Does COVID-19 Vaccination Protect Contact Persons? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Results
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Assessment of Study Quality
3. Results
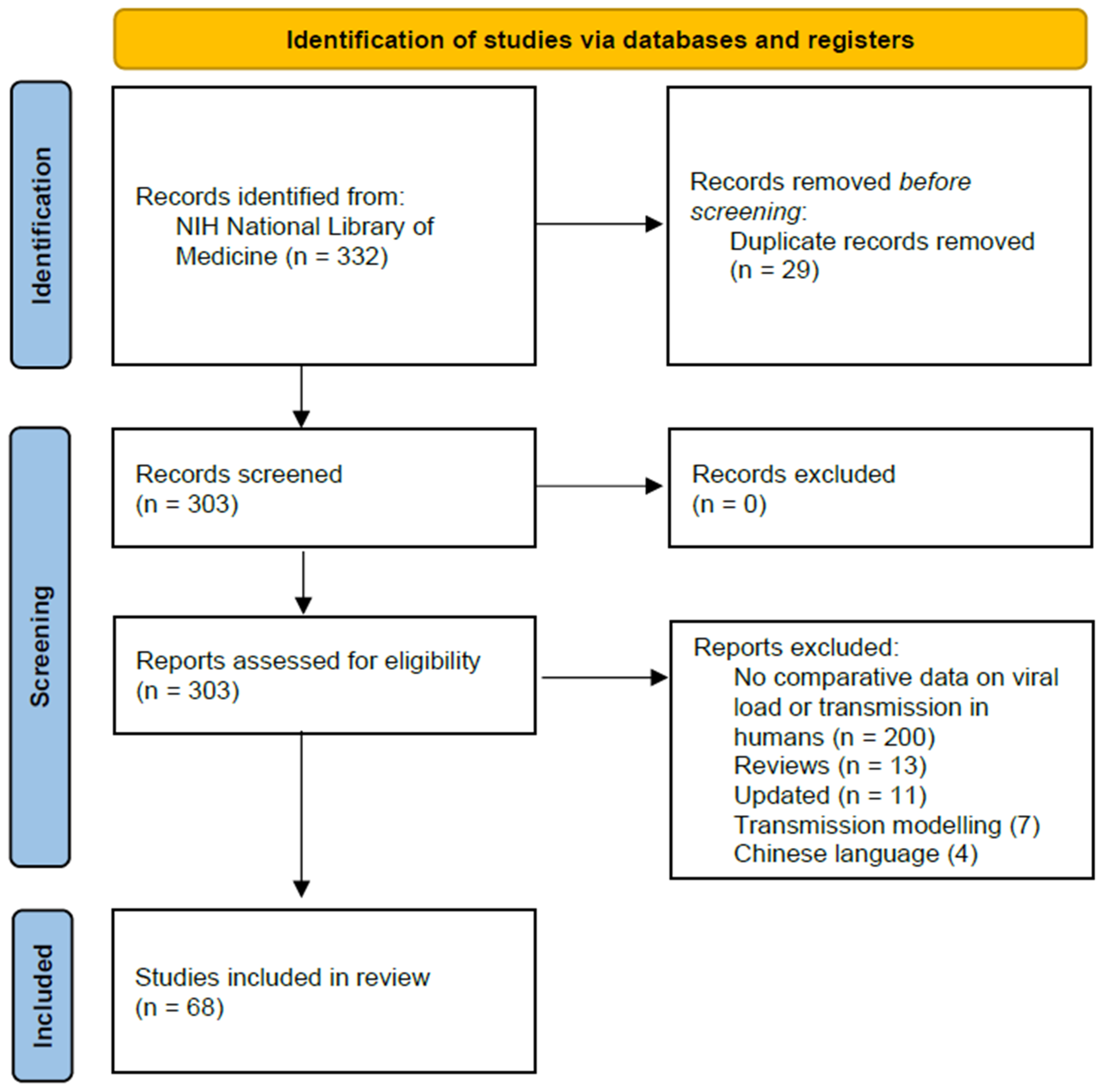
3.1. Search Results
3.2. Level of Viral Load
3.2.1. Ct Values According to Vaccination Status
3.2.2. Viral RNA Copies According to Vaccination Status
| SARS-CoV-2 Variant | Type of Gene | Sampling Period (Country) | Type of Population | Log10 Viral Copies per mL According to the Number of Vaccination Doses (Number of Samples) | p-Value | Source | |||
|---|---|---|---|---|---|---|---|---|---|
| None | 1 Dose | 2 Doses | 3 Doses | ||||||
| Delta | E gene | 6/21–12/21 (Switzerland) | Any individual with symptoms | Approx. 8.5 [SD: 0.8] * (127) | - | Approx. 8.2 [SD: 1.0] * (104) | - | 0.0002 | [18] |
| Delta | N gene | 12/20–4/22 (USA) | Healthcare workers, essential frontline workers, uninfected upon inclusion | 4.1 * (78) | - | 3.0 * (27) # 4.2 * (165) ## | 3.2 * (18) | “significant” n.s. n.s. | [51] |
| Delta | N gene | 10/20–8/21 (USA) | General population | 7.4 [IQR: 3.3–10.8] ** (36) | - | 4.7 [IQR: approx. 1.0–10.6] * (56) | - | <0.0001 | [14] |
| Mainly delta | N gene | 7/21–9/21 (France) | Patients | 7.1 [IQR: 5.7–7.9] ** (2619) | 7.0 ** [IQR: 5.6–7.8] ** (636) | 6.6 [IQR: 5.5–7.6] ** (520) | - | <0.0001 for delta variant (0 versus 2) | [50] |
| Mainly delta | N genes | 12/20–9/21 (USA) | General population | 1.1 [95% CI: approx. 0.7–1.6] * (160) | - | 0.3 * [95% CI: approx. 0.3–0.8] * (112) | - | 0.02 | [41] |
| Omicron | N gene | 12/20–4/22 (USA) | Healthcare workers, essential frontline workers, uninfected upon inclusion | 3.5 * (109) | - | 2.8 * (42) # 3.3 * (209) ## | 3.1 * (383) | “significant” n.s. n.s. | [51] |
| Omicron BA.1 | E gene | 12/21–2/22 (Switzerland) | General population | Approx. 7.6 [SD: approx. 0.8] * (33) | - | Approx. 7.8 [SD: approx. 0.8] * (91) | Approx. 7.7 [SD: approx. 0.7] * (30) | n.s. | [18] |
| Multiple variants | ORF1ab, N and S gene | 12/20–4/21 USA) | General population | 3.8 [SD: 1.7] * (155) | 2.3 [SD: 1.7] * (16) *** | - | - | “significant” | [53] |
| Multiple variants | N gene | 2/21–6/21 (USA) | General population and patients | 6.6 * (1061) | - | 6.6 * (121) | - | 0.99 | [45] |
| Multiple variants | Not described | 11/20–8/21 (USA) | Participants of the occupational health program of the National Basketball Association, infected upon inclusion | 8.0 [95% CI: 8.2–11.5] * (136) | - | 8.1 [95% CI: 7.9–11.5] * (37) | - | n.s. | [47] |
| Multiple variants | N gene | 9/20–9/21 (USA) | General population, infected non-hospitalized | 7.5 [IQR: 0.004–108.0] ** (52) | - | 1.0 [IQR: 0.2–32.0] ** (32) | - | 0.39 | [52] |
| Multiple variants | E gene | 9/20–9/21 (USA) | General population, infected non-hospitalized | 1.2 [IQR: 0.005–27.4] ** (52) | - | 0.6 [IQR: 0.07–23.3] ** (32) | - | 0.88 | [52] |
3.2.3. Infectious Virus According to Vaccination Status
3.3. Duration of Viral Detection
3.3.1. Detection of SARS-CoV-2 Genes
3.3.2. Detection of Infectious SARS-CoV-2
3.4. Transmission from COVID-19 Cases
| SARS-CoV-2 Variant | Study Period (Country) | Vaccination Status Index Cases (n) | Type of Contact | Secondary Attack Rate per Group | Proportion of Fully Vaccinated Contacts (Group) | p-Value | Source |
|---|---|---|---|---|---|---|---|
| Mainly alpha | 12/20–4/21 (Israel) | Unvaccinated (200) Fully vaccinated (15) | Household contacts of healthcare workers | 42.0% *** [95% CI: 38%–46%] 19.0% *** [95% CI: 9%–34%] | - | significant | [58] |
| Mainly alpha | 1/21–3/21 (Germany) | Unvaccinated (11) Fully vaccinated (5) | Household contacts | 66.7% 22.2% | 0% ** (all contacts) 0% ** (all contacts) | 0.046 | [48] |
| Alpha and delta | 1/21–8/21 (UK) | Unvaccinated (218,946) Partially vaccinated (91,394) Fully vaccinated (63,775) | “Close contacts” of infected adult patients | 46.4% 28.0% 26.8% | 27.8% (all contacts with PCR tests) | - * | [13] |
| Delta | 9/20–9/21 (UK) | Unvaccinated (63) Partially vaccinated (25) Fully vaccinated (50) | Mainly household contacts | 23.0% 37.1% 24.6% | 63.0% (contacts of unvaccinated index cases) 54.3% (contacts of partially vaccinated index cases) 62.3% (contacts of fully vaccinated index cases) | n.s. # | [61] |
| Delta | 2/21–5/21 (The Netherlands) | Unvaccinated (110,872) Partially vaccinated (2088) Fully vaccinated (622) | Household contacts | 30.8% 28.9% 11.2% | 2.1% (all contacts) | - | [62] |
| Delta | 6/21–10/21 (Denmark) | Unvaccinated (16,431) Fully vaccinated (8262) | Household contacts | 22.3% 20.1% | Correlation coefficient of 0.72 of the vaccination status between primary cases and contacts among individuals of ≥13 years of age | - | [63] |
| Delta | 6/21–7/21 (USA) | Unvaccinated (61) Fully and partially vaccinated (22) | Household contacts | 57.8% 16.7% | 35.7% (contacts of unvaccinated index cases) 75.0% (contacts of fully vaccinated index cases) | <0.01 | [64] |
| Delta | 6/21–10/21 (Denmark) | Unvaccinated (8611) Fully vaccinated, no previous infection (8765) Boosted (528) Unvaccinated, previous infection (145) Fully vaccinated, previous infection (58) | Household contacts | 21.5% 20.2% 18.0% 7.5% 7.5% | 48.7% (all contacts, delta and omicron) | - | [65] |
| Delta | 3/20–11/21 (Republic of Korea) | Unvaccinated (100) Partially vaccinated (10) Fully vaccinated (43) | Healthcare setting | 27.0% 20.0% 7.0% | - | 0.03 | [30] |
| Delta | 8/21–9/21 (The Netherlands) | Unvaccinated (2641) Partially vaccinated (540) Fully vaccinated (1740) | Household contacts | 17.7% 7.9% 12.4% | 35.3% (contacts of unvaccinated index cases) 67.3% (contacts of partially vaccinated index cases) 79.6% (contacts of fully vaccinated index cases) | - | [66] |
| Delta | 9/21–1/22 (Republic of Korea) | Partially or unvaccinated (30) Fully vaccinated (19) | “Close contacts” | 14.1% 3.7% | - | 0.001 | [15] |
| Omicron | 11/21–12/21 (Denmark) | Unvaccinated (1166) Fully vaccinated, no previous infection (6934) Boosted (468) Unvaccinated, previous infection (128) Fully vaccinated, previous infection (414) | Household contacts | 30.8% 29.5% 31.8% 15.0% 16.0% | 48.7% (all contacts, delta and omicron) | - | [65] |
| Omicron | 11/21–2/22 (USA) | Unvaccinated (36) Fully vaccinated (12) **** Boosted (57) | Household contacts | 63.9% 43.6% 42.7% | 35.1% (all contacts); an additional 26.0% were boosted. | - | [67] |
| Omicron | 12/21–1/22 (Germany) | Unvaccinated (202) Fully vaccinated (202) Boosted (204) | “Close contacts” | 47.9% 49.5% 34.8% | - | - | [68] |
| Multiple variants | 9/20–1/22 (USA) | Unvaccinated (21) Fully vaccinated (21) | Household contacts | 45.0% [95% CI: 29.0%–62.0%] 41.4% [95% CI: 25.0%–59.0%] | 20.0% (contacts of unvaccinated index cases) 85.3% (contacts of fully vaccinated index cases) | 0.60 | [69] |
| Multiple variants | 4/21–9/21 (Thailand) | Unvaccinated (177) Fully vaccinated (231) | Household contacts | 46.8% 50.8% | 3.1% (contacts of unvaccinated index cases) 14.7% (contacts of fully vaccinated index cases) | 0.177 | [70] |
| Multiple variants | 12/20–8/21 (Germany) | Unvaccinated (357) Fully vaccinated (357) | “close contacts” | 37.8% 10.1% | Significantly more secondary cases among unvaccinated contacts (p < 0.001) | <0.001 | [46] |
3.5. Previous COVID-19 Infection
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Vaccines and Immunization: What Is Vaccination? Available online: https://www.who.int/news-room/questions-and-answers/item/vaccines-and-immunization-what-is-vaccination (accessed on 1 August 2022).
- Korang, S.K.; von Rohden, E.; Veroniki, A.A.; Ong, G.; Ngalamika, O.; Siddiqui, F.; Juul, S.; Nielsen, E.E.; Feinberg, J.B.; Petersen, J.J.; et al. Vaccines to prevent COVID-19: A living systematic review with Trial Sequential Analysis and network meta-analysis of randomized clinical trials. PLoS ONE 2022, 17, e0260733. [Google Scholar] [CrossRef]
- Ghazy, R.M.; Ashmawy, R.; Hamdy, N.A.; Elhadi, Y.A.M.; Reyad, O.A.; Elmalawany, D.; Almaghraby, A.; Shaaban, R.; Taha, S.H.N. Efficacy and Effectiveness of SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 350. [Google Scholar]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef]
- Štěpánek, L.; Janošíková, M.; Nakládalová, M.; Ivanová, K.; Macík, J.; Boriková, A.; Vildová, H. Motivation for COVID-19 vaccination in priority occupational groups: A cross-sectional survey. Int. J. Environ. Res. Public Health 2021, 18, 11726. [Google Scholar]
- Bundesministerium für Gesundheit. Gemeinschaftsschutz durch Corona-Schutzimpfung. Available online: https://www.zusammengegencorona.de/impfen/gemeinschaftsschutz-solidaritaet-in-der-coronavirus-pandemie/ (accessed on 30 November 2022).
- Hütten, F. Weitergabe des Virus—Warum man mit einer Corona-Impfung auch andere schützt. Available online: https://www.derbund.ch/warum-man-mit-einer-corona-impfung-auch-andere-schuetzt-414879400098 (accessed on 30 November 2022).
- Zürcher, K.; Abela, I.A.; Stange, M.; Dupont, C.; Mugglin, C.; Egli, A.; Trkola, A.; Egger, M.; Fenner, L. Alpha variant coronavirus outbreak in a nursing home despite high vaccination coverage: Molecular, epidemiological and immunological studies. Clin. Infect. Dis. 2022, 77, ciab1005. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, T.H.; Lee, E.; Loeb, M.; Jeong, Y.S.; Kim, J.H.; Oh, S.M.; Cheong, S.; Park, H.; Jo, S.Y.; et al. A SARS-CoV-2 outbreak associated with vaccine breakthrough in an acute care hospital. Am. J. Infect. Control 2022, 50, 1006–1012. [Google Scholar] [CrossRef]
- Shitrit, P.; Zuckerman, N.S.; Mor, O.; Gottesman, B.S.; Chowers, M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Eurosurveillance 2021, 26, 2100822. [Google Scholar] [CrossRef]
- World Health Organization. Vaccine Efficacy, Effectiveness and Protection. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 30 November 2022).
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E.A. Effect of COVID-19 Vaccination on Transmission of Alpha and Delta Variants. N. Engl. J. Med. 2022, 386, 744–756. [Google Scholar] [CrossRef]
- Rife Magalis, B.; Rich, S.; Tagliamonte, M.S.; Mavian, C.; Cash, M.N.; Riva, A.; Marini, S.; Amador, D.M.; Zhang, Y.; Shapiro, J.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Delta Vaccine Breakthrough Transmissibility in Alachua County, Florida. Clin. Infect. Dis. 2022, 75, 1618–1627. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, J.Y.; Park, H.; Lim, S.Y.; Kim, J.; Bae, S.; Jung, J.; Kim, M.J.; Chong, Y.P.; Lee, S.O.; et al. Comparison of outward transmission potential between vaccinated and partially vaccinated or unvaccinated individuals with the SARS-CoV-2 delta variant infection. J. Infect. 2022, 85, e69. [Google Scholar] [CrossRef]
- Adams, C.; Chamberlain, A.; Wang, Y.; Hazell, M.; Shah, S.; Holland, D.P.; Khan, F.; Gandhi, N.R.; Fridkin, S.; Zelner, J.; et al. The Role of Staff in Transmission of SARS-CoV-2 in Long-term Care Facilities. Epidemiology 2022, 33, 669–677. [Google Scholar] [CrossRef]
- Hayek, S.; Shaham, G.; Ben-Shlomo, Y.; Kepten, E.; Dagan, N.; Nevo, D.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Barda, N. Indirect protection of children from SARS-CoV-2 infection through parental vaccination. Science 2022, 375, 1155–1159. [Google Scholar] [CrossRef]
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Jacquérioz, F.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022, 28, 1491–1500. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Coyle, P.; Hasan, M.R.; Yassine, H.M.; Benslimane, F.M.; Al-Khatib, H.A.; Al-Kanaani, Z.; et al. Relative infectiousness of SARS-CoV-2 vaccine breakthrough infections, reinfections, and primary infections. Nat. Commun. 2022, 13, 532. [Google Scholar] [CrossRef]
- Luo, C.H.; Morris, C.P.; Sachithanandham, J.; Amadi, A.; Gaston, D.C.; Li, M.; Swanson, N.J.; Schwartz, M.; Klein, E.Y.; Pekosz, A.; et al. Infection With the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Delta Variant Is Associated With Higher Recovery of Infectious Virus Compared to the Alpha Variant in Both Unvaccinated and Vaccinated Individuals. Clin. Infect. Dis. 2022, 75, e715–e725. [Google Scholar] [CrossRef]
- Muhsen, K.; Maimon, N.; Mizrahi, A.; Bodenneimer, O.; Cohen, D.; Maimon, M.; Grotto, I.; Dagan, R. Effectiveness of BNT162b2 mRNA COVID-19 vaccine against acquisitions of SARS-CoV-2 among health care workers in long-term care facilities: A prospective cohort study. Clin. Infect. Dis. 2022, 75, e755–e763. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Shrotri, M.; Krutikov, M.; Palmer, T.; Giddings, R.; Azmi, B.; Subbarao, S.; Fuller, C.; Irwin-Singer, A.; Davies, D.; Tut, G.; et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet. Infect. Dis. 2021, 21, 1529–1538. [Google Scholar] [CrossRef]
- Ioannou, P.; Karakonstantis, S.; Astrinaki, E.; Saplamidou, S.; Vitsaxaki, E.; Hamilos, G.; Sourvinos, G.; Kofteridis, D.P. Transmission of SARS-CoV-2 variant B.1.1.7 among vaccinated health care workers. Infect. Dis. 2021, 53, 876–879. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Amit, S.; Bergwerk, M.; Lipsitch, M.; Leshem, E.; Kahn, R.; Lustig, Y.; Cohen, C.; Doolman, R.; Ziv, A.; et al. Decreased infectivity following BNT162b2 vaccination: A prospective cohort study in Israel. Lancet Reg. Health-Eur. 2021, 7, 100150. [Google Scholar] [CrossRef]
- Acharya, C.B.; Schrom, J.; Mitchell, A.M.; Coil, D.A.; Marquez, C.; Rojas, S.; Wang, C.Y.; Liu, J.; Pilarowski, G.; Solis, L.; et al. Viral Load Among Vaccinated and Unvaccinated, Asymptomatic and Symptomatic Persons Infected With the SARS-CoV-2 Delta Variant. Open Forum Infect. Dis. 2022, 9, ofac135. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef]
- Chia, P.Y.; Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Chavatte, J.M.; Mak, T.M.; Cui, L.; Kalimuddin, S.; Chia, W.N.; Tan, C.W.; et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: A multicentre cohort study. Clin. Microbiol. Infect. 2022, 28, 612.e1–612.e7. [Google Scholar] [CrossRef]
- Fall, A.; Eldesouki, R.E.; Sachithanandham, J.; Morris, C.P.; Norton, J.M.; Gaston, D.C.; Forman, M.; Abdullah, O.; Gallagher, N.; Li, M.; et al. The displacement of the SARS-CoV-2 variant Delta with Omicron: An investigation of hospital admissions and upper respiratory viral loads. EBioMedicine 2022, 79, 104008. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.Y.; Park, H.; Park, S.; Lim, J.S.; Lim, S.Y.; Bae, S.; Lim, Y.-J.; Kim, E.O.; Kim, J.; et al. Transmission and Infectious SARS-CoV-2 Shedding Kinetics in Vaccinated and Unvaccinated Individuals. JAMA Netw. Open 2022, 5, e2213606. [Google Scholar] [CrossRef]
- Griffin, J.B.; Haddix, M.; Danza, P.; Fisher, R.; Koo, T.H.; Traub, E.; Gounder, P.; Jarashow, C.; Balter, S. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status—Los Angeles County, California, May 1-July 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1170–1176. [Google Scholar] [CrossRef]
- Sriraman, K.; Shaikh, A.; Vaswani, S.; Mestry, T.; Patel, G.; Sakthivel, S.; Oswal, V.; Kadam, P.; Nilgiriwala, K.; Shah, D.; et al. Impact of COVID-19 vaccination on transmission risk of breakthrough infections: Lessons from adapted N95 mask sampling for emerging variants and interventions. J. Med. Virol. 2022, 95, e28188. [Google Scholar] [CrossRef]
- Koh, T.; Ooi, X.Y.; Vasoo, S.; Yeo, S.C. Impact of Variant of Concern and Vaccination Status on COVID-19 Infection Virological Dynamics in End Stage Kidney Disease Patients Receiving Haemodialysis. Nephrology 2022, 27, 804–809. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Jiang, L.; Cheng, H.; Li, J.; Li, L.; Chen, Z.; Tang, F.; Fu, Y.; Jin, Y.; et al. Similar aerosol emission rates and viral loads in upper respiratory tracts for COVID-19 patients with Delta and Omicron variant infection. Virol. Sin. 2022, 37, 762–764. [Google Scholar] [CrossRef]
- Bollinger, M.; Saile, P.; Shapeton, A.D.; Kohl, M.; Kumle, B. Sensitivity of severe acute respiratory syndrome coronavirus type 2 rapid antigen point-of-care tests in vaccinated patients. Eur. J. Emerg. Med. 2022, 29, 285–290. [Google Scholar] [CrossRef]
- Elliott, P.; Haw, D.; Wang, H.; Eales, O.; Walters, C.E.; Ainslie, K.E.C.; Atchison, C.; Fronterre, C.; Diggle, P.J.; Page, A.J.; et al. Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant. Science 2021, 374, eabl9551. [Google Scholar] [CrossRef]
- Plante, J.A.; Machado, R.R.G.; Mitchell, B.M.; Shinde, D.P.; Walker, J.; Scharton, D.; McConnell, A.; Saada, N.; Liu, J.; Khan, B.; et al. Vaccination Decreases the Infectious Viral Load of Delta Variant SARS-CoV-2 in Asymptomatic Patients. Viruses 2022, 14, 2071. [Google Scholar] [CrossRef]
- Riemersma, K.K.; Haddock, L.A.; Wilson, N.A.; Minor, N.; Eickhoff, J.; Grogan, B.E.; Kita-Yarbro, A.; Halfmann, P.J.; Segaloff, H.E.; Kocharian, A.; et al. Shedding of infectious SARS-CoV-2 despite vaccination. PLoS Pathog. 2022, 18, e1010876. [Google Scholar] [CrossRef]
- Caserta, L.C.; Martins, M.; Cronk, B.; Anderson, R.; Eldridge, H.; Gallow, D.; Kruppa, F.; Plocharczyk, E.; Diel, D.G. Infection and Transmission of SARS-CoV-2 B.1.617.2 Lineage (Delta Variant) among Fully Vaccinated Individuals. Microbiol. Spectr. 2022, 10, e0056322. [Google Scholar] [CrossRef]
- Altawalah, H.; Alfouzan, W.; Al-Fadalah, T.; Ezzikouri, S. Diagnostic Performance of Automated SARS-CoV-2 Antigen Assay in Nasal Swab during COVID-19 Vaccination Campaign. Diagnostics 2021, 11, 2110. [Google Scholar] [CrossRef]
- Bramante, C.T.; Proper, J.L.; Boulware, D.R.; Karger, A.B.; Murray, T.; Rao, V.; Hagen, A.; Tignanelli, C.J.; Puskarich, M.; Cohen, K.; et al. Vaccination Against SARS-CoV-2 Is Associated With a Lower Viral Load and Likelihood of Systemic Symptoms. Open Forum Infect. Dis. 2022, 9, ofac066. [Google Scholar] [CrossRef]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef]
- Laura, L.; Dalmatin-Dragišić, M.; Martinović, K.; Tutiš, B.; Herceg, I.; Arapović, M.; Arapović, J. Does pre-existing immunity determine the course of SARS-CoV-2 infection in health-care workers? Single-center experience. Infection 2023, 51, 323–339. [Google Scholar] [CrossRef]
- Colavita, F.; Meschi, S.; Gruber, C.E.M.; Rueca, M.; Vairo, F.; Matusali, G.; Lapa, D.; Giombini, E.; De Carli, G.; Spaziante, M.; et al. Virological and Serological Characterisation of SARS-CoV-2 Infections Diagnosed After mRNA BNT162b2 Vaccination Between December 2020 and March 2021. Front. Med. 2022, 8, 815870. [Google Scholar]
- Servellita, V.; Morris, M.K.; Sotomayor-Gonzalez, A.; Gliwa, A.S.; Torres, E.; Brazer, N.; Zhou, A.; Hernandez, K.T.; Sankaran, M.; Wang, B.; et al. Predominance of antibody-resistant SARS-CoV-2 variants in vaccine breakthrough cases from the San Francisco Bay Area, California. Nat. Microbiol. 2022, 7, 277–288. [Google Scholar] [CrossRef]
- Hsu, L.; Grüne, B.; Buess, M.; Joisten, C.; Klobucnik, J.; Nießen, J.; Patten, D.; Wolff, A.; Wiesmüller, G.A.; Kossow, A.; et al. COVID-19 breakthrough infections and transmission risk: Real-world data analyses from Germany’s largest public health department (Cologne). Vaccines 2021, 9, 1267. [Google Scholar] [CrossRef]
- Kissler, S.M.; Fauver, J.R.; Mack, C.; Tai, C.G.; Breban, M.I.; Watkins, A.E.; Samant, R.M.; Anderson, D.J.; Metti, J.; Khullar, G.; et al. Viral Dynamics of SARS-CoV-2 Variants in Vaccinated and Unvaccinated Persons. N. Engl. J. Med. 2021, 385, 2489–2491. [Google Scholar] [CrossRef]
- Meyer, E.D.; Sandfort, M.; Bender, J.; Matysiak-Klose, D.; Dörre, A.; Bojara, G.; Beyrer, K.; Hellenbrand, W. BNT162b2 vaccination reduced infections and transmission in a COVID-19 outbreak in a nursing home in Germany, 2021. Influenza Other Respir. Viruses 2022, 17, e13051. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Waning of SARS-CoV-2 booster viral-load reduction effectiveness. Nat. Commun. 2022, 13, 1237. [Google Scholar] [CrossRef]
- Migueres, M.; Dimeglio, C.; Trémeaux, P.; Raymond, S.; Lhomme, S.; Da Silva, I.; Mendes, K.O.; Abravanel, F.; Félicé, M.P.; Mansuy, J.M.; et al. Influence of the Delta Variant and Vaccination on the SARS-CoV-2 Viral Load. Viruses 2022, 14, 323. [Google Scholar] [CrossRef]
- Thompson, M.G.; Yoon, S.K.; Naleway, A.L.; Meece, J.; Fabrizio, T.P.; Caban-Martinez, A.J.; Burgess, J.L.; Gaglani, M.; Olsho, L.E.W.; Bateman, A.; et al. Association of mRNA Vaccination With Clinical and Virologic Features of COVID-19 Among US Essential and Frontline Workers. JAMA 2022, 328, 1523–1533. [Google Scholar] [CrossRef]
- Garcia-Knight, M.; Anglin, K.; Tassetto, M.; Lu, S.; Zhang, A.; Goldberg, S.A.; Catching, A.; Davidson, M.C.; Shak, J.R.; Romero, M.; et al. Infectious viral shedding of SARS-CoV-2 Delta following vaccination: A longitudinal cohort study. PLoS Pathog. 2022, 18, e1010802. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef]
- Maier, H.E.; Plazaola, M.; Lopez, R.; Sanchez, N.; Saborio, S.; Ojeda, S.; Barilla, C.; Kuan, G.; Balmaseda, A.; Gordon, A. SARS-CoV-2 infection-induced immunity and the duration of viral shedding: Results from a Nicaraguan household cohort study. Influenza Other Respir. Viruses 2022, 17, e13074. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Y.; Wang, W.; Fang, F.; Zhang, W.; Zhu, Z.; Wan, Y. The impacts of vaccination status and host factors during early infection on SARS-CoV-2 persistence:a retrospective single-center cohort study. Int. Immunopharmacol. 2022, 114, 109534. [Google Scholar] [CrossRef]
- Ke, R.; Martinez, P.P.; Smith, R.L.; Gibson, L.L.; Achenbach, C.J.; McFall, S.; Qi, C.; Jacob, J.; Dembele, E.; Bundy, C.; et al. Longitudinal Analysis of SARS-CoV-2 Vaccine Breakthrough Infections Reveals Limited Infectious Virus Shedding and Restricted Tissue Distribution. Open Forum Infect. Dis. 2022, 9, ofac192. [Google Scholar] [CrossRef]
- Kolodziej, L.M.; van Lelyveld, S.F.L.; Haverkort, M.E.; Mariman, R.; Sluiter-Post, J.G.C.; Badoux, P.; de Koff, E.M.; Koole, J.C.D.; Miellet, W.R.; Swart, A.N.; et al. High Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Household Transmission Rates Detected by Dense Saliva Sampling. Clin. Infect. Dis. 2022, 75, e10–e19. [Google Scholar] [CrossRef]
- Layan, M.; Gilboa, M.; Gonen, T.; Goldenfeld, M.; Meltzer, L.; Andronico, A.; Hozé, N.; Cauchemez, S.; Regev-Yochay, G. Impact of BNT162b2 Vaccination and Isolation on SARS-CoV-2 Transmission in Israeli Households: An Observational Study. Am. J. Epidemiol. 2022, 191, 1224–1234. [Google Scholar] [CrossRef]
- McCormick, D.W.; Konkle, S.L.; Magleby, R.; Chakrabarti, A.K.; Cherney, B.; Lindell, K.; Namageyo-Funa, A.; Visser, S.; Soto, R.A.; Donnelly, M.A.P.; et al. SARS-CoV-2 infection risk among vaccinated and unvaccinated household members during the Alpha variant surge—Denver, Colorado, and San Diego, California, January–April 2021. Vaccine 2022, 40, 4845. [Google Scholar] [CrossRef]
- Salo, J.; Hägg, M.; Kortelainen, M.; Leino, T.; Saxell, T.; Siikanen, M.; Sääksvuori, L. The indirect effect of mRNA-based COVID-19 vaccination on healthcare workers’ unvaccinated household members. Nat. Commun. 2022, 13, 1162. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef]
- de Gier, B.; Andeweg, S.; Joosten, R.; ter Schegget, R.; Smorenburg, N.; van de Kassteele, J.; Hahné, S.J.M.; van den Hof, S.; de Melker, H.E.; Knol, M.J.; et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, The Netherlands, February to May 2021. Eurosurveillance 2021, 26, 2100640. [Google Scholar] [CrossRef]
- Lyngse, F.P.; Mølbak, K.; Denwood, M.; Christiansen, L.E.; Møller, C.H.; Rasmussen, M.; Cohen, A.S.; Stegger, M.; Fonager, J.; Sieber, R.N.; et al. Effect of vaccination on household transmission of SARS-CoV-2 Delta variant of concern. Nat. Commun. 2022, 13, 3764. [Google Scholar] [CrossRef]
- Baker, J.M.; Shah, M.M.; O’Hegarty, M.; Pomeroy, M.; Keiser, P.; Ren, P.; Weaver, S.C.; Maknojia, S.; Machado, R.R.G.; Mitchell, B.M.; et al. Primary and Secondary Attack Rates by Vaccination Status after a SARS-CoV-2 B.1.617.2 (Delta) Variant Outbreak at a Youth Summer Camp—Texas, June 2021. J. Pediatr. Infect. Dis. Soc. 2022, 11, piac086. [Google Scholar] [CrossRef]
- Lyngse, F.P.; Mortensen, L.H.; Denwood, M.J.; Christiansen, L.E.; Møller, C.H.; Skov, R.L.; Spiess, K.; Fomsgaard, A.; Lassaunière, R.; Rasmussen, M.; et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat. Commun. 2022, 13, 5573. [Google Scholar] [CrossRef]
- De Gier, B.; Andeweg, S.; Backer, J.A.; Hahné, S.J.M.; van den Hof, S.; de Melker, H.E.; Knol, M.J. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), the Netherlands, August to September 2021. Eurosurveillance 2021, 26, 2100977. [Google Scholar] [CrossRef]
- Baker, J.M.; Nakayama, J.Y.; O’Hegarty, M.; McGowan, A.; Teran, R.A.; Bart, S.M.; Mosack, K.; Roberts, N.; Campos, B.; Paegle, A.; et al. SARS-CoV-2 B.1.1.529 (Omicron) Variant Transmission Within Households—Four U.S. Jurisdictions, November 2021–February 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 341–346. [Google Scholar] [CrossRef]
- Grüne, B.; Grüne, J.; Kossow, A.; Joisten, C. Vaccination and Transmission Risk during the Outbreak of B.1.1.529 (Omicron). Vaccines 2022, 10, 1003. [Google Scholar] [CrossRef]
- Kelly, J.D.; Lu, S.; Anglin, K.; Garcia-Knight, M.; Pineda-Ramirez, J.; Goldberg, S.A.; Tassetto, M.; Zhang, A.; Donohue, K.; Davidson, M.C.; et al. Magnitude and determinants of SARS-CoV-2 household transmission: A longitudinal cohort study. Clin. Infect. Dis. 2022, 75, S193–S204. [Google Scholar] [CrossRef]
- Muadchimkaew, M.; Siripongboonsitti, T.; Wongpatcharawarakul, S.; Boonsankaew, C.; Tawinprai, K.; Soonklang, K.; Mahanonda, N. Effect of Inactivated SARS-CoV-2 Vaccines and ChAdOx1 nCoV-19 Vaccination to Prevent COVID-19 in Thai Households (VacPrevent trial). Int. J. Infect. Dis. 2022, 124, 190–198. [Google Scholar] [CrossRef]
- Ng, O.T.; Koh, V.; Chiew, C.J.; Marimuthu, K.; Thevasagayam, N.M.; Mak, T.M.; Chua, J.K.; Ong, S.S.H.; Lim, Y.K.; Ferdous, Z.; et al. Impact of Delta Variant and Vaccination on SARS-CoV-2 Secondary Attack Rate Among Household Close Contacts. Lancet Reg. Health West. Pac. 2021, 17, 100299. [Google Scholar] [CrossRef]
- Hsu, L.; Hurraß, J.; Kossow, A.; Klobucnik, J.; Nießen, J.; Wiesmüller, G.A.; Grüne, B.; Joisten, C. Breakthrough infections with the SARS-CoV-2 Delta variant: Vaccinations halved transmission risk. Public Health 2022, 204, 40–42. [Google Scholar] [CrossRef]
- Puhach, O.; Meyer, B.; Eckerle, I. SARS-CoV-2 viral load and shedding kinetics. Nat. Rev. Microbiol. 2023, 21, 147–161. [Google Scholar] [CrossRef]
- Li, D.; Li, A.E.; Li, Z.Q.; Bao, Y.; Liu, T.; Qin, X.R.; Yu, X.J. SARS-CoV-2 Delta Variant in Jingmen City, Hubei Province, China, 2021: Children Susceptible and Vaccination Breakthrough Infection. Front. Microbiol. 2022, 13, 856757. [Google Scholar] [CrossRef]
- Woodbridge, Y.; Amit, S.; Huppert, A.; Kopelman, N.M. Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infection. Nat. Commun. 2022, 13, 6706. [Google Scholar] [CrossRef]
- Hawken, S.E.; Sellers, S.A.; Smedberg, J.R.; Ward, J.D.; Elliott, A.M.; Whinna, H.C.; Fischer, W.A.; Miller, M.B. Longitudinal SARS-CoV-2 Testing among the Unvaccinated Is Punctuated by Intermittent Positivity and Variable Rates of Increasing Cycle Threshold Values. Microbiol. Spectr. 2022, 10, e0271521. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Mousa, A.; Winskill, P.; Watson, O.J.; Ratmann, O.; Monod, M.; Ajelli, M.; Diallo, A.; Dodd, P.J.; Grijalva, C.G.; Kiti, M.C.; et al. Social contact patterns and implications for infectious disease transmission—A systematic review and meta-analysis of contact surveys. eLife 2021, 10, e70294. [Google Scholar] [CrossRef]
- Tian, D.; Lin, Z.; Kriner, E.M.; Esneault, D.J.; Tran, J.; DeVoto, J.C.; Okami, N.; Greenberg, R.M.; Yanofsky, S.; Ratnayaka, S.; et al. Ct Values Do Not Predict Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmissibility in College Students. J. Mol. Diagn. 2021, 23, 1078–1084. [Google Scholar] [CrossRef]
- Boucau, J.; Marino, C.; Regan, J.; Uddin, R.; Choudhary, M.C.; Flynn, J.P.; Chen, G.; Stuckwisch, A.M.; Mathews, J.; Liew, M.Y.; et al. Duration of Shedding of Culturable Virus in SARS-CoV-2 Omicron (BA.1) Infection. N. Engl. J. Med. 2022, 387, 275–277. [Google Scholar] [CrossRef]
- Lim, D.S.; Choe, Y.J.; Kim, Y.M.; Lee, S.E.; Jang, E.J.; Kim, J.; Park, Y.-J. Household Secondary Attack Rates of SARS-CoV-2 Omicron Variant, South Korea, February 2022. Emerg. Infect. Dis. 2022, 28, 1731–1734. [Google Scholar] [CrossRef]
- Cai, H.; Bai, W.; Liu, S.; Liu, H.; Chen, X.; Qi, H.; Liu, R.; Cheung, T.; Su, Z.; Ng, C.H.; et al. Attitudes Toward COVID-19 Vaccines in Chinese Adolescents. Front. Med. 2021, 8, 691079. [Google Scholar] [CrossRef]
- Wright, E.; Pollard, G.; Robertson, H.; Anuradha, S. Household transmission of the Delta COVID-19 variant in Queensland, Australia: A case series. Epidemiol. Infect. 2022, 150, e173. [Google Scholar] [CrossRef]
- Freeman, D.; Loe, B.S.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Jenner, L.; Petit, A.; Lewandowsky, S.; Vanderslott, S.; et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 2022, 52, 3127–3141. [Google Scholar] [CrossRef]
- Hall, P.A.; Meng, G.; Sakib, M.N.; Quah, A.C.K.; Agar, T.; Fong, G.T. Do the vaccinated perform less distancing, mask wearing and hand hygiene? A test of the risk compensation hypothesis in a representative sample during the COVID-19 pandemic. Vaccine 2022, 41, 4027–4030. [Google Scholar] [CrossRef]
- Mongin, D.; Bürgisser, N.; Laurie, G.; Schimmel, G.; Vu, D.L.; Cullati, S.; Courvoisier, D.S. Effect of SARS-CoV-2 prior infection and mRNA vaccination on contagiousness and susceptibility to infection. Nat. Commun. 2023, 14, 5452. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Kampf, G. The epidemiological relevance of the COVID-19-vaccinated population is increasing. Lancet Reg. Health Eur. 2021, 11, 100272. [Google Scholar] [CrossRef]
- UK Health Security Agency. COVID-19 Vaccine Surveillance Report—Week 13. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1066759/Vaccine-surveillance-report-week-13.pdf (accessed on 20 December 2023).
| SARS-CoV-2 Variant | Type of Gene | Sampling Period (Country) | Type of Population | Ct Values According to Number of Applied Vaccine Doses (Number of Samples) | p-Value | Source | |||
|---|---|---|---|---|---|---|---|---|---|
| None | 1 Dose | 2 Doses | 3 Doses | ||||||
| Ancestral variant | E gene | 4/20–9/20 (Switzerland) | Any individual with symptoms | 13.9–26.6 * (118) | - | - | - | - | [18] |
| Mainly beta | N, ORF1ab and S gene | 2/20–7/21 (Qatar) | General population | 24.0 [95% CI: 23.8–24.2] ** (4035) | - | 25.0 [95% CI: 24.8–25.2] ** (4035 #) | - | <0.001 | [19] |
| Mainly beta | N, ORF1ab and S gene | 2/20–7/21 (Qatar) | General population | 26.8 [95% CI: 25.9–27.6] ** (265) | - | 30.3 [95% CI: 29.6–31.0] ** (265 ##) | - | <0.001 | [19] |
| Alpha | N gene | 1/21–9/21 (USA) | Patients | Approx. 20.9 *** (470) | - | Approx. 21.0 *** (94) | - | n.s. | [20] |
| Alpha | ORF1ab gene | 9/20–1/21 (Israel) | Healthcare workers | 26.7 [IQR: approx. 23–31] *** (40) | 32.0 [IQR: approx. 28–33.5] *** (20) | 0.008 | [21] | ||
| Alpha B.1.1.7 | N, ORF1ab and S gene | 12/10–5/21 (UK) | General population | 28.7 [IQR: 20.4–32.9] *** (10,853) | 31.6 (IQR: 26.6–33.7] *** (577) | 33.3 [IQR: 31.6–34.0] *** (56) | - | - | [22] |
| Alpha B.1.1.7 | Various genes ### | 12/20–3/21 (UK) | Nursing home residents | 26.6 [95% CI: 26.0–27.1] ** (552) | 25.9 [95% CI: 25.2–26.6] ** (411; 0–27 days after vaccination with dose 1) | - | - | 0.158 | [23] |
| Alpha B.1.1.7 | Various genes ### | 12/20–3/21 (UK) | Nursing home residents | 26.6 [95% CI: 26.0–27.1] ** (552) | 31.3 [95% CI: 29.6–33.0] ** (107; ≥28 days after vaccination with dose 1) | - | - | <0.0001 | [23] |
| Mainly alpha | N, ORF1ab and S gene | 1/21–4/21 (Greece) | Healthcare workers | 18.5 [IQR: 13.5–24.0] *** (31) | - | 18.5 [IQR: 16.0–26.0] *** (24) | - | 0.70 | [24] |
| Mainly alpha | N gene | 12/20–3/21 (Israel) | Healthcare workers | 22.2 [SD: 1.0] *** (163) | 27.3 [SD: 2.2] *** (31) | <0.001 | [25] | ||
| Delta | E gene | 6/21–12/21 (Switzerland) | Any individual with symptoms | 13.8–26.3 * (127) | - | 16.3–26.1 * (104) | - | 0.0002 | [18] |
| Delta | N gene | 6/21–8/21 (USA) | General population | 23.4 [IQR: approx. 19.0–28.5] *** (198) | - | 23.1 [IQR: approx. 17.0–27.0] *** (171) | - | 0.54 | [26] |
| Delta | N gene | 6/21–8/21 (USA) | General population (HYT cohort) | 25.4 [IQR: approx. 23.0–28.0] *** (375) | - | 25.5 [IQR: approx. 23.0–29.0] *** (125) | - | 0.80 | [26] |
| Delta | N gene | 1/21–9/21 (USA) | Patients | Approx. 21 *** (134) | 20.5 *** (117) | n.s. | [20] | ||
| Delta | RdRp gene | 6/21–9/21 (Israel) | Patients | 27.7 [SD: 5.0] ** (3100) | 26.9 [SD: 5.0] ** (12,934) | 29.1 [SD: 4.7] ** (519) | <0.05 (0 versus 2 doses) <0.001 (0 versus 3 doses) | [27] | |
| Delta | N gene | 6/21–9/21 (Israel) | Patients | 25.1 [SD: 5.0] ** (3100) | 25.4 [SD: 5.0] ** (12,934) | 27.5 [SD: 4.7] ** (519) | <0.05 (0 versus 2) <0.001 (0 versus 3) | [27] | |
| Delta | E gene | 6/21–9/21 (Israel) | Patients | 22.7 [SD: 4.9] ** (3100) | 22.9 [SD: 4.9] ** (12,934) | 25.2 [SD: 4.6] ** (519) | n.s. (0 versus 2) <0.001 (0 versus 3) | [27] | |
| Delta B.1.617.2 (early period) | N, ORF1ab and S gene | 12/10–5/21 (UK) | General population | 21.5 [IQR: 16.4–31.7] *** (75) | 30.1 [IQR: 18.6–31.7] *** (110) | 32.2 [IQR: 26.0–34.0] *** (104) | - | - | [22] |
| Delta B.1.617.2 (late period) | N, ORF1ab and S gene | 12/10–5/21 (UK) | General population | 25.7 [IQR: 19.1–30.8] *** (326) | 24.7 [IQR: 18.8–31.3] *** (705) | 25.3 [IQR: 19.1–31.3] *** (1593) | - | - | [22] |
| Delta | N and S gene | 4/21–6/21 (Singapore) | Patients | 18.8 [IQR: 14.9–22.7] *** (130) | - | 19.2 [IQR: 15.2–22.2] *** (71) | - | 0.929 | [28] |
| Delta | N gene | 11/21–12/21 (USA) | Patients | Approx. 18.5 [IQR: approx. 15.0–21.5] *** (400) | - | Approx. 17 [IQR: approx. 15.0–21.0] *** (230) | - | n.s. | [29] |
| Mainly delta | N and S gene | 3/20–11/21 (Republic of Korea) | Healthcare worker, inpatients, caregivers | 20 [IQR: 15.0–29.0] *** (109) | - | 19 [IQR: 16.0–24.0] *** (45) | - | 0.64 | [30] |
| Mainly delta (May 2021) | ORF1ab gene N gene | 5/21–7/21 (USA) | General population | 22.8 [IQR: approx. 18.0–31.0] *** (-) 24.0 [IQR: approx. 18.0–33.0] *** (-) | 36.6 [IQR: approx. 28.0–37.0] *** (-) 36.0 [IQR: approx. 33.0–37.0] *** (-) | 27.7 [IQR: approx. 23.0–37.0] *** (-) 30.6 [IQR: approx. 23.0–36.0] *** (-) | - - | - - | [31] |
| Mainly delta (July 2021) | ORF1ab gene N gene | 5/21–7/21 (USA) | General population | 18.8 [IQR: approx. 16.0–24.0] *** (-) 19.3 [IQR: approx. 16.0–25.0] *** (-) | 17.8 [IQR: approx. 16.0–23.0] *** (-) 18.6 [IQR: approx. 14.0–29.0] *** (-) | 19.0 [IQR: approx. 15.0–26.0] *** (-) 19.5 [IQR: approx. 16.0–23.0] *** (-) | - - | - - | [31] |
| Mainly delta | N and O genes | 7/21–9/21 (India) | Patients | 24 [IQR: 20.5–28.6] *** (14) | 19 [IQR: 17.0–23.0] *** (31) | 21 [IQR: 16.0–24.0] *** (50) | - | - | [32] |
| Delta | N and S gene | 7/21–11/21 (Republic of Korea) | Healthcare worker, inpatients, caregivers | 25 [IQR: 18.0–32.0] *** (28) | - | 19 [IQR: 16.0–26.0] *** (44) | - | 0.04 | [30] |
| Delta | Not described | 2/20–9/21 (Singapore) | Hemodialysis patients | 19.0 [SD: 3.0] ** (10) | - | 17.0 [SD: 3.5] ** (24) | - | 0.37 | [33] |
| Delta | ORF1ab gene | 2021 (China) | Patients | Approx. 28 [SD: approx. 7.5] ** (14) | - | Approx. 26 [SD: approx. 6.5] ** (6) | - | 0.528 | [34] |
| Delta | N gene | 2021 (China) | Patients | Approx. 27 [SD: approx. 7.5] ** (14) | - | Approx. 25 [SD: approx. 7.5] ** (6) | - | 0.427 | [34] |
| Delta | N gene | 11/21 (Germany) | Patients | 23.2 [SD: 6.0] ** (107) | - | 27.5 [SD: 6.1] ** (127) | - | 0.012 | [35] |
| Delta | ORF1a region | 11/21 (Germany) | Patients | 22.9 [SD: 6.1] ** (107) | - | 27.0 [SD: 6.0] ** (127) | - | 0.019 | [35] |
| Delta | N1 gene | 11/21 (Germany) | Patients | 24.4 [SD: 6.7] ** (107) | - | 23.8 [SD: 5.8] ** (127) | - | 0.80 | [35] |
| Delta | N2 gene | 11/21 (Germany) | Patients | 26.1 [SD: 5.8] ** (107) | - | 25.5 [SD: 5.0] ** (127) | - | 0.42 | [35] |
| Delta | E gene | 11/21 (Germany) | Patients | 24.6 [SD: 6.0] ** (107) | - | 23.8 [SD: 4.9] ** (127) | - | 0.37 | [35] |
| Delta | N gene | 6/21–7/21 (UK) | General population | 23.1 [95% CI: 20.3–25.8] **** (28) | 27.4 [95% CI: 24.8–30.0] **** (76) | 27.6 [95% CI: 25.5–29.7] **** (145) | - | 0.01 (2 versus 0) 0.04 (1 versus 0) | [36] |
| Delta | S gene | 6/21–8/21 (USA) | General population and patients | 19.0 [SD: 3.0] ** (59) | - | 20.0 [SD: 3.5] ** (28) | - | n.s. | [37] |
| Delta | N gene | 6/21–12/21 (USA) | General population | 22.9 [95% CI: 22.8–23.0] ** (11,084) | - | 22.1 [95% CI: 22.0–23.2] ** (9347) | - | <0.0001 | [38] |
| Delta | Not described | 7/21–8/21 (USA) | Patients | Approx. 23 *** (25) #### | - | Approx. 24 *** (47) | - | n.s. | [39] |
| Delta | RdRp/ORF1ab gene | 9/21–10/21 (Republic of Korea) | Healthcare workers (61), inpatients (18), caregivers (15) in an outbreak situation | 19.9 [SD: 5.4] ** (24) | - | 20.9 [SD: 6.3] ** (70) | - | 0.52 | [10] |
| Mainly delta | N gene | 5/21–7/21 (Kuwait) | General population | 19.7 [range: approx. 13.0–33.0] *** (91) | - | 19.6 [range: approx. 10.0–33.0] *** (59) | - | 0.42 | [40] |
| Mainly delta | N genes | 12/20–9/21 (USA) | General population | 23.1 [IQR: 19.4–29.0] *** (160) | - | 25.8 [IQR: 20.6–31.2] *** (112) | - | 0.02 | [41] |
| Mainly delta | Not described | 7/21 (USA) | General population | 21.5 [IQR: approx. 19.0–26.0] *** (84) ##### | 22.8 [IQR: approx. 18.0–27.0] *** (127) | n.s. | [42] | ||
| Omicron | N gene | 11/21–12/21 (USA) | Patients | Approx. 18.4 [IQR: approx.16.0–22.0] *** (166) | - | Approx. 17 [IQR: approx. 15.0–19.0] *** (229) | - | n.s. | [29] |
| Omicron BA.1 | E gene | 12/21–2/22 (Switzerland) | Any individual with symptoms | 16.6–26.7 * (33) | - | 14.6–26.7 * (121) ***** | n.s. | [18] | |
| Mainly omicron | RdRp gene | 1/22 (Bosnia and Herzegovina) | Healthcare workers (141) | Approx. 32.0 [IQR: approx. 28.0–34.0] *** (44) | - | Approx. 30.5 [IQR: approx. 27.5–33.0] (26) | - | n.s. | [43] |
| Mainly omicron | E gene | 1/22 (Bosnia and Herzegovina) | Healthcare workers (141) | Approx. 27.5 [IQR: approx. 22.0–29.0] *** (44) | - | Approx. 24.0 [IQR: approx. 22.0–27.0] (26) | - | n.s. | [43] |
| Mainly omicron | N gene | 1/22 (Bosnia and Herzegovina) | Healthcare workers (141) | Approx. 28.0 [IQR: approx. 26.0–32.0] *** (44) | - | Approx. 27.0 [IQR: approx. 25.5–30.5] (26) | - | n.s. | [43] |
| Multiple variants | ORF1ab gene | 12/20–3/21 (Italy) | General population | 19.4 [IQR: 18.0–28.7] *** (31) | - | 21.2 [IQR: 17.5–31.3] *** (54) | - | 0.20 | [44] |
| Multiple variants | N gene | 2/21–6/21 (USA) | General population and patients | 23.1 ** (1061) | - | 23.1 ** (121) | 0.99 | [45] | |
| Multiple variants | Not described | 12/20–8/21 (Germany) | General population, index cases | 25.7 [SD: 6.6] ** (287) | - | 29.5 [SD: 7.5] ** (300) | - | <0.001 | [46] |
| Multiple variants | Not described | 12/20–8/21 (Germany) | General population, contact persons of index cases | 25.6 [SD: 6.5] ** (270) | - | 26.2 [SD: 7.3] ** (56) | - | 0.599 | [46] |
| Multiple variants | Not described | 11/20–8/21 (USA) | Participants of the occupational health program of the National Basketball Association, infected upon inclusion | 20.7 [95% CI: 19.8–20.2] ** (136) | - | 20.5 [95% CI: 19.0–21.0] ** (37) | - | n.s. | [47] |
| SARS-CoV-2 Variant | Sampling Period (Country) | Type of Population | Log10 Infectious Virus Titer per ml According to Vaccination Status (Number of Samples) | p-Value | Source | |||
|---|---|---|---|---|---|---|---|---|
| None | 1 Dose | 2 Doses | 3 Doses | |||||
| Delta | 6/21–12/21 (Switzerland) | Any individual with symptoms | Approx. 2.5 [SD: 1.2] * (127) | - | Approx. 1.7 [SD: 1.3] * (104) | - | <0.0001 | [18] |
| Delta | 6/21–8/21 (USA) | Patients | Approx. 2.9 [SD: approx. 1.0] * (59) | - | Approx. 2.4 [SD: approx. 1.0] * (28) | - | <0.05 | [37] |
| Delta | 12/20–4/22 (USA) | Healthcare workers, essential frontline workers, uninfected upon inclusion | 4.8 * (36) | - | 3.8 * (9) # 4.1 * (61) ## | 3.1 * (10) | n.s. “significant” “significant” | [51] |
| Delta | 6/21–12/21 (USA) | General population | Approx. 2.3 ** (24) | - | Approx. 2.4 ** (23) | - | n.s. | [38] |
| Omicron BA.1 | 12/21–2/22 (Switzerland) | Any individual with symptoms | Approx. 1.6 [SD: 1.2] ** (33) | - | Approx. 1.7 [SD: 1.2] ** (91) | Approx. 0.9 [SD: 0.9] ** (30) | 0.038 | [18] |
| Multiple variants | 9/20–10/21 (USA) | General population, infected non-hospitalized | 3.9 [IQR: 0.0–5.4] ** (52) | - | 3.2 [IQR: 0.0–6.1] ** (32) | - | 0.60 | [52] |
| SARS-CoV-2 Variant | Type of Gene | Sampling Period (Country) | Type of Population | Duration of SARS-CoV-2 Detection (Days) According to Vaccination Status (Number of Samples) | p-Value | Source | |||
|---|---|---|---|---|---|---|---|---|---|
| None | 1 Dose | 2 Doses | Prior Infection | ||||||
| Delta | Not described | 2/20–9/21 (Singapore) | Hemodialysis patients | 32 [IQR: 30.0–34.0] * # (10) | - | 24 [IQR: 21.0–26.0] * # (24) | - | <0.01 | [33] |
| Delta | N and S gene | 9/21–1/22 (Republic of Korea) | Patients, infected upon inclusion | - | >26 [95% CI: 18–NA] * (30) ** | 15 [95% CI: 12–NA] * (19) | - | 0.15 | [15] |
| Delta | N and S gene | 3/20–11/21 (Republic of Korea) | Patients | ≥10 (28) | ≥10 (11) | ≥ 10 (6) | - | n.s. | [30] |
| Multiple variants | ORF1ab, N and S gene | 12/20–4/21 USA) | Healthcare workers, essential frontline workers, uninfected upon inclusion | 8.9 [SD: 10.2] *** (155) | 2.7 [SD: 3.0] *** (16) **** | - | - | significant | [53] |
| Multiple variants | Not described | 11/20–8/21 (USA) | Participants of the occupational health program of the National Basketball Association | 7.5 [95% CI: 6.8–8.2] *** (136) | - | 5.5 [95% CI: 4.6–6.5] *** (37) | - | significant | [47] |
| Multiple variants | N and E genes | 9/20–10/21 (USA) | General population, infected non-hospitalized | 9.0 [IQR: 7.0–12.0] * (52) | - | 8.0 [IQR: 4.5–12.0] * (32) | - | 0.52 | [52] |
| Multiple variants | ORF1ab and N gene | 5/20–3/22 (Nicaragua) | General population | 28.2 [IQR: 16.5–43.1] * | - | 12.9 [IQR: 7.6–19.8] * | significant | [54] | |
| Multiple variants | ORF1ab and N gene | 1/20–1/22 (China) | Hospitalized patients | 19.0 [IQR: 12.0–24.5] * (171) ***** Approx. 16 * ****** | - | 17.0 [IQR: 12.3–19.5] * (165) ***** Approx. 16 * ****** | - | 0.038 - | [55] |
| SARS-CoV-2 Variant | Sampling Period (Country) | Type of Population | Duration of SARS-CoV-2 Detection (Days) According to Vaccination Status (Number of Samples) | p-Value | Source | ||
|---|---|---|---|---|---|---|---|
| None | 1 Dose | 2 Doses | |||||
| Delta | 9/21–1/22 (Republic of Korea) | Patients, infected upon inclusion | - | 11 [95% CI: 9.0–15.0] * (30) ** | 5 [95% CI: 4.0–NA] * (19) | 0.0013 | [15] |
| Delta | 3/20–11/21 (Republic of Korea) | Inpatients | - | 4.4 [95% CI: 2.0–7.0] * (39) ** | 1.8 [95% CI: 1.0–NA] * (6) | 0.42 | [30] |
| Multiple variants | 9/20–10/21 (USA) | General population, infected non-hospitalized | 7.0 [IQR: 0.0–8.0] * (52) | - | 5.0 [IQR: 0.0–7.0] * (32) | 0.12 | [52] |
| Multiple variants | 12/20–3/21 (USA) | Students and university staff who tested positive | 2.8 *** (70) | - | 0.8 *** (12) | < 0.01 | [56] |
| SARS-CoV-2 Variant | Number of Samples (Reinfection in Unvaccinated) | Type of Control | Number of Samples from Control | Type of Gene | Ct Values (Reinfection in Unvaccinated) | Ct Values (Control Group) | p-Value | Source |
|---|---|---|---|---|---|---|---|---|
| Mainly beta | 1686 | Primary infection in unvaccinated | 1686 | N, ORF1ab and S gene | 29.9 * [IQR: 22.3–33.5] | 23.6 * [IQR: 18.5–29.9] | <0.001 | [19] |
| Mainly beta | 761 | BNT162b2 breakthrough infections | 761 | N, ORF1ab and S gene | 29.5 * [IQR: 22.0–33.4] | 26.4 * [IQR: 20.2–31.9] | <0.001 | [19] |
| Mainly beta | 85 | mRNA-1273 breakthrough infections | 85 | N, ORF1ab and S gene | 33.0 * [IQR: 29.4–34.4] | 31.6 * [IQR: 24.1–34.2] | <0.001 | [19] |
| Alpha B.1.1.7 | 68 | Primary infection in unvaccinated | 10,853 | N, ORF1ab and S gene | 32.8 * [IQR: 30.9–34.2] | 28.7 * [IQR: 20.4–32.9] | - | [22] |
| Alpha B.1.1.7 | 68 | BNT162b2 and ChAdOx1 breakthrough infections | 56 | N, ORF1ab and S gene | 32.8 * [IQR: 30.9–34.2] | 33.3 * [IQR: 31.6–34.0] | - | [22] |
| Delta B.1.617.2 (early period) | 5 | Primary infection in unvaccinated | 75 | N, ORF1ab and S gene | 30.8 * [IQR: 29.5–34.3] | 21.5 * [IQR: 16.4–31.7] | - | [22] |
| Delta B.1.617.2 (early period) | 5 | BNT162b2 and ChAdOx1 breakthrough infections | 104 | N, ORF1ab and S gene | 30.8 * [IQR: 29.5–34.3] | 32.2 * [IQR: 26.0–34.0] | - | [22] |
| Delta B.1.617.2 (late period) | 20 | Primary infection in unvaccinated | 326 | N, ORF1ab and S gene | 22.3 * [IQR: 16.5–30.3] | 25.7 * [IQR: 19.1–30.8] | - | [22] |
| Delta B.1.617.2 (late period) | 20 | BNT162b2 and ChAdOx1 breakthrough infections | 1593 | N, ORF1ab and S gene | 22.3 * [IQR: 16.5–30.3] | 25.3 * [IQR: 19.1–31.3] | - | [22] |
| Multiple variants | 443 ** | Primary infection in mostly unvaccinated | 302 *** | ORF1ab and N gene | 29.8 **** | 28.0 **** | 0.0004 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampf, G. Does COVID-19 Vaccination Protect Contact Persons? A Systematic Review. Hygiene 2024, 4, 23-48. https://doi.org/10.3390/hygiene4010003
Kampf G. Does COVID-19 Vaccination Protect Contact Persons? A Systematic Review. Hygiene. 2024; 4(1):23-48. https://doi.org/10.3390/hygiene4010003
Chicago/Turabian StyleKampf, Günter. 2024. "Does COVID-19 Vaccination Protect Contact Persons? A Systematic Review" Hygiene 4, no. 1: 23-48. https://doi.org/10.3390/hygiene4010003
APA StyleKampf, G. (2024). Does COVID-19 Vaccination Protect Contact Persons? A Systematic Review. Hygiene, 4(1), 23-48. https://doi.org/10.3390/hygiene4010003





