Antimicrobial Activity of Diffusible Substances Produced by Lactococcus lactis Against Bacillus cereus in a Non-Contact Co-Culture Model
Abstract
1. Introduction
2. Materials and Methods
2.1. The Co-Culture Model and Data Collection
2.2. Flow Cytometry Analysis
2.3. Preparation of Diffusible Substances
2.4. The Total Protein Content of Diffusible Substances
2.5. The Purification and Identification of Bacteriocin Fractions
2.6. Evaluation of the Antimicrobial Activity of Diffusible Substances
2.7. Data Processing and Statistical Analysis
3. Results and Discussion
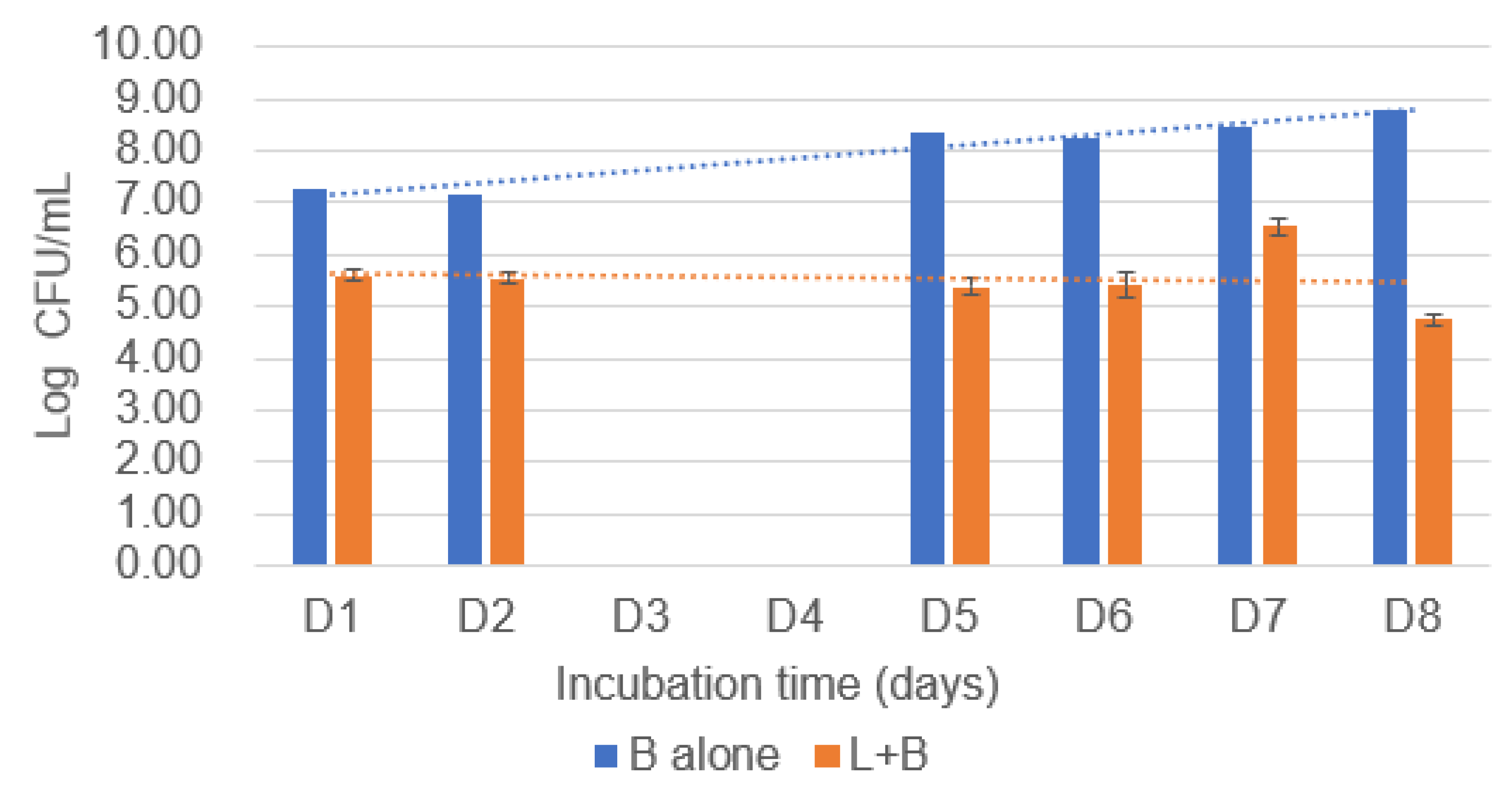
3.1. The CFU Data of Co-Culture Model from Plate Cultures
3.1.1. Counts of B. cereus Following Co-Culture
3.1.2. Counts of E. coli Following Co-Culture
3.2. The FCM Results of Co-Culture Model
3.2.1. Flow Cytometry Measurements of B. cereus
3.2.2. Flow Cytometry Measurements of E. coli
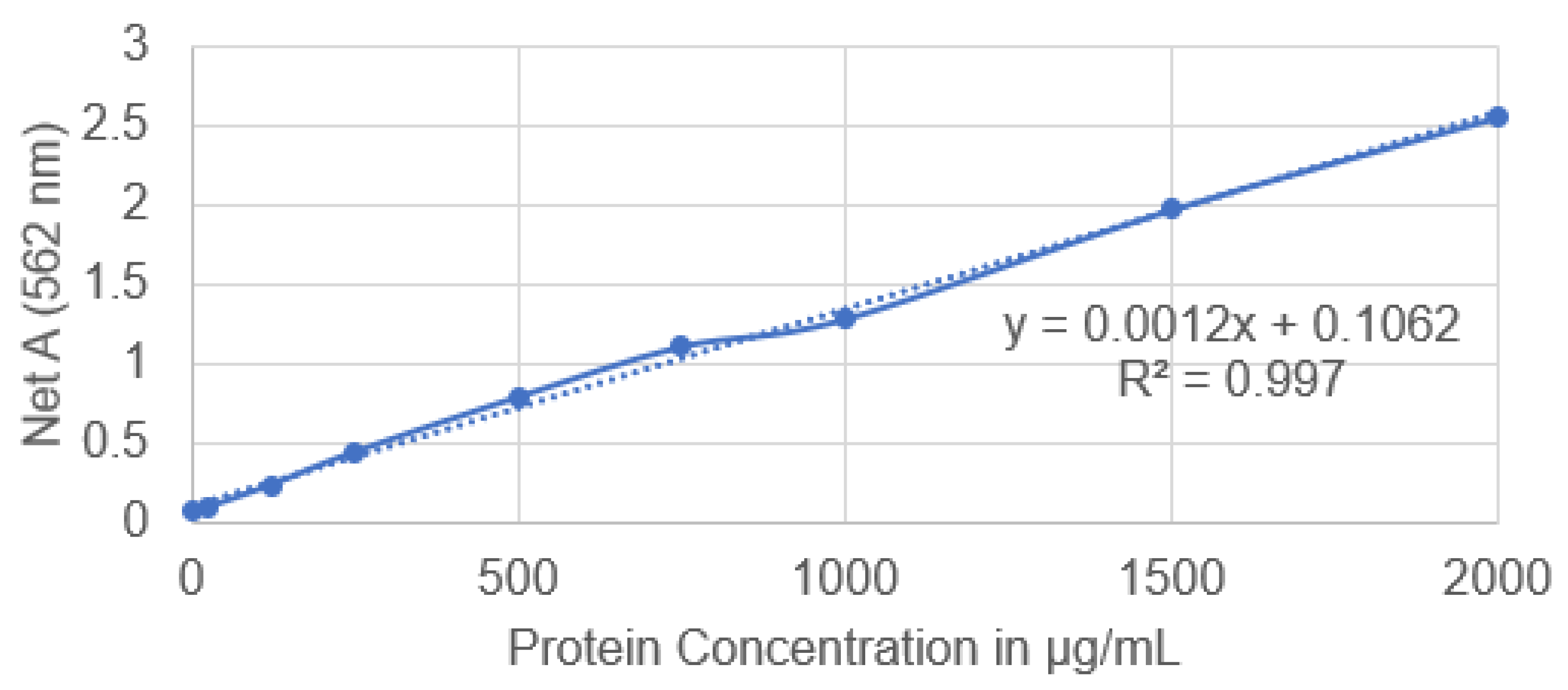
3.3. Protein Concentration Analysis of Extracted Diffusible Substances
3.4. The Compound Contained in the Diffusible Substance
3.5. The Antimicrobial Activity of Diffusible Substances
The Results of Disc Diffusion Assay
3.6. Limitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Samples | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
|---|---|---|---|---|---|---|---|
| L + B (1) | L | 6.38 | 6.18 | 6.48 | 6.43 | ||
| B | 5.28 | 4.62 | 5.14 | 4.32 | |||
| L + B (2) | L | 6.53 | 6.52 | 6.09 | 6.38 | ||
| B | 5.34 | 4.60 | 4.75 | 4.53 | |||
| L + B (3) | L | 6.56 | 6.82 | 6.41 | 6.37 | ||
| B | 5.00 | 4.72 | 5.34 | 5.08 | |||
| L + E (1) | L | 6.24 | 6.29 | 6.21 | 6.16 | ||
| E | 4.52 | NG | NG | NG | |||
| L + E (2) | L | 6.46 | 6.60 | 6.39 | 6.37 | ||
| E | 4.15 | NG | NG | NG | |||
| L + E (3) | L | 6.18 | 6.51 | 6.32 | 6.22 | ||
| E | 3.90 | NG | NG | NG | |||
| L alone | L | 6.36 | 6.45 | 6.37 | 6.31 | ||
| B alone | B | 7.18 | 7.23 | 7.48 | 7.46 | ||
| E alone | E | 7.19 | 7.92 | 7.12 | 8.11 |
References
- Lee, H.; Yoon, Y. Etiological agents implicated in foodborne illness world wide. Food Sci. Anim. Resour. 2021, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Foodborne Diseases; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/health-topics/foodborne-diseases#tab=tab_1 (accessed on 21 December 2023).
- Medeiros, L.; LeJeune, J. Bacillus cereus: A Foodborne Illness Confused with the 24-hour Flu. Ohio State University Extension. 2011. Available online: https://ohioline.osu.edu/factsheet/HYG-5576-11 (accessed on 21 December 2023).
- Ehling-Schulz, M.; Messelhäusser, U.; Granum, P.E. Bacillus cereus in milk and dairy production. In Rapid Detection, Characterization, and Enumeration of Foodborne Pathogens; Wiley: Hoboken, NJ, USA, 2011; pp. 275–289. [Google Scholar] [CrossRef]
- Dervyn, R.; Kavanaugh, D.W.; Cormontagne, D.; Glasset, B.; Ramarao, N. Identification of a new pathogenicity island within the large pAH187_270 plasmid involved in Bacillus cereus virulence. Front. Cell. Infect. Microbiol. 2022, 11, 788757. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.; Ornelis, V.F.; Madder, A.; Rajkovic, A. Bacillus cereus food intoxication and toxicoinfection. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3719–3761. [Google Scholar] [CrossRef] [PubMed]
- Schoeni, J.L.; Wong, A.C. Bacillus cereus food poisoning and its toxins. J. Food Prot. 2005, 68, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus food infection as multifactorial process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef]
- Glasset, B.; Sperry, M.; Dervyn, R.; Herbin, S.; Brisabois, A.; Ramarao, N. The cytotoxic potential of Bacillus cereus strains of various origins. Food Microbiol. 2021, 98, 103759. [Google Scholar] [CrossRef]
- Adamski, P.; Byczkowska-Rostkowska, Z.; Gajewska, J.; Zakrzewski, A.J.; Kłębukowska, L. Prevalence and Antibiotic Resistance of Bacillus sp. Isolated from Raw Milk. Microorganisms 2023, 11, 1065. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent advances in the application of the antimicrobial peptide nisin in the inactivation of spore-forming bacteria in foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Li, Y.; Hong, B.; Kang, D.; Ma, Y.; Wang, J. Two novel antimicrobial peptides against vegetative cells, spores and biofilm of Bacillus cereus. Food Control 2023, 149, 109688. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Omoregbe, O.; Hart, A.; Miri, T.; Eze, U.A.; Onyeaka, H. Applications of bacteriocins of lactic acid bacteria in biotechnology and food preservation: A bibliometric review. Open Microbiol. J. 2022, 16. [Google Scholar] [CrossRef]
- Mariam, S.H.; Zegeye, N.; Aseffa, A.; Howe, R. Diffusible substances from lactic acid bacterial cultures exert strong inhibitory effects on Listeria monocytogenes and Salmonella enterica serovar enteritidis in a co-culture model. BMC Microbiol. 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lindgren, S.E.; Dobrogosz, W.J. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol. Rev. 1990, 7, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Putra, T.F.; Suprapto, H.; Tjahjaningsih, W.; Pramono, H. The antagonistic activity of lactic acid bacteria isolated from peda, an Indonesian traditional fermented fish. IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 012060. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Garneau, S.; Martin, N.I.; Vederas, J.C. Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 2002, 84, 577–592. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Dang, H. Lactic acid bacteria and γ-aminobutyric acid and diacetyl. In Lactic Acid Bacteria; Springer: Singapore, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; de Melo, M.R.; da Silva, C.M.R.; Jain, S.; Dolabella, S.S. Nisin resistance in Gram-positive bacteria and approaches to circumvent resistance for successful therapeutic use. Crit. Rev. Microbiol. 2021, 47, 376–385. [Google Scholar] [CrossRef]
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 512–542. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- O’Reilly, C.; O’Connor, P.M.; O’Sullivan, Ó.; Rea, M.C.; Hill, C.; Ross, R.P. Impact of nisin on Clostridioides difficile and microbiota composition in a faecal fermentation model of the human colon. J. Appl. Microbiol. 2022, 132, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Vukomanović, M.; Žunič, V.; Kunej, Š.; Jančar, B.; Jeverica, S.; Podlipec, R.; Suvorov, D. Nano-engineering the antimicrobial spectrum of lantibiotics: Activity of nisin against gram negative bacteria. Sci. Rep. 2017, 7, 4324. [Google Scholar] [CrossRef] [PubMed]
- Raccach, M. Pediococcus. In Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 1–5. [Google Scholar]
- Singh, V.P. Recent approaches in food bio-preservation-a review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Ray, B. Nisin of Lactococcus lactis ssp. lactis as a food biopreservative. In Food Biopreservatives of Microbial Origin; CRC Press: Boca Raton, FL, USA, 2019; pp. 207–264. [Google Scholar]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Silva, C.C.; Silva, S.P.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- WISC. BD Accuri™ C6 Flow Cytometer Instrument Manual; WISC, 2017; Available online: https://bif.wisc.edu/wp-content/uploads/sites/389/2017/11/bd_accuri_c6_flow_cytometer_instrument_manual.pdf (accessed on 20 July 2022).
- BD. BD Accuri™ C6 Plus Flow Cytometer Optical Filter Guide; BD, 2016; Available online: https://www.bdbiosciences.com/content/dam/bdb/marketing-documents/BD-Accuri-C6-Plus-Filter-Guide.pdf (accessed on 20 July 2022).
- Serna-Cock, L.; Rojas-Dorado, M.; Ordoñez-Artunduaga, D.; García-Salazar, A.; García-González, E.; Aguilar, C.N. Crude extracts of metabolites from co-cultures of lactic acid bacteria are highly antagonists of Listeria monocytogenes. Heliyon 2019, 5, e02448. [Google Scholar] [CrossRef]
- Exalpha. Ammonium Sulfate Precipitation Protocol; Exalpha, 2022; Available online: http://www.exalpha.com/protocols/ammonium-sulfate-precipitation-protocol (accessed on 31 May 2022).
- Wingfield, P. Protein precipitation using ammonium sulfate. Curr. Protoc. Protein Sci. 2001, 13, A-3F. [Google Scholar] [CrossRef]
- Pongtharangkul, T.; Demirci, A. Evaluation of agar diffusion bioassay for nisin quantification. Appl. Microbiol. Biotechnol. 2004, 65, 268–272. [Google Scholar] [CrossRef]
- Thermo Scientific. Pierce™ BCA Protein Assay Kit; Thermo Scientific: Waltham, MA, USA, 2020; Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0011430_Pierce_BCA_Protein_Asy_UG.pdf (accessed on 7 June 2022).
- Batdorj, B.; Dalgalarrondo, M.; Choiset, Y.; Pedroche, J.; Métro, F.; Prevost, H.; Chobert, J.M.; Haertlé, T. Purification and characterization of two bacteriocins produced by lactic acid bacteria isolated from Mongolian airag. J. Appl. Microbiol. 2006, 101, 837–848. [Google Scholar] [CrossRef]
- Thermo Scientific. Chromeleon Chromatography Management System v6.80; Thermo Scientific: Waltham, MA, USA, 2006; Available online: https://tools.thermofisher.com/content/sfs/manuals/Man-Chromeleon-680-EN.pdf (accessed on 3 August 2022).
- Tendencia, E.A. Disk diffusion method. In Laboratory Manual of Standardized Methods for Antimicrobial Sensitivity Tests for Bacteria Isolated from Aquatic Animals and Environment; Aquaculture Department, Southeast Asian Fisheries Development Center: Iloilo, Philippines, 2004; pp. 13–29. [Google Scholar]
- Haindongo, N.; Anyogu, A.; Ekwebelem, O.; Anumudu, C.; Onyeaka, H. Antibacterial and antibiofilm effects of garlic (Allium sativum), ginger (Zingiber officinale) and mint (Mentha piperta) on Escherichia coli biofilms. Food Sci. Appl. Biotechnol. 2021, 4, 166–176. [Google Scholar] [CrossRef]
- Rollins, D.M.; Joseph, S.W. Minimal Inhibitory Concentration (MIC) (Broth Tube Dilution Method); University of Maryland: College Park, MD, USA, 2000; Available online: https://science.umd.edu/classroom/bsci424/LabMaterialsMethods/BrothTubeMIC.htm (accessed on 14 June 2022).
- Tenea, G.N.; Hurtado, P.; Ortega, C. Inhibitory effect of substances produced by native Lactococcus lactis strains of tropical fruits towards food pathogens. Prev. Nutr. Food Sci. 2018, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Modugno, C.; Kmiha, S.; Simonin, H.; Aouadhi, C.; Cañizares, E.D.; Lang, E.; André, S.; Mejri, S.; Maaroufi, A.; Perrier-Cornet, J.M. High pressure sensitization of heat-resistant and pathogenic foodborne spores to nisin. Food Microbiol. 2019, 84, 103244. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.; Field, D.; Rea, M.C.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocins: Novel solutions to age old spore-related problems? Front. Microbiol. 2016, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.J.; Nebe-Von-Caron, G. An industrial application of multiparameter flow cytometry: Assessment of cell physiological state and its application to the study of microbial fermentations. Cytom. J. Int. Soc. Anal. Cytol. 2001, 44, 179–187. [Google Scholar] [CrossRef]
- Pienaar, J.A.; Singh, A.; Barnard, T.G. The viable but non-culturable state in pathogenic Escherichia coli: A general review. Afr. J. Lab. Med. 2016, 5, 1–9. [Google Scholar] [CrossRef][Green Version]
- Ariana, M.; Hamedi, J. Enhanced production of nisin by co-culture of Lactococcus lactis sub sp. lactis and Yarrowia lipolytica in molasses based medium. J. Biotechnol. 2017, 256, 21–26. [Google Scholar] [CrossRef]

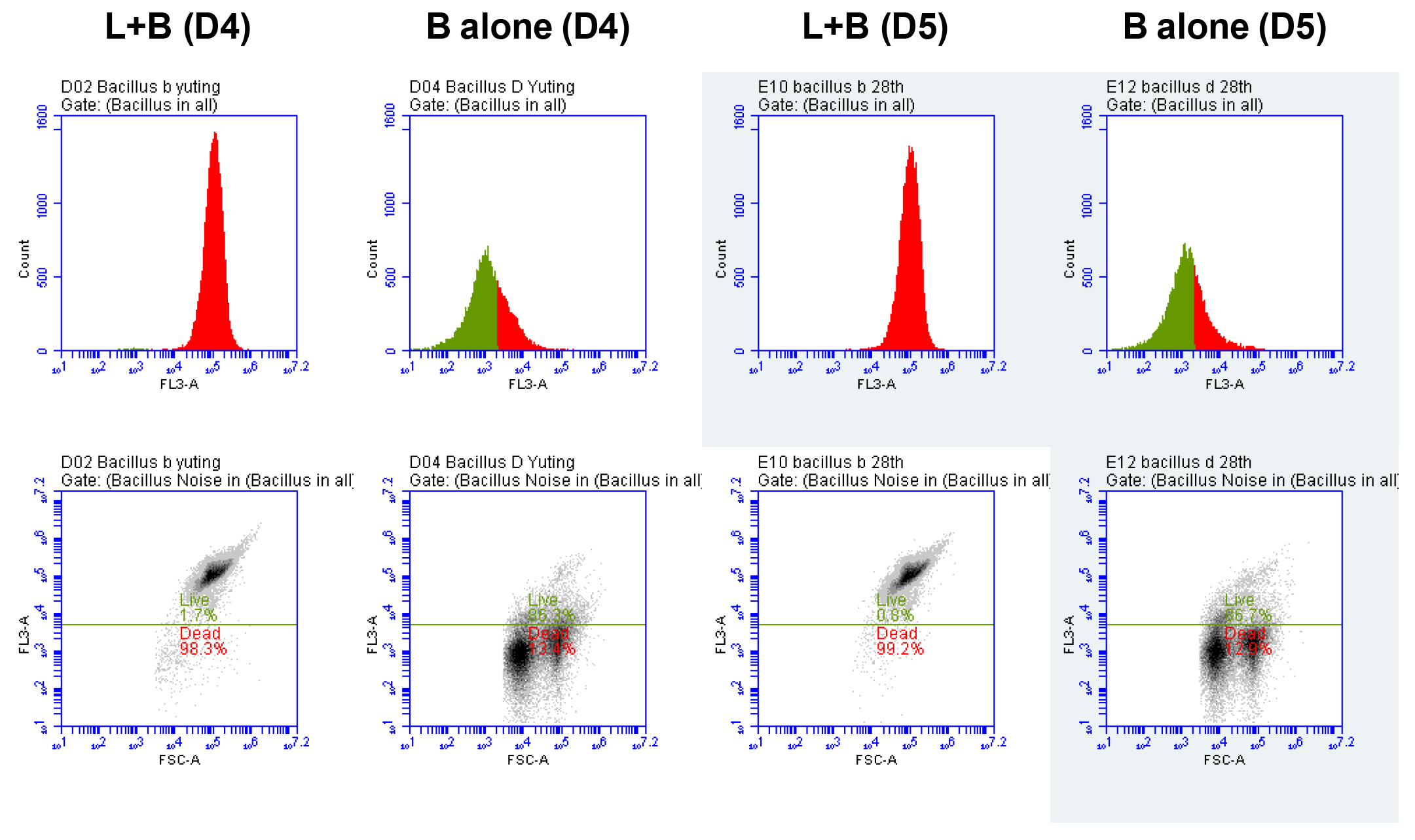


| L + B (D4) | B Alone (D4) | L + B (D5) | B Alone (D5) | |
|---|---|---|---|---|
| This plot | 24,852 | 21,253 | 24,972 | 21,224 |
| Dead cells | 24,422 | 2846 | 24,777 | 2732 |
| % Dead cells | 98.3% | 13.4% | 99.2% | 12.9% |
| Live cells | 425 | 18,337 | 191 | 18,403 |
| % Live cells | 1.7% | 86.3% | 0.8% | 86.7% |
| L + E (D4) | E Alone (D4) | L + E (D5) | E Alone (D5) | |
|---|---|---|---|---|
| This plot | 20,108 | 17,965 | 15,590 | 18,290 |
| Dead cells | 1710 | 214 | 2555 | 313 |
| % Dead cells | 8.5% | 1.2% | 16.4% | 1.7% |
| Live cells | 18,366 | 17,746 | 13,004 | 17,966 |
| % Live cells | 91.3% | 98.8% | 83.4% | 98.2% |
| Samples | OD (562 nm) | Protein Concentration (μg/mL) |
|---|---|---|
| Diffusible substances (supernatant) | 10.17 | 8386.50 |
| Diffusible substances (P) | 4.725 | 3849 |
| Diffusible substances (S) | 1.425 | 1099 |
| Control (1000 IU nisin pH 7.0) | 0.824 | 598.17 |
| Samples | Diameter |
|---|---|
| Control (1000 IU Nisin) | 12 ± 1 mm |
| Diffusible substances (P) | 16.5 ± 0.5 mm |
| Diffusible substances (S) | 11.5 ± 0.5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Akinsemolu, A.A.; Anumudu, C.K.; Miri, T.; Onyeaka, H. Antimicrobial Activity of Diffusible Substances Produced by Lactococcus lactis Against Bacillus cereus in a Non-Contact Co-Culture Model. Hygiene 2024, 4, 469-482. https://doi.org/10.3390/hygiene4040035
Han Y, Akinsemolu AA, Anumudu CK, Miri T, Onyeaka H. Antimicrobial Activity of Diffusible Substances Produced by Lactococcus lactis Against Bacillus cereus in a Non-Contact Co-Culture Model. Hygiene. 2024; 4(4):469-482. https://doi.org/10.3390/hygiene4040035
Chicago/Turabian StyleHan, Yuting, Adenike A. Akinsemolu, Christian K. Anumudu, Taghi Miri, and Helen Onyeaka. 2024. "Antimicrobial Activity of Diffusible Substances Produced by Lactococcus lactis Against Bacillus cereus in a Non-Contact Co-Culture Model" Hygiene 4, no. 4: 469-482. https://doi.org/10.3390/hygiene4040035
APA StyleHan, Y., Akinsemolu, A. A., Anumudu, C. K., Miri, T., & Onyeaka, H. (2024). Antimicrobial Activity of Diffusible Substances Produced by Lactococcus lactis Against Bacillus cereus in a Non-Contact Co-Culture Model. Hygiene, 4(4), 469-482. https://doi.org/10.3390/hygiene4040035











