Pneumonic Plague: Incidence, Transmissibility and Future Risks
Abstract
:1. Introduction
“Pneumonic plague must be considered highly contagious whenever it occurs, although person-to-person transmission is most likely in cold humid environments coupled with overcrowding.”
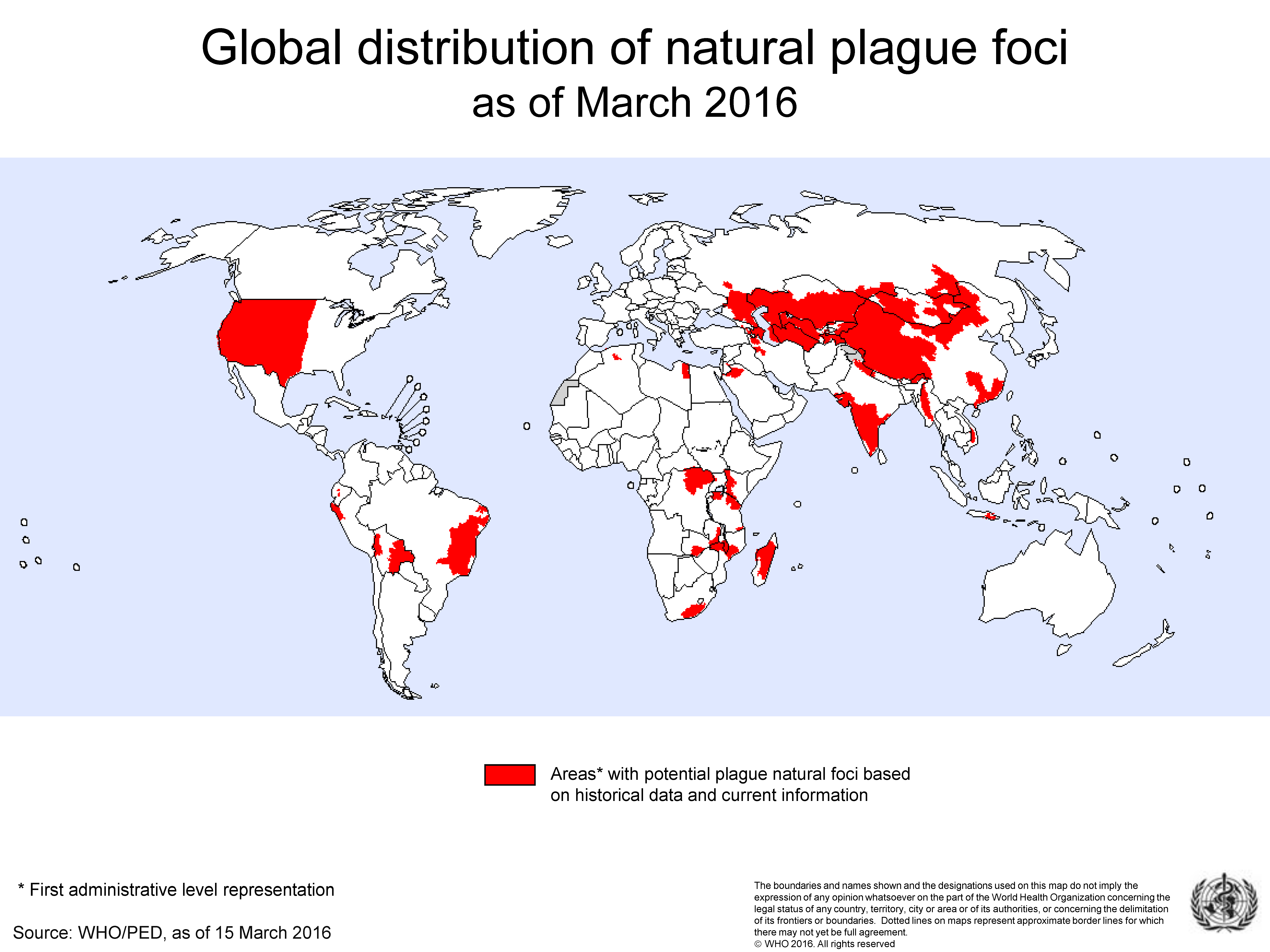
2. The Location and Frequency of PP Outbreaks
“Pneumonic plague is highly contagious. It is, however, rare (less than 3 per cent. of all cases) and plays a very small part in the general spread of the disease.”
“The incidence of pneumonic plague in India is generally below 1% and has never exceeded 3% in any year since 1895.”
“Hitherto the usual percentage of pneumonic cases in an epidemic has not been observed to be high… But the epidemic on the Gold Coast shows that that percentage is not an invariable standard of relationship between the one and the other, and that, under certain conditions, the bubonic may be displaced by the pneumonic form, which may, in its turn, occupy the whole field of the outbreak.”
3. The Infectivity of Pneumonic Plague
“…what impressed me… was not the number of the cases but their paucity. It seemed almost incredible that so many close contacts should escape infection; for example, two or three men who slept for several nights in the same hut as one dying from pneumonic plague.”
3.1. The Mechanism of Transmission and Factors Influencing Its Efficiency
“The idea that infection … is caused entirely by particles of sputum expectorated by the patient … is erroneous. It follows …that the wearing of masks and the proper covering of any surface of the skin where fresh abrasions are present are important… measures against plague infection”.
3.2. The Basic Reproductive Number (R0) and the Effective Reproductive Number (R or Rt)
3.3. Heterogenous Transmission and the 80:20 Rule
4. How PP Outbreaks Start
5. How PP Epidemics End
6. Future Risks
6.1. Anti-Microbial Resistance
6.2. Climate Change
6.3. Plague as a Bioweapon
6.4. Threats to First Responders and Health Workers
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Green, M.H. Editor’s introduction. In Pandemic Disease in the Medieval World: Rethinking the Black Death; Green, M.H., Ed.; ARC Medieval Press: Kalamazoo, MI, USA; Bradford, UK, 2014; pp. 27–62. [Google Scholar]
- Perry, R.D.; Fetherston, J.D. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef]
- Rasmussen, S.; Allentoft, M.E.; Neilsen, K.; Orlando, L.; Sikora, M.; Sjögren, K.-G.; Pedersen, A.G.; Schubert, M.; Van Dam, A.; Kapel, C.; et al. Early divergent strains of Yersinia pestis in Eurasia 5000 years ago. Cell 2015, 163, 571–582. [Google Scholar] [CrossRef] [Green Version]
- Susat, J.; Lübke, H.; Immel, A.; Brinker, U.; Macāne, A.; Meadows, J.; Steer, B.; Tholey, A.; Zagorska, I.; Gerhards, G.; et al. A 5000-year-old hunter-gatherer already plagued by Yersinia pestis. Cell Rep. 2021, 35, 109278. [Google Scholar] [CrossRef] [PubMed]
- Drancourt, M.; Houhamdi, L.; Raoult, D. Yersinia pestis as a telluric, human ectoparasite-borne organism. Lancet Infect. Dis. 2006, 6, 234–241. [Google Scholar] [CrossRef]
- Gage, K.L.; Beard, C.B. Plague. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 1078–1084. [Google Scholar]
- Strong, R.; Teague, O. Studies on pneumonic plague and plague immunisation. II Method of transmission of the infection in pneumonic plague and the manner of spread of the disease during the epidemic. Phillippine J. Sci. 1912, 7, 137–156. [Google Scholar]
- Erickson, D.L.; Hinnebusch, B. Pneumonic plague. In Microorganisms and Bioterrorism; Anderson, B., Friedman, H., Bendinelli, M., Eds.; Springer: Boston, MA, USA, 2006; pp. 155–179. [Google Scholar]
- Pechous, R.D.; Sivaraman, V.; Stasulli, N.M.; Goldman, W.E. Pneumonic plague: The darker side of Yersinia pestis. Trends Microbiol. 2016, 24, 190–197. [Google Scholar] [CrossRef]
- Dennis, T.D. Plague as a biological weapon. In Bioterrorism and Infectious Agents: A New Dilemma for the 21st Century; Fong, I.W., Alibek, K., Eds.; Springer Science and Business Media, Inc.: New York, NY, USA, 2009; pp. 7–70. [Google Scholar]
- Meyer, K. Pneumonic plague. Microbiol. Mol. Biol. Rev. 1961, 25, 249–261. [Google Scholar] [CrossRef]
- Andrianaivoarimanana, V.; Kreppel, K.; Elissa, N.; Duplantier, J.-M.; Carniel, E.; Rajerison, M.; Jambou, R. Understanding the persistence of plague foci in Madagascar. PLoS Neg. Trop. Dis. 2013, 7, e2382. [Google Scholar] [CrossRef] [Green Version]
- Seal, S.C. Epidemiological studies of plague in India 1. The present position. Bull. World Health Organ. 1960, 23, 283–292. [Google Scholar]
- Barberi, R.; Signoli, M.; Chevé, D.; Tzortzis, S.; Aboudharam, G.; Raoult, D.; Drancourt, M. Yersinia pestis: The natural history of plague. Clin. Microbiol. Rev. 2021, 34, e00044-19. [Google Scholar] [CrossRef] [PubMed]
- Oyston, P.C.F. Plague virulence. J. Med. Microbiol. 2001, 50, 1015–1017. [Google Scholar]
- Kool, J.L. Risk of Person-to-Person Transmission of Pneumonic Plague. Clin. Infect. Dis. 2005, 40, 1166–1172. [Google Scholar] [CrossRef]
- Dennis, D.T.; Gage, K.L.; Gratz, N.; Poland, J.D.; Tikhomirov, E. Plague Manual: Epidemiology, distribution, surveillance and control. World Health Organ. 1999, 32, 135–165. [Google Scholar]
- World Health Organisation. Case Management and chemoprophylaxis. In Proceedings of the Interregional Meeting on Prevention and Control of Plague, Section 6.1 Case Management, Antananarivo, Madagascar, 1–11 April 2006. [Google Scholar]
- Bevins, S.N.; Chandler, J.C.; Barrett, N.; Schmit, B.S.; Wiscomb, G.W.; Shriner, S.A. Plague exposure in mammalian wildlife across the Western United States. Vector Borne Zoonotic Dis. 2021, 21, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kutyrev, V.V.; Eroshenko, G.A.; Motin, V.L.; Nosov, N.Y.; Krasnov, J.M.; Kukleva, L.M.; Nikiforov, K.A.; Al’Khova, Z.V.; Oglodin, E.G.; Guseva, N.P. Phylogeny and classification of Yersinia pestis through the lens of strains from the plague foci of Commonwealth of Independent States. Front. Microbiol. 2018, 9, 1106. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Plague Fact Sheet. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/plague (accessed on 11 November 2021).
- Brygoo, E.R. Epidémiologie de la peste à Madagascar. Arch. Inst. Pasteur Madag. 1966, 35, 1–139. [Google Scholar]
- Migliani, R.; Chanteau, S.; Rahalison, L.; Ratsitorahina, M.; Boutin, J.P.; Ratsifasoamanana, L.; Roux, J. Epidemiological trends for human plague in Madagascar during the second half of the 20th century: A survey of 20 900 notified cases. Trop. Med. Int. Heal. 2006, 11, 1228–1237. [Google Scholar] [CrossRef]
- Boisier, P.; Rahalison, L.; Rasolomaharo, M.; Ratsitorahina, M.; Mahafaly, M.; Razafimahefa, M.; Duplantier, J.-M.; Ratsifasoamanana, L.; Chanteau, S. Epidemiologic features of four successive annual outbreaks of bubonic plague in Mahajanga, Madagascar. Emerg. Infect. Dis. 2002, 8, 311. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Plague Outbreak Madagascar: External Situation Report 14; World Health Organization Regional Office for Africa: Johannesberg, Germany, 2017; pp. 1–9. [Google Scholar]
- Randremanana, R.; Andrianaivoarimanana, V.; Nikolay, B.; Ramasindrazana, B.; Paireau, J.; Bosch, Q.A.T.; Rakotondramanga, J.M.; Rahajandraibe, S.; Rahelinirina, S.; Rakotomanana, F.; et al. Epidemiological characteristics of an urban plague epidemic in Madagascar, August–November, 2017: An outbreak report. Lancet Infect. Dis. 2019, 19, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Neerinckx, S.; Bertherat, E.; Leirs, H. Human plague occurrences in Africa: An overview from 1877 to 2008. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 97–103. [Google Scholar] [CrossRef]
- Wu, L.-T. A Treatise on Pneumonic Plague; Publications of the League of Nations Health Organisation: Geneva, Switzerland, 1926. [Google Scholar]
- Lamb, J.T.W. The Etiology and Epidemiology of Plague: A summary of the Work of the Plague Commission; Superintendent of Government Printing: Calcutta, India, 1908. [Google Scholar]
- Harvey, W.L. Report of the Municipal Commissioner on the Plague in Bombay for the Year Ending 31st May 1899; Times of India steam press: Bombay, India, 1899; pp. 158–159. [Google Scholar]
- Bannerman, W.B. The spread of plague in India. J. Hyg. 1906, 6, 179–211. [Google Scholar] [CrossRef]
- Gill, C.A. A note on the epidemiology of pneumonic plague. Ind. Med. Gaz. 1909, 44, 135–137. [Google Scholar] [CrossRef] [Green Version]
- Crawford, D.G. A report on the epidemic of plague in Hughli-Chinsura Municipality January to May 1905. Ind. Med. Gaz. 1905, 40, 371–377. [Google Scholar]
- Childe, L.F. Remarks on the occurrence of Plague Pneumonia. Brit. Med. J. 1897, 1, 1215–1216. [Google Scholar] [CrossRef] [Green Version]
- Nathan, R. The Plague in India 1896, 1897; Appendix II; Government Central Printing Office: Simla, India, 1898; Volume 2, pp. 59–106. [Google Scholar]
- McDonald, D. The Indian Medical Service. A short account of its achievements 1600–1947. Proc. R. Soc. Med. 1955, 49, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.A.; Bayliss, R.A. William John Ritchie Simpson (1855–1931): Public health and tropical medicine. Med. Hist. 1987, 31, 450–465. [Google Scholar] [CrossRef] [Green Version]
- Simpson, W.J. Report on Plague in the GOLD COAST in 1908; J. & A Churchill: London, UK, 1909. [Google Scholar]
- Wu, L.-T.; Chun, J.W.H.; Pollitzer, R. Clinical observations upon the Manchurian plague epidemic, 1920–1921. J. Hyg. 1923, 21, 298–306. [Google Scholar]
- Martini, E.; Petrie, G.F.; Stanley, A.; Strong, R.P. Report of the International Plague Conference Held at Mukden, April, 1911; Bureau of Printing: Manilla, Philippines, 1912. [Google Scholar]
- Wu, L.-T. Plague in the orient with special reference to the Manchurian outbreaks. J. Hyg. 1922, 21, 62–76. [Google Scholar]
- Mitra, A. The plague in Kashmir. Ind. Med. Gaz. 1907, 42, 133–138. [Google Scholar]
- M’Bokolo, E.M. Peste et société urbaine à Dakar: L’épidémie de 1914. Cah D’etudes Afrcanes 1982, 22, 13–46. [Google Scholar] [CrossRef]
- Cowling, P.; Moss, P. Infectivity of pneumonic plague. BMJ 1994, 309, 1369. [Google Scholar] [CrossRef] [Green Version]
- Gale, G.W. An outbreak of pneumonic plague in the Kalahari. S. Afr. Med. J. 1941, 25, 369–373. [Google Scholar]
- Wynne-Griffith, G. Pneumonic plague in Rangoon. Lancet 1948, 251, 625–627. [Google Scholar] [CrossRef]
- Hinckley, A.F.; Biggerstaff, B.J.; Griffith, K.S.; Mead, P.S. Transmission dynamics of primary pneumonic plague in the USA. Epidemiol. Infect. 2012, 140, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Joshi, K.; Thakur, J.S.; Kumar, R.; Singh, A.J.; Ray, P.; Jain, S.; Varma, S. Epidemiological features of pneumonic plague outbreak in Himachal Pradesh, India. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 455–460. [Google Scholar] [CrossRef]
- Ramasindrazana, B.; Andrianaivoarimanana, V.; Rakotondramanga, J.M.; Birdsell, D.N.; Ratsitorahina, M.; Rajerison, M. Pneumonic plague transmission, Moramanga, Madagascar, 2015. Emerg. Infect. Dis. 2017, 23, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cui, Y.; Wang, Z.; Wang, X.; Guo, Z.; Yan, Y.; Li, C.; Cui, B.; Xiao, X.; Yang, Y.; et al. A dog-associated primary pneumonic plague in Qinghai Province, China. Clin. Infect. Dis. 2011, 52, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Bazant, M.Z.; Bush, J.W.M. A guideline to limit airborne transmission of COVID–19. Proc. Natl. Acad. Sci. USA 2021, 118, e2018995118. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nature 2021, 19, 528–545. [Google Scholar] [CrossRef]
- Teague, O.; Barber, M.A. Studies on pneumonic plague and plague immunisation. III Influence of atmospheric temperature upon the spread of pneumonic plague. Phillippine J. Sci. 1912, 7, 157–172. [Google Scholar]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of respiratory viral infections. Ann. Rev. Virol. 2010, 7, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-T. The 1917–1918 Shansi epidemic (Pneumonic). Appendix I, Studies upon the plague situation in North China. Nat. Med. J. China 1929, 25, 373–385. [Google Scholar]
- Pollitzer, R. Plague studies. 8. Clinical Aspects. Bull. World Health Organ. 1953, 9, 59–129. [Google Scholar]
- Boone, S.A.; Gerba, C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Pittet, D.; Dharan, S.; Touveneau, S.; Sauvan, V.; Perneger, T.V. Bacterial contamination of the hands of hospital staff during routine patient care. Arch. Intern. Med. 1999, 159, 821–826. [Google Scholar] [CrossRef]
- Nicas, M.; Best, D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J. Occup. Environ. Hyg. 2008, 5, 347–352. [Google Scholar] [CrossRef]
- Rose, L.J.; Donlan, R.; Banerjee, S.N.; Arduino, M.J. Survival of Yersinia pestis on environmental surfaces. Appl. Environ. Microbiol. 2003, 69, 2166–2171. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.-T.; Chun, W.H.; Pollitzer, R. Plague in Manchuria: I. Observations made during and after the second Manchurian plague epidemic of 1920–1921. II. the rôle of the tarbagan in the epidemiology of plague. J. Hyg. 1923, 21, 307–358. [Google Scholar]
- Evans, C.M.; Egan, J.R.; Hall, I. Pneumonic plague in Johannesburg, South Africa, 1904. Emerg. Infect. Dis. 2018, 24, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Maule Clark, B.; Goldburg, S. Pneumonic plague: Recovery in a proved case. S. Afr. Med. J. 1943, 17, 57–60. [Google Scholar]
- Jullien, S.; de Silva, N.L.; Garner, P. Plague transmission from corpses and carcasses. Emerg. Infect. Dis. 2021, 27, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Heesterbeek, J.A.P.; Dietz, K. The concept of R0 in epidemic theory. Stat. Neerl. 1996, 50, 89–110. [Google Scholar] [CrossRef]
- Gostic, K.M.; McGough, L.; Baskerville, E.B.; Abbott, S.; Joshi, K.; Tedijanto, C.; Kahn, R.; Niehus, R.; Hay, J.A.; De Salazar, P.M.; et al. Practical considerations for measuring the effective reproductive number, Rt. PLoS Comput. Biol. 2020, 16, e1008409. [Google Scholar] [CrossRef] [PubMed]
- Gani, R.; Leach, S. Epidemiologic determinants for modeling pneumonic plague outbreaks. Emerg. Infect. Dis. 2004, 10, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Chowell, G. The effective reproduction number as a prelude to statistical estimation of time-dependent epidemic trends. Math. Stat. Estim. Approaches Epidemiol. 2009, 103–121. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; Schreiber, S.J.; Kopp, P.E.; Getz, W.M. Superspreading and the effect of individual variation on disease emergence. Nature 2005, 438, 355–359. [Google Scholar] [CrossRef]
- Delamater, P.L.; Street, E.J.; Leslie, T.F.; Yang, Y.T.; Jacobsen, K.H. Complexity of the basic reproduction number (R0). Emerg. Infect. Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Nishiura, H.; Schwehm, M.; Kakehashi, M.; Eichner, M. Transmission potential of primary pnemonic plague: Time inhomogeneous evaluation based on historical documents of the transmission network. J. Epidemiol. Community Health 2006, 60, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Tsuzuki, S.; Lee, H.; Miura, F.; Chan, Y.H.; Jung, S.-M.; Akhmetzhanov, A.R.; Nishiura, H. Dynamics of the pneumonic plague epidemic in Madagascar. Euro. Surveill. 2017, 22, 17-00710. [Google Scholar]
- Nguyen, V.K.; Parra-Rojas, C.; Hernandez-Vargas, E.A. The 2017 plague outbreak in Madagascar: Data descriptions and epidemic modelling. Epidemics 2018, 25, 20–25. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Dye, C.; Etard, J.-F.; Smith, T.; Charlwood, J.D.; Garnett, G.P.; Hagan, P.; Hii, J.L.K.; Ndhlovu, P.D.; Quinnell, R.J.; et al. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proc. Natl. Acad. Sci. USA 1997, 94, 338–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvani, A.P.; May, R.M. Epidemiology: Dimensions of superspreading. Nature 2005, 438, 293–295. [Google Scholar] [CrossRef]
- Edwards, D.A.; Man, J.C.; Brand, P.; Katstra, J.P.; Sommerer, K.; Stone, H.A.; Nardell, E.; Scheuch, G. Inhaling to mitigate exhaled bioaerosols. Proc. Natl. Acad. Sci. USA 2004, 101, 17383–17388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellogg, W.H. An epidemic of pneumonic plague. Am. J. Public Health 1920, 10, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickie, W.M. Plague in California 1900–1925. Plague Pathology and Bacteriology. In Proceedings of the Conference of State & Provincial Health Authorities of North America, Atlantic City, NJ, USA, 21–22 May 1926; pp. 30–78. [Google Scholar]
- Wu, L.-T. The second pneumonic plague epidemic in Manchuria, 1920–1921: I. A general survey of the outbreak and its course. J. Hyg. 1923, 21, 262–288. [Google Scholar]
- Seal, S.C.; Prasad, G. Further notes on the incidence of pneumonic plague cases in Gaya (Bihar). Ind. Med. Gaz. 1949, 84, 408–413. [Google Scholar]
- Pollitzer, R. A review of recent literature on plague. Bull. World Health Organ. 1960, 23, 313–400. [Google Scholar]
- Simpson, W.J. A Treatise on Plague Dealing with the Historical, Epidemiological, Clinical, Therapeutic and Preventative Aspects of the Disease; Cambridge University Press: Cambridge, UK, 1905; pp. 211–212. [Google Scholar]
- Baltazard, M.; Bahmanyar, M.; Mofidi, C.; Seydian, B. Le foyer de Peste du Kurdistan 1. Recherches dans le foyer. Bull. Org. Mond. Sante. 1952, 5, 441–472. [Google Scholar]
- Brygoo, E.R.; Gonon, M. L’épidémie de peste pulmonaire de Doany en octobre 1957. Arch. Inst. Pasteur. 1958, 13, 865–936. [Google Scholar]
- Loukaskin, A.S. The Tarbagan or the Transbaikalian Marmot as a carrier of plague. In Proceedings of the Extrait des Comptes Rendus du XIIème Congres International de Zoologie, Lisbonne, Portugal; 1935; pp. 2097–2111. [Google Scholar]
- Wu, L.-T. North Manchuria Plague Prevention Service Reports (1911–1913); Lien-Teh, W., Ed.; Cambridge University Press: Cambridge, UK, 1914. [Google Scholar]
- Li, M.; Song, Y.; Li, B.; Yang, R.; Jiang, L.; Yang, R. Asymptomatic Yersinia pestis infection, China. Emerg. Infect. Dis. 2005, 11, 1494–1496. [Google Scholar] [CrossRef]
- Gupta, M.L.; Sharma, A. Pneumonic Plague, Northern India, 2002. Emerg. Infect. Dis. 2007, 13, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Wild, M.A.; Walburger, M.A.; Higgins, C.L.; Callahan, M.; Czarnecki, L.A.; Lawaczeck, E.W.; Levy, C.E.; Patterson, J.G.; Sunenshine, R.; et al. Primary pneumonic plague contracted from a mountain lion carcass. Clin. Infect. Dis. 2009, 49, e33–e38. [Google Scholar] [CrossRef] [Green Version]
- Gage, K.L.; Dennis, D.T.; Orloski, K.A.; Ettestad, P.; Brown, T.L.; Reynolds, P.J.; Pape, W.J.; Fritz, C.L.; Carter, L.G.; Stein, J.D. Cases of cat-associated human plague in the Western US, 1977–1998. Clin. Infect. Dis. 2000, 30, 893–900. [Google Scholar] [CrossRef]
- Dutt, A.K.; Akhtar, R.; McVeigh, M. Surat plague of 1994 re-examined. Southeast Asian J. Trop. Med. Public Health 2006, 37, 755–760. [Google Scholar] [PubMed]
- Butler, T. Plague history: Yersin’s discovery of the causative bacterium in 1894 enabled, in the subsequent century, scientific progress in understanding the disease and the development of treatments and vaccines. Clin. Microbiol. Infect. 2014, 20, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Singh, A.K. Plague vaccine: Recent progress and prospects. NPJ Vaccines 2019, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, P.J.; Brockhurst, M.A.; Harrison, E. Sampling the mobile gene pool: Innovation via horizontal gene transfer in bacteria. Phil. Trans. R. Soc. B 2017, 372, 20160424. [Google Scholar] [CrossRef] [Green Version]
- Galimand, M.; Guiyoule, A.; Gerbaud, G.; Rasoamanaana, B.; Chanteau, S.; Carniel, E.; Courvalin, P. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 1997, 337, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Guiyoule, A.; Gerbaud, G.; Buchrieser, C.; Galimand, M.; Rahalison, L.; Chanteau, S.; Courvalin, P.; Carniel, E. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 2001, 7, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinnebusch, B.J.; Rosso, M.-L.; Schwan, T.G.; Carniel, E. High-frequency conjugative transfer of antibiotic resistance genes to Yersinia pestis in the flea midgut. Mol. Microbiol. 2002, 2, 249–354. [Google Scholar]
- Dai, R.; He, J.; Zha, X.; Wang, Y.; Zhang, X.; Gao, H.; Yang, X.; Li, J.; Xin, Y.; Wang, Y.; et al. A novel mechanism of streptomycin resistance in Yersinia pestis: Mutation in the rpsL gene. PLoS Negl. Trop. Dis. 2021, 15, e0009324. [Google Scholar] [CrossRef]
- Andrianalvoarimanana, V.; Wagner, D.M.; Birdsell, D.N.; Nikolay, B.; Rakotoarimanana, F.; Randriantseheno, L.N.; Vogler, A.J.; Sahl, J.W.; Hall, C.M.; Somprasong, N.; et al. Transmission of antimicrobial resistant Yersinia pestis during a pneumonic plague outbreak. Clin. Infect. Dis. 2021, 20, ciab606. [Google Scholar] [CrossRef] [PubMed]
- Urich, S.K.; Chalcraft, L.; Schriefer, M.E.; Yockey, B.M.; Petersen, J.M. Lack of antimicrobial resistance in Yersinia pestis isolates from 17 countries in the Americas, Africa, and Asia. Antimicrob. Agents Chemother. 2012, 56, 555–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.B. Epidemics on the move: Climate change and infectious disease. PLoS Biol. 2020, 18, e3001013. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.P.H.; Lee, H.F. Pre-industrial plague transmission mediated by the synergistic effect of temperature and aridity index. BMC Infect. Dis. 2018, 18, 134. [Google Scholar] [CrossRef]
- Tennant, W.S.D.; Tildesley, M.J.; Spencer, S.E.F.; Keeling, M.J. Climate drivers of plague epidemiology in British India, 1898–1949. Proc. R. Soc. B 2020, 287, 20200538. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.; Koopmans, M.; Morton, A.; Van Baal, P. The economics of improving global infectious disease surveillance. BMJ Glob. Health 2021, 6, e006597. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Tegnell, A.; Baka, A.; Van Loock, F.; Hendriks, J.; Werner, A.; Maidhof, H.; Gouvras, G. Bichat guidelines for the clinical management of plague and bioterrorism-related plague. Euro. Surveill. 2004, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Inglesby, T.V.; Grossman, R.; O’Toole, T. A plague on your city: Observations from TOPOFF. Clin. Infect. Dis. 2001, 32, 436–445. [Google Scholar]
- Reidel, S. Biological warfare and bioterrorism: A historical review. BUMC Proc. 1974, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Wheelis, M. Biological warfare at the 1346 siege of Caffa. Emerg. Infect. Dis. 2004, 8, 971–975. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Health Aspects of Chemical and Biological Weaponry; World Health Organisation: Geneva, Switzerland, 1970. [Google Scholar]
- Mitchell, F.K. The plague in Cape Town in 1901 and its subsequent establishment as an endemic disease in South Africa. S. Afr. Med. J. 1983, 63, 17–19. [Google Scholar]
- Murdock, J.R. Pneumonic plague in Ecuador during 1939. Public Health Rep. 1940, 55, 2172–2178. [Google Scholar] [CrossRef]
- Farrar, R. Plague in Manchuria. J. R. Soc. Med. 1912, 5, 1–24. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Atshabar, B.B.; Begon, M.; Belmain, S.R.; Bertherat, E.; Carniel, E.; Gage, K.L.; Leirs, H.; Rahalison, L. Plague: Past, present, and future. PLoS Med. 2008, 5, e3.009–e3.013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, C. Pneumonic Plague: Incidence, Transmissibility and Future Risks. Hygiene 2022, 2, 14-27. https://doi.org/10.3390/hygiene2010002
Evans C. Pneumonic Plague: Incidence, Transmissibility and Future Risks. Hygiene. 2022; 2(1):14-27. https://doi.org/10.3390/hygiene2010002
Chicago/Turabian StyleEvans, Charles. 2022. "Pneumonic Plague: Incidence, Transmissibility and Future Risks" Hygiene 2, no. 1: 14-27. https://doi.org/10.3390/hygiene2010002
APA StyleEvans, C. (2022). Pneumonic Plague: Incidence, Transmissibility and Future Risks. Hygiene, 2(1), 14-27. https://doi.org/10.3390/hygiene2010002




