1. Introduction
Bhutan is a conservation stronghold for seven nonhuman primates, with perhaps the most notable taxon being the golden langur (
Trachypithecus geei). This species is endangered [
1] and for the past two review cycles has appeared on the International Union for the Conservation of Nature’s top 25 most endangered primates [
2]. Golden langurs are endemic to western Assam in northeast India [
3] and to six districts in Bhutan at elevations ranging from 199–2600 m asl [
4,
5]. In India, the species can be found in degraded forest fragments with secondary forest growth [
6,
7]. The species’ total number is estimated at ~5141 individuals in India [
2]. In Bhutan, national parks provide intact, strictly protected habitats, and parks are connected by forested corridors (biological corridors (BCs)). Golden langurs also range in anthropogenically altered and impacted environments, where they can be found along roads, near farms, and near construction projects such as hydroelectric dams and quarries (human settlements (HSs)) [
8]. BCs are designed to conserve meta-populations of wide-ranging species and to promote the gene flow for all taxa [
9]. Sustainable development and use of natural resources, including dams constructed for hydropower, are permitted in HSs [
10]. In Bhutan, it is important to study golden langurs living in HSs because this is where the majority of the population is found [
5]. Golden langurs in Bhutan total ~2439 individuals [
5], a number that is considerably lower than previous estimates [
11], and only 33% live in national parks [
5].
Habitats and associated dispersal options influence golden langurs’ group sizes, densities, and compositions [
12]. Golden langurs have been observed living in uni-male/multi-female groups of 3–9 individuals, bi-male/multi-female groups of 8–15 individuals, and multi-male/multi-female groups of varying sizes [
2] (
Figure 1). All-male bands of two to five individuals and solo males have also been reported [
2]. The uni-male/multi-female social structure is considered to be the most common and stable group formation [
12,
13]. In a study based at Royal Manas National Park (Zhemgang, Bhutan), researchers documented an overall average group size of 7.14 individuals, and 78% of groups observed were uni-male/multi-female [
4]. Shil and colleagues [
12] found significant differences in golden langur average group sizes at forest-core, forest-edge, and plantation sites in Assam, with the largest groups (average of 13.9 individuals) in plantations. In human-altered habitats, langurs live in larger groups with higher densities [
12,
14]. For example, we observed a group of 25 golden langurs ranging near a hydroelectric dam construction site in Trongsa district, Bhutan. Anthropic environments can alter the environment in ways that influence dispersal from birth groups and that increase mortality risks from injuries due to electrocution, car collisions, and domestic dogs [
6,
12].
Sleep sites are an important aspect of primates’ habitats to study, record, and protect because nonhuman primates spend nearly half of their lives there [
15,
16,
17]. Knowledge of the tree species that golden langurs prefer for sleeping will aid in the development of conservation policies that protect preferred trees across golden langurs’ distribution. Qualities of sleep sites have been explained in terms of predator avoidance, food access, parasite avoidance, comfort/thermoregulation, and range/resource defense [
18,
19,
20,
21]. At sleep sites, primates may have a reduced ability to detect predators, so they may choose sleep sites with characteristics that reduce the likelihood of predation (e.g., chimpanzees (
Pan troglodytes)) [
22]. Studying golden langur–predator interactions adds to our understanding of their vulnerability to and avoidance of predators and, if langurs avoid sites with high predator density, they may seek refuge near settlements, thereby accelerating human–wildlife conflict. Golden langurs reportedly prefer to sleep in tall trees to avoid natural predators [
2]. Golden langurs are described as being strictly arboreal during 99% of their active time [
2], so one might expect golden langurs to sleep in the forest canopy. Aspects of sleep sites (e.g., connectivity to neighboring trees, location in the canopy, and branching pattern) influence predators’ entry to sites and primates’ escape routes from them. Leopards, pythons, and raptors are among Asian primates’ natural predators [
23]. Chetry and colleagues [
2] report leopards, wild dogs (dhole), and pythons as golden langurs’ main predators. For golden langurs living near people, domestic dogs are also predators, and additional causes of mortality include electrocution on power lines, roadkill, and retaliatory killing by farmers whose crops have been damaged or destroyed by langurs [
24,
25,
26].
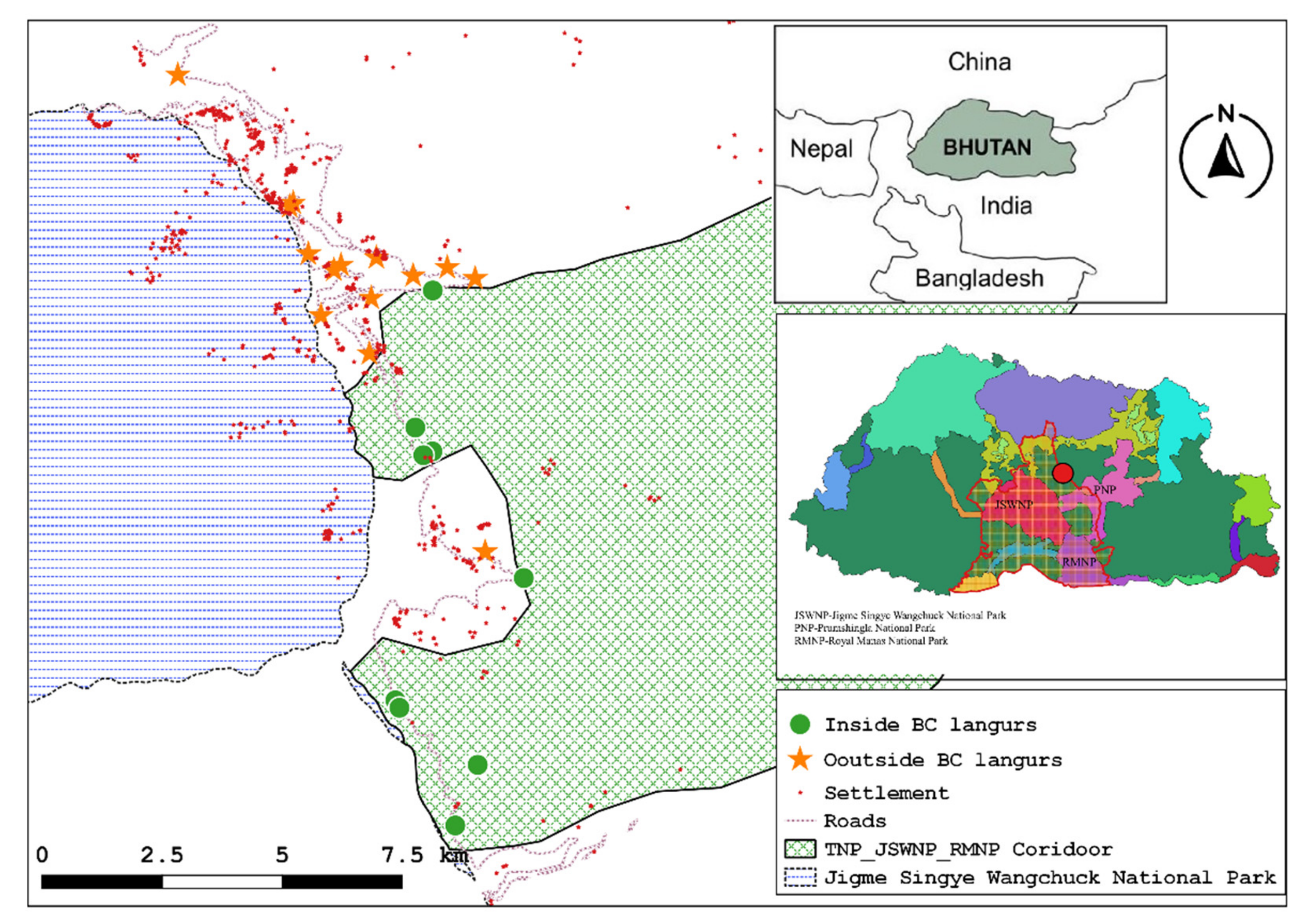
Information on the distribution and numbers of Bhutan’s golden langurs is now available, but few studies have focused on golden langur ecology, particularly the characteristics of sleep sites. We studied golden langurs ranging in Langthel subdistrict, Trongsa district, central Bhutan. Our data collection occurred between late fall and early spring, prior to the COVID-19 pandemic travel shutdown. For the first time, we provide preliminary, descriptive data from Bhutan on golden langur sleep sites and context on predation and other environmental factors that may influence sleep site selection. Based on prior observations [
4,
12], we hypothesized that group sizes and compositions would differ for golden langurs living in a BC and those living near HSs and predicted that group sizes would be larger and would more often include more than one male for groups near the HSs.
4. Discussion
Data on Bhutan’s golden langurs living outside of BCs and national parks are urgently needed because the majority of the population is found in these landscapes, which often have marked anthropic impacts [
6,
25]. We studied golden langurs living near an HS and in a BC in Trongsa district, central Bhutan. As has been documented at other sites in Bhutan and India [
4,
12,
14], our golden langur groups living near the HS were significantly larger and included more adult males relative to adult females. Habitat quality and golden langur group sizes appeared to be inversely proportional: when forest habitat quality deteriorates, the number of individuals in each group increases [
6]. A similar pattern has been noted in other golden langur studies: group sizes are larger when langurs live near people. In human-altered landscapes such as plantations and farms, this might occur due to a lack of dispersal options, increased reliance on crops, and an associated reluctance to disperse from natal groups, or in response to scramble competition. In their study of golden langurs in Assam, Shil and colleagues [
12] found increasing average group sizes in forest-core (average 7.4 individuals per group), forest-edge (average 12.7 individuals per group), and plantation (average 13.9 individuals per group) sites. They noted that larger groups might occur in more fragmented habitats as a response to scramble competition. They also found that while group size did seem to be influenced by forest type, age/sex ratios within groups were not, perhaps because larger groups tended to include more adult males. In their study of golden langurs in Bhutan, Lhendup and colleagues [
4] found that average group sizes were significantly larger in disturbed (average 7.67 individuals per group) versus undisturbed (average 6.76 individuals per group) forests. Group compositions near HSs may reflect reliance on clumped and/or predictable distributions of nutrient-dense food resources (such as occurs in orchards or along roadways; see [
39]), altered dispersal patterns compared to the natal group, a lack of dispersal options due to the scarcity of suitable habitats, and distinct predation patterns. The adult to immature ratio we calculated was low in the HS compared to the BC and compared to values calculated in Assam (0.6, 0.9, and 0.9 in forest core, edge, and plantation, respectively) [
12] and Bhutan (2.12 and 1.86 in undisturbed and disturbed sites, respectively) [
4]. Bhutan is perhaps distinct compared to Assam in terms of the presence of an intact predator array in both HSs and BCs, whereas predators may be hunted out or otherwise absent in disturbed, fragmented, or extensively altered habitats in Assam. The existence of predators, particularly those that prey on immature individuals, may explain the low numbers of immature individuals we observed in HS groups, coupled with other mortality risks associated with roads, powerlines, and areas where people use dogs to protect crops.
We recorded the presence of leopards, raptors, and, for groups living near the HS, domestic dogs near golden langur sleep sites. Leopards, dholes, and snakes such as pythons are documented predators of golden langurs [
2], and raptors are common ambush predators of primates foraging on branches [
40]. We tallied the frequencies of leopard signs, counted numbers of raptors hovering and within a 30 m radius of sleep sites, and recorded seven incidents of python encounters with local people, so predators were present at our study area; however, we saw no cases of predation on golden langurs. Langurs may be susceptible to predators when they are solitary, as occurs when adult males migrate between groups, when they are located toward the periphery of a group, or during periods of reduced vigilance, as occurs at sleep sites.
Domestic dogs are also golden langur predators and may target or have greater hunting success with smaller, immature individuals; for example, Chetry and co-workers [
24] report seven golden langur deaths from domestic dogs in one year, of which five were juvenile langurs. We did not observe any golden langur mortality directly attributable to domestic dogs, but we did observe a domestic dog eating a golden langur that had been electrocuted on powerlines. Conversely, natural predators such as leopards may be deterred from hunting near HSs, although farmers reported to us the presence of leopards in or near fields, and one farmer described to us how a leopard killed his adult dog, which the farmer had formerly relied on to drive langurs from his orchard, and we recovered the skull of an adult golden langur from a tree that may have been a leopard prey cache site.
The tree species Sapium insigne (synonym of Falconeria insignis, classified in the spurge family Euphorbiaceae) appears to be particularly important to conserve in golden langur habitats, as this tree was most often used for sleeping in our dataset. Additionally, langurs most often used emergent trees rooted in ground with strong or moderate slopes and oriented south or southeast, perhaps because these trees provided good visibility of the surrounding area. Langurs most often slept in the mid-region of the crown in trees that were 11 m tall or taller with girths exceeding 26 cm. Individuals of the langur group usually spread among several, most often three, neighboring trees to sleep, but once we saw the entire group sleep in the crown of one tree.
Anecdotally, we think that golden langurs prefer Sapium insigne as sleeping sites because this species has spreading and open crown shapes and can accommodate almost all family members in the same tree crown. Golden langurs living near human settlements may prefer Sapium insigne as people do not consider these species good for timber, so these trees are rarely cut down by local people. In the BC, our anecdotal data indicated that golden langurs also prefer to sleep in Calicarpa arborea and Schima wallichiana trees, both of which are timber species used by local people, but their extraction is regulated by policies in force in protected areas. We were not able to test for sleep site preferences because we had not yet assessed the distribution of tree species in the BC and near the HS; we collected a few sleep-site data points and our data collection spanned one season due to COVID-19 restrictions on travel.
All sleep sites we recorded were trees. One sleep tree was reused in our study period (of the species
Sapium insigne), but other sleep sites appeared to be single-use. This infrequent reuse of sleep sites has been taken by previous researchers as evidence of predation avoidance being the primary driver of sleep site selection, as opposed to access to food resources or other causes [
19,
20]. At Nonggang Nature Reserve (China),
Trachypithecus francoisi exclusively slept on cliffs (23 sleep sites; 17 ledges and 6 caves) and reused seven of these sites more than nine times each [
19]. Similarly,
T. leucocephalus in Fusui Nature Reserve (China) used 18 sleep sites (1 ledge and 17 caves), and all sleep sites were reused more than once [
20]. Our results should be interpreted with caution as the COVID 19 pandemic shortened our field season and meant that our dataset spanned late fall to early spring. Golden langur ranging patterns and sleep sites are likely impacted by season. Although we lacked sufficient data to compare the characteristics of sleep trees near the HS with those in the BC, our preliminary data indicated that predation, which included natural predators and domestic dogs near the HS, drove sleep site selection at both locations. Although cliffs and caves exist in Trongsa district in both the BC and HS, we did not observe golden langurs using them as sleep sites in our dataset; however, two of us (RG and KD) have observed langurs on cliffs during the day using them as salt licks (
Figure 6), so it seems likely that additional study will show golden langurs’ use of cliffs and caves, too.
In addition to death due to predation, golden langurs living near people experience mortality due to electrocution on power lines and vehicle collisions [
6], which increases the costs golden langurs experience from living near people [
39]. We observed both causes of mortality during the short duration of our study. If this mortality is biased toward a particular sex or age class, it could contribute to the differences observed in the group composition of langurs living in anthropic versus less disturbed habitats. In Trongsa district, golden langurs are often spotted on or near roadways, and they sometimes cross roads on the ground when the canopy is bisected by a roadway. At higher elevations, the use of salt to melt roadway ice attracts grey langurs (
Semnopithecus schistaceus) to the road as a salt lick, and at lower altitudes, such as our study site, on cool or cold days, golden langurs sometimes bask in the sun on the warm asphalt. Increased use of signage and installation of speed bumps and insulated wires would greatly reduce golden langur mortality for those populations living near anthropic environments.
Thinley and colleagues [
25] noted that, at some sites, farmers engage in retaliatory killing of crop-reliant golden langurs. We did not observe this cause of golden langur mortality during our study, but we note that subsistence farmers in Bhutan are increasingly vulnerable to food insecurity due to temperature and rainfall changes associated with climate change [
41]. Currently, farmers are adapting to these changes by increasing their amounts of off-farm labor and by working on others’ farms to supplement income, but one study recorded increased wild collection and livestock grazing to supplement what is produced on farms [
41]. This precarious farming situation and people’s behavioral adaptations seem likely to increase golden langurs’ reliance on crops and encounters between langurs and people. Our ongoing research focuses on assessing costs and benefits for people [
26] and for golden langurs [
39] living in close association with people.