4-Year Study in Monitoring the Presence of Legionella in the Campania Region’s Healthcare Facilities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Type of Facilities Analyzed, and Collected Samples
2.2. Sample Collection
2.3. Microbiological Analysis and Identification
2.4. Data Analysis
3. Results
Colonization by Legionella: Levels of Contamination and Species Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younas, F.; Soltanmohammadi, N.; Knapp, O.; Benz, R. The major outer membrane protein of Legionella pneumophila Lpg1974 shows pore-forming characteristics similar to the human mitochondrial outer membrane pore, hVDAC1. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.G.; Fernandes, H.S.; Melo, A.; Sousa, S.F.; Simões, L.C.; Simões, M. LegionellaDB—A database on. Trends Microbiol. 2021, 29, 863–866. [Google Scholar] [CrossRef]
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and legionnaires’ disease 25 Years of investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef] [PubMed]
- Herpers, B.L.; Bossink, A.W.; Cohen Stuart, J.W.; Hustinx, W.N.; Thijsen, S.F. A patient with Legionella pneumophila serogroup-3 pneumonia, detected by PCR. Ned. Tijdschr. Geneeskd. 2005, 149, 2009–2012. Available online: https://pubmed.ncbi.nlm.nih.gov/16171114 (accessed on 14 November 2022).
- Khanna, N.; Meikle, A.; Gillespie, L.; Edwards, G.; Lindsay, D. Legionella pneumophila serogroup 3 infection Importance of serology. Scott. Med. J. 2012, 57, 182. [Google Scholar] [CrossRef]
- Ito, A.; Ishida, T.; Washio, Y.; Yamazaki, A.; Tachibana, H. Legionella pneumonia due to non-Legionella pneumophila serogroup 1 Usefulness of the six-point scoring system. BMC Pulm. Med. 2017, 17, 211. [Google Scholar] [CrossRef]
- Ito, A.; Ishida, T.; Tachibana, H.; Ito, Y.; Takaiwa, T.; Fujii, H.; Hashimoto, T.; Nakajima, H.; Amemura-Maekawa, J. A case of community-acquired pneumonia due to Legionella pneumophila serogroup 9 wherein initial treatment with single-dose oral azithromycin appeared useful. Jpn. J. Infect. Dis. 2017, 70, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, K.Y.; Hsueh, P.R.; Yang, P.C. Severe community-acquired pneumonia due to Legionella pneumophila serogroup 6. J. Formos. Med. Assoc. 2006, 105, 256–262. [Google Scholar] [CrossRef]
- Legionella Generalità. Available online: https://www.iss.it/documents/20126/1989545/Legionella_generalit_PDF.doc/4dad36bb-ded6-0988-1e62-cd6ac8d464e2?t=1575701441520&download=true (accessed on 30 September 2024).
- Lombardi, A.; Borriello, T.; De Rosa, E.; Di Duca, F.; Sorrentino, M.; Torre, I.; Montuori, P.; Trama, U.; Pennino, F. Environmental Monitoring of Legionella in Hospitals in the Campania Region A 5-Year Study. Int. J. Environ. Res. Public Health 2023, 20, 5526. [Google Scholar] [CrossRef]
- Scanlon, M.M.; Gordon, J.L.; McCoy, W.F.; Cain, M.F. Water management for construction: Evidence for risk characterization in community and healthcare settings: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 2168. [Google Scholar] [CrossRef] [PubMed]
- Straus, W.L.; Plouffe, J.F.; File, T.M.; Lipman, H.B.; Hackman, B.H.; Salstrom, S.J.; Benson, R.F.; Breiman, R.F. Risk factors for domestic acquisition of Legionnaires disease. Arch. Intern. Med. 1996, 156, 1685–1692. [Google Scholar] [CrossRef]
- Viasus, D.; Di Yacovo, S.; Garcia-Vidal, C.; Verdaguer, R.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Community-acquired Legionella pneumophila pneumonia A single-center experience with 214 hospitalized sporadic cases over 15 years. Medicine 2013, 92, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Campese, C.; Bitar, D.; Jarraud, S.; Maine, C.; Forey, F.; Etienne, J.; Desenclos, J.C.; Saura, C.; Che, D. Progress in the surveillance and control of Legionella infection in France, 1998–2008. Int. J. Infect. Dis. 2011, 15, e30–e37. [Google Scholar] [CrossRef][Green Version]
- Den Boer, J.W.; Nijhof, J.; Friesema, I. Risk factors for sporadic community-acquired Legionnaires’ disease. A 3-year national case–control study. Public Health 2006, 120, 566–571. [Google Scholar] [CrossRef]
- Marston, B.J.; Lipman, H.B.; Breiman, R.F. Surveillance for Legionnaires’ disease Risk factors for morbidity and mortality. Arch. Intern. Med. 1994, 154, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Diederen, B.M.W. Legionella spp. and Legionnaires’ disease. J. Infect. 2008, 56, 1–12. [Google Scholar] [CrossRef]
- Di Onofrio, V.; Pagano, M.; Santulli, M.; Rossi, A.; Liguori, R.; Di Dio, M.; Liguori, G. Contamination of Hotel Water Distribution Systems by Legionella Species: Environmental Surveillance in Campania Region, South Italy. Microorganisms 2023, 11, 1840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sciuto, E.L.; Laganà, P.; Filice, S.; Scalese, S.; Libertino, S.; Corso, D.; Faro, G.; Coniglio, M.A. Environmental Management of Legionella in Domestic Water Systems: Consolidated and Innovative Approaches for Disinfection Methods and Risk Assessment. Microorganisms 2021, 9, 577. [Google Scholar] [CrossRef]
- Italian Health Ministry Guidelines for Prevention and Control of Legionellosis. 2015. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2362_allegato.pdf (accessed on 14 November 2022).
- Field, B.S. Minimizing the Risk of Legionellosis Associated with Building Water Systems. Ashrae Guidline. 2000. Available online: https://cir.nii.ac.jp/crid/1570291225346474368 (accessed on 14 November 2022).
- Muzzi, A.; Cutti, S.; Bonadeo, E.; Lodola, L.; Monzillo, V.; Corbella, M.; Scudeller, L.; Novelli, V.; Marena, C. Prevention of nosocomial legionellosis by best water management Comparison of three decontamination methods. J. Hosp. Infect. 2020, 105, 766–772. [Google Scholar] [CrossRef]
- Viasus, D.; Gaia, V.; Manzur-Barbur, C.; Carratalà, J. Legionnaires’ disease Update on diagnosis and treatment. Infect. Dis. Ther. 2022, 11, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Cagarelli, R.; Caraglia, A.; La Mura, S.; Mele, G.; Ottaviani, M.; Pompa, M.; Ricci, M.L.; Rota, M.C.; Scaturro, M.; Venditti, M.; et al. Linee Guida per la Prevenzione ed il Controllo della Legionellosi. Conferenza Stato-Regioni del 07/05/2015, Repertorio Atti n.: 79/ CSR del 07/05/2015. 2015. Available online: http://old.iss.it/binary/iss4/cont/C_17_pubblicazioni_2362.pdf (accessed on 23 February 2023).
- Linee Guida Recanti Indicazioni ai Laboratori con Attività di Diagnosi Microbiologica e Controllo Ambientale della Legionellosi. Gazzetta Ufficiale della Repubblica Italiana (Serie Generale), Numero 29. 2005. Available online: http://old.iss.it/binary/iss4/cont/29_02_05.1144152818.pdf (accessed on 13 January 2005).
- ISO/IEC 17025; Legionella pneumophila Identification in Drinking Water Samples. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO/IEC 11731; Water Quality Enumeration of Legionella. International Organization for Standardization: Geneva, Switzerland, 2017.
- Ricketts, K.D.; Joseph, C.; on behalf of the European Working Group for Legionella Infections. Travel Associated Legionnaires’ Disease in Europe 2003. Eurosurveillance 2004, 9, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Torre, I.; Diana, M.V.; Iervolino, C.; Borriello, T.; Imperato, O.C.; Maccarino, S.; Pennino, F. Legionella contamination in hospitals of the Campania Region Five years of environmental surveillance results. Ann. Di Ig. 2014, 26, 89–96. [Google Scholar] [CrossRef] [PubMed]
- UNI EN ISO 19458:2006; Qualità dell’acqua–Campionamento per Analisi Microbiologiche. Ente Italiano di Unificazione: Milano, Italy, 2006.
- WHO. Coronavirus Disease (COVID-19) Weekly Epidemiological Updates and Monthly Operational Updates. Situation Report-25. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports# (accessed on 27 February 2023).
- Proctor, C.R.; Rhoads, W.J.; Keane, T.; Salehi, M.; Hamilton, K.; Pieper, K.J.; Whelton, A.J. Considerations for large building water quality after extended stagnation. AWWA Water Sci. 2020, 2, e1186. [Google Scholar] [CrossRef] [PubMed]
- Protocollo Condiviso di Aggiornamento delle Misure per il Contrasto e il Contenimento della Diffusione del Virus SARS-CoV-2/COVID-19 Negli Ambienti di Lavoro. 2021. Available online: https://www.lavoro.gov.it/notizie/Documents/Aggiornamento-Protocollo-generale-COVID-6-aprile-2021.pdf (accessed on 6 April 2021).
- Graham, F.F.; Hales, S.; White, P.S.; Baker, M.G. Review Global seroprevalence of legionellosis-a systematic review and meta-analysis. Sci. Rep. 2020, 10, 7337. [Google Scholar] [CrossRef]
- Stojek, N.M.; Wójcik-Fatla, A.; Dutkiewicz, J. Efficacy of the detection of Legionella in hot and cold water samples by culture and PCR. II. Examination of native samples from various sources. Ann. Agric. Environ. Med. 2012, 19, 295–298. Available online: https://pubmed.ncbi.nlm.nih.gov/22742805/ (accessed on 16 January 2023).
- Boppe, I.; Bedard, E.; Taillandier, C.; Lecellier, D.; Nantel-Gauvin, M.A.; Villion, M.; Prévost, M. Investigative approach to improve hot water system hydraulics through temperature monitoring to reduce building environmental quality hazard associated to Legionella. Build. Environ. 2016, 108, 230–239. [Google Scholar] [CrossRef]
- Falkinham JO 3rd Hilborn, E.D.; Arduino, M.J.; Pruden, A.; Edwards, M.A. Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 2015, 123, 749–758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Giglio, O.; Fasano, F.; Diella, G.; Lopuzzo, M.; Napoli, C.; Apollonio, F.; Montagna, M.T. Legionella and legionellosis in touristic-recreational facilities: Influence of climate factors and geostatistical analysis in Southern Italy (2001–2017). Environ. Res. 2019, 178, 108721. [Google Scholar] [CrossRef]

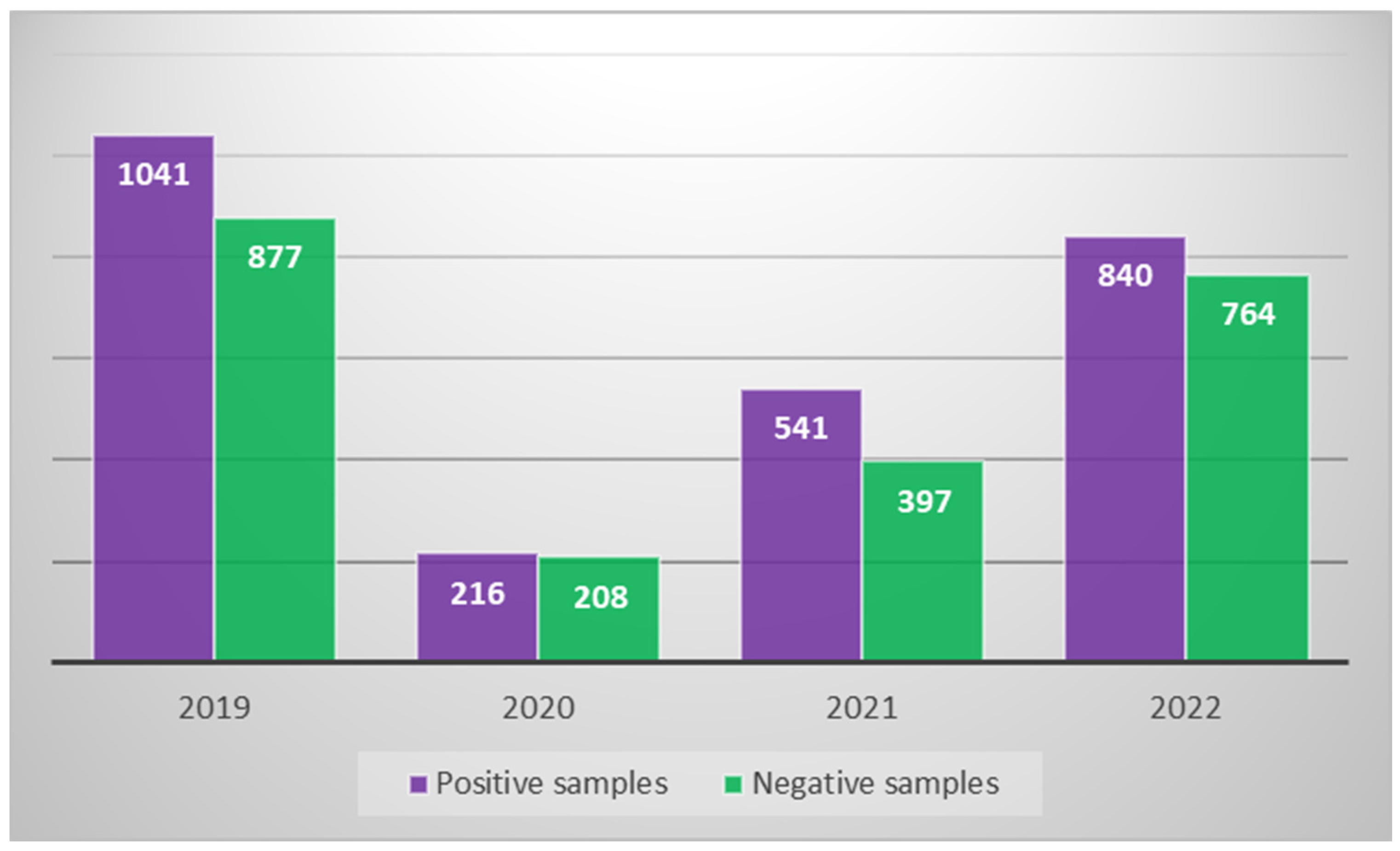
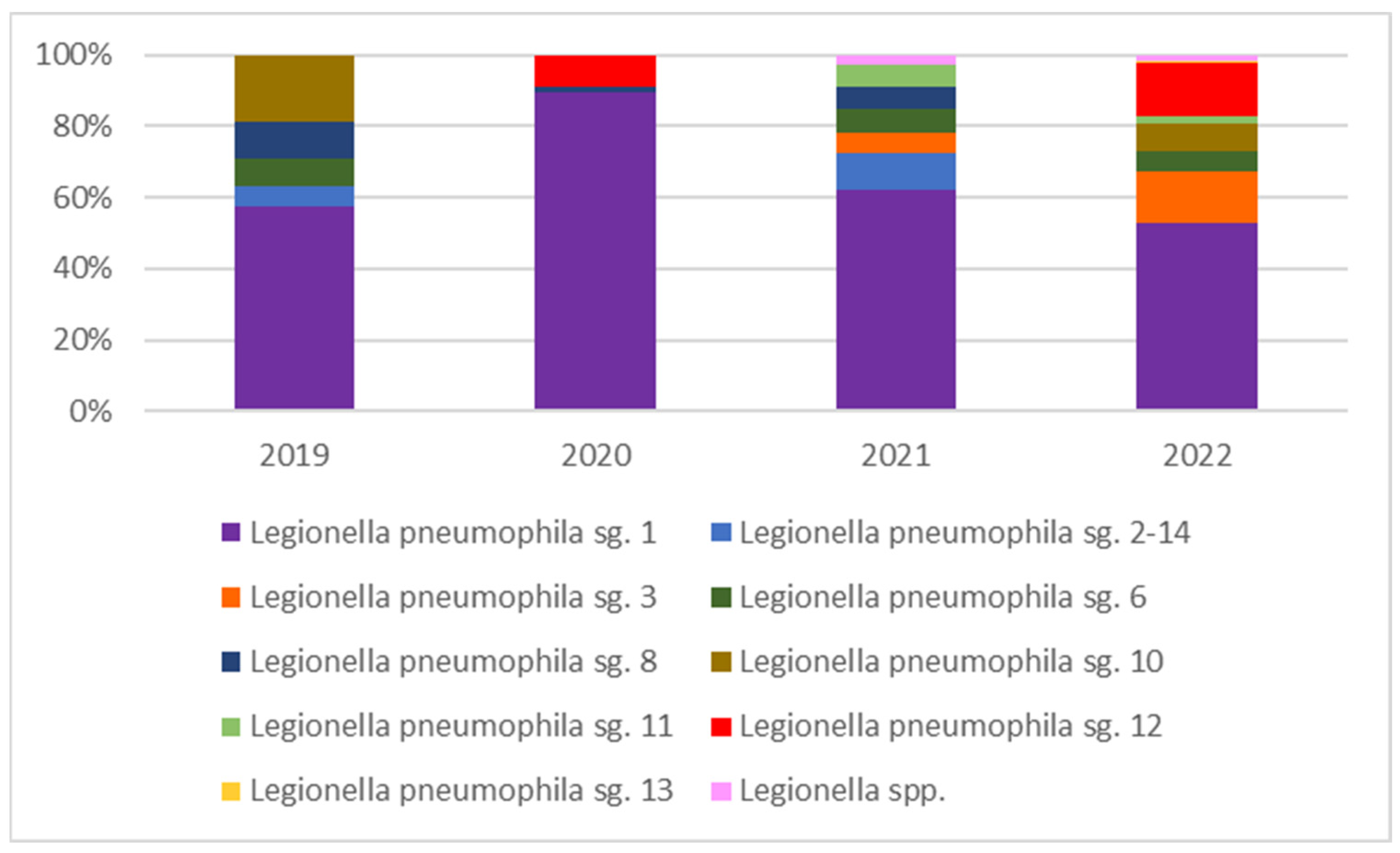
| Year | Sample Source | Number of Positive Samples Broken Down by Sample Source | Percent Positivity by Sample Source Type | Bacteria Count (CFU/L) (Average Value) | Minimum and Maximum Bacterial Count Value (CFU/L) |
|---|---|---|---|---|---|
| 2019 2020 2021 2022 | Cold Water | 127 24 74 85 | 11.8% | 65,866.43 5700.00 1037.50 1980.00 | 3 × 10–10.2 × 103 |
| 2019 2020 2021 2022 | Hot Water | 838 179 437 656 | 79.9% | 104,553.92 21,257.69 4904.08 12,568.67 | 102–1.5 × 106 |
| 2019 2020 2021 2022 | Thermal Water | 76 13 30 99 | 8.3% | 9816.67 135,000.00 925.00 2938.46 | 3 × 102–13.5 × 104 |
| Year | Facilities Analyzed | Samples Collected | Positive Samples | Bacteria Count (CFU/L) |
|---|---|---|---|---|
| 2019 | 41 | 1918 | 1041 | In 259 samples, between 10 and 103 In 547 samples, between 103 and 104 In 235 samples, between 104 and 105 |
| 2020 | 21 | 424 | 216 | In 183 samples, between 102 and 103 In 33 samples, between 104 and 105 |
| 2021 | 21 | 938 | 541 | In 519 samples, between 102 and 103 In 22 samples, between 103 and 104 |
| 2022 | 33 | 1604 | 840 | In 757 samples, between 10 and 103 In 83 samples, between 104 and 105 |
| Year | Highest Counts (CFU/L) | N° of Facilities with Positive Samples/n° of Hotel Examined |
|---|---|---|
| 2019 | 1.5 × 106 | 17/41 |
| 2020 | 4 × 105 | 9/21 |
| 2021 | 8.5 × 104 | 12/21 |
| 2022 | 6 × 105 | 15/33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Dio, M.; Santulli, M.; Pagano, M.; Rossi, A.M.; Liguori, R.; Liguori, G.; Di Onofrio, V. 4-Year Study in Monitoring the Presence of Legionella in the Campania Region’s Healthcare Facilities. Hygiene 2025, 5, 16. https://doi.org/10.3390/hygiene5020016
Di Dio M, Santulli M, Pagano M, Rossi AM, Liguori R, Liguori G, Di Onofrio V. 4-Year Study in Monitoring the Presence of Legionella in the Campania Region’s Healthcare Facilities. Hygiene. 2025; 5(2):16. https://doi.org/10.3390/hygiene5020016
Chicago/Turabian StyleDi Dio, Mirella, Marco Santulli, Mariangela Pagano, Anna Maria Rossi, Renato Liguori, Giorgio Liguori, and Valeria Di Onofrio. 2025. "4-Year Study in Monitoring the Presence of Legionella in the Campania Region’s Healthcare Facilities" Hygiene 5, no. 2: 16. https://doi.org/10.3390/hygiene5020016
APA StyleDi Dio, M., Santulli, M., Pagano, M., Rossi, A. M., Liguori, R., Liguori, G., & Di Onofrio, V. (2025). 4-Year Study in Monitoring the Presence of Legionella in the Campania Region’s Healthcare Facilities. Hygiene, 5(2), 16. https://doi.org/10.3390/hygiene5020016







