial, fungal, but not mammalian cells [42]. Adding polyproline chains to the C-terminal end of human LZ increased its hydrophobicity, enhancing its bactericidal activity against E. coli [43]. LZ data from two separate studies reveal that the concentration of LZ in breast milk initially decreases from colostrum (0.36–0.37 g/L) to transitional milk (0.27–0.30 ± 0.01 g/L) and early mature milk (0.24–0.30 ± 0.01 g/L, approximately in days 15–42). Subsequently, the concentration increases in late mature milk (0.33–0.35 ± 0.01 g/L in days 29/43–56), reaching its peak (0.83 ± 0.05–0.89 g/L) between days 57 and 84 postpartum [44].
The antimicrobial protein lactoperoxidase (LPO) is in mature human milk in two enzyme forms with molecular weights of 80 kDa and 100 kDa. LPO has a structure with alpha helices, beta strands, a hydrophobic core around a heme group, and two cavities for iron binding and substrate interaction. It was measured in 26 samples of mature milk, with a mean concentration of 0.77 ± 0.38 mg/L [45,46]. It was stable at pH 4.0 and resistant to proteases, suggesting that it retains antimicrobial mechanism activity even in the infant’s digestive system [47]. The antimicrobial mechanism involves the catalysis of thiocyanate (SCN−) oxidation in the presence of hydrogen peroxide (H2O2), producing antimicrobial compounds; adding LPO to a mixture of thiocyanate and hydrogen peroxide significantly enhances its antimicrobial activity. Without LPO, the mixture showed a negligible antimicrobial effect, but with LPO, it effectively eradicated Streptococcus, Streptococcus sanguinis, and Candida albicans [48].
Calprotectin is an antimicrobial heterodimer protein composed of the S100A8 and S100A9 proteins, often referred to as MRP-8 and MRP-14. They can chelate metal ions, particularly manganese, and impair the growth of pathogens to protect against neonatal infections, such as sepsis [49]. The levels of S100A8/A9 are significantly elevated in breast milk following birth, particularly in mothers who delivered vaginally and at term. These elevated levels are crucial in inhibiting the growth of pathogenic microorganisms like Staphylococcus aureus and Streptococcus agalactiae, as these bacteria rely on manganese for their development and proliferation; removing S100A8/A9 from breast milk diminishes its antimicrobial activity [50].
Osteopontin (OPN) is an antimicrobial protein highly post-translationally modified with critical roles in human health. Bovine milk OPN contains 22 phosphorylated residues, while human milk OPN has ~25, primarily on serines and threonines [51,52]. The concentration of OPN varies significantly across species and lactation stages. In bovine milk, OPN levels average around 23 mg/L but can range from 0.4 to 67.8 mg/L depending on factors like parity and days in milk [53,54]. Human milk, however, contains much higher concentrations, ranging from 48 to 334 mg/L, with levels typically decreasing postpartum. Geographic differences also influence OPN levels, with higher concentrations observed in Asian populations compared to Danish mothers [55,56]. These modifications, catalyzed by FAM20C, enhance interactions with integrins (αvβ3, α9β1) via key domains like RGD and SVVYGLR [57,58]. Proteolytic cleavage generates N-terminal fragments resistant to digestion, enabling intestinal absorption via integrin-mediated transcytosis [59,60,61,62].
Advanced techniques such as ELISA and mass spectrometry (UHPLC-MS/MS) are used for quantification, though molecular heterogeneity poses challenges. For instance, the peptide GDSVVYGLR is often used in MS/MS analyses, but phosphorylation can interfere with accurate measurements [63,64,65]. OPN regulates gene expression (ILF-2, IL-2), supports beneficial gut bacteria (Bifidobacterium), and inhibits pathogens (S. aureus) [66,67]. The protein’s resistance to gastrointestinal digestion and ability to modulate immune responses further highlight its functional versatility in health and disease [62,63,64,65,66,67,68,69].
Finally, our recent results show the presence of soluble bactericidal/permeability-increasing protein (BPI) and leukocytes and epithelial cells containing it, primarily during the colostrum stage. When using BPI at concentrations typical of colostrum, we found that it inhibits the growth of two E. coli strains [70] and confirms the presence of BPI in breast milk but found in extracellular vesicles obtained from breast milk [71].
3. Antimicrobial Peptides in Human Breast Milk
Breast milk contains antimicrobial peptides generated through the proteolytic cleavage of breast milk proteins (Figure 1). Among these, lactoferricin (Lfcin) is derived from LF with an amphipathic structure and a high positive charge effective against bacteria, fungi, parasites, and viruses; neutralizes bacterial toxins; and exhibits an amphipathic structure and positive charge that enables interaction with negatively charged pathogen membranes. Upon electrostatic attraction, the hydrophobic residues, particularly tryptophans, insert into the membrane, leading to membrane destabilization, depolarization, increased membrane permeability, and the inhibition of DNA, RNA, and protein synthesis, resulting in bacterial death [72]. Variants include LfcinB (bovine), forming a loop stabilized by a disulfide bond, and LfcinH (human), which retains a helical structure in membrane-mimetic environments. Its mechanism involves electrostatic interaction with bacterial membranes, followed by the insertion of hydrophobic residues (especially tryptophans), which destabilizes the membrane, causes depolarization, and inhibits the synthesis of DNA, RNA, and proteins [73].
Synthetic human lactoferrin derivatives such as hLF(1-11) have been developed, retaining and enhancing these antimicrobial properties. hLF(1-11) acts by disrupting bacterial membrane potential and interfering with metabolic processes essential for bacterial viability, showing 100-fold greater efficacy against MRSA in murine models compared to natural lactoferrin [74]. Similarly, in humans, HLR1r, a designed lactoferrin-derived peptide, effectively targets Gram-positive and Gram-negative bacteria and Candida albicans by membrane permeabilization and intracellular killing mechanisms [75]. Another lactoferrin-derived peptide, lactoferrampin (LFampH), exhibits antimicrobial action through its amphipathic alpha-helical structure. Structural modifications, including lysine addition at the C-terminus and the Asp17Asn mutation, enhance electrostatic interaction with microbial membranes, leading to membrane insertion and microbial death, particularly effective against Candida albicans [76].
Casein breast milk protein generates microbicidal peptides like the peptide β-casein 197 and the β-casein 65. These inhibit the growth of E. coli, S. aureus, and Y. enterocolitica, primarily through membrane permeabilization with no evidence of DNA binding. They are also effective against common pathogenic bacteria (E. coli and S. aureus), primarily through membrane permeabilization [77,78].
Cationic host defense peptides (CHDPs), also known as antimicrobial peptides (AMPs), include cathelicidins and defensins, according to their structure—cathelicidin LL-37 in human milk hCAP18/LL-37 and hBD-1 mRNA significantly increase expression levels at 30 and 60 days postpartum, with an estimated concentration of 32 µM. Additionally, LL-37 demonstrated antimicrobial activity against S. aureus, group A. Additionally, LL-37 can modulate inflammatory signaling pathways by binding to lipopolysaccharides (LPSs), preventing excessive immune activation [79].
At the same time, human β-defensin-1 (HBD-1), three HBD-1 variants (4015.6, 4637.3, and 5068.8 daltons) were measured with concentrations ranging from 1 to 10 µg/mL and exhibited potent antimicrobial activity against E. coli. Still, they showed no significant effect on L. monocytogenes or group B Streptococcus, indicating selectivity towards Gram-negative bacteria by binding to bacterial membranes and disrupting their integrity. In contrast, no significant activity against Gram-positive Listeria monocytogenes or group B Streptococcus was observed, highlighting specificity in target recognition [80].
Another defensin, the human β-defensin-2 (HBD-2), was more abundant in colostrum (8.52 µg/mL) than in mature milk (0.97 µg/mL) in 100 Venezuelan donors. It demonstrates potent activity against enteric (Salmonella spp., E. coli) and nosocomial pathogens (Serratia marcescens, Pseudomonas aeruginosa, Acinetobacter baumannii) by similar membrane-disruptive mechanisms [81].
Altogether, antimicrobial proteins and peptides (APPs) in breast milk inhibit critical pathogens such as S. epidermidis, S. aureus, E. coli, and S. agalactiae, which are leading causes of late-onset sepsis (LOS) in preterm infants (<32 weeks). Decreasing concentrations of LF, HBD-1, HBD-2, and α-defensin 5 by day 21 of life have been linked to increased susceptibility to LOS, suggesting that sustained exposure to APPs enhances immune protection and modulates gut microbiota, promoting neonatal health [82]. Notably, human defensin 5 (HD5), expressed by Paneth cells and present in breast milk, exhibits potent antimicrobial activity and potential antitumor properties. HD5 can permeabilize cancer cell membranes, induce apoptosis, and suppress tumor-promoting inflammation, highlighting its possible role in neonatal immune defense and oncological protection [83].
4. Selective Antimicrobial Activity of Breast Milk Peptides Supporting Neonatal Microbiota
Probiotic bacteria like Bifidobacterium bifidum var. pennsylvanicus depend on iron for growth, with ferrous iron-reversing inhibition caused by chelators such as α, α’-bipyridyl or lactoferrin (LF) [84]. These antimicrobial effects are influenced by processing; Bifidobacterium breve showed limited growth in pasteurized donor milk (PDHM) and no growth in unpasteurized human donor milk (UDHM), suggesting that pasteurization reduces the availability of probiotic-supportive components [85].
In contrast, through iron sequestration, LF may act as a prebiotic-like substrate and carbon source for Bifidobacterium. Bovine LF (bLF) induces the expression of genes involved in oligosaccharide metabolism in B. infantis, such as fucosidases and solute-binding proteins specific for human milk oligosaccharides (HMOs) [86]. Moreover, peptides generated by LF hydrolysis promote the growth of B. infantis and B. breve, in contrast to native human LF, which appears to favor B. bifidum. These peptides exhibit enhanced bifidogenic activity and resistance to gastrointestinal digestion, suggesting they may be responsible for LF’s microbial effects [87,88]. Another bifidogenic peptide is the synthetic bovine LF pepsin hydrolysate (bLFH), produced through trypsin and pepsin digestion, which supports the growth of B. breve, B. bifidum, B. infantis, and especially B. longum [89]. The structure of bLFH, a disulfide-linked heterodimer, is critical for activity; a mixture of the same amino acids without structural conformation showed no effect [90].
At the molecular level, LF-derived peptides such as lactoferricin B (LFcin B) exert antimicrobial activity by disrupting bacterial membranes through electrostatic interactions with anionic phospholipids and insertion of hydrophobic residues. This mechanism compromises membrane integrity and interferes with bacterial DNA, RNA, and protein synthesis. LF also binds bacterial lipopolysaccharides (LPSs), neutralizing endotoxins and inhibiting TLR4-mediated proinflammatory signaling pathways. LFcin B’s effect varies depending on bacterial strain and growth medium; Gram-positive bacteria are generally more susceptible than Gram-negative strains. Notably, Pseudomonas fluorescens, Enterococcus faecalis, and B. bifidum exhibit higher resistance, likely due to differences in membrane structure and permeability [91].
Bifidobacterium’s relative resistance to LF peptides like LFcin B is likely a result of membrane adaptations and efficient metal uptake strategies that allow it to thrive in iron-limited environments. These mechanisms help maintain its dominance and beneficial role in the intestinal ecosystem [90].
In a transgenic mouse model expressing recombinant human lysozyme (rhLZ), bacterial strains such as B. infantis, B. breve, B. longum, and Lactobacillus acidophilus remained resistant at lysozyme concentrations of 5, 50, and 500 mg/L—even after exposure to heat or chemical treatment. Only when treated with trypsin was lysis significantly enhanced, except in B. longum [92]. In human milk, Bifidobacterium species are generally resistant to lysozyme, whereas some Clostridia strains are sensitive [93].
The bifidogenic activity of defensins and cathelicidins remains poorly characterized. While some defensins inhibit beneficial bacteria, others selectively support commensal growth, indicating a nuanced, context-dependent interaction rather than contradiction. While hBD-1 showed no inhibitory effect, hBD-2 and hBD-3 inhibited some Lactobacillus and Bifidobacterium strains, with hBD-3 being more potent [94]. These peptides interact with microbial membranes by forming pores and modulate epithelial immune signaling. hBD-2 has been shown to suppress proinflammatory genes such as TLR7 and IRAK while enhancing the expression of antimicrobial effectors like LL-37 and NF-κBp65, reflecting a role in immune regulation. Additionally, hBD-2 and hBD-3 show selective activity against oral and intestinal pathogens. While aerobic bacteria were uniformly susceptible, resistance among anaerobes, including strains from breast milk, was variable, indicating that susceptibility is more strain-specific than species-dependent [95]. Finally, the antimicrobial activity of hBD-1, hBD-2, hBD-3, and lysozyme increases under acidic conditions like the intestinal pH of neonates (~pH 5–6), while LL-37 activity decreases. This pH-dependent functionality is essential, as the neonatal gut shifts from near-neutral pH at birth to more acidic values upon colonization, affecting the bioactivity of these peptides [96].
These findings highlight the critical role of antimicrobial proteins and peptides—especially lactoferrin and its hydrolyzed derivatives—in neonatal immune defense. Their mechanisms of action include membrane disruption, immune modulation through signaling pathways such as TLR and NF-κB, and selective support of beneficial microbes. Their bifidogenic and anti-inflammatory effects position them as potential therapeutic agents for promoting intestinal health and microbial balance in newborns (Figure 2).
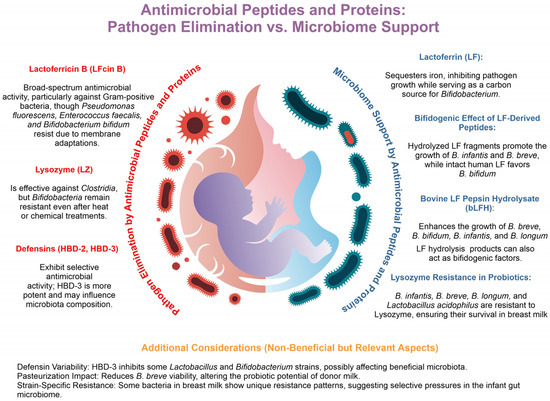
Figure 2.
Antimicrobial peptides and proteins: pathogen elimination vs. microbiome support. Antimicrobial peptides and proteins (APPs) in breast milk eliminate pathogens while supporting beneficial microbiota. Lactoferricin B (LFcin B) shows broad-spectrum antimicrobial activity, though strains like Pseudomonas fluorescens, Enterococcus faecalis, and Bifidobacterium bifidum resist it through membrane adaptations. Lysozyme (LZ) targets Clostridia but spares bifidobacteria, maintaining microbiota balance. Human β-defensins 2 and 3 (HBD-2, HBD-3) selectively inhibit pathogens, with HBD-3 showing greater potency. Additionally, lactoferrin (LF) sequesters iron, limiting pathogen growth while serving as a carbon source for Bifidobacterium. Hydrolyzed LF fragments promote the growth of B. infantis and B. breve, while intact LF favors B. bifidum. Bovine lactoferrin pepsin hydrolysate (bLFH) further supports bifidobacterium proliferation, and natural LZ resistance in B. infantis, B. breve, B. longum, and Lactobacillus acidophilus ensures their survival. However, HBD-3 may inhibit beneficial strains, and pasteurization can reduce B. breve viability, highlighting the delicate balance APPs maintain between immune protection and microbiome support.
5. Milk Formulas Supplemented with Antimicrobial Proteins and Peptides
The undeniable benefit of breastfeeding to the newborn’s health is due to its complex composition, which no formula or feeding technology has yet to match. However, the study of antimicrobial proteins and peptides and their interaction with microbiome and immune neonatal system shed light on advances in artificial feeding and neonatal health (Figure 3) [97]. The factors influencing the neonatal health gut microbiome highlight the impact of birth mode, antibiotic exposure, feeding (breastfeeding vs. formula), and environment. Breastfeeding promotes the growth of beneficial bacteria like Bifidobacterium, while formula feeding can alter the microbiota [98].
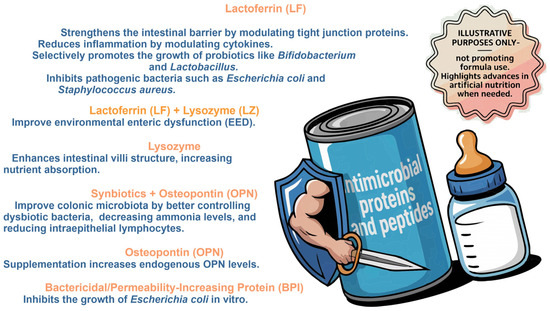
Figure 3.
Milk formulas supplemented with antimicrobial proteins and peptides. The effects of lactoferrin (Lf), lysozyme (Lz), symbiotics + Osteopontin (OPN), and bactericidal/permeability-increasing protein (BPI) on gut microbiota and neonatal health are illustrated based on in vitro and in vivo studies. The importance of these components in modulating the microbiota, improving the intestinal barrier, and reducing inflammation is highlighted. (Image for illustrative purposes does not promote formula use).
Supplementing formula with human milk oligosaccharides (HMOs) is standard and studied. HMOs influence the development of gut microbiota and the immune system because they possess anti-infective and immunomodulatory properties and promote the growth of beneficial bacteria like Bifidobacterium, a signal of a healthy microbiome [16,99].
The supplementation of infant formula with lactoferrin (Lf) promotes gut homeostasis by stimulating epithelial maturation and providing metabolizable glycans that serve as carbon sources for probiotics-promoting beneficial bacteria such as Bifidobacterium and Lactobacillus. LF also enhances gene expression in energy metabolism and protein syn-thesis; modulates immune responses by increasing TLR-2, IgG, and IL-10 levels; and inhibits pathogens such as E. coli and S. typhimurium. Especially in its low iron-saturated form, Lf limits iron availability to pathogens and binds to bacterial LPSs, destabilizing the outer membranes of bacteria [100]. In microbiome stability, in the LACUNA trial, stool samples were analyzed from infants under 31 weeks of gestational age, randomly assigned to receive either probiotics alone or probiotics plus bLf. The results showed that bLf can alter microbial diversity or taxonomic composition and increase intestinal microbiota diversity in neonates [101]. However, according to genetic analysis, a 6-month Peruvian study on infants aged 12–18 months found that oral lactoferrin administration did not significantly alter the diversity or composition of their gut microbiota and suggests that, in this population, oral lactoferrin had no notable effect on the intestinal microbiome [102]. Differences between the studies likely stem from variations in infant age, microbiota maturity, and intervention context; the former involved preterm neonates receiving probiotics and bLf in a hospital setting, while the latter examined older, healthy infants without probiotics, suggesting that microbiota maturity and co-interventions may influence the detectability of bLf effects.
Lactoferrin supplementing formulas like the duo lactoferrin and lysozyme supplementation in Malawian children improve environmental enteric dysfunction (EED). The supplemented group showed an initial improvement in an EED marker and a significant reduction in hospitalizations and acute malnutrition; so, lactoferrin and lysozyme may benefit intestinal health and prevent severe EED complications in young children [103]. These proteins in breast milk modulate microbiota by reducing pathogenic bacteria and promoting beneficial bacteria such as Bifidobacteriaceae and Lactobacillaceae. Lactoferrin reduces inflammation by modulating cytokines, while lysozyme enhances intestinal villi structure, increasing nutrient absorption [104]. However, the temperature affects the levels and stability; both proteins significantly decreased after 6 h at room temperature and refrigeration but remained stable for 6 days when frozen at −20 °C, preserving these bioactive components [105].
Recent studies with the piglet’s model have shown that supplementing infant formula with a multi-strain synbiotic and osteopontin improves colonic microbiota (a better control of dysbiotic bacteria, decreased ammonia, and intraepithelial lymphocytes). At the molecular level, the synbiotic alone (SYN group) upregulated jejunal genes for intestinal maturation and nutrient transport and downregulated genes for pathogen and inflammatory responses, genes related to intestinal maturation (ALPI, SI) and nutrient transport (SLC13A1, SLC15A1, SLC5A1, SLC7A8), as well as the underexpression of genes involved in the response to pathogens (GBP1, IDO, TLR4) or the inflammatory response (IDO, IL-1B, TGF-β1) [106]. Other studies show plasma OPN levels are higher in infants fed breast milk (BF) or OPN-supplemented formulas (F65 and F130) compared to unsupplemented formulas (F0). Small amounts of bovine OPN (bOPN) were detected in plasma, suggesting partial absorption. OPN supplementation increased endogenous OPN levels, indicating that dietary OPN stimulates production [57]. Although studied in animal models, symbiotics and osteopontin have shown promise in intestinal maturation, nutrient absorption, and immune modulation, and increased immune marker levels following supplementation indicate a biological response in human infants. However, due to physiological differences between species, further studies are needed to evaluate their efficacy and safety in newborns.
Commercial formula milk supplemented with the recombinant antimicrobial protein bactericidal/permeability-increasing protein (BPI) recently detected in human breast milk at a concentration of colostrum (1.645 µg/mL) inhibits the growth of two different strains of E. coli in vitro. However, the implementation of an in vivo model is yet to confirm this [70].
Finally, no evidence exists of the supplementation effect of defensins or cathelicidins formula. However, hBD-2 levels varied significantly among groups, with higher levels in the formula-predominant group compared to the exclusive breast milk group. This showed a positive correlation between hBD-2 levels and feeding patterns. These results suggest that breast milk promotes a more balanced intestinal environment, whereas formula feeding may be associated with a heightened inflammatory response [107].
6. Modulation of the Immune System by Antimicrobial Peptides
Antimicrobial proteins and peptides, mainly lactoferrin, have been used to improve infant feeding alternatives, and other responses have been observed during the process in different cells and systems (Table 1). During 90 days of intervention with an Lf-enriched supplement (400 mL/day), the participants showed significant anthropometric improvements: weight gain and height increase. At the immunological level, a marked reduction in IL-10 and improved IL-6/IL-10, particularly in severe malnutrition, enhances growth and modulates immune responses [108].
Bovine lactoferrin absorption is crucial for immune function development. It could be improved in microencapsulated form (Inferrin™). Serum lactoferrin levels were taken before and after the intervention to assess absorption. A significant increase in plasma lactoferrin was observed in the group receiving the microencapsulated formulation compared to a placebo, suggesting that microencapsulation enhances intestinal absorption by improving stability. Additionally, this formulation has demonstrated a favorable safety profile, with no adverse effects reported and a reduction in proinflammatory cytokines (IL-6 and TNF-α), indicating anti-inflammatory activity and good immunological tolerability. It reduces immune cell activation (CD69+ in CD4+ and CD8+) and positively modulates the gut microbiome by decreasing infection-related bacteria and increasing beneficial ones, with a decrease in Euryarchaeota, Acidobacteria, Chloroflexi, and Nitrospirae and an increase in Firmicutes and Bacteroidetes [109]. On the other hand, in lactoferrin structural integrity, a study demonstrated that lactoferrin (Lf) complexes with α-lactalbumin (α-Lac), whey protein hydrolysates (WPH), and skim milk powder (MP) enhance resistance to digestive enzymes (pepsin/pancreatin) maintained binding to intestinal cells’ Lf receptor (LfR), promoting proliferation and differentiation, strengthened the gut barrier and antimicrobial activity against E. coli and anti-inflammatory effects (reducing IL-1β, IL-6, TNF-α) [93]. The immunomodulatory capacity of lactoferrin also depends on its iron-binding state, either native (nLf) or iron-saturated (sLf). In a mouse model of antibiotic-induced dysbiosis, both forms of lactoferrin helped restore the abundance of beneficial bacterial families such as Bacteroidaceae, Prevotellaceae, and Rikenellaceae, which had been reduced by clindamycin treatment. Additionally, lactoferrin administration led to a decreased expression of Toll-like receptors TLR2, TLR8, and TLR9, supporting its role in promoting anti-inflammatory microbial populations and modulating innate immune recognition pathways [110]. On the other hand, iron-free lactoferrin (apo-LF) protects against LPS-induced intestinal inflammation by modulating NF-κB and PPAR pathways. It found that apo-LF significantly reduces proinflammatory cytokines (TNF-α, IL-6, IL-1β) while increasing IFN-γ, and inhibits the NF-κB signaling pathways (NF-κB, TNF-α, IL-1β) [111].
In addition to cytokines and immune receptors, lactoferrin affects dendritic cell development and behavior. Bovine lactoferrin (LF) modulates the differentiation of human monocytes into tolerogenic dendritic cells (moDCs) through LPS-independent mechanisms and reduces the expression of the maturation markers CD1a and HLA-DR while increasing the production of IL-6 and IL-8 during differentiation. Furthermore, LF-treated moDCs showed hyporesponsiveness to TLR ligands (LPS, Poly I: C/R848), with a decreased expression of CD83/CD86 and decreased production of IL-10 and IL-12p70 [112].
The lactoferrin response in plasmacytoid dendritic cells (pDCs), a key factor in antiviral immunity, which detect viruses through TLR7 and TLR9 receptors for the release of interferon-α (IFN-α), is enhanced when lactoferrin is simultaneously present with viral single-stranded RNA. It activates pDCs through a molecular mechanism involving TLR7/9 signaling and the use of human PBMCs incubated with LF, its hydrolysate (LFH), or LFcin together with single-stranded RNA, which increases IFN-α secretion and upregulates the expression of the activation markers CD86 and HLA-DR. The cationic charge of LF/LFcin likely promotes interactions with viral nucleic acids, amplifying TLR7/9 signaling. These results position LFs as potent immunomodulators with therapeutic potential for viral infections [113].
In contrast, the effects of human lactoferrin (hLF) on neonatal and adult monocyte-derived macrophages (moMφs). hLF attenuated the production of proinflammatory cytokines (TNF-α, IL-1β, IL-6) and reduced Toll-like receptor (TLR) signaling by decreasing ERK1/2 and NF-κB phosphorylation. It also lowered the expression of activation markers (CD40, HLA-DR) and phagosome acidification, suggesting a shift towards an “anergic” rather than an “anti-inflammatory” state [114]. Also, the effect of lactoferrin (LF) from breast milk on macrophages, focusing on prostaglandin E2 (PGE2) secretion assays, showed that LF dose-dependently inhibits PGE2 production in macrophages under basal conditions [115].
In rhesus monkeys, supplementation with bovine OPN in infant formulas modified the intestinal transcriptome, stimulated intestinal cell proliferation and maturation, regulated genes such as MAPK13 and NF-kB, and increased the secretion of IL-18, a key cytokine in the immune response, reduces inflammation and increases infection resistance by lowering TNF-α levels and boosting IFN-γ production. These findings support using OPNs in infant formulas to improve infant development and health [116].
Human beta-defensin 2 (HBD-2) modulates the immune response in breast and intestinal epithelial cells. In the CaCo-2 cell line (intestine), HBD-2 reduced the expression of TLR-7, IRAK, and alpha-defensins while increasing LL37 and NFκBp65. These effects suggest that HBD-2 contributes to increase the antimicrobial peptide cathelicin and its benefits [117].

Table 1.
Immune modulation by antimicrobial proteins and peptides to promote neonatal health. The following summary highlights studies focused on immune modulation to promote neonatal health, emphasizing the effects of antimicrobial proteins and peptides like lactoferrin, osteopontin, and human beta-defensin 2. It details their impact on immune cells, cytokine regulation, gut barrier reinforcement, microbiota modulation, and antimicrobial activity.
Table 1.
Immune modulation by antimicrobial proteins and peptides to promote neonatal health. The following summary highlights studies focused on immune modulation to promote neonatal health, emphasizing the effects of antimicrobial proteins and peptides like lactoferrin, osteopontin, and human beta-defensin 2. It details their impact on immune cells, cytokine regulation, gut barrier reinforcement, microbiota modulation, and antimicrobial activity.
| Protein/Peptide | Effect Produced and Key Mechanisms | Target Immune Cells/Structures | Altered Markers or Functions | |
|---|---|---|---|---|
| Lactoferrin (Bovine) | Improves infant feeding, promotes growth (weight gain, height increase), modulates immune responses by reducing IL-10 and enhancing IL-6/IL-10 ratio, particularly in severe malnutrition | General immune system | Decreased IL-10, improved IL-6/IL-10 ratio, increased weight and height [108] | |
| Lactoferrin (Bovine) | Enhances intestinal absorption, reduces immune cell activation (CD69+ in CD4+ and CD8+), modulates gut microbiome by decreasing pathogenic bacteria and increasing beneficial bacteria (Firmicutes, Bacteroidetes) | Intestinal cells, gut microbiota | Decreased CD69+ (CD4+, CD8+), reduction in pathogenic bacteria, increase in beneficial bacteria [109] | |
| Lactoferrin (Bovine) | Strengthens gut barrier, antimicrobial activity against E. coli, and anti-inflammatory effects (reduces IL-1β, IL-6, TNF-α), promotes intestinal cell proliferation and differentiation | Intestinal epithelial cells | Decreased IL-1β, IL-6, TNF-α; strengthened gut barrier [92] | |
| Lactoferrin (Bovine) | Restores gut microbiota balance, reduces TLR2/8/9 expression, increases anti-inflammatory bacteria and recognition receptors for pathogens | Intestinal immune cells | Decreased TLR2/8/9 expression, restoration of anti-inflammatory bacteria [110] | |
| Lactoferrin (Bovine) | Protects against LPS-induced intestinal inflammation, modulates NF-κB and PPAR pathways, reduces proinflammatory cytokines (TNF-α, IL-6, IL-1β), increases IFN-γ | Intestinal cells | Decreased TNF-α, IL-6, IL-1β; increased IFN-γ [111] | |
| Lactoferrin (Bovine) | Modulates dendritic cell differentiation into tolerogenic cells, reduces TLR ligand response and proinflammatory cytokine production | Dendritic cells (moDC) | Decreased CD1a, HLA-DR, CD83/CD86, IL-10, IL-12p70; increased IL-6, IL-8 [112] | |
| Lactoferrin (Bovine) | Restores gut microbiota balance, reduces TLR2/8/9 expression, increases anti-inflammatory bacteria and recognition receptors for pathogens | Intestinal immune cells | Decreased TLR2/8/9 expression, restoration of anti-inflammatory bacteria [110] | |
| Lactoferrin (Bovine) | Enhance antiviral immunity in plasmacytoid dendritic cells (pDCs), promotes TLR7/9 signaling with viral RNA, increases IFN-α secretion, boosts activation markers | Plasmacytoid dendritic cells (pDCs) | Increased IFN-α, CD86, HLA-DR [117] | |
| Lactoferrin (Human) | Attenuates proinflammatory cytokine production (TNF-α, IL-1β, IL-6), reduces TLR signaling by decreasing ERK1/2 and NF-κB phosphorylation, shifts macrophages towards an “anergic” state | Monocyte-derived macrophages (moMφ) | Decreased TNF-α, IL-1β, IL-6; reduced TLR signaling (ERK1/2, NF-κB) [114] | |
| Lactoferrin (Bovine) | Inhibits prostaglandin E2 (PGE2) production in macrophages, modulates immune response in basal conditions | Macrophages | Decreased PGE2 production [115] | |
| Osteopontin (Bovine) | Modifies intestinal transcriptome, stimulates intestinal cell proliferation and maturation, reduces inflammation, boosts IL-18, IFN-γ production | Intestinal cells | Increased IL-18, decreased TNF-α, increased IFN-γ [116] | |
| Human Beta-Defensin-2 (Human) | Modulates immune response in epithelial cells, increases antimicrobial peptides (cathelicin), and modulates inflammation by reducing TLR-7, IRAK, alpha-defensins while increasing LL37 and NFκBp65 | Epithelial cells (intestinal and mammary) | Decreased TLR-7, IRAK, alpha-defensins; increased LL37, NFκBp65 [117] | |
7. The Protective Role of Antimicrobial Peptides in Breastfeeding Mothers
While breastfeeding sustains infant growth and immunity, it exposes the mammary tissue to infections like mastitis. In this regard, the antimicrobial peptides and proteins are recognized for their importance in breast milk for infant health. Still, few studies have shown its direct role in mammary tissue defense (Table 2) [118,119]. To assess subclinical mastitis (SCM), a breast inflammation without apparent symptoms, breast milk from 305 European mothers was analyzed, revealing a 35.4% prevalence during the first postpartum month and milk-increased total proteins and, interestingly, lactoferrin [120].

Table 2.
Antimicrobial proteins and peptides in mammary tissue: roles in infection defense and inflammation regulation. A summary of key antimicrobial proteins and peptides found in human milk, transgenic cows, and lactating mice, detailing their functions in the mammary tissue and their impact on infection and inflammation. These proteins contribute to bacterial clearance, antimicrobial action, immune receptor activation, and inflammation regulation, playing an essential role in maternal and neonatal health.
In the subclinical mastitis model with six transgenic cows producing recombinant human lactoferrin (rhLf) infected with Staphylococcus chromogenes, a common subclinical mastitis pathogen, all transgenic cows became infected but showed no clinical signs. Transgenics cleared bacteria faster, reduced inflammation, and showed no systemic response protected against clinical symptoms and prolonged inflammation but did not prevent initial infection [121]. In contrast, recombinant human lactoferrin (rhLf) in transgenic cows’ milk against E. coli mastitis failed to prevent infection but remained stable and mitigated systemic inflammation (lower cortisol/haptoglobin). The findings indicate that, despite its LPS-neutralizing and immunomodulatory effects, it is ineffective in enhancing E. coli mastitis resistance [122].
The expression of antimicrobial peptides (AMPs), including cathelicidins and defensins, has been demonstrated in mammary glands and human milk. Human cathelicidin hCAP18/LL-37 and hBD-1 mRNA levels significantly increase at 30 and 60 days postpartum, while the murine orthologue CRAMP is found in the mammary tissues of lactating mice. Lactation-associated hormones, such as prolactin and glucocorticoids, regulate this expression. It can be enhanced by local inflammatory signals, including TNF-α and IL-1β, strengthening the innate defense functions of mammary epithelial cells. Additionally, LL-37 exhibits antimicrobial activity against S. aureus, group A Streptococcus, and E. coli in an ionic environment similar to human milk [81]. Interestingly, cathelicidin LL-37 also interacts with the mTOR signaling pathway, a key regulator of immune cell growth, metabolism, and autophagy. Through Toll-like receptor (TLR) engagement and EGFR activation, LL-37 can modulate mTOR activity, influencing epithelial repair, immune activation, and even tumor progression, depending on the cellular context [81]. Immunohistochemistry studies revealed strong HBD-1 expression in mammary epithelium during lactation, with lower levels in non-lactating women, suggesting hormonal regulation. HBD-1 displayed a potent antimicrobial activity against E. coli but showed a limited effect against L. monocytogenes and group B Streptococcus, indicating selectivity for Gram-negative bacteria [80]. Moreover, HBD-1, present in human milk, modulates the immune response in mammary epithelial cells (MCF-7), inducing the expression of TLR-7, MyD88, and IRAK while silencing LARC, contributing to the fine-tuning of immune activation [117].
8. Conclusions
Human milk is a fundamental pillar for infant health, providing essential nutrients and a sophisticated arsenal of antimicrobial peptides and proteins (APPs) that work in conjunction with a dynamic community of beneficial microorganisms. We highlight the diverse array of antimicrobial proteins, including lactoferrin and lysozymes, and beneficial antimicrobial peptides for the newborn, mainly those produced by proteolytic protein cleavage in breast milk, such as lactoferrin-derived peptides, as well as the family of defensins and cathelicidins and their contributions to combating pathogens and modulating the neonatal immune response. Critically, we highlighted the growing understanding of the synergistic relationship between these APPs and the breast milk microbiota. This complex interaction promotes a healthy gut microbiome, characterized by the proliferation of beneficial bacteria and the suppression of harmful ones, ultimately contributing to long-term infant well-being. Recognizing the vital role of these bioactive components opens avenues for improving artificial feeding strategies through targeted supplementation, especially when breastfeeding is not feasible. Furthermore, the benefits extend beyond the infant’s gut and immune system, potentially impacting breast tissue health. Continued research into the limited amount of research conducted to understand the complex molecular interactions within breast milk promises to shed even more light on its remarkable protective properties and inform strategies to optimize infant health outcomes globally.
Author Contributions
Conceptualization, A.S.A.-D. and M.G.-L.; investigation, A.S.A.-D. and M.G.-L.; writing—original draft preparation, A.S.A.-D. and S.R.A.-R.; writing—review and editing, S.R.A.-R., A.S.A.-D. and M.G.-L.; supervision, A.S.A.-D. and M.G.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, R.; Ware, J.; Chen, A.; Nelson, J.M.; Kmet, J.M.; Parks, S.E.; Morrow, A.L.; Chen, J.; Perrine, C.G. Breastfeeding and post-perinatal infant deaths in the United States, a national prospective cohort analysis. Lancet Reg. Health Am. 2022, 5, 100094. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.C.; Stewart, C.J. Role of breastfeeding in disease prevention. Microb. Biotechnol. 2024, 17, e14520. [Google Scholar] [CrossRef]
- Ware, J.L.; Li, R.; Chen, A.; Nelson, J.M.; Kmet, J.M.; Parks, S.E.; Morrow, A.L.; Chen, J.; Perrine, C.G. Associations between breastfeeding and post-perinatal infant deaths in the U.S. Am. J. Prev. Med. 2023, 65, 763–774. [Google Scholar] [CrossRef]
- Phukan, D.; Ranjan, M.; Dwivedi, L.K. Impact of timing of breastfeeding initiation on neonatal mortality in India. Int. Breastfeed. J. 2018, 13, 27. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.M.; Nazar, A.M. Breastfeeding, the immune response, and long-term health. J. Am. Osteopath. Assoc. 2006, 106, 203–207. [Google Scholar] [PubMed]
- Qi, C.; Tu, H.; Zhou, J.; Tu, R.; Chang, H.; Chen, J.; Hu, H.; Yu, R.; Sun, J. Widespread vertical transmission of secretory immunoglobulin A coated trace bacterial variants from the mother to infant gut through breastfeeding. Food Funct. 2022, 13, 11543–11554. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; de La Cochetiere, M.F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Meng, L.; Xie, H.; Li, Z.; Tye, K.D.; Fan, G.; Huang, T.; Yan, H.; Tang, X.; Luo, H.; Xiao, X. Gut-mammary pathway: Breast milk microbiota as a mediator of maternal gut microbiota transfer to the infant gut. J. Funct. Foods. 2025, 124, 106620. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp. Mol. Med. 2020, 52, 1288–1297. [Google Scholar] [CrossRef]
- Dai, D.L.Y.; Petersen, C.; Hoskinson, C.; Del Bel, K.L.; Becker, A.B.; Moraes, T.J.; Mandhane, P.J.; Finlay, B.B.; Simons, E.; Kozyrskyj, A.L.; et al. Breastfeeding enrichment of B. longum subsp. infantis mitigates the effect of antibiotics on the microbiota and childhood asthma risk. Med 2023, 4, 92–112.e5. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Chassard, C. New insights in gut microbiota establishment in healthy breast-fed neonates. PLoS ONE 2012, 7, e44595. [Google Scholar] [CrossRef]
- Nadimpalli, M.L.; Bourke, C.D.; Robertson, R.C.; Delarocque-Astagneau, E.; Manges, A.R.; Pickering, A.J. Can breastfeeding protect against antimicrobial resistance? BMC Med. 2020, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Newburg, D.S. Human milk glycoproteins protect infants against human pathogens. Breastfeed. Med. 2013, 8, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef]
- Pessentheiner, A.R.; Spann, N.J.; Autran, C.A.; Oh, T.G.; Grunddal, K.V.; Coker, J.K.; Painter, C.D.; Ramms, B.; Chiang, A.W.; Wang, C.-Y.; et al. The human milk oligosaccharide 3′-sialyllactose reduces low-grade inflammation and atherosclerosis development in mice. JCI Insight 2024, 9, e181329. [Google Scholar] [CrossRef]
- Johnson-Hence, C.B.; Gopalakrishna, K.P.; Bodkin, D.; Coffey, K.E.; Burr, A.H.P.; Rahman, S.; Rai, A.T.; Abbott, D.A.; Sosa, Y.A.; Tometich, J.T.; et al. Stability and heterogeneity in the antimicrobiota reactivity of human milk-derived immunoglobulin A. J. Exp. Med. 2023, 220, e20220839. [Google Scholar] [CrossRef]
- Salerno-Goncalves, R.; Safavie, F.; Fasano, A.; Sztein, M.B. Free and complexed-secretory immunoglobulin A triggers distinct intestinal epithelial cell responses. Clin. Exp. Immunol. 2016, 185, 338–347. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, S.; Liu, Y.; Scott, A.L.; Yang, J.; Wan, F. Complement in breast milk modifies offspring gut microbiota to promote infant health. Cell 2024, 187, 750–763.e20. [Google Scholar] [CrossRef]
- Suwaydi, M.A.; Gridneva, Z.; Perrella, S.L.; Wlodek, M.E.; Lai, C.T.; Geddes, D.T. Human Milk Metabolic Hormones: Analytical Methods and Current Understanding. Int. J. Mol. Sci. 2021, 22, 8708. [Google Scholar] [CrossRef]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of human milk bioactives on infants’ gut and immune health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, F.; Grimm, V.J.; Hinrichs, J. Iron saturation and binding capacity of lactoferrin—Development and validation of a colorimetric protocol for quality control. Food Chem. 2025, 463 Pt 3, 141365. [Google Scholar] [CrossRef]
- Yen, C.C.; Shen, C.J.; Hsu, W.H.; Chang, Y.H.; Lin, H.T.; Chen, H.L.; Chen, C.M. Lactoferrin: An iron-binding antimicrobial protein against Escherichia coli infection. Biometals 2011, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Capobianco, D.; Campagna, G.; Laforgia, N.; Drimaco, P.; Dileone, A.; Baldassarre, M.E. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals 2014, 27, 1077–1086. [Google Scholar] [CrossRef]
- Talbert, J.A.; Lu, J.; Spicer, S.K.; Moore, R.E.; Townsend, S.D.; Gaddy, J.A. Ameliorating adverse perinatal outcomes with lactoferrin: An intriguing chemotherapeutic intervention. Bioorganic Med. Chem. 2022, 74, 117037. [Google Scholar] [CrossRef]
- Lu, J.; Francis, J.D.; Guevara, M.A.; Moore, R.E.; Chambers, S.A.; Doster, R.S.; Eastman, A.J.; Rogers, L.M.; Noble, K.N.; Manning, S.D.; et al. Antibacterial and anti-biofilm activity of the human breast milk glycoprotein lactoferrin against Group B Streptococcus. Chembiochem 2021, 22, 2124–2133. [Google Scholar] [CrossRef]
- Zhang, R.; Ying, E.; Wu, X.; Qin, H.; Guo, Y.; Guo, X.; Yu, Z.; Chen, J. A systematic review and meta-analysis of breastfeeding and neurodevelopmental outcomes in preterm infant. Front. Public. Health 2024, 12, 1401250. [Google Scholar] [CrossRef] [PubMed]
- Favara, G.; Maugeri, A.; Barchitta, M.; Lanza, E.; Magnano San Lio, R.; Agodi, A. Maternal lifestyle factors affecting breast milk composition and infant health: A systematic review. Nutrients 2024, 17, 62. [Google Scholar] [CrossRef]
- Dallas, D.C.; Guerrero, A.; Khaldi, N.; Castillo, P.A.; Martin, W.F.; Smilowitz, J.T.; Bevins, C.L.; Barile, D.; German, J.B.; Lebrilla, C.B. Extensive in vivo human milk peptidomics reveals specific proteolysis yielding protective antimicrobial peptides. J. Proteome Res. 2013, 12, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Beverly, R.L.; Woonnimani, P.; Scottoline, B.P.; Lueangsakulthai, J.; Dallas, D.C. Peptides from the intestinal tract of breast milk-fed infants have antimicrobial and bifidogenic activity. Int. J. Mol. Sci. 2021, 22, 2377. [Google Scholar] [CrossRef]
- Battersby, A.J.; Khara, J.; Wright, V.J.; Levy, O.; Kampmann, B. Antimicrobial proteins and peptides in early life: Ontogeny and translational opportunities. Front. Immunol. 2016, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Virtanen, A.; Dodson, R. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Groves, M.L. The Isolation of a Red Protein from Milk. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lönnerdal, B.; German, J.B.; et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell. Proteom. 2012, 11, M111.015248. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Hirai, Y.; Kawakata, N.; Satoh, K.; Ikeda, Y.; Hisayasu, S.; Orimo, H.; Yoshino, Y. Concentrations of lactoferrin and iron in human milk at different stages of lactation. J. Nutr. Sci. Vitaminol. 1990, 36, 531–544. [Google Scholar] [CrossRef]
- André, G.O.; Politano, W.R.; Mirza, S.; Converso, T.R.; Ferraz, L.F.; Leite, L.C.; Darrieux, M. Combined effects of lactoferrin and lysozyme on Streptococcus pneumoniae killing. Microb. Pathog. 2015, 89, 7–17. [Google Scholar] [CrossRef]
- Ito, Y.; Kwon, O.H.; Ueda, M.; Tanaka, A.; Imanishi, Y. Bactericidal activity of human lysozymes carrying various lengths of polyproline chain at the C-terminus. FEBS Lett. 1997, 415, 285–288. [Google Scholar] [CrossRef]
- Montagne, P.; Cuillière, M.L.; Molé, C.; Béné, M.C.; Faure, G. Microparticle-enhanced nephelometric immunoassay of lysozyme in milk and other human body fluids. Clin. Chem. 1998, 44, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Montagne, P.; Cuillière, M.L.; Molé, C.; Béné, M.C.; Faure, G. Changes in Lactoferrin and Lysozyme Levels in Human Milk During the First Twelve Weeks of Lactation. In Bioactive Components of Human Milk; Newburg, D.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 501. [Google Scholar] [CrossRef]
- Shin, K.; Hayasawa, H.; Lönnerdal, B. Purification and quantification of lactoperoxidase in human milk with use of immunoadsorbents with antibodies against recombinant human lactoperoxidase. Am. J. Clin. Nutr. 2001, 73, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Tomita, M.; Lönnerdal, B. Identification of lactoperoxidase in mature human milk. J. Nutr. Biochem. 2000, 11, 94–102. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.K.; Kaushik, S.; Sinha, M.; Singh, R.P.; Sharma, P.; Sirohi, H.; Kaur, P.; Singh, T.P. Lactoperoxidase: Structural insights into the function, ligand binding and inhibition. Int. J. Biochem. Mol. Biol. 2013, 4, 108–128. [Google Scholar] [PubMed]
- Schubert, R.; Schwahn, C.; Kramer, A.; Below, H. Effect of lactoperoxidase on the antimicrobial effectiveness of the thiocyanate hydrogen peroxide combination in a quantitative suspension test. BMC Microbiol. 2009, 9, 134. [Google Scholar] [CrossRef]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef]
- Pirr, S.; Richter, M.; Fehlhaber, B.; Pagel, J.; Härtel, C.; Roth, J.; Vogl, T.; Viemann, D. High Amounts of S100-Alarmins Confer Antimicrobial Activity on Human Breast Milk Targeting Pathogens Relevant in Neonatal Sepsis. Front. Immunol. 2017, 8, 1822. [Google Scholar] [CrossRef]
- Sørensen, E.S.; Højrup, P.; Petersen, T.E. Posttranslational Modifications of Bovine Osteopontin: Identification of Twenty-Eight Phosphorylation and Three O-Glycosylation Sites. Protein Sci. 1995, 4, 2040–2049. [Google Scholar] [CrossRef]
- Christensen, B.; Nielsen, M.S.; Haselmann, K.F.; Petersen, T.E.; Sørensen, E.S. Post-Translationally Modified Residues of Native Human Osteopontin Are Located in Clusters: Identification of 36 Phosphorylation and Five O-Glycosylation Sites and Their Biological Implications. Biochem. J. 2005, 390, 285–292. [Google Scholar] [CrossRef]
- Christensen, B.; Zachariae, E.D.; Poulsen, N.A.; Buitenhuis, A.J.; Larsen, L.B.; Sørensen, E.S. Factors Influencing Milk Osteopontin Concentration Based on Measurements from Danish Holstein Cows. J. Dairy Res. 2021, 88, 89–94. [Google Scholar] [CrossRef]
- Dudemaine, P.L.; Thibault, C.; Alain, K.; Bissonnette, N. Genetic Variations in the SPP1 Promoter Affect Gene Expression and the Level of Osteopontin Secretion into Bovine Milk. Anim. Genet. 2014, 45, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.; Jacobsen, L.N.; Ze, X.; Husby, S.; Ueno, H.M.; Nojiri, K.; Kobayashi, S.; Kwon, J.; Liu, X.; Yan, S.; et al. Osteopontin Levels in Human Milk Vary Across Countries and Within Lactation Period: Data From a Multicenter Study. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 250–256. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, X.; Wang, Y.; Bai, S.; Lai, J.; Tong, X.; Xing, Y. Longitudinal Changes of Lactopontin (Milk Osteopontin) in Term and Preterm Human Milk. Front. Nutr. 2022, 9, 962802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Osteopontin in Human Milk and Infant Formula Affects Infant Plasma Osteopontin Concentrations. Pediatr. Res. 2019, 85, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Schytte, G.N.; Christensen, B.; Bregenov, I.; Kjøge, K.; Scavenius, C.; Petersen, S.V.; Enghild, J.J.; Sørensen, E.S. FAM20C Phosphorylation of the RGDSVVYGLR Motif in Osteopontin Inhibits Interaction with the Avβ3 Integrin. J. Cell. Biochem. 2020, 121, 4809–4818. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wen, J.; Wiley, S.E.; Worby, C.A.; Kinch, L.N.; Xiao, J.; Grishin, N.V.; Dixon, J.E. Secreted Kinase Phosphorylates Extracellular Proteins That Regulate Biomineralization. Science 2012, 336, 1150–1153. [Google Scholar] [CrossRef]
- Christensen, B.; Schack, L.; Kläning, E.; Sørensen, E.S. Osteopontin Is Cleaved at Multiple Sites Close to Its Integrin-Binding Motifs in Milk and Is a Novel Substrate for Plasmin and Cathepsin D. J. Biol. Chem. 2010, 285, 7929–7937. [Google Scholar] [CrossRef]
- Christensen, B.; Sørensen, E.S. Osteopontin Is Highly Susceptible to Cleavage in Bovine Milk and the Proteolytic Fragments Bind the AVβ3-Integrin Receptor. J. Dairy Sci. 2014, 97, 136–146. [Google Scholar] [CrossRef]
- Christensen, B.; Karlsen, N.J.; Jørgensen, S.D.S.; Jacobsen, L.N.; Ostenfeld, M.S.; Petersen, S.V.; Müllertz, A.; Sørensen, E.S. Milk Osteopontin Retains Integrin-Binding Activity after in Vitro Gastrointestinal Transit. J. Dairy Sci. 2020, 103, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, J.; Jiang, Y.; Tong, W.; Lai, S.; Ren, Y. Quantitative Determination of Osteopontin in Bovine, Buffalo, Yak, Sheep and Goat Milk by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry and Stable Isotope Dimethyl Labeling. Food Chem. 2021, 343, 128489. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, A.; Lai, S.; Yuan, Q.; Jia, X.; Wang, P.; Zhang, Y. Longitudinal Changes in the Concentration of Major Human Milk Proteins in the First Six Months of Lactation and Their Effects on Infant Growth. Nutrients 2021, 13, 1476. [Google Scholar] [CrossRef]
- Goonatilleke, E.; Huang, J.; Xu, G.; Wu, L.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Human Milk Proteins and Their Glycosylation Exhibit Quantitative Dynamic Variations during Lactation. J. Nutr. 2019, 149, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Aasmul-Olsen, K.; Henriksen, N.L.; Nguyen, D.N.; Heckmann, A.B.; Thymann, T.; Sangild, P.T.; Bering, S.B. Milk Osteopontin for Gut, Immunity and Brain Development in Preterm Pigs. Nutrients 2021, 13, 2675. [Google Scholar] [CrossRef]
- Das, S.; Song, Z.; Han, H.; Ge, X.; Desert, R.; Athavale, D.; Babu Komakula, S.S.; Magdaleno, F.; Chen, W.; Lantvit, D.; et al. Intestinal Osteopontin Protects From Alcohol-Induced Liver Injury by Preserving the Gut Microbiome and the Intestinal Barrier Function. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 813–839. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, R.; Lönnerdal, B. Assessment of Bioactivities of the Human Milk Lactoferrin-Osteopontin Complex in Vitro. J. Nutr. Biochem. 2019, 69, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Kvistgaard, A.S.; Peerson, J.M.; Donovan, S.M.; Peng, Y. Growth, Nutrition, and Cytokine Response of Breast-Fed Infants and Infants Fed Formula With Added Bovine Osteopontin. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 650–657. [Google Scholar] [CrossRef]
- Aquino-Domínguez, A.S.; Romero-Tlalolini, M.L.A.; Torres-Aguilar, H.; Aguilar-Ruiz, S.R. Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets. Int. J. Mol. Sci. 2021, 22, 10230. [Google Scholar] [CrossRef]
- Van Herwijnen, M.J.C.; Zonneveld, M.I.; Goerdayal, S.S.; Nolte-‘t Hoen, E.N.M.; Garssen, J.; Stahl, B.; Maarten Altelaar, A.F.; Redegeld, F.A.; Wauben, M.H.M. Comprehensive Proteomic Analysis of Human Milk-Derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Wang, S.; Qiao, X.; Zhao, B.; Bai, J.; Zhao, Y.; Shi, Y. Lactoferricin, an antimicrobial motif derived from lactoferrin with food preservation potential. Crit. Rev. Food Sci. Nutr. 2024, 64, 9032–9044. [Google Scholar] [CrossRef]
- Nibbering, P.H.; Ravensbergen, E.; Welling, M.M.; van Berkel, L.A.; van Berkel, P.H.; Pauwels, E.K.; Nuijens, J.H. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Björn, C.; Mahlapuu, M.; Mattsby-Baltzer, I.; Håkansson, J. Anti-infective efficacy of the lactoferrin-derived antimicrobial peptide HLR1r. Peptides 2016, 81, 21–28. [Google Scholar] [CrossRef]
- Haney, E.F.; Nazmi, K.; Lau, F.; Bolscher, J.G.; Vogel, H.J. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 2009, 91, 141–154. [Google Scholar] [CrossRef]
- Fu, Y.; Ji, C.; Chen, X.; Cui, X.; Wang, X.; Feng, J.; Li, Y.; Qin, R.; Guo, X. Investigation into the antimicrobial action and mechanism of a novel endogenous peptide β-casein 197 from human milk. AMB Express 2017, 7, 119. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Yan, X.; Yu, Q.; Hu, X.; Chen, X.; Han, S. Antibacterial function and mechanism of peptide β-casein 65 action in breast milk. Authorea 2023. preprint. [Google Scholar] [CrossRef]
- Murakami, M.; Dorschner, R.A.; Stern, L.J.; Lin, K.H.; Gallo, R.L. Expression and secretion of cathelicidin antimicrobial peptides in murine mammary glands and human milk. Pediatr. Res. 2005, 57, 10–15. [Google Scholar] [CrossRef]
- Jia, H.P.; Starner, T.; Ackermann, M.; Kirby, P.; Tack, B.F.; McCray, P.B., Jr. Abundant human beta-defensin-1 expression in milk and mammary gland epithelium. J. Pediatr. 2001, 138, 109–112. [Google Scholar] [CrossRef]
- Baricelli, J.; Rocafull, M.A.; Vázquez, D.; Bastidas, B.; Báez-Ramirez, E.; Thomas, L.E. β-defensin-2 in breast milk displays a broad antimicrobial activity against pathogenic bacteria. J. Pediatr. 2015, 91, 36–43. [Google Scholar] [CrossRef]
- Trend, S.; Strunk, T.; Hibbert, J.; Kok, C.H.; Zhang, G.; Doherty, D.A.; Richmond, P.; Burgner, D.; Simmer, K.; Davidson, D.J.; et al. Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PLoS ONE 2015, 10, e0117038. [Google Scholar] [CrossRef]
- Chang, X.Y.; Zhang, M.W.; Zhang, L.J.; Chai, L.Q. The Function and Modification of Human Defensin 5. Protein Pept. Lett. 2023, 30, 830–840. [Google Scholar] [CrossRef]
- Bezkorovainy, A.; Topouzian, N. The effect of metal chelators and other metabolic inhibitors on the growth of Bifidobacterium bifidus var. Pennsylvanicus. Clin. Biochem. 1981, 14, 135–141. [Google Scholar] [CrossRef]
- Woodman, T.; Strunk, T.; Patole, S.; Hartmann, B.; Simmer, K.; Currie, A. Effects of lactoferrin on neonatal pathogens and Bifidobacterium breve in human breast milk. PLoS ONE 2018, 13, e0201819. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Nwosu, C.; Ruiz-Moyano, S.; Aldredge, D.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Endo-β-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol. Cell. Proteom. 2012, 11, 775–785. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Lactoferrin and bifidobacteria. Biometals 2014, 27, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Liepke, C.; Adermann, K.; Raida, M.; Mägert, H.J.; Forssmann, W.G.; Zucht, H.D. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 2002, 269, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Ohashi, M.; Tanaka, T.; Kumura, H.; Kim, G.Y.; Kwon, I.K.; Goh, J.S.; Shimazaki, K. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals 2004, 17, 279–283. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Sato, T.; Xiao, J.Z.; Abe, F.; Iwatsuki, K. Isolation of a bifidogenic peptide from the pepsin hydrolysate of bovine lactoferrin. Appl. Environ. Microbiol. 2013, 79, 1843–1849. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef]
- Heine, W.; Braun, O.H.; Mohr, C.; Leitzmann, P. Enhancement of lysozyme trypsin-mediated decay of intestinal bifidobacteria and lactobacilli. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 54–58. [Google Scholar] [CrossRef]
- Rockova, S.; Rada, V.; Marsik, P.; Vlkova, E.; Bunesova, V.; Sklenar, J.; Splichal, I. Growth of bifidobacteria and clostridia on human and cow milk saccharides. Anaerobe 2011, 17, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Tian, F.; Cao, R.M.; Li, J.; Wu, S.M.; Guo, X.K.; Chen, T.X. Antimicrobial activity of human β-defensins against lactic acid bacteria. Nat. Prod. Res. 2015, 29, 2164–2166. [Google Scholar] [CrossRef]
- Joly, S.; Maze, C.; McCray, P.B., Jr.; Guthmiller, J.M. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J. Clin. Microbiol. 2004, 42, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Okuda, D.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 2005, 40, 123–132. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Farver, M.; Smilowitz, J.T. The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants. Nutr. Metab. Insights 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef]
- Wang, K.; Xia, X.; Sun, L.; Wang, H.; Li, Q.; Yang, Z.; Ren, J. Microbial Diversity and Correlation between Breast Milk and the Infant Gut. Foods 2023, 12, 1740. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef]
- Grzywacz, K.; Butcher, J.; Li, J.; Barrington, K.; Mohamed, I.; Stintzi, A. Bovine Lactoferrin Supplementation Does Not Disrupt Microbiota Development in Preterm Infants Receiving Probiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 216–222. [Google Scholar] [CrossRef]
- González, L.; Paredes Sosa, J.L.; Mosquito, S.; Filio, Y.; Romero, P.E.; Ochoa, T.J.; Tsukayama, P. Oral lactoferrin administration does not impact the diversity or composition of the infant gut microbiota in a Peruvian cohort. Microbiol. Spectr. 2023, 11, e0009623. [Google Scholar] [CrossRef]
- Cheng, W.D.; Wold, K.J.; Bollinger, L.B.; Ordiz, M.I.; Shulman, R.J.; Maleta, K.M.; Manary, M.J.; Trehan, I. Supplementation With Lactoferrin and Lysozyme Ameliorates Environmental Enteric Dysfunction: A Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Gastroenterol. 2019, 114, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.D.; Wold, K.J.; Benzoni, N.S.; Thakwalakwa, C.; Maleta, K.M.; Manary, M.J.; Trehan, I. Lactoferrin and lysozyme to reduce environmental enteric dysfunction and stunting in Malawian children: Study protocol for a randomized controlled trial. Trials 2017, 18, 523. [Google Scholar] [CrossRef] [PubMed]
- Kasiati; Pertami, S.B.; Utomo, A.; Arifah, S. Stability of lactoferrin and lysozyme in human milk at various temperatures and duration of storage. Malays. J. Nutr. 2021, 27, 271–278. [Google Scholar] [CrossRef]
- Ferreres-Serafini, L.; Martín-Orúe, S.M.; Sadurní, M.; Jiménez, J.; Moreno-Muñoz, J.A.; Castillejos, L. Supplementing infant milk formula with a multi-strain synbiotic and osteopontin enhances colonic microbial colonization and modifies jejunal gene expression in lactating piglets. Food Funct. 2024, 15, 6536–6552. [Google Scholar] [CrossRef]
- Corebima, B.I.R.V.; Rohsiswatmo, R.; Gayatri, P.; Patole, S. Fecal human β-defensin-2 (hBD-2) levels and gut microbiota patterns in preterm neonates with different feeding patterns. Iran. J. Microbiol. 2019, 11, 151–159. [Google Scholar]
- Widjaja, N.A.; Hamidah, A.; Purnomo, M.T.; Ardianah, E. Effect of lactoferrin in oral nutrition supplement (ONS) towards IL-6 and IL-10 in failure to thrive children with infection. F1000Research 2023, 12, 897. [Google Scholar] [CrossRef]
- Dix, C.; Wright, O. Bioavailability of a Novel Form of Microencapsulated Bovine Lactoferrin and Its Effect on Inflammatory Markers and the Gut Microbiome: A Pilot Study. Nutrients 2018, 10, 1115. [Google Scholar] [CrossRef]
- Bellés, A.; Aguirre-Ramírez, D.; Abad, I.; Parras-Moltó, M.; Sánchez, L.; Grasa, L. Lactoferrin modulates gut microbiota and Toll-like receptors (TLRs) in mice with dysbiosis induced by antibiotics. Food Funct. 2022, 13, 5854–5869. [Google Scholar] [CrossRef]
- Wu, H.; Fan, L.; Gao, Y.; Wang, J.; Zheng, N. The Protective Effects of Iron Free Lactoferrin on Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Modulating the NF-κB/PPAR Signaling Pathway. Foods 2022, 11, 3378. [Google Scholar] [CrossRef]
- Perdijk, O.; van Neerven, R.J.J.; van den Brink, E.; Savelkoul, H.F.J.; Brugman, S. Bovine Lactoferrin Modulates Dendritic Cell Differentiation and Function. Nutrients 2018, 10, 848. [Google Scholar] [CrossRef]
- Kubo, S.; Miyakawa, M.; Tada, A.; Oda, H.; Motobayashi, H.; Iwabuchi, S.; Tamura, S.; Tanaka, M.; Hashimoto, S. Lactoferrin and its digestive peptides induce interferon-α production and activate plasmacytoid dendritic cells ex vivo. Biometals 2023, 36, 563–573. [Google Scholar] [CrossRef]
- Wisgrill, L.; Wessely, I.; Spittler, A.; Förster-Waldl, E.; Berger, A.; Sadeghi, K. Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages. Clin. Exp. Immunol. 2018, 192, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Bartal, L.; Padeh, S.; Passwell, J.H. Lactoferrin inhibits prostaglandin E2 secretion by breast milk macrophages. Pediatr. Res. 1987, 21, 54–57. [Google Scholar] [CrossRef]
- Jiang, R.; Lönnerdal, B. Effects of Milk Osteopontin on Intestine, Neurodevelopment, and Immunity. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Stroinigg, N.; Srivastava, M.D. Modulation of toll-like receptor 7 and LL-37 expression in colon and breast epithelial cells by human beta-defensin-2. Allergy Asthma Proc. 2005, 26, 299–309. [Google Scholar]
- Hassiotou, F.; Geddes, D.T. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Adv. Nutr. 2015, 6, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Saifi, F.; Jeoboam, B.; Demory Beckler, M.; Costin, J.M. The Association Between Lactational Infective Mastitis and the Microbiome: Development, Onset, and Treatments. Cureus 2024, 16, e62717. [Google Scholar] [CrossRef]
- Samuel, T.M.; De Castro, C.A.; Dubascoux, S.; Affolter, M.; Giuffrida, F.; Billeaud, C.; Picaud, J.C.; Agosti, M.; Al-Jashi, I.; Pereira, A.B.; et al. Subclinical Mastitis in a European Multicenter Cohort: Prevalence, Impact on Human Milk (HM) Composition, and Association with Infant HM Intake and Growth. Nutrients 2020, 12, 105. [Google Scholar] [CrossRef]
- Simojoki, H.; Hyvönen, P.; Orro, T.; Pyörälä, S. High concentration of human lactoferrin in milk of rhLf-transgenic cows relieves signs of bovine experimental Staphylococcus chromogenes intramammary infection. Vet. Immunol. Immunopathol. 2010, 136, 265–271. [Google Scholar] [CrossRef]
- Hyvönen, P.; Suojala, L.; Orro, T.; Haaranen, J.; Simola, O.; Røntved, C.; Pyörälä, S. Transgenic cows that produce recombinant human lactoferrin in milk are not protected from experimental Escherichia coli intramammary infection. Infect. Immun. 2006, 74, 6206–6212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).